Introduction
Bronchial asthma, commonly abbreviated to asthma, is
one of the most common respiratory diseases, which is characterized
by infiltration of the airways with mast cells, eosinophils and
activated T helper lymphocytes (1). Asthma affects >300 million
individuals worldwide, and by 2025 the prevalence is predicted to
increase by 100 million (2,3). At
present, inhaled corticosteroids are the standard therapy for
persistent asthma; however, the antioxidant effects of
corticosteroids are not satisfactory. Furthermore, this treatment
is hindered when steroid dependence or steroid resistance occurs
(4). Therefore, the development of
novel and efficient therapeutic strategies is of great significance
in the control of asthma.
Vitamin D3 is a fat-soluble vitamin that can be
produced in the skin, liver and kidney, or can be absorbed from
food. Vitamin D3 is widely known to act as a regulator of calcium
homeostasis, and has an important role in bone formation and
resorption (5). Furthermore, it
has been reported that vitamin D3 exhibits several pharmaceutical
properties, including anti-inflammatory (6), anticancer (7–9),
antimicrobial (10) and
immunoregulatory activities (11,12).
Recently, Rigo et al (13)
reported that vitamin D3 is associated with modulation of the
innate immune defense of airway epithelium, and vitamin D3
deficiency has been shown to result in the exaggerated features of
airway disease in ovalbumin (OVA)-induced asthmatic mice (14). In addition, Lai et al
(15) reported that the
biologically active metabolite of vitamin D3, 1,25-dihydroxyvitamin
D3, could protect OVA-sensitized mice from airway remodeling.
Another study also demonstrated that 1,25-dihydroxyvitamin D3 may
attenuate airway inflammation in asthmatic rats (16). These findings suggest that vitamin
D3 may contribute to the control of asthma; however, the underlying
mechanism remains to be fully elucidated.
In the present study, mice were sensitized to OVA,
and were then treated with the biologically active form of vitamin
D3, 1,25-dihydroxyvitamin D3, in order to examine the protective
effects of vitamin D3 on murine asthma. The results demonstrated
that treatment with vitamin D3 reduced airway inflammation in
asthmatic mice via the inhibition of inflammatory cell
infiltration. Airway remodeling was also alleviated in the vitamin
D3-treated group, as characterized by decreased α-smooth muscle
actin (α-SMA) and hydroxyproline levels, collagen deposition and
goblet cell hyperplasia. Furthermore, OVA-induced activation of
transforming growth factor-β (TGF-β)/Smad was inhibited following
vitamin D3 treatment. Vitamin D3 treatment also alleviated
oxidative stress via activation of the NF-E2-related factor 2
(Nrf2)/heme oxygenase-1 (HO-1) signaling pathway. These data
preliminarily revealed the mechanisms by which vitamin D3 protects
against airway damage in OVA-induced asthma.
Materials and methods
OVA-induced murine model of asthma
A total of 36 BALB/c female mice (age, 8–10 weeks;
weight, 22±2 g) were purchased from Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China) and housed in a controlled
environment with 40–50% humidity at 22±1°C under a 12-h light/dark
cycle. All mice had access to food and water ad libitum. All
animal experiments in the present study were conducted in
compliance with the Guide for the Care and Use of Laboratory
Animals, and were approved by the Institutional Animal Care and Use
Committee of Harbin Medical University (Harbin, China). Asthma was
induced using OVA as previously described (15). Briefly, mice were sensitized with
50 µg OVA (Sigma-Aldrich, St. Louis, MO, USA), which was
emulsified with 5 mg aluminum hydroxide (Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China), via intraperitoneal injection on days
0, 7 and 14. From day 15, the mice received an airway challenge
with 1% OVA for 30 min, three times a week for 9 weeks.
Animal groups and vitamin D3
treatment
Following a week of adjustable feeding, the mice
were randomly divided into three groups (n=12/group): i) Control
group, mice received the same volume of phosphate-buffered saline
(PBS) by intraperitoneal injection, and did not undergo airway
challenge; ii) OVA group, mice were injected intraperitoneally with
300 µl PBS containing 0.9% ethanol 30 min prior to each
airway challenge; iii) vitamin D3 group, mice were
intraperitoneally injected with 100 ng 1,25-dihydroxyvitamin D3
(Sigma-Aldrich) dissolved in ethanol, and further diluted to 0.9%
by 300 µl PBS, 30 min prior to each airway challenge. A
total of 24 h after the last challenge, mice were anesthetized with
3.0 ml/kg 10% chloral hydrate (Sinopharm Chemical Reagent Co.,
Ltd.) by intraperitoneal injection and lungs were lavaged with 1.5
ml saline three times; the bronchoalveolar lavage fluid (BALF) was
collected for further analysis. Subsequently, the mice were
sacrificed by 5.0 ml/kg 10% chloral hydrate, and the lungs were
harvested for further experimentation.
Analysis of BALF
BALF samples were centrifuged at 156 × g for 10 min
at 4°C, the supernatant was stored at −80°C, and the cell pellet
was resuspended in 1 ml PBS. The number of total cells was counted
using a hemocytometer, and the remaining cells were centrifuged
onto slides and stained with Wright-Giemsa in order to count the
number of eosinophils.
Measurement of immunoglobulin (Ig)E,
TGF-β1 and calcium
The levels of total IgE and TGF-β1 in the BALF
samples were measured using ELISA kit for Immunoglobulin E and
ELISA kit for TGF-β1 (USCN Life Science, Inc., Wuhan, China)
according to the manufacturer's protocol. The optical density (450
nm) was measured using a microplate reader (ELx800; Biotek
Instruments, Inc., Winooski, VT, USA), and the concentrations of
IgE and TGF-β1 was calculated from a standard curve. The serum
levels of calcium were measured using a calcium colorimetric assay
kit (Sigma-Aldrich) according to the manufacturer's protocol.
