Introduction
Hyperlipidemia is a group of disorders characterized
by elevated concentrations of circulating lipids, including
cholesterol, cholesterol esters, phospholipids and triglycerides.
It often results from delayed or defective clearance, or
overproduction of very low-density lipoprotein (VLDL) by the liver,
which is subsequently transformed into low-density lipoprotein
(LDL). Excessive intake of saturated fats increases lipid
production in the liver via a molecular mechanism involving protein
activators (1). Notably,
hyperlipidemia is a major modifiable risk factor for
atherosclerosis and cardiovascular disease, including coronary
heart disease.
In the present study, 4 herbs were combined to form
HVC1, based on the theory of Korean medicine. HVC1 is composed of
Prunus yedoensis bark (Rosaceae), Rheum palmatum
rhizome (Polygonaceae), Coptis chinensis rhizome
(Ranunculaceae) and Scutellaria baicalensis radix
(Lamiaceae). P. yedoensis bark (Rosaceae) is a traditional
medicine used to treat cough, urticaria, pruritus, dermatitis,
asthma and measles (2,3). It has been reported that P.
yedoensis bark induced vasorelaxation in isolated rat thoracic
aorta rings through increased nitric oxide (NO) formation (4). Additionally, it was demonstrated that
prunetin, a component of P. yedoensis bark, efficiently
suppressed adipogenesis and obesity, as well as serum lipid levels
(5). R. palmatum is used
most frequently in the weight-reducing formulae of traditional
Chinese medicine. Recently, rhein, one of the major components of
Rheum palmatum, has been shown to be an inhibitor of 3T3-L1
adipocyte differentiation (6).
Moreover, rhein has been reported to have pharmacological and
biochemical effects on the inhibition of liver fibrosis and insulin
sensitization (7–9), and to prevent hepatic steatosis
through LXR inhibition in a high-fat diet-induced obese mouse model
(10). It has been reported that
C. chinensis rhizome and its primary active component,
berberine, have anti-obesity activity. C. chinensis rhizome
and berberine significantly reduce blood glucose and lipid levels
in high-fat diet-fed mice and increase mRNA expression of
AMP-activated protein kinase (AMPK) to promote mitochondrial energy
metabolism (11). R.
palmatum rhizome and anthraquinones significantly reduce blood
levels of total cholesterol (TC), triglycerides, high-density
lipoprotein (HDL) and LDL (12).
S. baicalensis radix protects against high fat and alcoholic
liver damage through the inhibition of
3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA R) expression in
the liver (13). In addition, the
primary active compounds of S. baicalensis radix, baicalein
and wogonin have been reported to attenuate obesity, dyslipidemia
and diabetes (14).
Based on previous data, HVC1 was developed to treat
or prevent hyperlipidemic diseases (15) and the present study was designed to
examine the hypolipidemic effects of HVC1 in a high cholesterol
diet (HCD)-induced hyperlipidemia rat model.
Materials and methods
Reagents
The normal diet (ND) and HCD were obtained from
Research Diets (New Brunswick, NJ, USA). The P-5′ adenosine
monophosphate-activated protein kinase (P-5′ AMPK; Thr 172; cat.
no. sc-33524), AMPK (cat. no. sc-398861) and β-actin (cat. no.
sc-81178) monoclonal antibodies were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). Horseradish
peroxidase-conjugated anti-mouse (cat. no. 315-035-003) and
anti-rabbit (cat. no. 111-035-003) were purchased from Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). All other
reagents were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Preparation of HVC1
HVC1 consists of P. yedoensis Matsum bark,
R. palmatum L. rhizome, C. chinensis Franch. rhizome
and S. baicalensis Georgi radix. The herbs were purchased
from Dongwoodang Co., Ltd. (Yeongcheon, Korea). Professor Ho-Young
Choi of Kyung Hee University identified the plants. Voucher
specimens of C. chinensis rhizome (CC001), S.
baicalensis radix (SB001), R. palmatum rhizome (RP001),
and P. yedoensis bark (PY001) were deposited at the College
of Korean Medicine, Kyung Hee University (Seoul, Korea). Each herb
was used at a ratio of 2:2:1:1 (600:600:300:300 g). The herbs were
extracted using 30% (v/v) ethanol in water at 60°C for 8 h. The
extracts were filtered through 10-μm cartridge paper, and
the ethanol was removed by vacuum rotary evaporation (EYELA; Tokyo,
Japan). The concentrates were freeze-dried, and the yield was
calculated to be 13%. The powders were dissolved in distilled water
for the experiments, and the residual powder was stored at
−20°C.
Animal experiments
Sprague-Dawley (SD) rats (n=36; 4 weeks, male,
200–230 g) were purchased from Daehan Biolink (Daejeon, Korea) and
maintained under constant conditions (temperature, 20–25°C;
humidity, 40–60%; 12 h light/dark cycle). The rats were adapted to
the feeding conditions for 1 week and then given free access to
food and tap water for 8 weeks. The rats were randomly assigned to
6 groups (n=6): ND group, HCD group, and treatment groups fed HCD
with simvastatin (10 mg/kg) or HVC1 (10, 50 or 250 mg/kg). With the
exception of the ND group, all of the rats consumed a HCD (D12108)
containing 20.1% saturated fat, 1.37% cholesterol and 0% sodium
cholate. HVC1 was orally administered to the treated groups, and
the untreated groups received the same volume (500 μl) of
physiological saline for 8 weeks. Body weight and dietary intake
were recorded every week. After 8 weeks, the animals were fasted
overnight. The following day, they were anesthetized with Zoletil
(50 mg/kg; Virbac; Carros Cedex, France), and blood samples were
collected by cardiac puncture using a 3 ml syringe with 23 G
needle. Immediately following collection of blood sample, the rats
were sacrificed by exsanguination under anesthesia with Zoletil (60
mg/kg). To remove clots, whole blood were separated by
centrifugation at 1,200 × g, for 10 min (4°C) and supernatants were
maintained at 2–8°C. The liver and aorta were excised, rinsed,
weighed and directly stored at −80°C until analyzed. All procedures
were conducted in accordance with the National Institutes of Health
guidelines and approved by the Ethical Committee for Animal Care
and the Use of Laboratory Animals, Sangji University (Wonju, Korea;
reg. no. 2014-05).
