Introduction
Acute kidney injury (AKI), referred to acute renal
failure, is a type of clinical syndrome caused by multiple factors.
Different clinical manifestations occur and the predominant
characteristic is a decrease in the rate of glomerular filtration
(1). AKI is a common clinical
critical disease, with incidence rates as high as 2/3 in AKI
disease. Three major parallel complications can occur, including
respiratory failure and shock, in AKI patients, and these are
independent risk factors to predict the mortality of patients with
AKI. According to a previous report, the incidence of AKI in
hospitals was 4.05% and the mortality rate was as high as 12.7%
(2). In China's comprehensive
hospitals, patients with AKI accounted for 3.19% of hospitalized
patients (3). In the USA in 2011,
AKI medical costs were as much as $4.7 billion (4). Although modern medical treatment
conditions and medical technology have improved, and a variety of
alternative treatments exist, AKI morbidity and mortality remains
high. This is a difficult problem worldwide (5).
The mechanism of ischemia/reperfusion-induced acute
kidney injury (I/R-IAKI) remains to be fully elucidated. It is
known that at least three factors are involved: Reactive oxygen
species, neutrophils and the Complement system (6). Previous research has demonstrated
that I/R-IAKI is closely associated with the generation of reactive
oxygen free radicals (7). It was
also confirmed that oxygen free radicals in the I/R-IAKI serve a
highly important role (8).
I/R-IAKI generates oxygen free radicals, which have strong
oxidation activity, and can damage biological molecules,
particularly lipids (9). Cell
membrane and lipid peroxidation of unsaturated fatty acids on
mitochondrial and lysosomal membranes, can lead to damage to the
structure and function of renal tubular cells (9). Eventually lipid peroxide breaks down
into malondialdehyde (MDA), and MDA also serves a cytotoxic effect,
adding to I/R injury.
Ursolic acid is widely distributed in nature and
exists in numerous plants, including hawthorn, bearberry, Chinese
elder herb, fructus ligustri lucidi, lanatoside kiwi fruit,
dogwood, wild rose fruit, Chinese sage, gentiana, Prunella
vulgaris, spreading hedyotis herb, chrysanthemum cuckoo, azalea
and forsythia (10). Ursolic acid
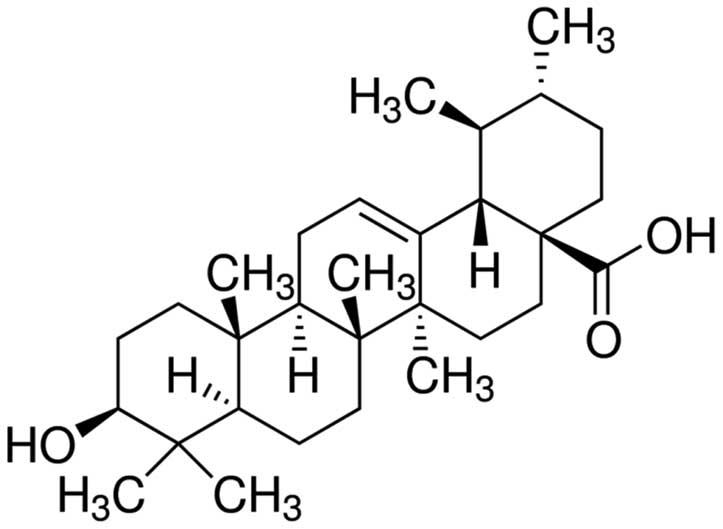
is a pentacyclic triterpene compound, with the molecular formula
for C30H48O3 and a molecular
weight of 456.68 (Fig. 1). Certain
Chinese herbal medicines containing ursolic acid exert a variety of
effects, including effects on blood stasis, as an analgesic, as an
anti-inflammatory, and as a protective agent in the liver (11). Modern pharmacological studies have
found that ursolic acid has extensive biological effects,
particularly in antitumor, antioxidant, resistance to viral
hepatitis and anti-inflammatory (12). The present study hypothesized that
ursolic acid may affect I/R-IAKI, thus functioning in antioxidant,
anti-inflammatory and the STAT3/NF-κB signaling pathway. The
present study aimed to demonstrate the renoprotective effects of
ursolic acid on I/R-IAKI through oxidative stress, inflammation and
inhibiting STAT3 and NF-κB activities in rat.
Materials and methods
Reagents
Ursolic acid (purity 90%) and sodium pentobarbital
were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Angiotensin-II kits were obtained from SpiBio (Bertin-Pharma, Lille
Cedex, France). Tumor necrosis factor (TNF)α, interleukin (IL)-6,
IL-1β and nuclear factor (NF)-κB p65 unit, microvessel density and
superoxide dismutase (SOD) specific enzyme-linked immunosorbent
assay (ELISA) kits were obtained from Jiancheng Bioengineering
Institute (Nanjing, China).
Animals and I/R-IAKI model
A total of 30 male Sprague-Dawley rats (age,
2-month-old; weight, 230–300 g), were purchased from the Animal
Experiment Center of Wuhan University (Wuhan, China) maintained
under a 12 h dark/light cycle at 23–24°C with a relative humidity
of 40–60%. All studies performed on animals were approved by the
Institutional Animal Care and the local Ethics Committee.
Sprague-Dawley rats were anaesthetized using sodium pentobarbital
(40 mg/kg, Sigma Chemical Co.). A right nephrectomy was performed
from a dorsal incision. Additionally, by means of dorsal incision,
the left kidney and renal vessels were exposed and occluded with a
vascular clip. After 45–90 min clamping, the vascular clip was
removed and I/R-IAKI model rat was successful created.
Experimental design
The 30 rats were divided into three groups
(n=10/group): i) Sham group with sham-operated rats administered
with saline; ii I/R-IAKI group where the I/R-IAKI model rats were
administered with saline; iii) ursolic acid group where the
I/R-IAKI model rats were administered with 10 mg/kg ursolic acid
twice daily for 4 days (13).
Blood pressure measurement
Sprague-Dawley rats were individually caged at 37°C
for 30 min and were subsequently transferred to a fixed measurement
cage following warming at 37°C. Blood pressure was measured using a
Narcosystem Device (North Dakota). The Sprague-Dawley rats were
restrained and body length and weight were measured.
