Introduction
One of the common types of malignant cancer
affecting the head and neck is laryngeal cancer. As these types of
cancer are largely derived from the skin of the larynx, the
majority are cases of laryngeal squamous cell carcinoma (LSCC).
While early-stage LSCC is often curable with surgery or
radiotherapy, patients with advanced carcinoma have poor long-term
survival rates (1,2), as LSCCs are often difficult to remove
completely, and cancer recurrence following treatment remains a
significant obstacle.
Despite advances in anticancer therapeutics, the
outcome for advanced LSCC patients has not improved in the last two
decades. This is due to the fact that the molecular mechanisms
involved in the initiation and progression of LSCC remain largely
unknown (3). Therefore,
understanding the molecular mechanisms of LSCC progression is
crucial for improving therapies and the long-term prognosis of
patients.
One pathway that has been implicated in cell
survival, angiogenesis and resistance to therapy in various
different tumors is the Notch signaling pathway (4). Notch signaling is crucial during
embryonic development and in adult life. For the pathway to be
activated, one of the four Notch receptors expressed in mammals
(Notch 1–4) will interact with one of the five known Notch ligands,
jagged 1, jagged 2, Delta-like (DLL)1, DLL3 or DLL4, present on an
adjacent cell. Following ligand binding, the intracellular domain
of Notch (NICD) is cleaved by metalloproteases and γ-secretases.
The NICD then translocates from the plasma membrane to the nucleus,
where it can drive the expression of numerous genes, including
hes-related family bHLH transcription factor with YRPW motif 1, hes
family bHLH transcription factor 1, v-myc avian
myelocytomatosis viral oncogene homolog (Myc), cyclin D1 and B-cell
CLL/lymphoma 2 (Bcl-2), which regulate multiple cellular processes,
including cell proliferation, differentiation, stem cell
maintenance and apoptosis.
Notch pathway signaling has been previously reported
be tumor-suppressive and oncogenic (5,6).
Aberrant activation of this pathway has been associated with
tumorigenesis in prostate cancer development and metastasis
(7,8). Similarly, expression of the Notch
ligand jagged 1 is correlated with increased aggressiveness of
gastric cancer and poor patient survival rates (9). Dysregulation of Notch signaling has
been previously reported in human hematological malignancies and in
various solid tumors (6),
including cases of T-cell (10–12)
and B-cell (13–15) carcinoma. Additionally, Notch
signaling maintains the survival of stem and precursor cells in
multiple tissues, including the gut and glandular tissues, and
thus, also maintains the survival of cancer stem cells (16).
Notch signaling has also been previously
demonstrated to promote tissue differentiation, notably, in the
squamous epithelia of various organs and the skin. Therefore,
mutations that inactivate crucial components of the Notch signaling
pathway, including mutations in the Notch 1, 2 or 3 receptors, may
result in squamous cell carcinomas, including LSCC. However, the
precise mechanisms of the Notch pathway in LSCC development remain
unclear.
Multiple studies have reported that the Notch 2
signaling pathway in particular has important potential oncogenic
or tumor-suppressing actions in several hematologic malignancies,
including multiple myeloma, B cell chronic lymphocytic leukemia, B
and T cell acute lymphoblastic leukemia, and also in various solid
tumors, including glioblastoma and breast, cervical, colon,
pancreatic, skin and small cell lung cancer (17,18).
Consequently, the current study analyzed the expression of Notch 2
in clinical LSCC samples using quantum dots (QDs)-based
immunofluorescence histochemical staining. Additionally, knockdown
of NOTCH2 was performed in a Hep-2 cell line using short hairpin
RNA (shRNA). These studies aimed to elucidate the effects of Notch
2 on the proliferation, migration and invasion of LSCC.
Materials and methods
Patients
In total, 95 laryngeal carcinoma samples with
adjacent non-tumor tissues were obtained from patients who had
undergone surgery between 2009 and 2013 at the Renmin Hospital of
Wuhan University (Wuhan, China). All paraffin-embedded tissue
specimens were analyzed and reconfirmed by two experienced
pathologists. The clinicopathologic characteristics of the patients
were squamous cell carcinoma. All patients underwent selective neck
lymph node dissection and lymph node tissues were analyzed by
pathologists. If the clinicopathologic characteristics of lymph
nodes tissue were positive, the tissue was confirmed as LSCC with
lymph node metastasis. If the clinicopathologic characteristics of
the lymph node tissue was negative, the tissue of patients was
confirmed as LSCC without lymph node metastasis. The present study
was approved by the Ethics Committee of Renmin Hospital of Wuhan
University, and written informed consent was obtained from each
participant.