Serum analysis
Serum concentrations of TC, LDL cholesterol, and HDL
cholesterol were determined by enzymatic methods with HDL and
LDL/VLDL Quantification Colorimetric/Fluorometric kit (cat. no.
K613-100; BioVision, Milpitas, CA, USA).
Histological analysis
The liver and aorta from a representative rat in
each group were fixed in 10% buffered formalin, embedded in
paraffin, and cut into 5-μm sections. Certain sections were
stained with hematoxylin and eosin (H&E) for histological
examination of fat droplets. Samples of aorta were stained with Oil
red O, as previously described (16). Images were acquired using an SZX10
microscope (Olympus; Tokyo, Japan).
Western blot analysis
Liver tissues were homogenized in the protein
extraction solution, PRO-PREP (Intron Biotechnology, Gyeongi-do,
Korea), and then incubated for 25 min on ice. The samples were
centrifuged at 16,000 × g (4°C) for 5 min, and the supernatant was
transferred to a new 1.5-ml tube. The protein concentration was
determined using the Bio-Rad protein assay reagent, according to
the manufacturer's instructions (Bio-Rad Laboratories, Hercules,
CA, USA). Protein samples were immunoblotted onto a polyvinylidene
difluoride (PVDF) membrane following separation on a 10% sodium
dodecyl sulfate (SDS)-polyacrylamide gel. The membranes were
blocked with a 5% skimmed milk solution for 1 h and incubated
overnight with a primary antibody (1:1,000 dilution). The
immunoblots were washed 3 times with Tween 20/Tris-buffered saline
(TTBS) and incubated with the corresponding secondary antibody
(1:2,000 dilution) for 1 h at room temperature. After washing 3
times with TTBS, the immunoblots were developed using enhanced
chemiluminescence and X-ray film (Amersham Life Science,
Buckinghamshire, UK).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The liver tissues were homogenized, and total RNA
was isolated using the Easy-Blue Reagent according to the
manufacturer's instructions (Intron Biotechnology). Total RNA was
quantified using an Epoch micro-volume spectrophotometer system
(BioTek Instruments, Winooski, VT, USA). In brief, total RNA was
converted to cDNA using a high-capacity cDNA reverse transcription
kit (Applied Biosystems, Foster City, CA, USA) and thermo-cycler
(Gene Amp PCR system 9700; Applied Biosystems) with the following
program: Initiation for 10 min at 25°C, followed by incubation at
50°C for 90 min and at 85°C for 5 min. qPCR analysis was conducted
using a Step One Plus Real-time PCR system (StepOne software
version 2.3; Applied Biosystems). SYBR Green master mix and primers
were used for PCR analysis of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), peroxisome proliferator-activated receptor-γ
(PPAR-γ), HMG-CoA R and low-density lipoprotein receptor (LDLR).
The PCR cycling parameters were as follows: 10 min at 95°C; 40
cycles of 5 sec at 95°C and 45 sec at 60°C; and a final melting
curve of 15 sec at 95°C, 1 min at 60°C, and 15 sec at 95°C. All
primer sequences are shown in Table
I. Gene expression was calculated according to the Cq
method.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Forward primer | Reverse primer |
|---|
| PPAR-γ |
5′-ATCGAGTGCCGAGTCTGTGG-3′ |
5′-GCAAGGCACTTCTGAAACCG-3′ |
| HMG-CoA R |
5′-TGTTGGAGTGGCAGGACCTC-3′ |
5′-GGCACCTCCACCAAGACTGA-3′ |
| LDL receptor |
5′-CTCACTTCCGCTGCAACTCC-3′ |
5′-CCACAGTGGAACTCGAGGGA-3′ |
| GAPDH |
5′-TGATTCTACCCACGGCAAGT-3′ |
5′-AGCATCACCCCATTTGATGT-3′ |
High Performance Liquid Chromatography
(HPLC) analysis of HVC1
HVC1 (100 mg) was precisely weighed and dissolved in
10 ml methanol (HPLC grade, J.T. Baker Co. Ltd., Center Valley, PA,
USA), followed by filtration through a 0.45-μm
polyvinylidene difluoride syringe filter (Waters, Corporation,
Milford, CT, USA). Standards used in the HPLC analysis of HVC1 were
as follows: Sennoside A and sennoside B (Rhubarb standards;
Sigma-Aldrich); coptisine, berberine and wogonin (C.
chinensis standards; Sigma-Aldrich); baicalin, and baicalein
(S. baicalensis standards; Sigma-Aldrich); prunetin (P.
yedoensis standard; Sigma-Aldrich); and genistein-7-glucose and
prunetin-5-glucose (P. yedoensis standards; directly
isolated). The standards (1 mg) were dissolved at a concentration
of 100 μg/ml, and the HPLC chromatograms of the standards
were obtained. The HPLC apparatus was a Gilson system equipped with
a 234 Autosampler, a UV/VIS-155 detector, and a 321 HPLC Pump
(Gilson, Seoul, Korea). A Luna 4.60×264 mm C18
reversed-phase column with 5-mm particles (Phenomenex, CA, USA) was
used. The mobile phase consisted of 0.1% formic acid (A) and
acetonitrile (HPLC grade, J.T. Baker Co. Ltd.) (B) in a ratio
specified by the following binary gradient with linear
interpolation: 0 min 20% B, 60 min 30% B, 70 min 60% B and 100 min
70% B. The column eluent was monitored at UV 250 nm, following
which all solvents were degassed with a micromembrane filter
(Advantec, Tokyo, Japan). Chromatography was performed at room
temperature at a flow rate of 0.5 ml/min, and 10 μl was
analyzed for 100 min.