Lung histological and immunohistochemical
analyses
For histological analysis, lung tissues were fixed
with 10% buffered neutral formalin, embedded in paraffin, cut into
5-µm sections, and stained with hematoxylin and eosin
(H&E) using 0.2% hematoxylin for 5 min and 0.35% eosin for 3
min; periodic acid-Schiff (PAS), using 0.5% Schiff staining
solution for 15 min; or Masson, using 1% hematoxylin for 6 min and
ponceaux-acid fuchsin staining solution (0.7% ponceaux, 0.3% acid
fuchsin) for 1 min. For immunohistochemical analysis, the sections
were initially incubated with anti-α-SMA mouse monoclonal antibody
(1:200; Wuhan Boster Biological Technology, Ltd., Wuhan, China;
cat. no. BM0002), anti-Nrf2 mouse polyclonal antibody (1:50; Wuhan
Boster Biological Technology, Ltd.; cat. no. BA3790) or anti-HO-1
mouse polyclonal antibody (1:200; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; cat. no. sc-390991) at 4°C overnight, and were
then incubated with horseradish peroxidase (HRP)-conjugated goat
anti-mouse (1:50; cat. no. A0286) and goat anti-rabbit (1:50; cat.
no. A0277) secondary antibodies (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min at 37°C. Positive staining
was detected with HRP-conjugated streptavidin, visualized with
3,3′-diaminobenzidine and counterstained with hematoxylin. Finally,
the sections were mounted, cover-slipped, and examined under a
light microscope (DP73; Olympus Corporation, Tokyo, Japan). The
extent of lung infiltration as detected by H&E staining, and
goblet cell hyperplasia as detected by PAS staining were graded
using a semi-quantitative scoring system (17). The collagen area of similar size
bronchioles and the α-SMA positive area were analyzed using
Image-Pro Plus 4.5 (Media Cybernetics, Inc., Rockville, MD, USA)
and are presented as positive area/total area of bronchioles
(18) The integrated optical
density (IOD) of Nrf2 and HO-1 was also measured by Image-Pro Plus
4.5, and the mean density was calculated as IOD / area.
Western blot analysis
Total proteins were extracted using
radioimmunoprecipitation assay lysis solution (Beyotime Institute
of Biotechnology). Nuclear and cytoplasmic protein extracts were
prepared using a Nuclear and Cytoplasmic Protein Extraction kit
(Beyotime Institute of Biotechnology). Protein concentration was
determined using the BCA Protein assay kit (Beyotime Institute of
Biotechnology) and 40 µg protein were separated by 5, 10 or
14% sodium dodecyl-sulfate polyacrylamide gel electrophoresis, and
were transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Bedford, MA, USA). After blocking with 5% fat-free milk,
the membranes were immunoblotted with primary antibodies as
follows: Mouse α-SMA (1:400), rabbit HO-1 (1:200; Santa Cruz
Biotechnology, Inc.; cat. no. sc-10789), mouse Nrf2 (1:400), rabbit
TGF-β1 (1:200; Santa Cruz Biotechnology, Inc.; cat. no. sc-146),
rabbit Smad2 (1:500, BIOSS, Beijing, China; cat. no. bs-0718R),
rabbit phosphorylated (p)-Smad2 (1:500; BIOSS; cat. no. bs-5618R),
rabbit Smad3 (1:500; BIOSS; cat. no. bs-3484R), rabbit p-Smad3
(1:500; BIOSS; cat. no. bs-5459R), mouse β-actin (1:1,000; Santa
Cruz Biotechnology, Inc.; cat. no. sc-47778) and rabbit histone H3
(1:1,000; BIOSS; cat. no. bs-17422R) at 4°C overnight.
Subsequently, the membranes were incubated with HRP-conjugated goat
anti-rabbit (1:5,000; cat. no. A0216) or goat anti-mouse (1:5,000;
cat. no. A0208) IgG (Beyotime Institute of Biotechnology) at 37°C
for 45 min. The specific bands were visualized using an enhanced
chemiluminescence (ECL) kit (7Sea Biotech, Shanghai, China)
according to the manufacturer's protocol. The semi-quantitative
analysis was performed using the Gel-Pro Analyzer version 3.0
(Media Cybernetics, Inc.), β-actin or histone H3 was employed as an
internal control and the relative protein levels were quantified by
the comparison to the control group.
Measurement of hydroxyproline, HO-1,
malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione
(GSH) in lungs
Lung tissues from each group were homogenized in
pre-cooled PBS, and were frozen in liquid nitrogen and thawed three
times. Following centrifugation at 10,000 × g for 10 min at 4°C,
the supernatant was collected, in order to determine the
concentrations of hydroxyproline, MDA and GSH, and the activity of
SOD. HO-1 activity was evaluated by determining the amount of
bilirubin, as previously described (19). All measurements were performed
using commercial kits (Hydroxyproline assay kit, Malondialdehyde
assay kit, Glutathione Peroxidase assay kit, Total Superoxide
Dismutase assay kit, and Total Bilirubin kit) obtained from Nanjing
Jiancheng Bioengineering Institute (Jiangsu, China) according to
the manufacturer's manual.
Electrophoretic mobility shift assay
(EMSA)
To detect the DNA-binding activity of Nrf2, EMSA was
performed using a Chemiluminescent EMSA kit (Viagene Biotech, Inc.,
Tampa, FL, USA). Briefly, 25 µg nuclear protein from each
group were combined with biotin-labeled double-stranded
oligonucleotide probes containing the specific recognition sequence
of Nrf2 (sense strand, 5′-GGGGAACCTGTGCTGAGTCACTGGA-3′; anti-sense
strand: 5′-TCCAGTGACTCAGCACAGGTTCCCC-3′). Following separation on a
6.5% polyacrylamide gel, the complex was transferred onto a nylon
membrane and was exposed to ultraviolet light for 10 min.
Subsequently, the specific bands were detected using HRP-conjugated
streptavidin and an ECL kit (7Sea Biotech).
Statistical analysis
Data are expressed as mean ± standard deviation.