Laryngeal carcinoma specimens and cell
lines
The Hep-2 cell line was obtained from the China
Center for Type Culture Collection (Wuhan, China). Hep-2 cells were
cultured in RPMI-1640 (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and incubated in a humidified
atmosphere with 5% CO2 at 37°C.
RNA interference of NOTCH2 using
shRNA
The enhanced green fluorescent protein
(EGFP)-V-RS-Notch 2 shRNA plasmids were synthesized by Wuhan XiMa
Technologies Co., Ltd. (Wuhan, China). Cells were transfected with
shRNA vectors using Lipofectamine RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. The structure of the shRNA plasmid was as follows:
Stop-mir30-flanking-shRNA1-mir30-flanking-EG FP-CMV-U6-shRNA2-stop.
The shRNA sequences were as follows: Notch2 shRNA1,
5′-TGGAGGTCTCAGTGGATATAA-3′; and Notch2 shRNA2,
5′-AAGATCCTGTTAGACCATTT-3′. Three treatments were designed for the
current study. Untreated Hep-2 cells were considered the blank
control and termed the non-transfected group. The cells transfected
with the EGFP-V-RS-negative shRNA plasmid, containing non-specific
shRNA, and the EGFP-V-RS-Notch 2 shRNA plasmid, containing the
Notch 2-specific shRNA, were termed the negative-shRNA and Notch
2-shRNA groups, respectively.
QDs-based immunofluorescence
histochemistry
Tissue sections were deparaffinized in xylene and
rehydrated in a graded ethanol series. For antibody binding, slides
were first incubated with 2% bovine serum albumin (BSA; Gibco
Thermo Fisher Scientific, Inc.) at 37°C for 30 min, and then
incubated with primary rabbit anti-prostate stem cell antigen
antibody (rabbit polyclonal anti-Notch2 antibody; cat. no.
SAB4502020, Sigma-Aldrich, St. Louis, MO, USA, 1:100) diluted in
Tris-buffered saline (TBS) overnight at 4°C. Slides were then
washed in TBS. Negative control samples were prepared in parallel,
in which the primary antibody was replaced by TBS.
For QD conjugation, slides were incubated with 2%
BSA at 37°C for 10 min, and then incubated with ZnS-capped CdSe QDs
conjugated anti-rabbit IgG probes (Wuhan Jiayuan Quantum Dot
Technological Development Co., Ltd., Wuhan, China), with an
emission wavelength of 605 nm, diluted 1:50 in 2% BSA for 30 min at
37°C. Following incubation, the slides were vigorously washed with
TBS, mounted with neutral glycerol, and stored at 4°C until
observation.
The QDs were excited by blue light (excitation
wavelength of 450–480 nm under U-MWB filters) and the subsequent
emission of red light was monitored. The immunohistochemical
staining was observed under a light microscope. Positive cells
expressing Notch 2 exhibited brown-yellow staining and were
granular in appearance. During the observation period, all labeled
slides were stored at 4°C, primarily to prevent the drying of
tissues. The stained samples were scored using the extensional
standard as follows: i) Number of positively stained cells ≤5%
scored 0, 6–25% scored 1, 26–50% scored 2, 51–75% scored 3, >75%
scored 4; and ii) intensity of stain, colorless scored 0,
pallide-flavens scored 1, yellow scored 2, brown scored 3. The
staining score was obtained by multiplying the number of positively
stained cells and the intensity of stain, and stratified as either
negative (<3 score) or strong (≥3 score) (19).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed to cDNA with the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol. The synthesized cDNA was used as the
template to detect the expression of the genes of interest by
RT-qPCR with SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.).
Primer sequences were as follows: NOTCH2, forward
5′-ATCCCACAAAGCCTAGCACC-3′, reverse 5′-CCTTGTCCCTGAGCAACCAT-3′; and
GAPDH, forward 5′-GAAAGCCTGCCGGTGACTAA-3′, reverse
5′-AGGAAAAGCATCACCCGGAG-3′. qPCR was conducted using LightCycler
Technology (LC-96; Roche, Mannheim, Germany). Cycling conditions
included reverse transcription at 50°C for 30 min, denaturation at
95°C for 15 min, 40 cycles of 94°C for 30 sec, 55°C for 30 sec, and
68°C for 2 min, and a final extension step at 68°C for 10 min. PCR
experiments were repeated 3 times. Data were analyzed according to
the 2−ΔΔCq method (20).