Renal functioning evaluation
Following anesthesia with 5% chloral hydrate, blood
samples were extracted from the rat tail vein into test tubes and
centrifuged at 3,000 × g for 10 min at 4°C. Following
centrifugation, the supernatants were collected and the serum
creatinine and cystatin-C levels were measured using a
Roche/Hitachi 917 autoanalyzer (Roche Diagnostics, Indianapolis,.
IN, USA), according to the manufacturer's protocol.
Measurement of angiotensin-II
Blood samples were extracted from the tail vein into
test tubes and centrifuged at 3,000 × g for 10 min at 4°C.
Following centrifugation, the supernatants were collected and the
levels of angiotensin-II were measured using a specific ELISA
(SpiBio), according to the manufacturer's protocol.
Measurement of inflammatory status and
oxidative stress
Blood samples were extracted from the tail vein into
test tubes and centrifuged at 3,000 × g for 10 min at 4°C.
Following centrifugation, the supernatants were collected and the
levels of TNF-α, IL-6, IL-1β, IL-10, MDA, SOD and NF-κB p65 unit
were measured using specific ELISA kits (Jiancheng Bioengineering
Institute), according to the manufacturer's protocols.
Western blotting analysis of STAT3
The rats were sacrificed by brief halothane overdose
and renal tissues samples were homogenated with
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Jiangsu, China). The solution was centrifuged at
3,000 × g for 10 min at 4°C. Following centrifugation, the
supernatants were collected and the protein contents were measured
using a Coomassie brilliant blue assay (Beyotime Institute of
Biotechnology). Equivalent quantities (40 µg) of protein
were separated by electrophoresis using 12% sodium dodecyl
sulfate-poly-acrylamide gels and were subsequently transferred onto
nitrocellulose filter membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% non-fat milk for 1 h at
37°C. Following blocking, the membranes were incubated with the
corresponding primary antibodies against anti-STAT3 (1:2,000; cat.
no. sc-8001) and anti-β-actin (cat. no. 130656; 1:5,000) both from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) overnight at
4°C. Following this, the membranes were washed with Tris-buffered
saline with Tween 20 and incubated with goat anti-rabbit secondary
antibodies (1:5,000; BestBio, Inc., Shanghai, China; cat. no.
BB-2202-1) for 2–3 h at room temperature. The bands were visualized
using enhanced chemiluminescence detection (Amersham, Arlington
Heights, IL, USA).
Measurement of caspase-3
The rats were sacrificed by brief halothane overdose
and renal tissues samples were homogenated with
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Jiangsu, China). The solution was centrifuged at
3,000 × g for 10 min at 4°C. Following centrifugation, the
supernatants were collected and the protein contents were measured
using a Coomassie brilliant blue assay (Beyotime Institute of
Biotechnology). Equivalent quantities of protein (50 µg)
were incubated with Ac-LEHD-pNA (Beyotime Institute of
Biotechnology) at 37°C for 2 h in the dark and the caspase-3 level
was measured at an absorbance of 405 nm.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Statistical analyses were performed where appropriate
with the Student-Newman-Keuls method using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Ursolic acid demonstrated no effect on
blood pressure monitoring
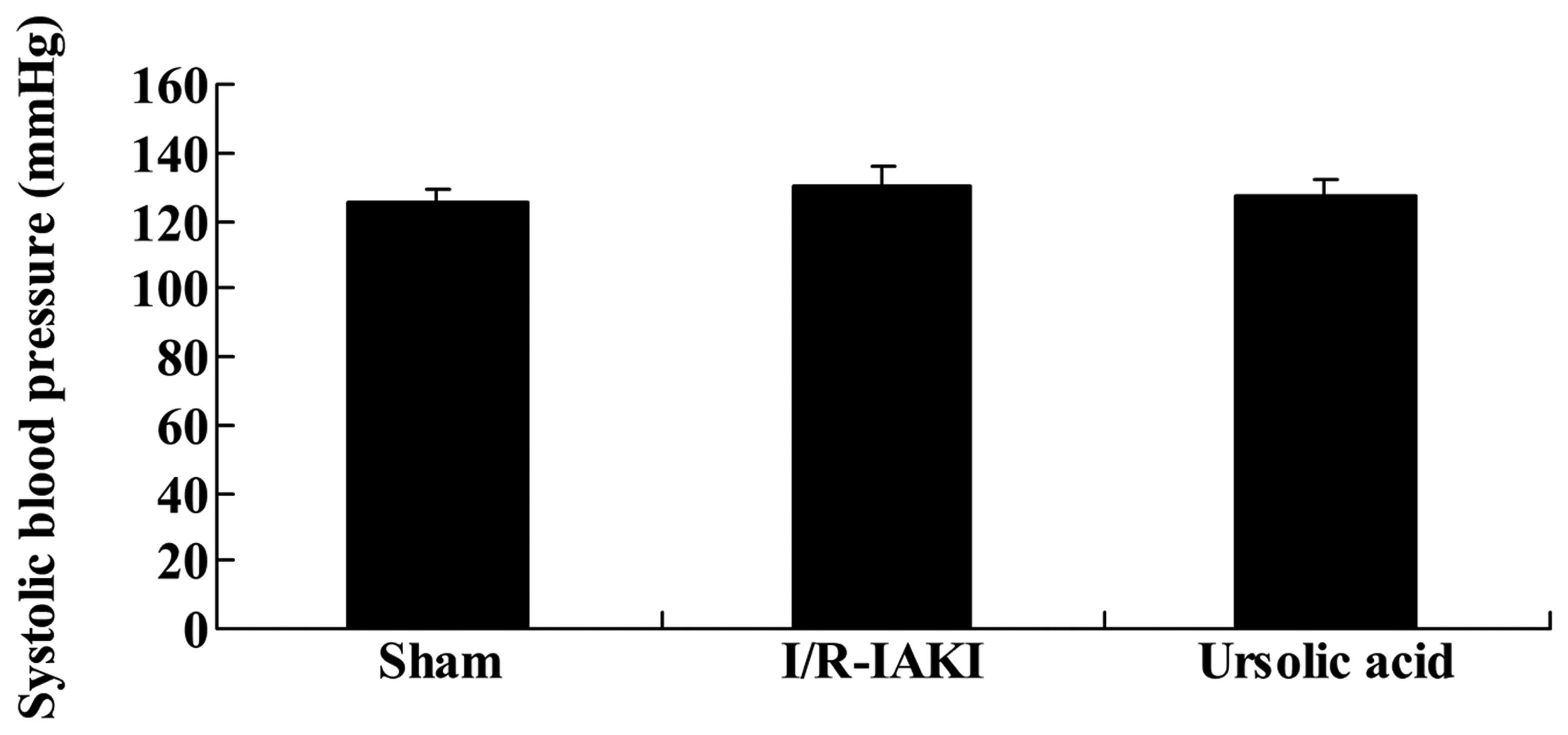
To assess the renoprotective effects of ursolic acid
on I/R-IAKI, blood pressure monitoring was performed. As shown in
Fig. 2, no significant changes
were determined between the groups (P=0.911).