Differences between the groups were compared by one-way analysis of
variance followed by Bonferroni post-hoc test, or nonparametric
Kruskal-Wallis test followed by Dunn's multiple comparisons test
using SPSS version 16.0 software for Windows (SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
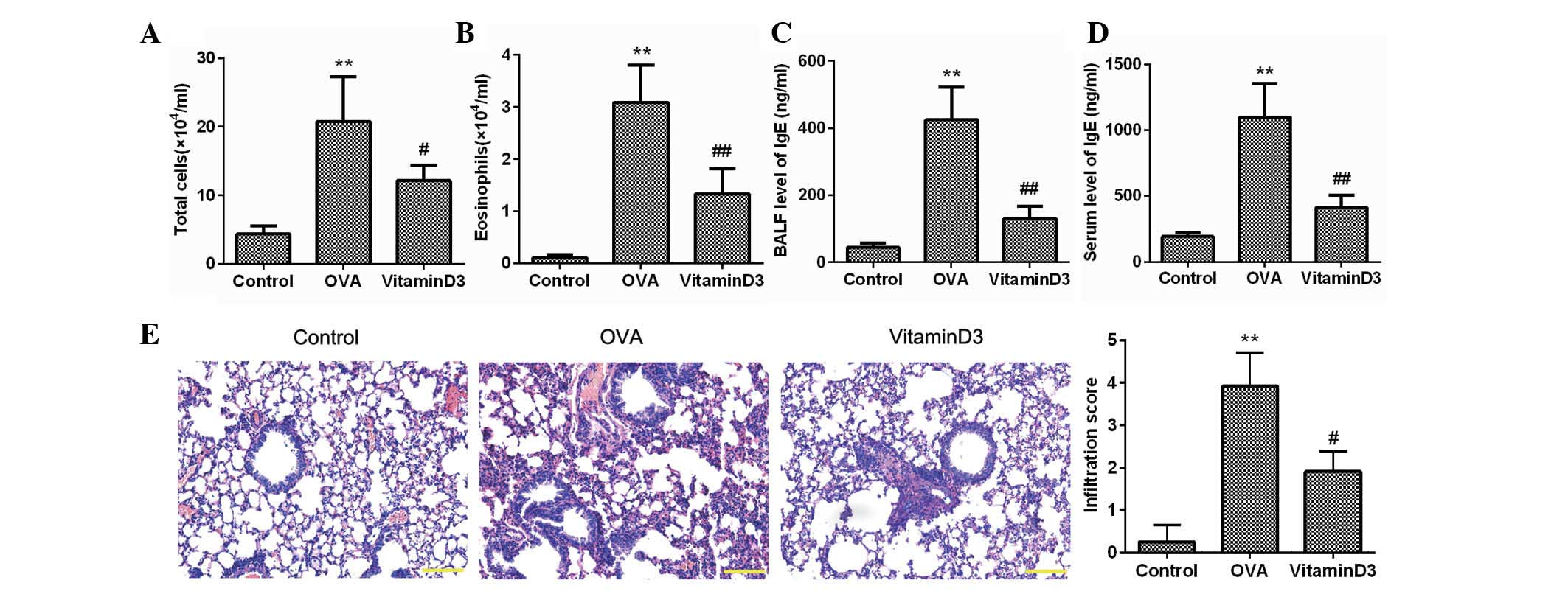
Vitamin D3 inhibits OVA-induced airway
inflammation
To investigate the effects of vitamin D3 on asthma,
mice were sensitized and challenged with OVA, and the inflammatory
cells in the BALF were counted (Fig.
1A and B). As compared with the control group, the number of
total cells and eosinophils in the OVA group was significantly
increased (P<0.05), whereas these were markedly decreased by
vitamin D3 treatment (P<0.05). The concentration of IgE, which
is another feature of asthma, was also determined; as shown in
Fig. 1C and D, the elevated levels
of IgE in the BALF and serum of the OVA group were significantly
reduced following vitamin D3 treatment. H&E staining further
confirmed that inflammatory cells intensively infiltrated the
peribronchial space of OVA-challenged mice, which was accompanied
by thickening of the lung mesenchyme; however, these alterations
were markedly alleviated by treatment with vitamin D3 (Fig. 1E). These results clearly indicate
that vitamin D3 may effectively inhibit OVA-induced airway
inflammation.
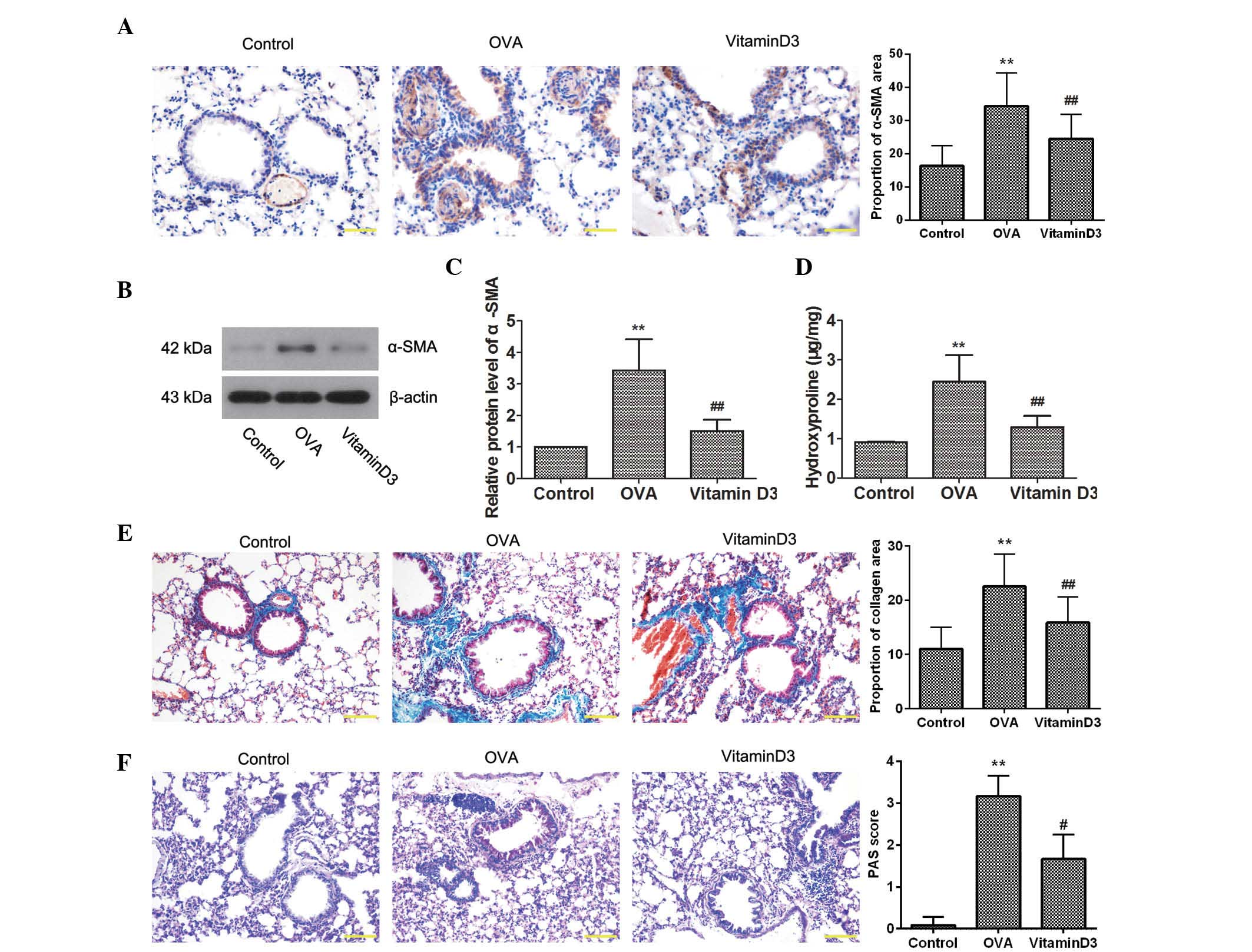
Vitamin D3 attenuates OVA-induced airway
remodeling
To determine the effects of vitamin D3 on
OVA-induced airway remodeling, the expression of α-SMA, a marker of
smooth muscle cells, was detected by immunohistochemistry. As shown
in Fig. 2A, OVA challenge resulted
in a marked increase in the expression of α-SMA, which was
decreased by vitamin D3 treatment. This result was further
confirmed by western blotting (Fig. 2B
and C). In addition, the amount of hydroxyproline was
determined, in order to assess collagen content (Fig. 2D); OVA challenge significantly
elevated hydroxyproline levels (P<0.05), which were reversed
following treatment with vitamin D3 (P<0.05). Consistent with
these results, Masson's staining indicated that OVA-induced
collagen deposition in the lungs was markedly reduced by vitamin D3
treatment (Fig. 2E). Furthermore,
PAS staining revealed that OVA-induced goblet cell hyperplasia in
the airway epithelium was inhibited by vitamin D3 (Fig. 2F). Collectively, these results
suggest that vitamin D3 attenuates OVA-induced airway
remodeling.