Statistical analysis
All the values are expressed as the mean ± standard
error of the mean. Data were analyzed using one-way analysis of
variance with Dunnett's test. Statistical analysis was performed
using GraphPad Prism (version 5; Graphpad Software Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
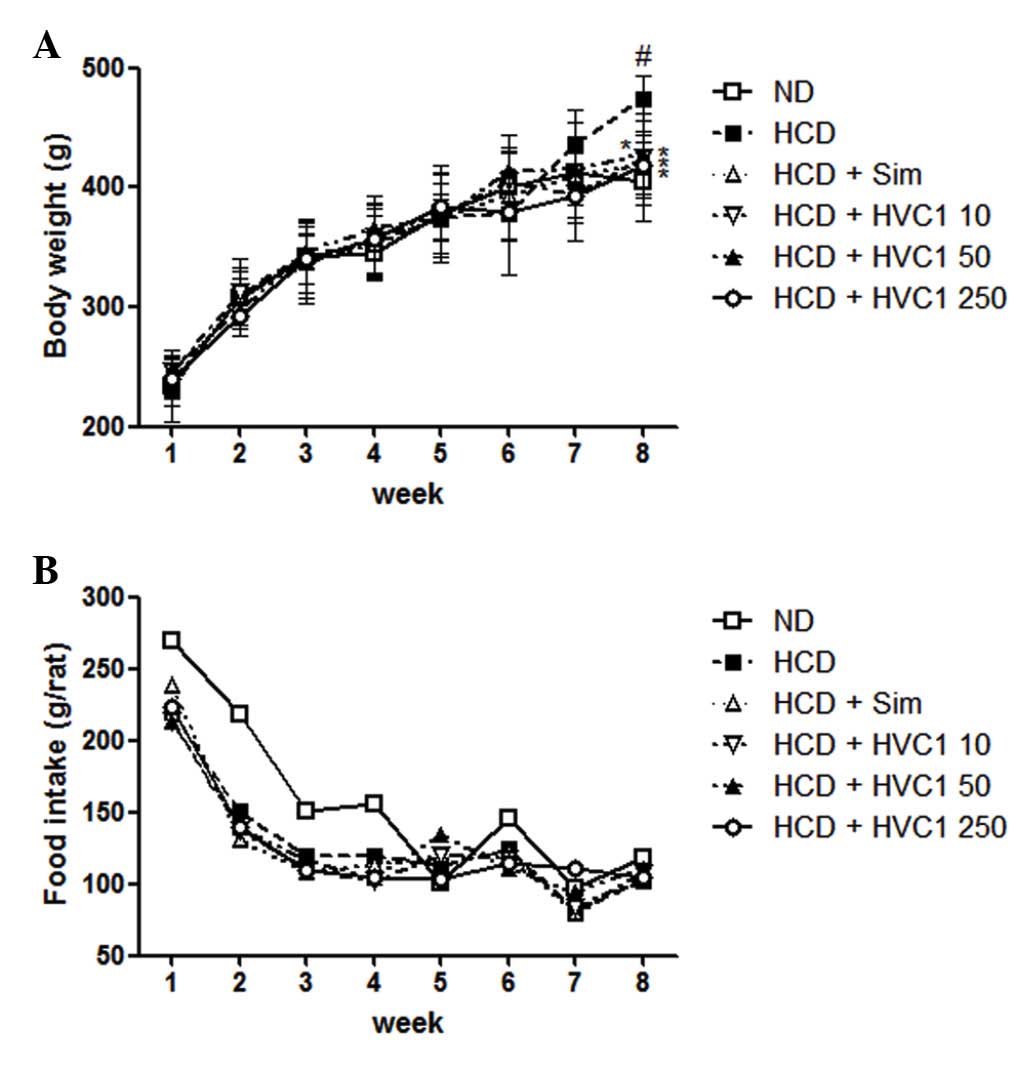
Effects of HVC1 on body weight gains
There was a significant difference in body weight
between the ND and HCD groups. The HVC1-treated groups had a
significantly lower weight when compared with the HCD group, as
shown in Fig. 1. The HCD group
gained an average of 202.5 g, whereas the HVC1 treated groups (10,
50, or 250 mg/kg) gained only 160.2, 176.6 or 172.2 g,
respectively, after 8 weeks (Fig.
1A). During the experimental period, there were no significant
differences in food and water intake in the HCD group compared with
the other treatment groups (Fig.
1B).
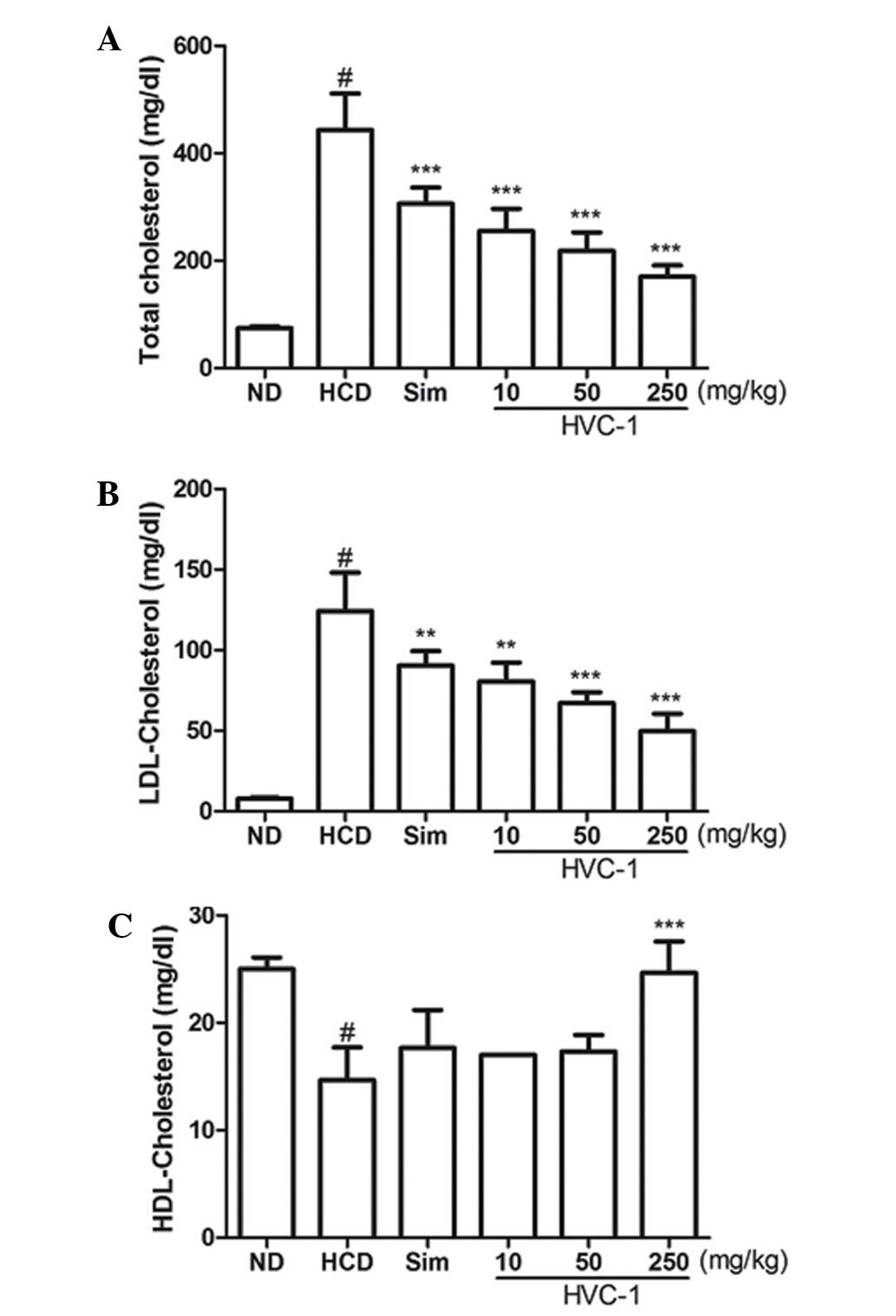
Effects of HVC1 on serum cholesterol
levels
To determine the effect of HVC1 on HCD-induced
changes in serum cholesterol levels, serum samples were prepared