Ursolic acid exerted a renoprotective
effect on renal functioning
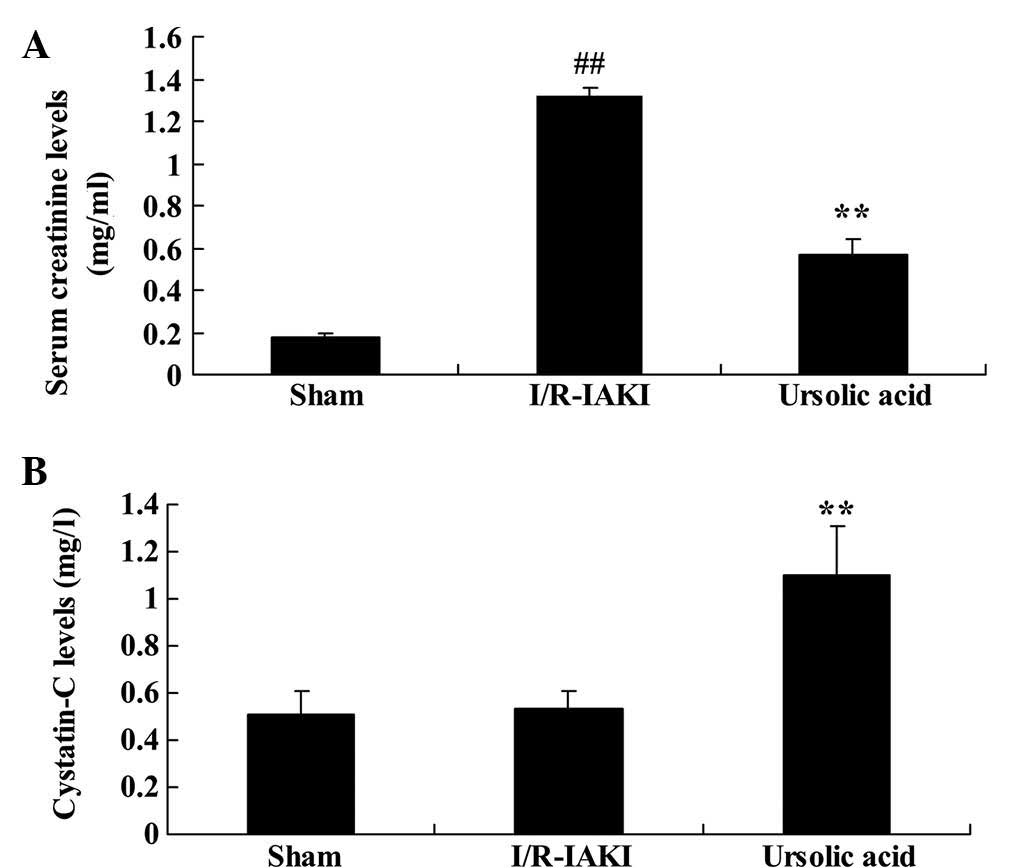
The present study next probed the renoprotective
effects of ursolic acid on I/R-IAKI renal functioning. I/R-IAKI
significantly increased the levels of serum creatinine in rats
compared with that of the sham group (Fig. 3; P=0.0001). However, I/R-IAKI
revealed no effect on the serum levels of cystatin-C (Fig. 3). Additionally, the increased serum
creatinine was effectively inhibited and the serum cystatin-C
levels were effectively increased by treatment with ursolic acid,
as compared with those of the I/R-IAKI group (Fig. 3; P=0.0009).
Ursolic acid decreased levels of
angiotensin-II
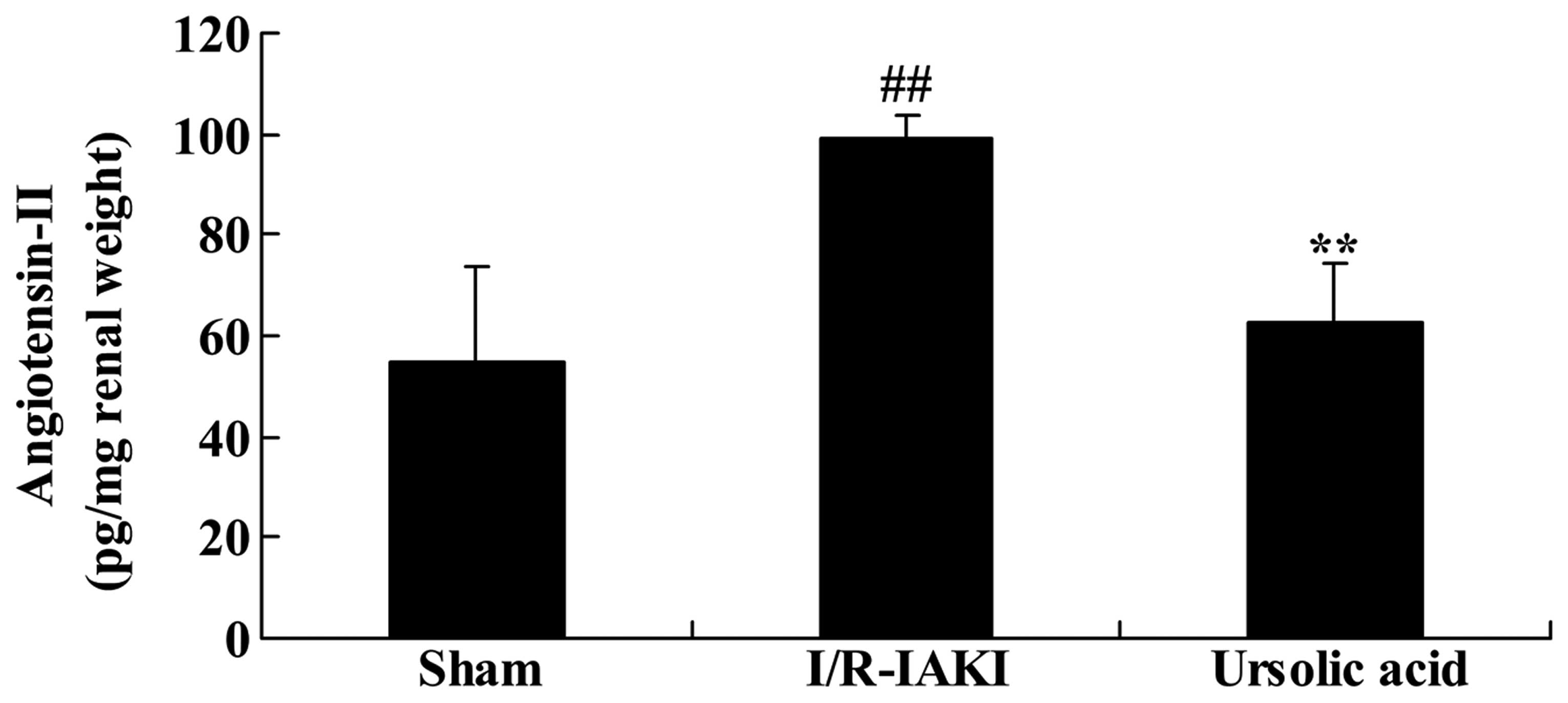
To investigate the renoprotective effects of ursolic
acid on I/R-IAKI, the serum angiotensin-II level was determined in
the present study. I/R-IAKI increased the serum levels of