Cell viability assay
Cells were transfected with shRNA for 24 h, then
plated in 96-well culture plates. At the same time each day for
three consecutive days, the original culture medium was removed,
and 10 µl cell counting kit-8 (CCK-8) dye (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) and 90 µl fresh
RPMI-1640 were added to each well. The cells were incubated at 37°C
for 1 h. Absorbance (450 nm) of the medium was measured with an
ELx-800 plate reader (BioTek China, Beijing, China). Representative
data for three independent experiments are presented.
Apoptosis assay
Cellular apoptosis was analyzed using the FACScan
instrument (BD Biosciences, San Jose, CA, USA). The cells were
harvested and washed in phosphate-buffered saline, then
subsequently stained with annexin V-allophycocyanin and propidium
iodide 7-amino actinomycin D (BD Pharmingen, San Diego, CA, USA).
Cells were quantified using a BD FACSVerse flow cytometer (BD
Biosciences). Quantification of the apoptotic fraction included
early and late apoptotic cells. All data were analyzed using Kaluza
software 1.1 (Beckman Coulter, Brea, CA, USA). Representative data
for three independent experiments are presented.
Scratch wound healing motility assay
Cells were transfected with shRNA for 24 h and then
plated in 6-well culture plates. A 'scratch' was created by running
a pipette tip through the plate. Cells were then cultured under
standard conditions for a further 24 h. Plates were washed twice
with fresh medium to remove non-adherent cells and then images were
captured. The number of cells that had migrated from the edge of
the wound were counted. Results were expressed as the average
number of cells per field. Representative data for three
independent experiments are presented.
In vitro cell invasion assay
Transwell membranes (Corning Incorporated, Corning,
NY, USA) were coated with Matrigel (2.5 mg/ml). Cells were
transfected with shRNA for 24 h, then serum starved for 8 h and
collected in RPMI-1640 medium containing 3% FBS. Cells were seeded
onto the upper wells of pre-coated Transwell inserts in the same
medium at a density of 1.0×105 cells/well. Lower wells
of pre-coated Transwell inserts contained 800 µl RPMI-1640
supplemented with 10% FBS. After 48 h, membranes were swabbed with
a Q-tip and stained with crystal violet prior to cell counting
under a microscope. Representative data for three independent
experiments are presented.
In vitro cell migration assay
Cell migration assays were also performed using the
Transwell membranes. The procedure used for this assay was similar
to that of the cell invasion assay, except that the Transwell
membrane was not coated with Matrigel. Three to four fields on each
filter were scored under an inverted microscope in each experiment.
Representative data for three independent experiments are
presented.
Western blot assay
Total protein from Hep-2 cells transfected with the
plasmids was extracted using radioimmunoprecipitation assay buffer
(1 mM MgCl2, 10 mM Tris-HCl pH 7.4, 1% Triton X-100,
0.1% SDS, 1% NP-40). The sample was then centrifuged at 4°C, 12,000
× g for 15 min. The supernatant was isolated and protein expression
was analyzed by western blot analysis. The amount of protein loaded
for each lane was quantified at 25 µg. Total protein
extracts were separated on 12% SDS-PAGE gels and transferred to
polyvinylidene difluoride membranes. GAPDH was used as a loading
control. The membrane was blocked using 5% skimmed milk powder at
4°C for 60 min. The membranes were immunoblotted with the following
primary antibodies: Anti-p-ERK (1:1,000 rabbit monoclonal antibody,
cat. no. 4695, Cell Signaling Technology, Danvers, MA, USA,);
anti-t-ERK (rabbit monoclonal antibody, cat. no. 5013, Cell
Signaling Technology, 1:1,000); anti-p-AKT (rabbit monoclonal, cat.
no. 4060, Cell Signaling Technology, 1:1,000); t-AKT (rabbit
monoclonal antibody, cat. no. 4691, Cell Signaling Technology,
Inc., 1:1,000); anti-c-myc (rabbit monoclonal antibody, cat. no.