from each group and cholesterol levels were analyzed by enzymatic
methods. The concentrations of serum TC and LDL cholesterol were
significantly increased by a HCD. Administration of HVC1
significantly and dose-dependently suppressed the elevation of
serum TC and LDL cholesterol levels (Fig. 2A and B). The serum level of TC in
the HCD group was 443.3±67.7 mg/dl, and 10, 50 and 250 mg/kg HVC1
reduced TC levels to 255.0±41.6, 218.0±34.6 and 170.3±21.0 mg/dl,
respectively (Fig. 2A). The serum
level of LDL cholesterol in the HCD group was 124.3±23.7 mg/dl and
10, 50 and 250 mg/kg HVC1 reduced LDL levels to 80.7±11.6, 67.0±6.9
and 49.7±11.0 mg/dl, respectively (Fig. 2B). There was a dose-dependent
response with HVC1 for TC and LDL cholesterol levels; HVC1
exhibited a greater effect than that of simvastatin. In addition,
the administration of HVC1 (250 mg/kg) also significantly reversed
the HCD-induced reduction of serum HDL cholesterol levels (Fig. 1C). Serum levels of HDL cholesterol
in the HCD group and HVC1 group (250 mg/kg) were 14.7±3.1 and
24.7±2.9 mg/dl, respectively (Fig.
2C). Based on this data, HVC1 more effectively inhibited
HCD-induced changes in serum cholesterol levels than the
hypolipidemic drug, simvastatin.
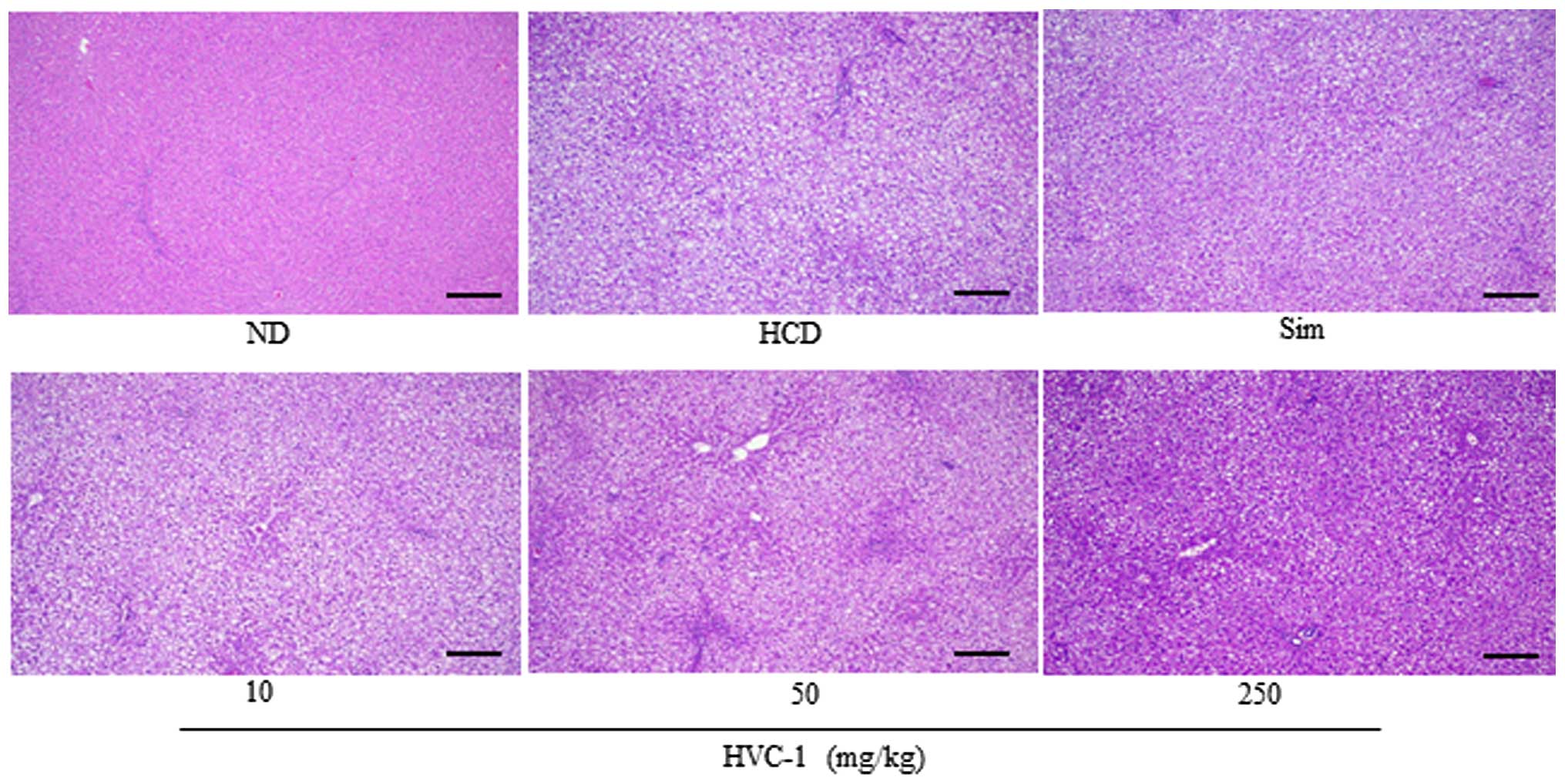
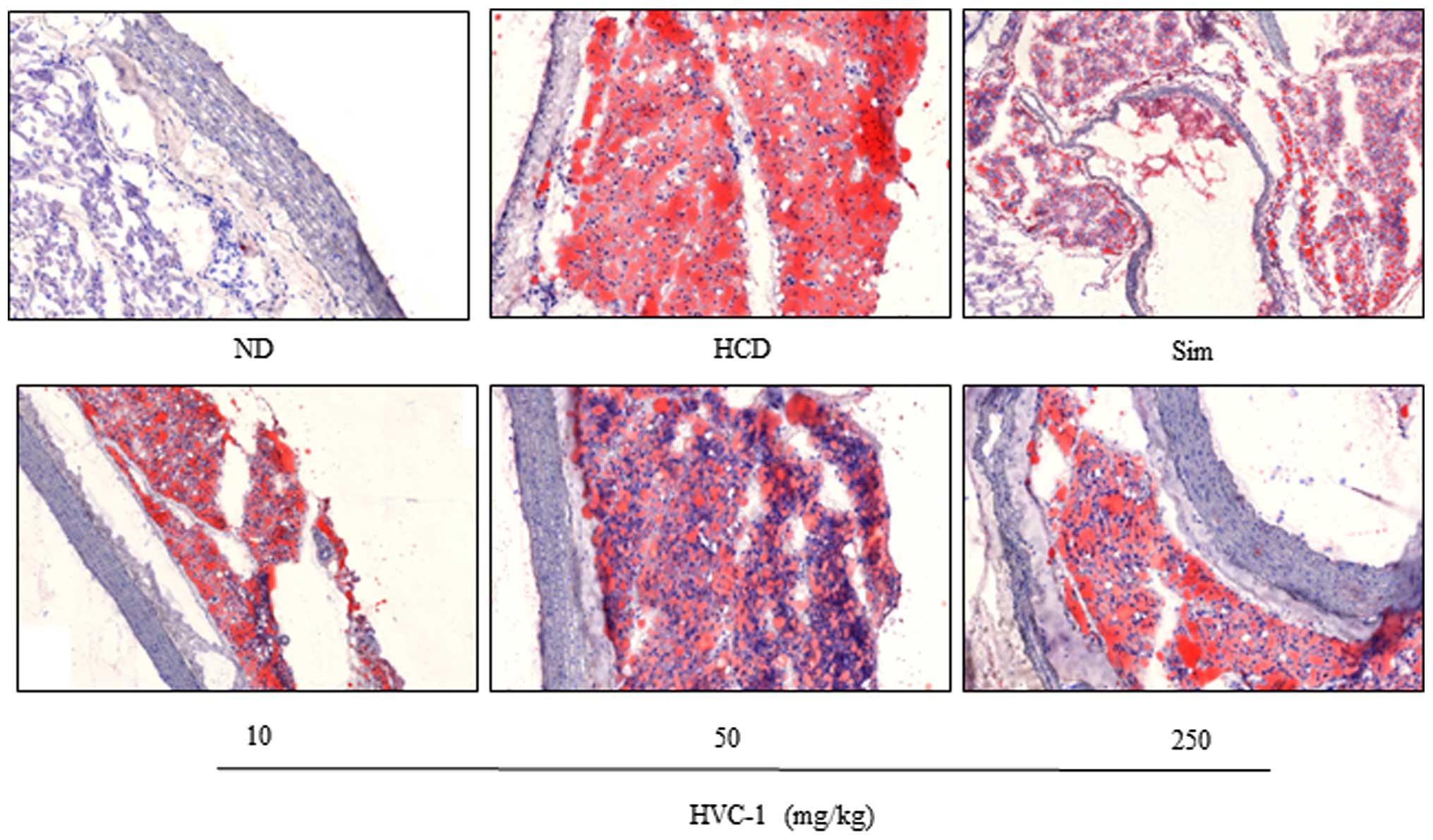
Effects of HVC1 on lipid accumulation in
the liver and aorta
To identify the effect of HVC1 on HCD-induced lipid
accumulation in the liver and aorta, tissue samples were prepared
from each group and stained with H&E or Oil red O. As shown in
Fig. 3 the H&E staining
results for liver tissues demonstrated that in the HCD group, lipid
droplets appeared as small vacuoles within liver cells. Enlargement
of lipid droplets was more pronounced in the liver tissue of rats
in the HCD group than the HVC1 group (250 mg/kg). In addition,
lipid accumulation in the aorta was more visible in the HCD group
than in the HVC1-treated groups (Fig.
4). Thus, the results for each representative tissue clearly
showed that lipid accumulation in the liver and aorta was higher in
the HCD group than in the HVC1 group (250 mg/kg).