angiotensin-II in the rats compared with that of the sham group
(Fig. 4; P=0.0038). Treatment with
ursolic acid suppressed the enhancement of serum angiotensin-II
levels in I/R-IAKI rats (Fig. 4;
P=0.0042), when compared with the I/R-IAKI rats.
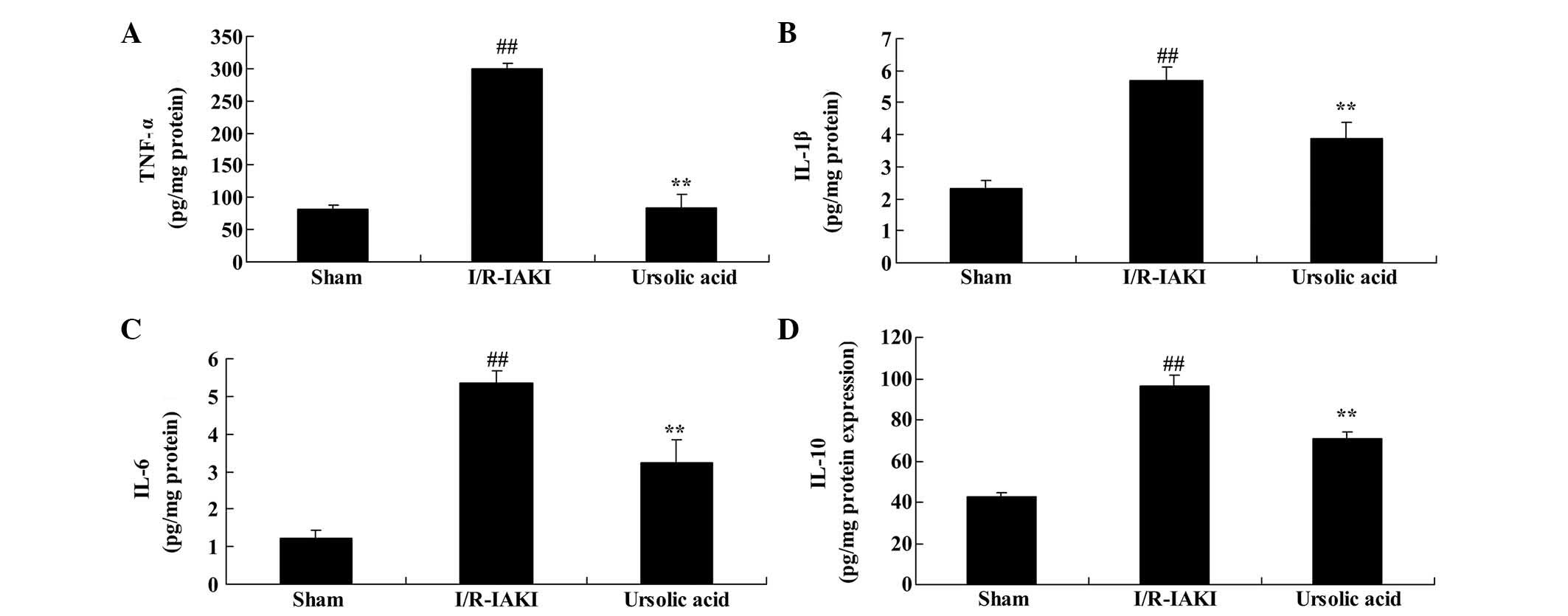
Ursolic acid reduced inflammation
To further determine the renoprotective effects of
ursolic acid on I/R-IAKI, the serum levels of TNF-α, IL-6, IL-1β
and IL-10 were measured following injury. I/R-IAKI significantly
increased the serum levels of TNF-α, IL-6, IL-1β and IL-10 compared
with the sham group (Fig. 5;
P=0.0009, P=0.0051, P=0.0007 and P=0.0076, respectively). As
presented in Fig. 5, pre-treatment
with ursolic acid significantly reduced the I/R-IAKI-induced serum
levels of TNF-α, IL-6, IL-1β and IL-10 in the I/R-IAKI rats
(P=0.0011, P=0.0066, P=0.0053 and P=0.0072, respectively).