13987, Cell Signaling Technology, 1:1,000); anti-Bax (rabbit
monoclonal antibody, cat. no. 5023, Cell Signaling Technology,
Inc., 1:1,000); anti-Bcl-2 (rabbit monoclonal antibody, cat. no.
4223, Cell Signaling Technology, Inc., 1:1,000). The membranes were
then washed three times using TBS with 0.1% Tween 20, and incubated
with Donkey anti-rabbit IgG secondary antibody (cat. no. ab175731;
Abcam, Cambridge, MA, USA; 1:10,000). Enhanced chemiluminescence
detection (Cell Signaling Technology) was used to observe the
blots. The densitometry of the bands was quantified using the Image
J version 1.38X software (imagej.nih.gov/ij). Western blotting was performed in
triplicate.
Statistical analysis
The statistical analysis of Notch 2 QDs-based
immunofluorescence histochemistry was performed using the Wilcoxin
rank-sum test. The other results were analyzed using one-way
analysis of variance followed by Bonferonni's method. The
statistical analyses were performed using SPSS software (version
17.0; SPSS, Inc., Chicago, IL, USA) P<0.05 was considered to
indicate a statistically significant difference.
Results
Notch 2 protein levels upregulated in
LSCC
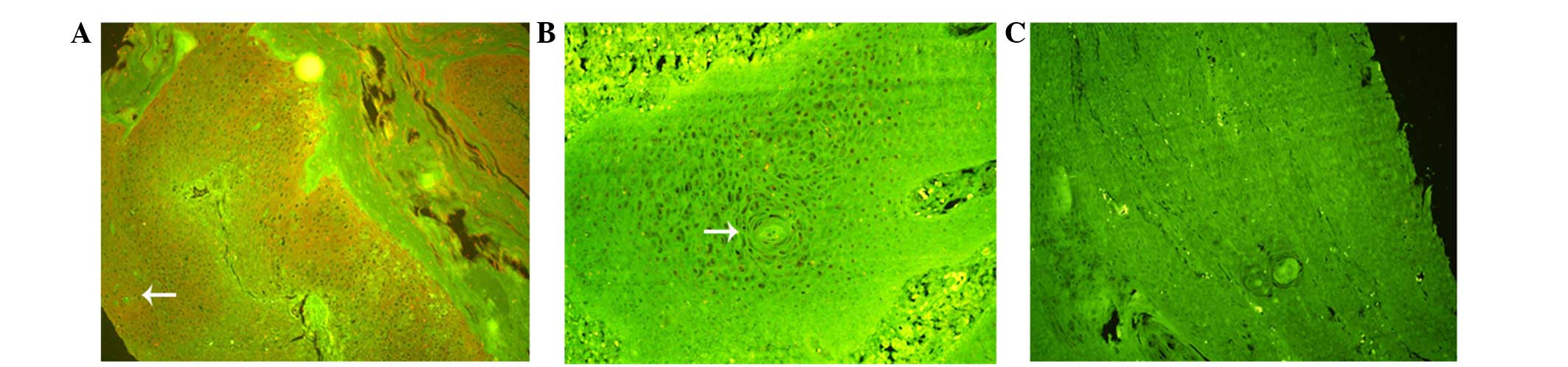
The present study examined the expression of Notch 2
in a total of 126 samples from LSCC tissues and vocal cord polyps.
Among the primary LSCC tissues, 83/95 (87.4%) cases exhibited Notch
2 expression, whereas, 10/31 (32.3%) cases from vocal cord polyps
exhibited Notch 2 expression (Table
I). QDs staining demonstrated that the Notch 2 protein is
located in the cytoplasm and the nucleus (Fig. 1). Notch 2 expression was absent or
low in vocal cord polyps compared with LSCC samples. The protein
expression rates of Notch 2 in LSCC samples with (P=0.001) and
without (P=0.001) lymph node metastasis were increased compared
with the levels in vocal cord polyp samples (Table I). Additionally, the expression of
Notch 2 was increased in tissue samples from LSCC with lymph node
metastasis compared with those without metastasis (P=0.037), which
indicates that Notch 2 may be important in lymph node metastasis of
LSCC.
 | Table IExpression of Notch 2 in LSCC tissue
and samples from vocal cord polyps. |
Table I
Expression of Notch 2 in LSCC tissue
and samples from vocal cord polyps.