Effects of HVC1 on the mRNA expression of
hepatic PPAR-γ, HMG-CoA R and LDLR
To investigate the effects of HVC1 on the mRNA
expression of hepatic PPAR-γ, HMG-CoA R and LDLR, RT-qPCR was
performed. As shown in Fig. 5A and
B, the mRNA expression of PPAR-γ and HMG-CoA R was upregulated
in the HCD group as compared with the ND group, and administration
of HVC1 significantly and dose-dependently suppressed the mRNA
expression of PPAR-γ and HMG-CoA R. In addition, administration of
HVC1 (250 mg/kg) significantly reversed the HCD-induced reduction
of LDLR mRNA expression (Fig. 5C).
Notably, HVC1 had a more potent effect than simvastatin.
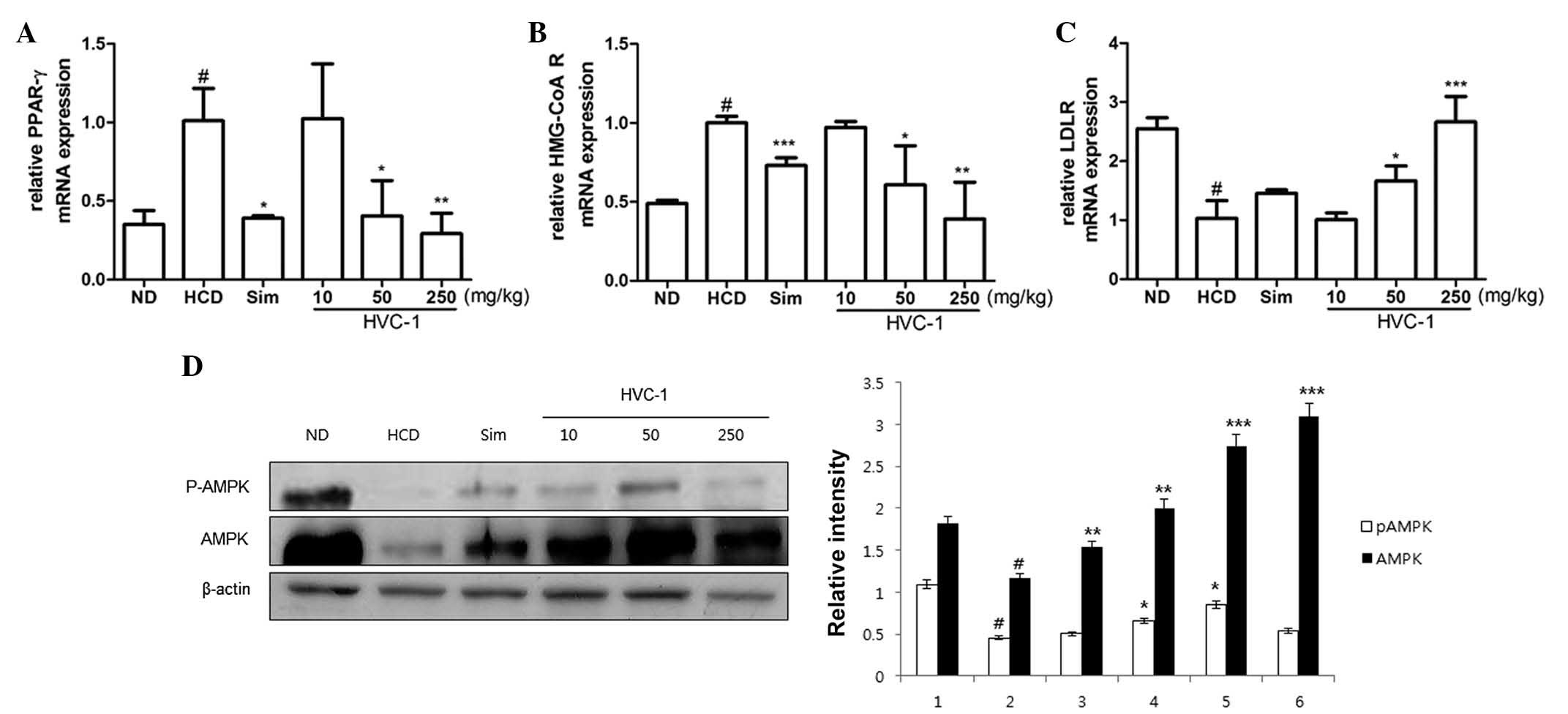 | Figure 5Effects of HVC1 on mRNA expression of
PPAR-γ, HMG-CoA R and LDLR, and the activation of AMPK in the liver
tissue. Total RNA was prepared for the real-time-PCR analysis of
(A) PPAR-γ, (B) HMG-CoA R and (C) LDLR gene expression from liver
tissues. Reverse transcription-quantitative polymerase chain
reaction analysis was conducted using a Step One Plus Real-time PCR
system. (D) The liver tissue was homogenized and total protein was
prepared. Western blot analysis was performed using specific
antibodies. β-actin was used as an internal control. ND, Normal
diet group; HCD, High-cholesterol diet group; Sim, Simvastatin (10
mg/kg) treated with HCD group; HVC1, HVC1 treated with HCD group.
The values are represented as the mean ± standard error of the mean
(n=6). #P<0.05, compared to ND;
*P<0.05, **P<0.01 and
***P<0.001 compared to HCD. PPAR-γ, peroxisome
proliferator-activated receptor-γ; HMG-CoA R,
3-hydroxy-3-methylglutaryl-CoA reductase; LDLR, low-density
lipoprotein receptor; AMPK, AMP-activated protein kinase; p-,
phosphorylated. |
Effects of HVC1 on the activation of
AMPK
Since AMPK has been implicated in lipid metabolism
(17), it was investigated whether
HVC1 could inhibit HCD-induced dephosphorylation of AMPK.
Phosphorylation and total protein levels of AMPK were decreased by
HCD and HVC1 significantly reversed these effects (Fig. 5D).