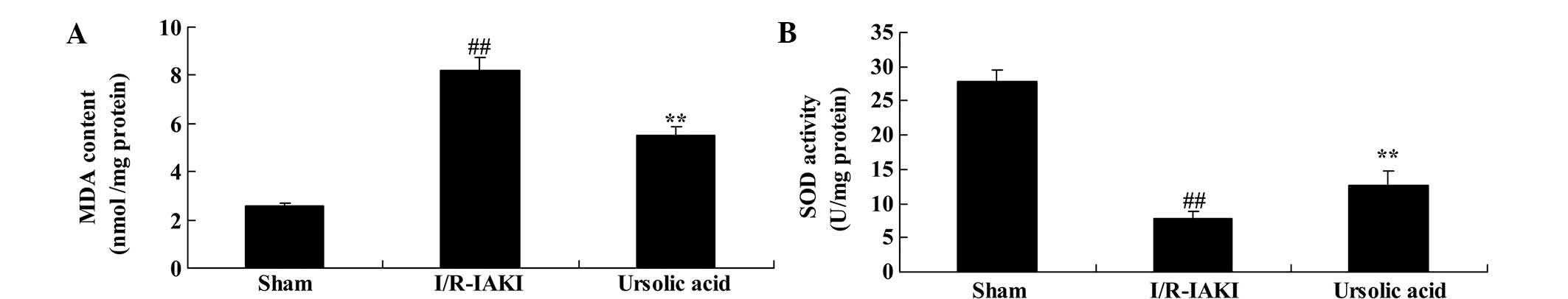
Ursolic acid reduced oxidative
stress
Additionally, the present study determined the serum
levels of MDA and SOD following injury. As shown in Fig. 6, I/R-IAKI caused increased serum
levels of MDA and suppressed the serum levels of SOD in rats
(P=0.0039 and P=0.0026, respectively). Oxidative stress was
effectively reduced following treatment ursolic acid (Fig. 6; P=0.0016 and P=0.0079,
respectively).
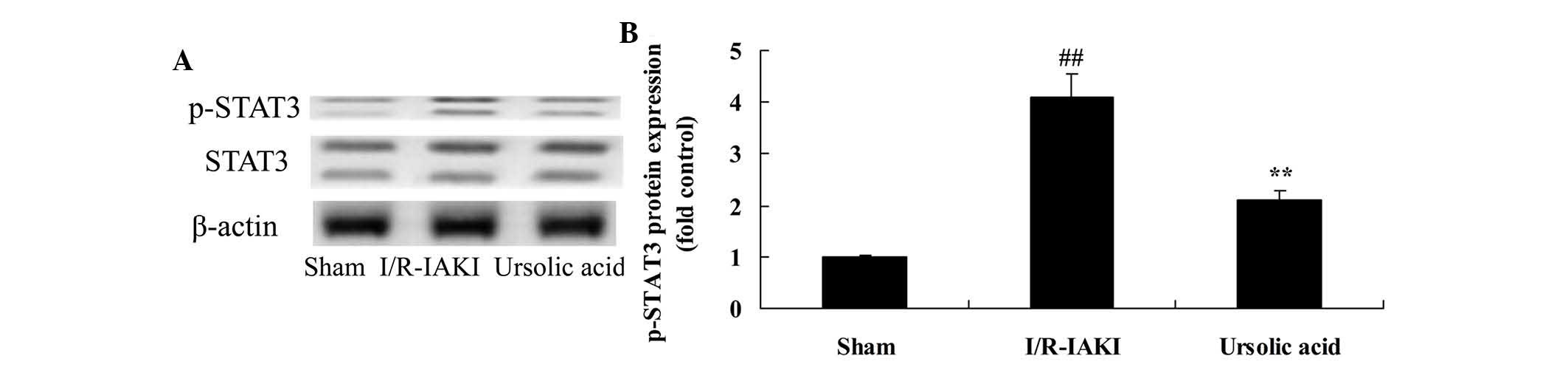
Ursolic acid reduced levels of STAT3
To further investigate the renoprotective effects of
ursolic acid on I/R-IAKI, the protein expression levels of
phosphorylated (p)-STAT3 were measured following injury. I/R-IAKI
rats exhibited a significant progressive increase in the protein
expression levels of p-STAT3 compared with that of the sham group
(Fig. 7; P=0.0011). Following
ursolic acid treatment, the protein expression levels of p-STAT3
were significantly reduced in I/R-IAKI rats (Fig. 7; P=0.0044).
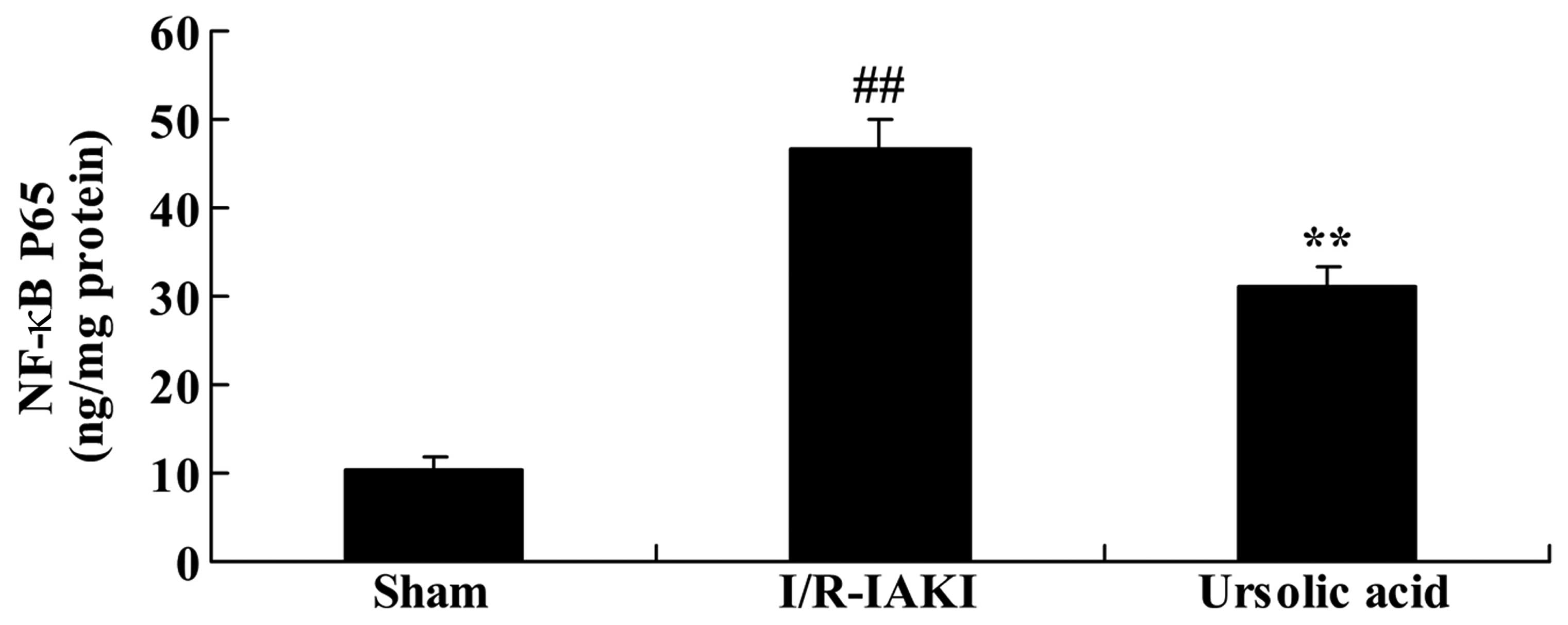
Ursolic acid decreased NF-κB expression
in the I/R-IAKI rats
The levels of NF-κB were measured following injury.