| Sample | Total, n | Negative, n (%) | Positive, n
(%) | P-value |
|---|
| Vocal cord
polyp | 31 | 21 (67.74) | 10 (32.26) | |
| LSCC without lymph
node metastasis | 72 | 12 (16.67) | 60 (83.33) | <0.05a |
| LSCC with lymph
node metastasis | 23 | 0 (0.00) | 23 (100.00) | <0.05a,b |
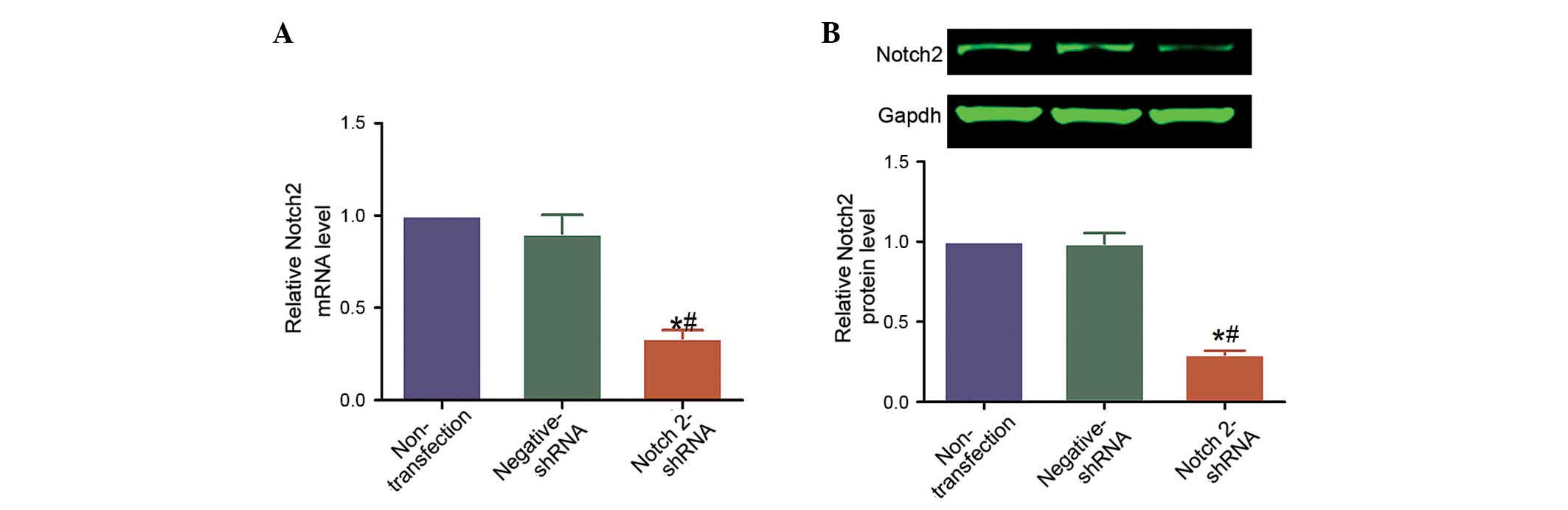
Notch 2 protein expression is stably and
effectively downregulated by shRNA in Hep-2 laryngeal cancer
cells
The efficacy of shRNA-mediated knockdown of NOTCH2
in Hep-2 cells and the successful transfection of Notch2-shRNA
plasmid transfection into Hep-2 cells were analyzed using RT-qPCR
and western blotting. NOTCH2 mRNA levels were reduced by 82.0 and
80.3% in Notch2-shRNA-transfected cells compared with cells that
were non-transfected or transfected with negative-shRNA,
respectively (P=0.011 and P=0.013, respectively; Fig. 2A). Similarly, Notch 2 protein
levels were reduced by 82.0 and 80.3% (P=0.011 and P=0.013,
respectively; Fig. 2B).
Additionally, no significant difference was observed in the levels
of mRNA or protein between the cells that were non-transfected and
those transfected with negative-shRNA (P>0.05; Fig. 2).