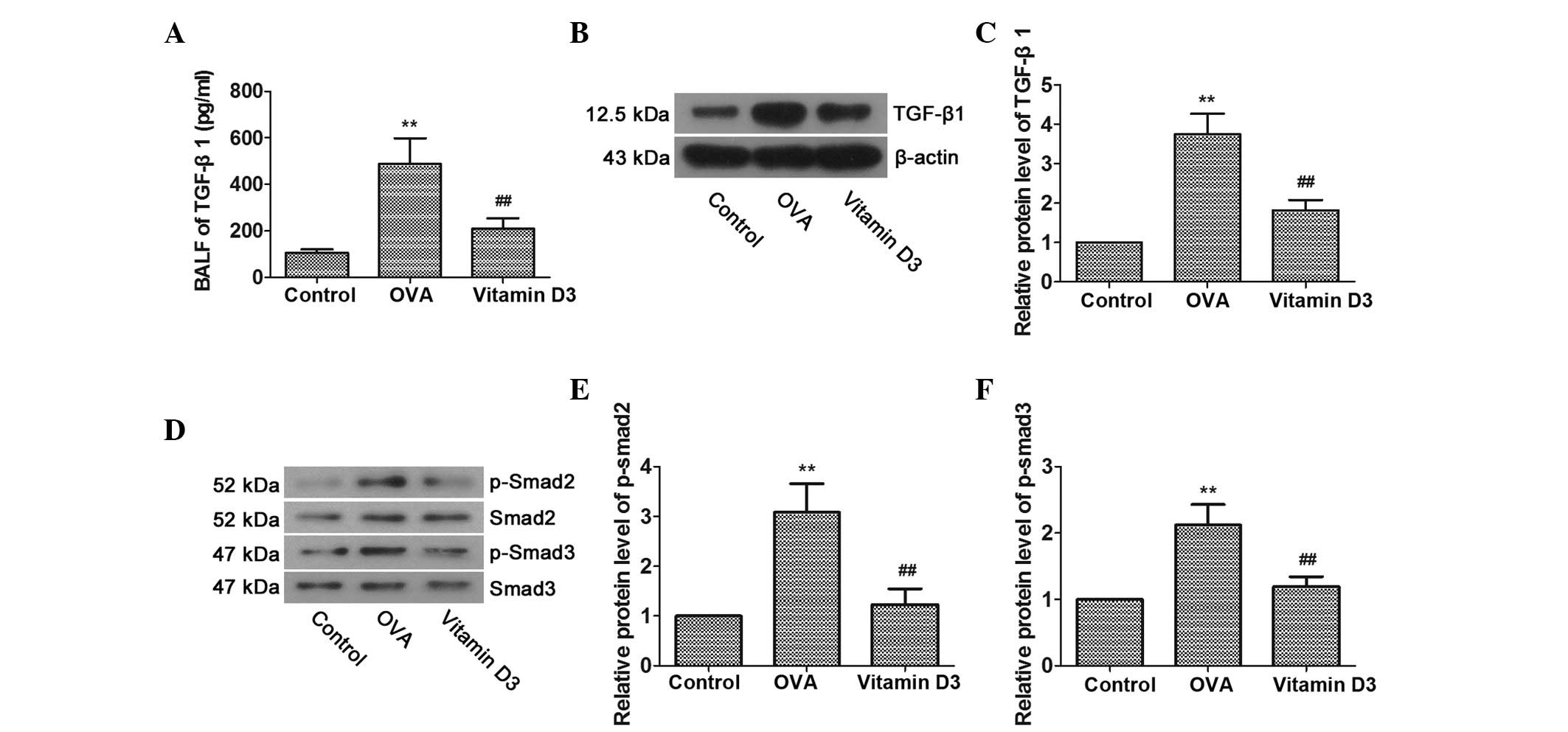
Vitamin D3 suppresses OVA-induced
activity of the TGF-β/Smad pathway
Previous studies have suggested that TGF-β1 is an
important mediator of airway disease (20,21);
therefore, the activities of TGF-β1 and members of its signal
transducing system, Smad2 and Smad3, were determined, in order to
investigate whether TGF-β/Smad pathway inhibition is involved in
the protective effects of vitamin D3 on OVA-induced airway
remodeling. As shown in Fig. 3A–C,
OVA challenge resulted in a significant increase in the expression
of TGF-β1 in BALF and lung tissues; however, it was markedly
reversed by vitamin D3 treatment. Furthermore, the levels of
p-Smad2 and p-Smad3 in the lungs were markedly upregulated by OVA
challenge and were further dampened by vitamin D3 treatment
(Fig. 3D–F). Overall, these data
indicate that the TGF-β/Smad pathway is associated with vitamin
D3-mediated protection of OVA-induced airway remodeling.