In the I/R-IAKI rats, NF-κB level was significantly augmented
compared with the rats of the sham group (Fig. 8; P=0.0016). Following treatment
with ursolic acid, the increased level of NF-κB was significantly
inhibited compared to that of the I/R-IAKI group (Fig. 8; P=0.0003).
Ursolic acid reduced the increase in
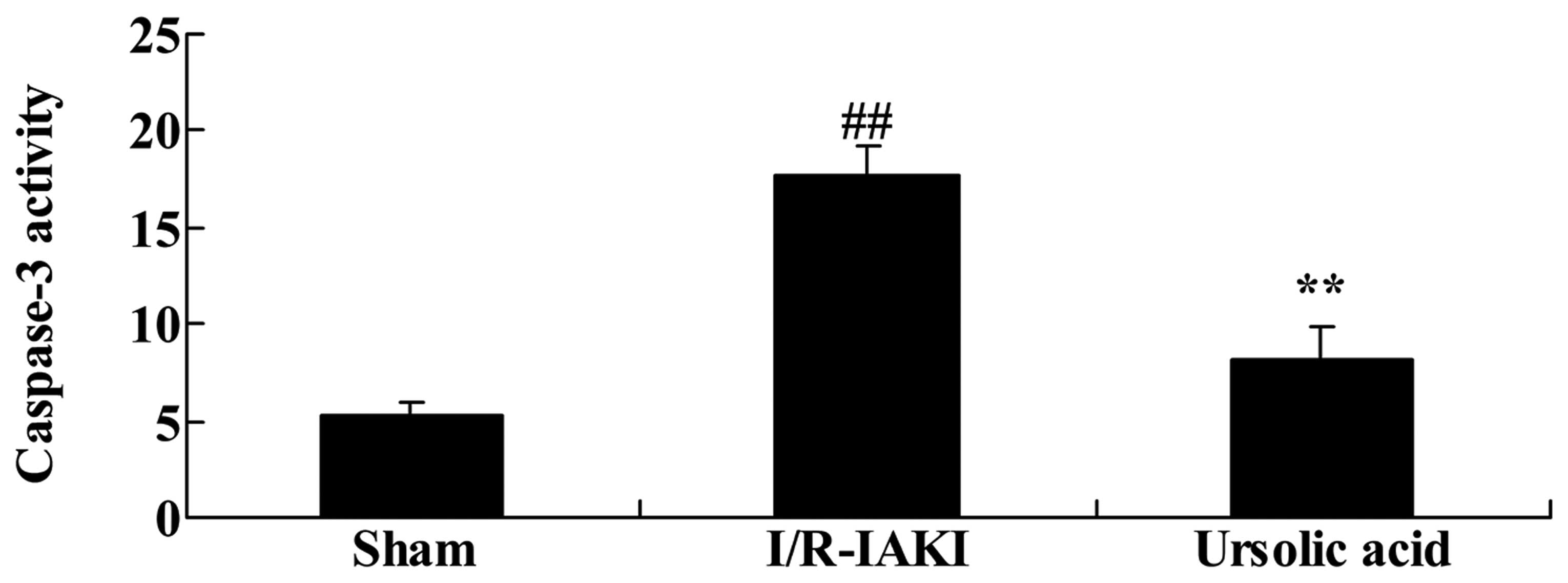
caspase-3 observed in I/R-IAKI rats
To further understand the renoprotective effects of
ursolic acid on I/R-IAKI, the caspase-3 activity was assessed
following injury. As shown in Fig.
9, I/R-IAKI caused an increase in caspase-3 activity in rats
compared with that of the sham group (Fig. 9; P=0.00032). Following treatment
with ursolic acid, the increase in caspase-3 activity was
significantly weakened in the I/R-IAKI rats (Fig. 9; P=0.00071).
Discussion
At present, the underlying mechanisms of I/R-IAKI
remains unclear. In the I/R-IAKI process, a large number of free
radicals are generated, resulting in calcium overload and cell
apoptosis. This interferes with energy metabolism. Neutrophil
infiltration is important role in vascular endothelial injury
(14). Existing literature
reported that I/R-IAKI free radical oxygen outbreak is important
for pathogenesis (15). In the
present study, the results confirmed that pre-treatment with
ursolic acid caused no affect on blood pressure monitoring and
increased renal functioning in I/R-IAKI rat, resulting in a
significant change that may decrease the I/R-IAKI-induced serum
angiotensin-II level. Li et al (16) reported that ursolic acid protected
the brain against ischemic injury in mice. Tundis et al
(17) reported that the isolation
of ursolic acid inhibits angiotensin-converting enzyme inhibitory
activity (17). A number of
results suggested that the protective effect of ursolic acid can be
used for the treatment of I/R-IAKI.
The I/R-IAKI systemic response may be an initial
cause of inflammation. Kidney I/R injury can lead to kidney
synthesis of pro-inflammatory factors, including IL-6, IL-1 and
TNF-α. This can also quickly produce a variety of chemokines,
including keratinocyte-derived chemokine, a rat chemotactic factor
similar to that of the IL-8 that can also be used as an I/R-IAKI
biochemical marker (18). In the
present experimental setting, ursolic acid treatment significantly
reduced the I/R-IAKI-induced expression of TNF-α, IL-6, IL-1β and
IL-10 in I/R-IAKI rats. Senthil et al (19) indicated that ursolic acids protects
against isoproterenol-induced myocardial ischemia through
antioxidant and anti-inflammatory functions in rats (19). This accumulation suggested that the
anti-inflammatory effect of ursolic acid is associated with
protection against I/R-IAKI.