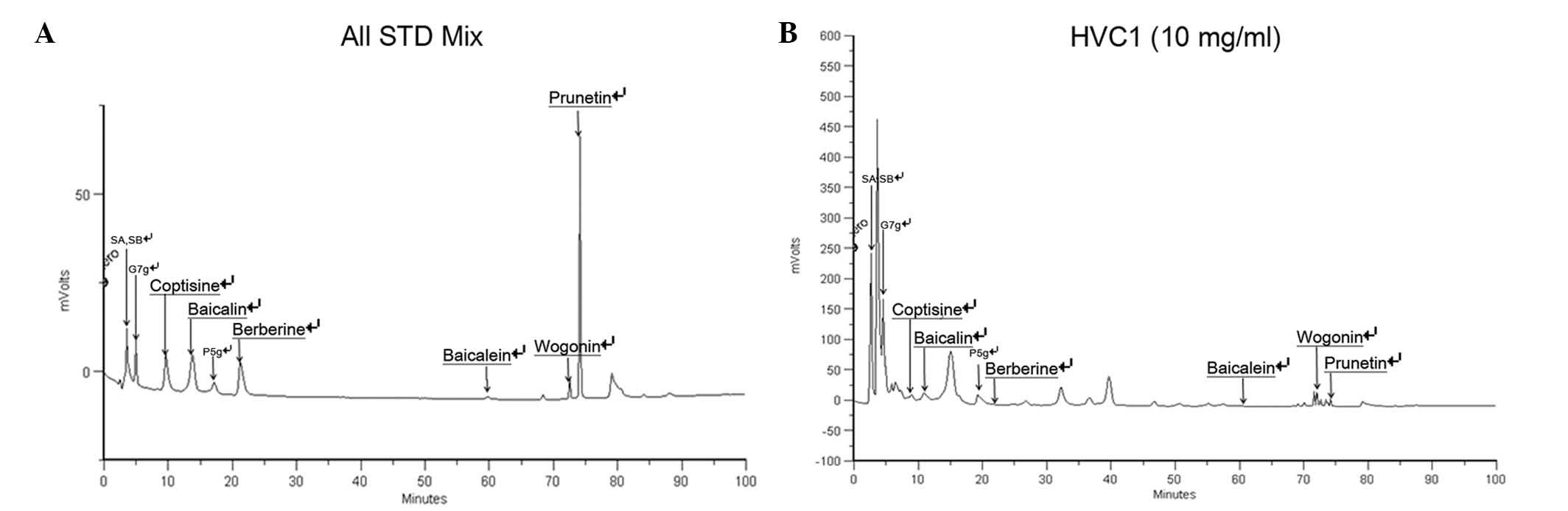
Standard material analysis
For standardization, HPLC analysis was performed.
The retention time of the sample mixture was as follows: 3.49 min
for sennoside A and sennoside B, 4.98 min for genistein-7-glucose,
9.61 min for coptisine, 13.78 min for baicalin, 17.18 min for
prunetin-5-glucose, 21.22 min for berberine, 59.76 min for
baicalein, 72.53 min for wogonin, 74.12 min for prunetin (Fig. 6A and B).
Discussion
In the present study, the hypolipidemic effects of
HVC1 against HCD-induced hyperlipidemia in rats were investigated.
HVC1 significantly reduced serum lipid levels and inhibited the
expression of PPAR-γ, HMG-CoA R and LDLR.
Increased serum concentrations of LDL cholesterol
and triglycerides are atherogenic and have been recognized as a
risk factor for cardiovascular diseases (18). An increased HDL level has been
considered cardioprotective. In this study, HVC1 decreased TC and
LDL, and increased HDL in the serum of HCD fed rats. In the same
manner, lipid accumulation in the aorta was less visible in the
HVC1-treated groups than in the HCD group. HVC1 consists of R.
palmatum rhizome, P. yedoensis bark, C. chinensis
rhizome and S. baicalensis radix, all of which are known to
positively affect lipid metabolism. According to the present study,
these four herbs or active compounds may be associated with the
powerful effect of HVC1.
Generally, the liver is considered an essential
organ in lipid metabolism. As shown in Fig. 3, HCD induced lipid accumulation in
the liver tissue, but treatment with HVC1 suppressed lipid droplet
content in liver tissue. Hepatic lipid metabolism is a highly
co-ordinated process, in which numerous pathways are regulated by
transcription (19). PPAR-γ is a
nuclear receptor and ligand-activated transcription factor that is
involved in the expression of lipogenic enzymes, such as acetyl CoA
carboxylase and fatty acid synthetase (20). HMG-CoA R is a transmembrane protein
that is implicated in the synthesis of lipids. It is the
rate-limiting step in cholesterol synthesis and represents the
major target for the cholesterol-lowering drugs, statins (21). Inhibition of the HMG-CoA R induces
the expression of LDLR in the liver (22). LDLR is a cell-surface receptor that
increases the catabolism of plasma LDL and lowers the plasma
concentration of cholesterol. LDL-cholesterol binds to the LDLR, is
internalized in a process known as endocytosis, and prevents the
LDL diffusing around the membrane surface (23). Endocytosis occurs predominantly in
the liver, which removes ~70% of LDL from the circulation (24). In this study, the mRNA expression
of PPAR-γ, HMG-CoA R and LDLR were significantly and
dose-dependently recovered by HVC1. These results indicate that
HVC1 could reduce serum lipid levels and fat accumulation in the
liver and aorta through the regulation of gene expression.
AMPK is a well-known regulator of lipid metabolism
in the liver, and is also involved in cellular energy homeostasis
(19). It is known that AMPK is
regulated by phosphorylation and inactivates HMG-CoA R, a key
enzyme in cholesterol synthesis in the liver (25). To investigate a possible mechanism
for the hypolipidemic effects of HVC1, the phosphorylation and
total protein level of AMPK were examined. HVC1 significantly
reversed the reduction of AMPK phosphorylation, as well as the
reduction of total AMPK protein levels. This data indicates that
HVC1 could regulate AMPK at a transcriptional or translational
level. Therefore, it may also be possible that HVC1 exerts its
hypolipidemic effects through the regulation of AMPK.