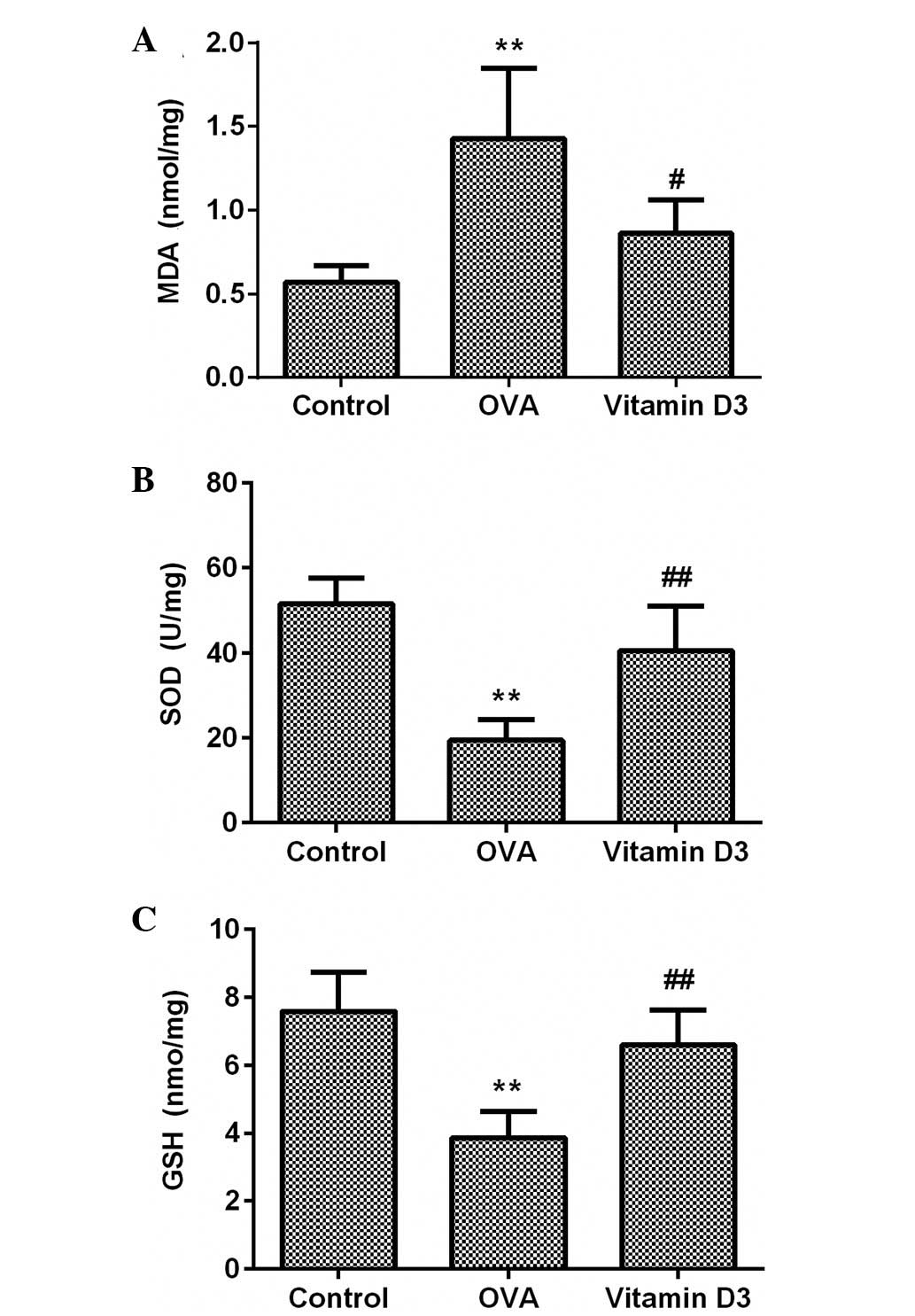
Vitamin D3 inhibits OVA-induced oxidative
stress via activation of the Nrf2/HO-1 signaling pathway
To evaluate the effects of vitamin D3 on OVA-induced
oxidative stress, MDA and GSH content, and SOD activity were
detected in the lungs using commercial kits. As shown in Fig. 4, MDA content was significantly
increased in the OVA group (P<0.05), which was accompanied by a
marked decrease in SOD activity and GSH content (P<0.05);
however, these effects were significantly attenuated by vitamin D3
treatment.
The Nrf2/HO-1 signaling pathway has a pivotal role
in antioxidant reactions (22,23);
therefore, to investigate whether it is associated with the
protective effects of vitamin D3 on OVA-induced oxidative damage to
the lungs, the expression and activity of Nrf2 and HO-1 were
examined. As expected, immunohistochemistry demonstrated that the
expression of Nrf2 was significantly increased in the bronchial
epithelial cells of the OVA group (Fig. 5A; P<0.01), and was further
upregulated in the vitamin D3 group (P<0.01). In addition,
nuclear Nrf2 levels were significantly higher in the OVA group
compared with the control group (Fig.
5B; P<0.01), and were further upregulated following vitamin
D3 treatment (P<0.01). Similar results were observed by EMSA
(Fig. 5C); Nrf2 binding activity
was elevated in the OVA group, and it was intensified by vitamin D3
treatment. In accordance with Nrf2, HO-1 levels were also markedly
upregulated in airway epithelial cells from the OVA group, the
levels of which were further enhanced by vitamin D3 treatment
(Fig. 5D and E). Furthermore, OVA
challenge resulted in a marked increase in HO-1 activity, which was
markedly enhanced by vitamin D3 treatment (Fig. 5F). These data indicate that vitamin
D3 protects airways from OVA-induced oxidative injury, at least
partially via activation of the Nrf2/HO-1 signaling pathway.