Under normal physiological conditions, the body
maintains normal metabolic processes and can produce the correct
quantity of free radicals. A large number of free radicals has a
damaging effect to the body. However, a large number of enzymes can
remove free radicals from the body exerting antioxidant properties
to prevent damage due to free radicals (20). The radical scavenging enzyme
system, including SOD, MDA, hydrogen peroxide enzyme peptide,
glutathione peroxidase and I/R-IAKI, cause cytotoxicity damage
factors besides hypoxemia, can also directly induce inflammatory
reactions in renal parenchymal cells resulting in ischemic acute
renal failure (21). The present
study quantified the serum levels of MDA and SOD, and these were
significantly suppressed by treatment with ursolic acid in the
I/R-IAKI rat. Ma et al (22) suggested that ursolic acid protects
against CCl4-induced oxidative stress and inflammation
of the liver in mice. Ramachandran et al (23) concluded that ursolic acid protects
against exposure to ultraviolet B-induced reactive oxygen species
through modulation of antioxidants (23). The present results indicated that
the protective effect of ursolic acid on I/R-IAKI is correlated
with the modulation of oxidative stress.
JAKs (JAK1, JAK2, JAK3 and TYK2) and STATs protein
family (STAT1, STAT2, STAT3, STAT4, STAT5a and STAT5b, STAT6) have
been identified as signal transduction pathways in cells (24). Numerous cytokines, including IFN,
IL-2, IL-4, IL-6 and CNTF, and growth factors, including EGF, PDGF
and CSF, all using the signal transduction pathway induced cell
proliferation, differentiation and apoptosis. These proteins may
serve a special and pleiotropic biological function on immune
regulation, hematopoietic stem cells, cancer, nervous system and
embryonic development (25).
Previous studies have shown that the JAK/STAT signal transduction
pathways are involved in the development of the central nervous
system and nerve cell proliferation, survival, differentiation, and
they are closely associated with brain tumors, central nervous
system diseases, including cerebral ischemia, and pathological
physiological processes (26). The
present study found that ursolic acid treatment reduced the
increase of the protein expression levels of p-STAT3 in I/R-IAKI
rats. Pathak et al (27)
suggested that ursolic acid suppresses the cell proliferation of
multiple myeloma cells and inhibits STAT3 activation (27). Ma et al (28) suggested that ursolic acid protects
against oxidative DNA damage and inflammation by inhibiting the
STAT3 and NF-κB activities (28).
Taken collectively, ursolic acid may protect against
I/R-IAKI-induced oxidative damage and inflammation by inhibiting
STAT3.
It was previously shown in animal models that
hypoxemia can lead to the rapid activation of the NF-κB signaling
system, causing the transcription and synthesis of pro-inflammatory
factors (29). It was also shown
that in the stage of the ischemia, the NF-κB signaling system has
been activated and that signaling peaked after 15 min reperfusion,
indicating that the NF-κB signaling system may be very close to the
release of inflammation triggered by renal epithelial cell damage
signaling system (30). The
results from the present study indicated that treatment with
ursolic acid significantly reduced the increasing NF-κB and
caspase-3 level in the I/R-IAKI rat. Ma et al (28) suggested that ursolic acid protects
against oxidative DNA damage and inflammation by inhibiting the
activities of STAT3 and NF-κB. Lu et al (31) suggested that ursolic acid improves
cognitive impairments via NF-κB-mediated inflammatory pathways in
mice. By contrast, the present study found that NF-κB-mediated
inflammatory pathways may be an important mechanims by which
ursolic acid protects against I/R-IAKI.
In conclusion, the present study demonstrated that
the renoprotective effects of ursolic acid on I/R-IAKI occur via
oxidative stress, inflammation and inhibition of the activities of
STAT3 and NF-κB. Ursolic acid may serve a potentially important
role in the treatment of I/R-IAKI.
References
|
1
|
Kirkendall ES, Spires WL, Mottes TA,
Schaffzin JK, Barclay C and Goldstein SL: Development and
performance of electronic acute kidney injury triggers to identify
pediatric patients at risk for nephrotoxic medication-associated
harm. Appl Clin Inform. 5:313–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Renhua L, Miaolin C, Junlin W, Qingwei W,
Xiaoping X, Huili D, Weiming Z, Zhaohui N, Jiaqi Q and Yan Y: The
level of the biomarkers at the time of nephrology consultation
might predict the prognosis of acute kidney injury in hospitalized
patients. Blood Purif. 38:89–95. 2014.PubMed/NCBI
|
|
3
|
Li W, Qian J, Liu X, Zhang Q, Wang L, Chen
D and Lin Z: Management of severe crush injury in a front-line tent
ICU after 2008 Wenchuan earthquake in China: An experience with 32
cases. Crit Care. 13:R1782009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai TT, Patel UD, Chang TI, Kennedy KF,
Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC,
Rumsfeld JS and Spertus JA: Contemporary incidence, predictors and
outcomes of acute kidney injury in patients undergoing percutaneous
coronary interventions: Insights from the NCDR Cath-PCI registry.
JACC Cardiovasc Interv. 7:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou C, Wang R, Ding Y, Du L, Hou C, Lu D,
Hao L and Lv W: Prognostic factors for acute kidney injury
following transarterial chemoembolization in patients with
hepatocellular carcinoma. Int J Clin Exp Pathol. 7:2579–2586.
2014.PubMed/NCBI
|
|
6
|
Chiang WC, Chien CT, Lin WW, Lin SL, Chen
YM, Lai CF, Wu KD, Chao J and Tsai TJ: Early activation of
bradykinin B2 receptor aggravates reactive oxygen species
generation and renal damage in ischemia/reperfusion injury. Free
Radic Biol Med. 41:1304–1314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du Y, Hou L, Guo J, Sun T, Wang X and Wu
Y: Renal neutrophil gelatinase-associated lipocalin and kidney
injury molecule-1 expression in children with acute kidney injury
and Henoch-Schönlein purpura nephritis. Exp Ther Med. 7:1130–1134.