In conclusion, HVC1 effectively suppressed serum
lipid levels and fat accumulation in the liver and aorta of rats
with HCD-induced hyperlipidemia. The mechanisms underlying the
hypolipidemic effect of HVC1 appear to involve the recovery of
PPAR-γ, HMG-CoA R and LDLR expression through the induction of
AMPK. The findings clearly demonstrate that HVC1 has a potent
hypolipidemic effect, and suggests that HVC1 should be evaluated as
a potential treatment for hyperlipidemia.
Acknowledgments
This study was supported by a grant from the Korea
Health-care Technology R&D Project, Ministry of Health &
Welfare, Republic of Korea (grant no. B110081).
References
|
1
|
Saha SA and Arora RR: Hyperlipidaemia and
cardiovascular disease: Do fibrates have a role? Curr Opin Lipidol.
22:270–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn D: Illustrated book of Korean
medicinal herbs. Kyohak Publishing; 1998
|
|
3
|
Kim J: Illustrated natural drugs
encyclopedia, colorth edition ed. Namsandang; 1997
|
|
4
|
Lee K, Ham I, Yang G, Lee M, Bu Y, Kim H
and Choi HY: Vaso-relaxant effect of Prunus yedoensis bark. BMC
Complement Altern Med. 13:312013. View Article : Google Scholar
|
|
5
|
Ahn TG, Yang G, Lee HM, Kim MD, Choi HY,
Park KS, Lee SD, Kook YB and An HJ: Molecular mechanisms underlying
the anti-obesity potential of prunetin, an O-methylated isoflavone.
Biochem Pharmacol. 85:1525–1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Zhang XL, Tao RY, Niu YJ, Chen XG,
Tian JY and Ye F: Rhein, an inhibitor of adipocyte differentiation
and adipogenesis. J Asian Nat Prod Res. 13:714–723. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Q, Lu G, Shen HM, Chung MC and Ong
CN: Anti-cancer properties of anthraquinones from rhubarb. Med Res
Rev. 27:609–630. 2007. View Article : Google Scholar
|
|
8
|
Guo MZ, Li XS, Xu HR, Mei ZC, Shen W and
Ye XF: Rhein inhibits liver fibrosis induced by carbon
tetrachloride in rats. Acta Pharmacol Sin. 23:739–744.
2002.PubMed/NCBI
|
|
9
|
Choi SB, Ko BS, Park SK, Jang JS and Park
S: Insulin sensitizing and alpha-glucoamylase inhibitory action of
sennosides, rheins and rhaponticin in Rhei Rhizoma. Life Sci.
78:934–942. 2006. View Article : Google Scholar
|
|
10
|
Sheng X, Wang M, Lu M, Xi B, Sheng H and
Zang YQ: Rhein ameliorates fatty liver disease through negative
energy balance, hepatic lipogenic regulation, and immunomodulation
in diet-induced obese mice. Am J Physiol Endocrinol Metab.
300:E886–E893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie W, Gu D, Li J, Cui K and Zhang Y:
Effects and action mechanisms of berberine and Rhizoma coptidis on
gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS
One. 6:e245202011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie HC and Shang J: Study on the
extraction process of total anthraquinones in Radix et Rhizoma Rhei
and their antilipemic effects. Afr J Tradit Complement Altern Med.
11:358–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee IS, Park S, Park K and Choue R:
Hepatoprotective activity of scutellariae radix extract in mice fed
a high fat diet with chronic alcohol exposure. Phytother Res.
25:1348–1353. 2011.PubMed/NCBI
|
|
14
|
Bak EJ, Kim J, Choi YH, Kim JH, Lee DE,
Woo GH, Cha JH and Yoo YJ: Wogonin ameliorates hyperglycemia and
dyslipidemia via PPARα activation in db/db mice. Clin Nutr.
33:156–163. 2014. View Article : Google Scholar
|
|
15
|
Nunnari JJ, Zand T, Joris I and Majno G:
Quantitation of oil red O staining of the aorta in
hypercholesterolemic rats. Exp Mol Pathol. 51:1–8. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee K, Kim B, Hur H, Chinannai KS, Ham I
and Choi HY: Antihypertensive effect of the GaMiSamHwangSaSimTang
in spontaneous hypertensive rats. Evid Based Complement Alternat
Med. 2015:8023682015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Unger RH: The hyperleptinemia of
obesity-regulator of caloric surpluses. Cell. 117:145–146. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boruah DC, Devi R, Tamuli S, Kotoky J and
Sharma DK: Hypolipidemic activity of crude polyphenols from the
leaves of Clerodendron colebrookianum Walp in cholesterol fed rats.
J Food Sci Technol. 51:3333–3340. 2014. View Article : Google Scholar
|
|
19
|
Jump DB, Tripathy S and Depner CM: Fatty
acid-regulated transcription factors in the liver. Annu Rev Nutr.
33:249–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walczak R and Tontonoz P: PPARadigms and
PPARadoxes: Expanding roles for PPARgamma in the control of lipid
metabolism. J Lipid Res. 43:177–186. 2002.PubMed/NCBI
|
|
21
|
Istvan ES and Deisenhofer J: Structural
mechanism for statin inhibition of HMG-CoA reductase. Science.
292:1160–1164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rudling M: Hepatic mRNA levels for the LDL
receptor and HMG-CoA reductase show coordinate regulation in vivo.
J Lipid Res. 33:493–501. 1992.PubMed/NCBI
|
|
23
|
Südhof TC, Goldstein JL, Brown MS and
Russell DW: The LDL receptor gene: A mosaic of exons shared with
different proteins. Science. 228:815–822. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pieper-Fürst U and Lammert F: Low-density
lipoprotein receptors in liver: Old acquaintances and a newcomer.
Biochim Biophys Acta. 1831:1191–1198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim CT, Kola B and Korbonits M: AMPK as a
mediator of hormonal signalling. J Mol Endocrinol. 44:87–97. 2010.
View Article : Google Scholar
|