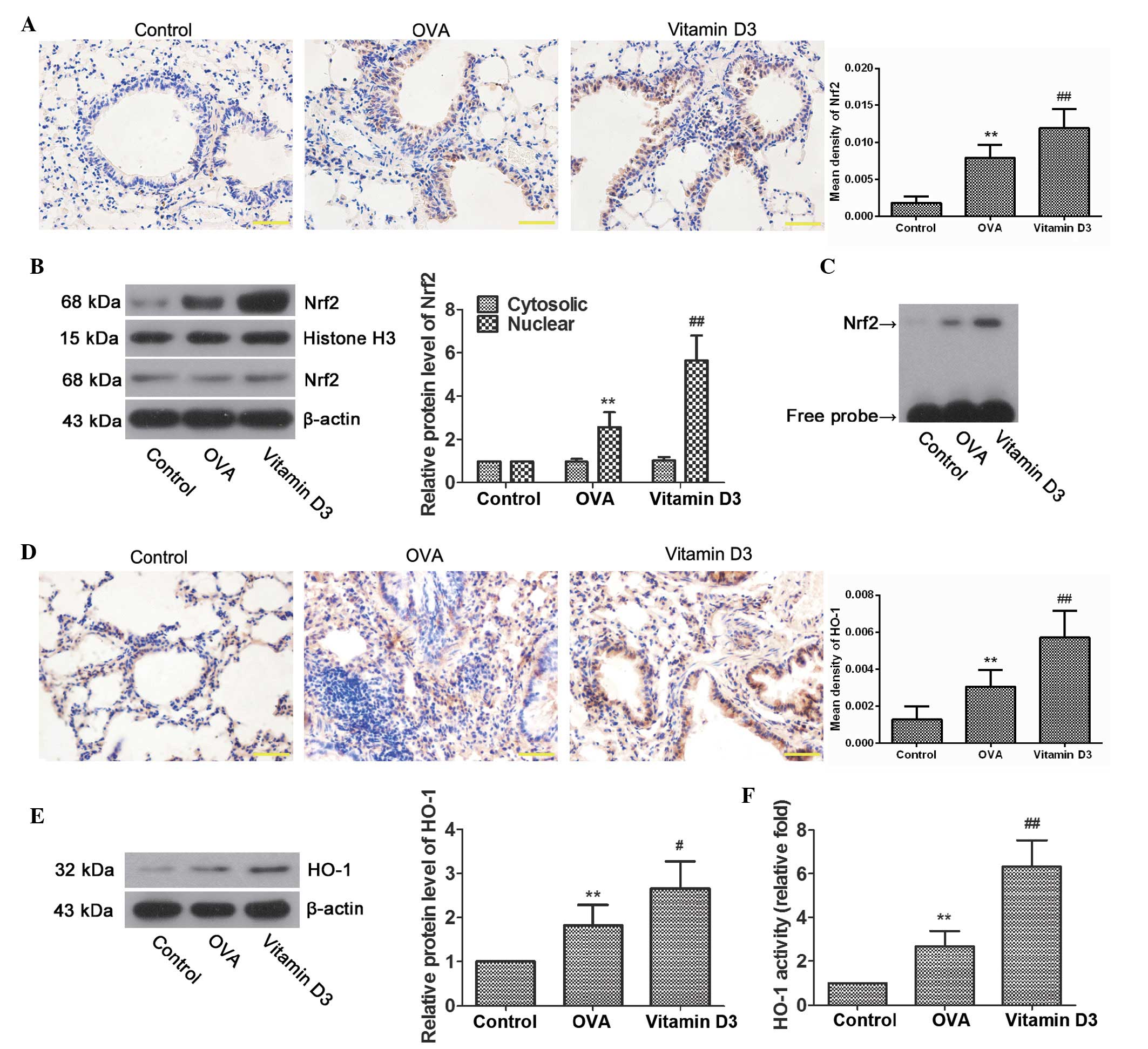 | Figure 5Effects of vitamin D3 on NF-E2-related
factor 2 (Nrf2)/heme oxygenase-1 (HO-1) activity in lungs induced
by ovalbumin (OVA) challenge. (A) Representative
immunohistochemical images of Nrf2, and the mean density. (B)
Representative bands of Nrf2 in the cytoplasm and nucleus, as
examined by western blotting, and relative protein levels of Nrf2
in lung tissues. (C) Nrf2 activity, as determined by
electrophoretic mobility shift assay. (D) Representative
immunohistochemical images of HO-1, and the mean density. (E)
Representative protein bands of HO-1, as determined by western
blotting, and relative protein levels of HO-1 in lung tissues. (F)
HO-1 activity, as evaluated by bilirubin content, and expressed as
relative fold. Data are presented as the mean ± standard deviarion
of three repeat experiments, n=6. **P<0.01 vs. the
control group; #P<0.05 and ##P<0.01 vs.
the OVA group. |
Vitamin D3 protects asthmatic airways,
accompanied by hypercalcemia
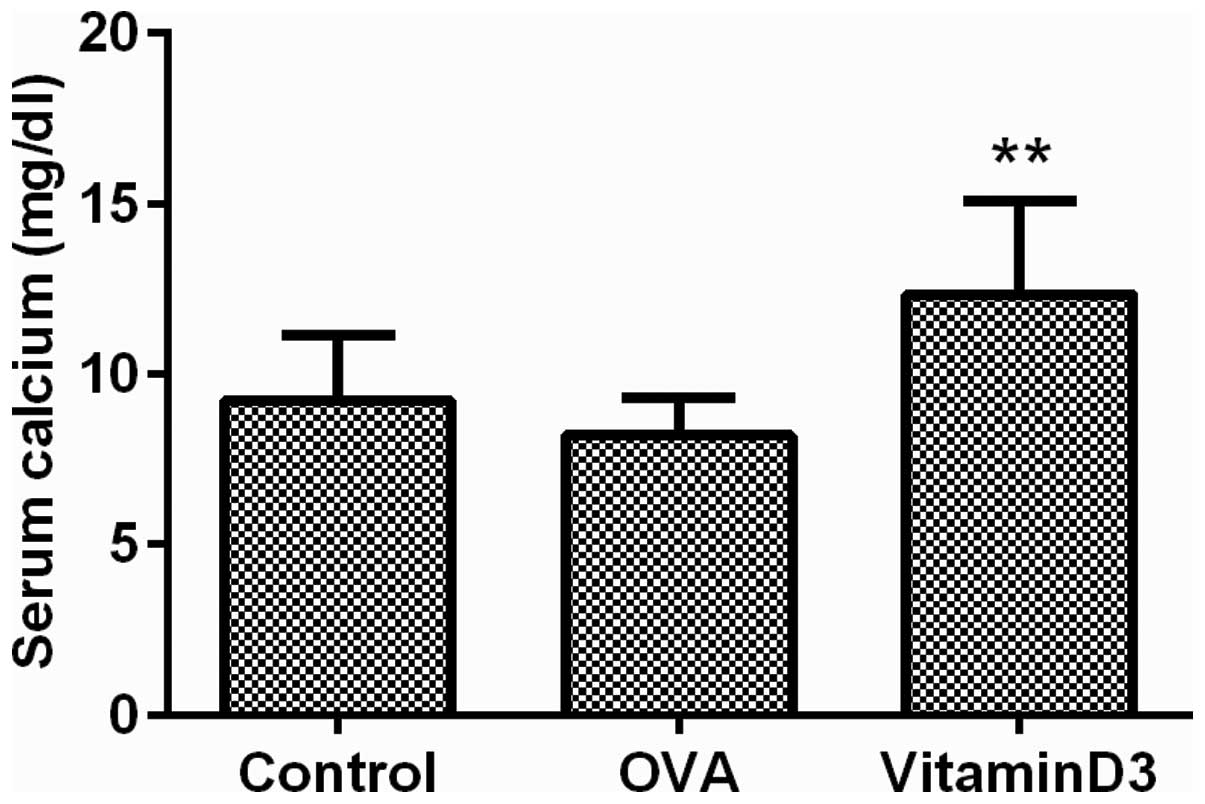
A previous study demonstrated that high doses of
vitamin D3 may result in a surfeit of calcium in the blood
(24); therefore, the serum levels
of calcium were measured to evaluate the effects of vitamin D3 on
blood calcium levels. The results demonstrated that calcium
concentration in the vitamin D3 group was significantly increased
(Fig. 6; P<0.01). These results
suggest that the asthma protective effects of vitamin D3 are
accompanied by hypercalcemia.