2014.PubMed/NCBI
|
|
8
|
Wang Z, Ge Y, Bao H, Dworkin L, Peng A and
Gong R: Redox-sensitive glycogen synthase kinase 3β-directed
control of mitochondrial permeability transition: Rheostatic
regulation of acute kidney injury. Free Radic Biol Med. 65:849–858.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung FL, Zhu TY, Au-Yeung KK, Siow YL and
O K: Enhanced MCP-1 expression during ischemia/reperfusion injury
is mediated by oxidative stress and NF-kappaB. Kidney Int.
62:1160–1170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Li JS, Zhang X, Wu YJ, Huang K and
Zheng L: Ursolic acid inhibits early lesions of diabetic
nephropathy. Int J Mol Med. 26:565–570. 2010.PubMed/NCBI
|
|
11
|
Gong YY, Liu YY, Yu S, Zhu XN, Cao XP and
Xiao HP: Ursolic acid suppresses growth and adrenocorticotrophic
hormone secretion in AtT20 cells as a potential agent targeting
adrenocorticotrophic hormone-producing pituitary adenoma. Mol Med
Rep. 9:2533–2539. 2014.PubMed/NCBI
|
|
12
|
Novotný L, Vachálková A and Biggs D:
Ursolic acid: An anti-tumorigenic and chemopreventive activity.
Minireview Neoplasma. 48:241–246. 2001.
|
|
13
|
Hu Z, Gu Z, Sun M, Zhang K, Gao P, Yang Q
and Yuan Y: Ursolic acid improves survival and attenuates lung
injury in septic rats induced by cecal ligation and puncture. J
Surg Res. 194:528–536. 2015. View Article : Google Scholar
|
|
14
|
Lien YH, Lai LW and Silva AL: Pathogenesis
of renal ischemia/reperfusion injury: Lessons from knockout mice.
Life Sci. 74:543–552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia Y, Rao J, Yao A, Zhang F, Li G, Wang X
and Lu L: Lithium exacerbates hepatic ischemia/reperfusion injury
by inhibiting GSK-3β/NF-κB-mediated protective signaling in mice.
Eur J Pharmacol. 697:117–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Zhang X, Cui L, Wang L, Liu H, Ji H
and Du Y: Ursolic acid promotes the neuroprotection by activating
Nrf2 pathway after cerebral ischemia in mice. Brain Res.
1497:32–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tundis R, Nadjafi F and Menichini F:
Angiotensin-converting enzyme inhibitory activity and antioxidant
properties of Nepeta crassifolia Boiss & Buhse and Nepeta
binaludensis Jamzad. Phytother Res. 27:572–580. 2013. View Article : Google Scholar
|
|
18
|
Hagiwara M, Shen B, Chao L and Chao J:
Kallikrein-modified mesenchymal stem cell implantation provides
enhanced protection against acute ischemic kidney injury by
inhibiting apoptosis and inflammation. Hum Gene Ther. 19:807–819.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Senthil S, Chandramohan G and Pugalendi
KV: Isomers (oleanolic and ursolic acids) differ in their
protective effect against isoproterenol-induced myocardial ischemia
in rats. Int J Cardiol. 119:131–133. 2007. View Article : Google Scholar
|
|
20
|
Tawfik MK: Renoprotective activity of
telmisartan versus pioglitazone on ischemia/reperfusion induced
renal damage in diabetic rats. Eur Rev Med Pharmacol Sci.
16:600–609. 2012.PubMed/NCBI
|
|
21
|
Jin X, Zhang Y, Li X, Zhang J and Xu D:
C-type natriuretic peptide ameliorates ischemia/reperfusion-induced
acute kidney injury by inhibiting apoptosis and oxidative stress in
rats. Life Sci. 117:40–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma JQ, Ding J, Zhang L and Liu CM: Ursolic
acid protects mouse liver against CCl4-induced oxidative stress and
inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol.
37:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramachandran S and Prasad NR: Effect of
ursolic acid, a triterpenoid antioxidant, on ultraviolet-B
radiation-induced cytotoxicity, lipid peroxidation and DNA damage
in human lymphocytes. Chem Biol Interact. 176:99–107. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Yuan W, Liu Y, Zhang Y, Wang Z,
Zhou X, Ning G, Zhang L, Yao L, Feng S and Kong X: The role of the
JAK-STAT pathway in neural stem cells, neural progenitor cells and
reactive astrocytes after spinal cord injury. Biomed Rep.
3:141–146. 2015.PubMed/NCBI
|
|
25
|
Hsieh HL and Yang CM: Role of redox
signaling in neuroinflammation and neurodegenerative diseases.
Biomed Res Int. 2013:4846132013. View Article : Google Scholar
|
|
26
|
Susnik N, Sörensen-Zender I, Rong S, von
Vietinghoff S, Lu X, Rubera I, Tauc M, Falk CS, Alexander WS, Melk
A, et al: Ablation of proximal tubular suppressor of cytokine
signaling 3 enhances tubular cell cycling and modifies macrophage
phenotype during acute kidney injury. Kidney Int. 85:1357–1368.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pathak AK, Bhutani M, Nair AS, Ahn KS,
Chakraborty A, Kadara H, Guha S, Sethi G and Aggarwal BB: Ursolic
acid inhibits STAT3 activation pathway leading to suppression of
proliferation and chemosensitization of human multiple myeloma
cells. Mol Cancer Res. 5:943–955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma JQ, Ding J, Xiao ZH and Liu CM: Ursolic
acid ameliorates carbon tetrachloride-induced oxidative DNA damage
and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB
activities. Int Immunopharmacol. 21:389–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang KK, Liu LB, Li H, Mei J, Shao J, Xie
F, Li MQ and Li DJ: TSLP induced by estrogen stimulates secretion
of MCP-1 and IL-8 and growth of human endometrial stromal cells
through JNK and NF-κB signal pathways. Int J Clin Exp Pathol.
7:1889–1899. 2014.
|
|
30
|
Cao CC, Ding XQ, Ou ZL, Liu CF, Li P, Wang
L and Zhu CF: In vivo transfection of NF-kappaB decoy
oligodeoxynucleotides attenuate renal ischemia/reperfusion injury
in rats. Kidney Int. 65:834–845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu J, Wu DM, Zheng YL, Hu B, Cheng W,
Zhang ZF and Shan Q: Ursolic acid improves high fat diet-induced
cognitive impairments by blocking endoplasmic reticulum stress and
IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in
mice. Brain Behav Immun. 25:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|