Discussion
The results of the present study demonstrated that
vitamin D3 effectively suppressed airway inflammation in a murine
model of asthma. In addition, the overexpression of α-SMA,
hydroxyproline and collagen deposition in the airways of asthmatic
mice was reversed by vitamin D3 treatment. Vitamin D3 treatment
also suppressed the OVA-induced activation of TGF-β/Smad.
Furthermore, vitamin D3 suppressed airway oxidative stress in
asthmatic mice, alongside increased activation of the Nrf2/HO-1
pathway. Taken together, these results suggested that vitamin D3
may protect airway injury from allergic asthma via the suppression
of TGF-β/Smad signaling and activation of the Nrf2/HO-1 pathway;
therefore, vitamin D3 may be considered effective in the control of
asthma.
Epidemiological studies have demonstrated that
vitamin D deficiency is a cause of increased asthma prevalence
(25); however, Wittke et
al (26) reported that vitamin
D receptor deficiency blocked asthma-induced airway injury and
suggested that the vitamin D endocrine system is implicated in the
pathogenesis of asthma. Subsequently, other studies have
demonstrated that the active metabolite of vitamin D has a
protective effect on asthmatic rodents (15,16).
Airway inflammation, eosinophil infiltration and IgE production are
prominent features of asthma (27). In the present study, asthma was
induced by OVA challenge, as evidenced by an increased number of
eosinophils, upregulated IgE levels in BALF and increased
inflammatory cell infiltration in the airways. Conversely, these
alterations were markedly reversed following vitamin D3 treatment.
These results further confirmed that vitamin D3 may protect airways
from OVA-induced inflammatory damage.
Airway remodeling is the most common
pathophysiological feature of asthma, which is characterized by
subepithelial fibrosis and airway collagen deposition (28). Furthermore, goblet cell hyperplasia
that leads to excessive mucin secretion is also a typical
pathological alteration common in asthma (29). The present study detected a marked
increase in α-SMA, airway collagen deposition and goblet cell
hyperplasia in OVA-challenged mice; however, these effects were all
significantly reduced by vitamin D3 treatment. These results are
consistent with a previous study, which suggested that vitamin D3
could protect airways from asthmatic remodeling (15). The TGF-β/Smad pathway has been
demonstrated to be a main mediator in asthmatic lung remodeling,
due to its effect on epithelial alterations, subepithelial
fibrosis, goblet cell hyperplasia and smooth muscle proliferation
(20,30). A clinical study reported that
TGF-β/Smad signaling was significantly activated in bronchial
biopsy specimens from asthmatic subjects, and was closely related
with basement membrane thickness (31). The results of the present study
demonstrated that an OVA challenge resulted in the obvious
activation of TGF-β/Smad signaling, which was markedly suppressed
by vitamin D3 treatment. Therefore, vitamin D3 may attenuate
OVA-induced airway remodeling via the inhibition of TGF-β/Smad
signaling.
Mounting evidence has demonstrated that oxidative
stress is involved in increased lipid peroxidation, aggravated
airway reactivity and overexpression of chemoattractants in asthma
(32,33). In the present study, OVA challenge
significantly increased the levels of MDA, which is the end product
of lipid peroxidation, and decreased the activity of antioxidant
enzymes, SOD and GSH. However, these changes were markedly
attenuated by vitamin D3 treatment. These results suggested that
vitamin D3 may limit airway damage by reducing oxidative stress;
these results were in accordance with those of previous studies,
which suggested that vitamin D3 is a potent antioxidant (34,35).
In addition, the present study detected enhanced Nrf2/HO-1 pathway
activity in the airways of the vitamin D3 treatment group. Nrf2 is
a cellular sensor of oxidative stress, which is associated with
transcriptional activation of antioxidant-response element genes
(23). Nrf2 deficiency has been
reported to result in an exaggerated airway inflammation in
asthmatic mice (36). HO-1 is
considered an important endogenous antioxidant and cytoprotective
enzyme, which may be upregulated by Nrf2 (37,38).
Therefore, the airway protective effects of vitamin D3 in
OVA-induced asthma may partly depend on activation of the Nrf2/HO-1
pathway. However, the asthmatic protective effects of vitamin D3
are accompanied by a non-lethal, but significant increase in serum
calcium levels.
In conclusion, the present study reported a
protective role of vitamin D3 in allergic asthma. In addition,
inhibition of TGF-β/Smad signaling and activation of the Nrf2/HO-1
pathway are potential mechanisms by which vitamin D3 protects
against asthma-induced airway damage. However, treatment with
vitamin D3 may result in hypercalcemia.
References
|
1
|
Hamid Q and Tulic M: Immunobiology of
asthma. Ann Rev Physiol. 71:489–507. 2009. View Article : Google Scholar
|
|
2
|
Masoli M, Fabian D, Holt S and Beasley R;
Global Initiative for Asthma (GINA) Program: The global burden of
asthma: Executive summary of the GINA dissemination committee
report. Allergy. 59:469–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou W and Nie X: Afzelin attenuates
asthma phenotypes by downregulation of GATA3 in a murine model of
asthma. Mol Med Rep. 12:71–76. 2015.PubMed/NCBI
|
|
4
|
Barnes PJ: Corticosteroid resistance in
patients with asthma and chronic obstructive pulmonary disease. J
Allergy Clin Immunol. 131:636–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dusso AS, Brown AJ and Slatopolsky E:
Vitamin D. Am J Physiol Renal Physiol. 289:F8–F28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nebel D, Svensson D, Arosenius K, Larsson
E, Jönsson D and Nilsson BO: 1α,25-dihydroxyvitamin D3 promotes
osteogenic activity and downregulates proinflammatory cytokine
expression in human periodontal ligament cells. J Periodontal Res
Dec. 12:2014.Epub ahead of print.
|
|
7
|
Kelsey L, Katoch P, Ray A, Mitra S,
Chakraborty S, Lin MF and Mehta PP: Vitamin D3 regulates the
formation and degradation of gap junctions in androgen-responsive
human prostate cancer cells. PloS One. 9:e1064372014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robsahm TE, Tretli S, Dahlback A and Moan
J: Vitamin D3 from sunlight may improve the prognosis of breast-,
colon- and prostate cancer (Norway). Cancer Causes Control.
15:149–158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pálmer HG, González-Sancho JM, Espada J,
Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros
AG, Lafarga M and Muñoz A: Vitamin D(3) promotes the
differentiation of colon carcinoma cells by the induction of
E-cadherin and the inhibition of beta-catenin signaling. J Cell
Biol. 154:369–387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang TT, Nestel FP, Bourdeau V, Nagai Y,
Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S and
White JH: Cutting edge: 1,25-dihydroxyvitamin D3 is a direct
inducer of antimicrobial peptide gene expression. J Immunol.
173:2909–2912. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Penna G and Adorini L: 1
Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation,
activation, and survival of dendritic cells leading to impaired
alloreactive T cell activation. J Immunol. 164:2405–2411. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen S, Sims GP, Chen XX, Gu YY, Chen S
and Lipsky PE: Modulatory effects of 1,25-dihydroxyvitamin D3 on
human B cell differentiation. J Immunol. 179:1634–1647. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rigo I, McMahon L, Dhawan P, Christakos S,
Yim S, Ryan LK and Diamond G: Induction of triggering receptor
expressed on myeloid cells (TREM-1) in airway epithelial cells by
1,25(OH) vitamin D3. Innate Immun. 18:250–257. 2012.
View Article : Google Scholar
|
|
14
|
Gorman S, Tan DH, Lambert MJ, Scott NM,
Judge MA and Hart PH: Vitamin D(3) deficiency enhances
allergen-induced lymphocyte responses in a mouse model of allergic
airway disease. Pediatr Allergy Immunol. 23:83–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai G, Wu C, Hong J and Song Y:
1,25-Dihydroxyvitamin D(3) (1,25-(OH)(2)D(3)) attenuates airway
remodeling in a murine model of chronic asthma. J Asthma.
50:133–140. 2013. View Article : Google Scholar
|
|
16
|
Zhou Y, Zhou X and Wang X:
1,25-Dihydroxyvitamin D3 prevented allergic asthma in a rat model
by suppressing the expression of inducible nitric oxide synthase.
Allergy Asthma Proc. 29:258–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terawaki K, Yokomizo T, Nagase T, Toda A,
Taniguchi M, Hashizume K, Yagi T and Shimizu T: Absence of
leukotriene B4 receptor 1 confers resistance to airway
hyperresponsiveness and Th2-type immune responses. J Immunol.
175:4217–4225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le AV, Cho JY, Miller M, McElwain S,
Golgotiu K and Broide DH: Inhibition of allergen-induced airway
remodeling in Smad 3-deficient mice. J Immunol. 178:7310–7316.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao P, Nussler A, Liu L, Hao L, Song F,
Schirmeier A and Nussler N: Quercetin protects human hepatocytes
from ethanol-derived oxidative stress by inducing heme oxygenase-1
via the MAPK/Nrf2 pathways. J Hepatol. 47:253–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halwani R, Al-Muhsen S, Al-Jahdali H and
Hamid Q: Role of transforming growth factor-β in airway remodeling
in asthma. Am J Respir Cell Mol Biol. 44:127–133. 2011. View Article : Google Scholar
|
|
21
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Ann Rev Immunol. 24:99–146. 2006. View Article : Google Scholar
|
|
22
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaspar JW, Niture SK and Jaiswal AK: Nrf2:
INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med.
47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zella JB, McCary LC and DeLuca HF: Oral
administration of 1,25-dihydroxyvitamin D3 completely protects NOD
mice from insulin-dependent diabetes mellitus. Arch Biochem
Biophys. 417:77–80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Litonjua AA and Weiss ST: Is vitamin D
deficiency to blame for the asthma epidemic? J Allergy Clin
Immunol. 120:1031–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wittke A, Weaver V, Mahon BD, August A and
Cantorna MT: Vitamin D receptor-deficient mice fail to develop
experimental allergic asthma. J Immunol. 173:3432–3436. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodrigo GJ, Rodrigo C and Hall JB: Acute
asthma in adults: A review. Chest. 125:1081–1102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holgate ST: What does inflammation and
airway remodelling mean? Clinical & Experimental Allergy
Reviews. 1:59–61. 2001. View Article : Google Scholar
|
|
29
|
Fahy JV: Remodeling of the airway
epithelium in asthma. Am J Respir Crit Care Med. 164:S46–S51. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makinde T, Murphy RF and Agrawal DK: The
regulatory role of TGF-beta in airway remodeling in asthma. Immunol
Cell Biol. 85:348–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sagara H, Okada T, Okumura K, Ogawa H, Ra
C, Fukuda T and Nakao A: Activation of TGF-beta/Smad2 signaling is
associated with airway remodeling in asthma. J Allergy Clin
Immunol. 110:249–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Riedl MA and Nel AE: Importance of
oxidative stress in the pathogenesis and treatment of asthma. Curr
Opin Allergy Clin Immunol. 8:49–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andreadis AA, Hazen SL, Comhair SA and
Erzurum SC: Oxidative and nitrosative events in asthma. Free Radic
Biol Med. 35:213–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bao BY, Ting HJ, Hsu JW and Lee YF:
Protective role of 1 alpha, 25-dihydroxyvitamin D3 against
oxidative stress in nonmalignant human prostate epithelial cells.
Int J Cancer. 122:2699–2706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamden K, Carreau S, Jamoussi K, Miladi S,
Lajmi S, Aloulou D, Ayadi F and Elfeki A: 1 Alpha,25
dihydroxyvitamin D3: Therapeutic and preventive effects against
oxidative stress, hepatic, pancreatic and renal injury in
alloxan-induced diabetes in rats. J Nutr Sci Vitaminol (Tokyo).
55:215–222. 2009. View Article : Google Scholar
|
|
36
|
Rangasamy T, Guo J, Mitzner WA, Roman J,
Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN and
Biswal S: Disruption of Nrf2 enhances susceptibility to severe
airway inflammation and asthma in mice. J Exp Med. 202:47–59. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010. View Article : Google Scholar
|
|
38
|
Lim S, Groneberg D, Fischer A, Oates T,
Caramori G, Mattos W, Adcock I, Barnes PJ and Chung KF: Expression
of heme oxygenase isoenzymes 1 and 2 in normal and asthmatic
airways: Effect of inhaled corticosteroids. Am J Respir Crit Care
Med. 162:1912–1918. 2000. View Article : Google Scholar : PubMed/NCBI
|