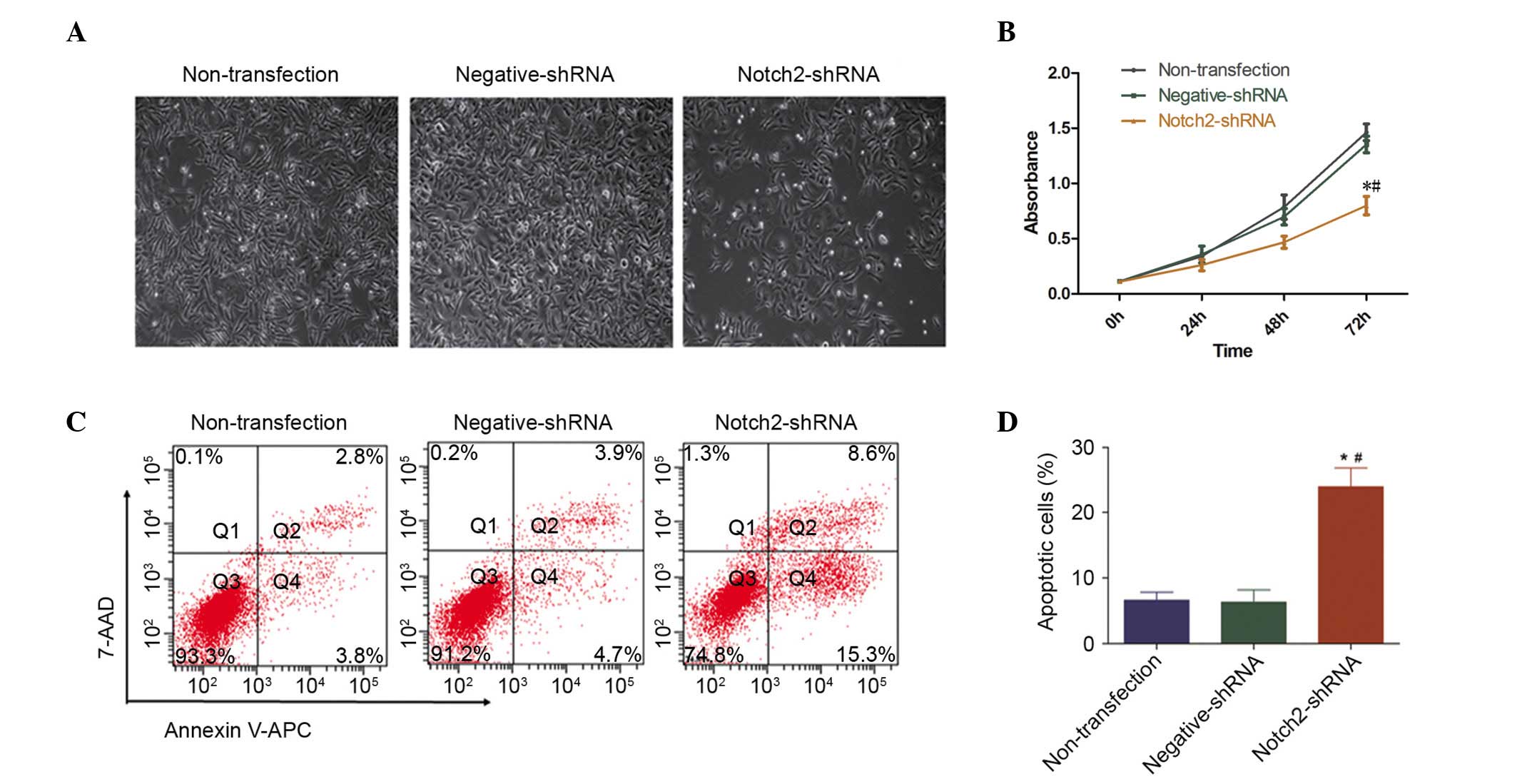
NOTCH2 knockdown decreases cell
proliferation and increases cell apoptosis in Hep-2 cells
The present study investigated the effect of Notch 2
on the cell morphology, proliferation and apoptosis of LSCC.
Following NOTCH2 knockdown, the Hep-2 cells underwent morphological
changes, consistent with reduced cell numbers in culture (Fig. 3A). Similarly, NOTCH2 knockdown
significantly inhibited the growth of the Hep-2 cells compared with
negative-shRNA and non-transfected cells, as measured using CCK-8
reagent (P=0.028 and P=0.046, respectively; Fig. 3B). In addition to the decreased
percentage of proliferating cells, the percentage of apoptotic
cells were increased, compared with negative-shRNA and
non-transfected cells, as measured by flow cytometry (both P=0.003;
Fig. 3C and D).
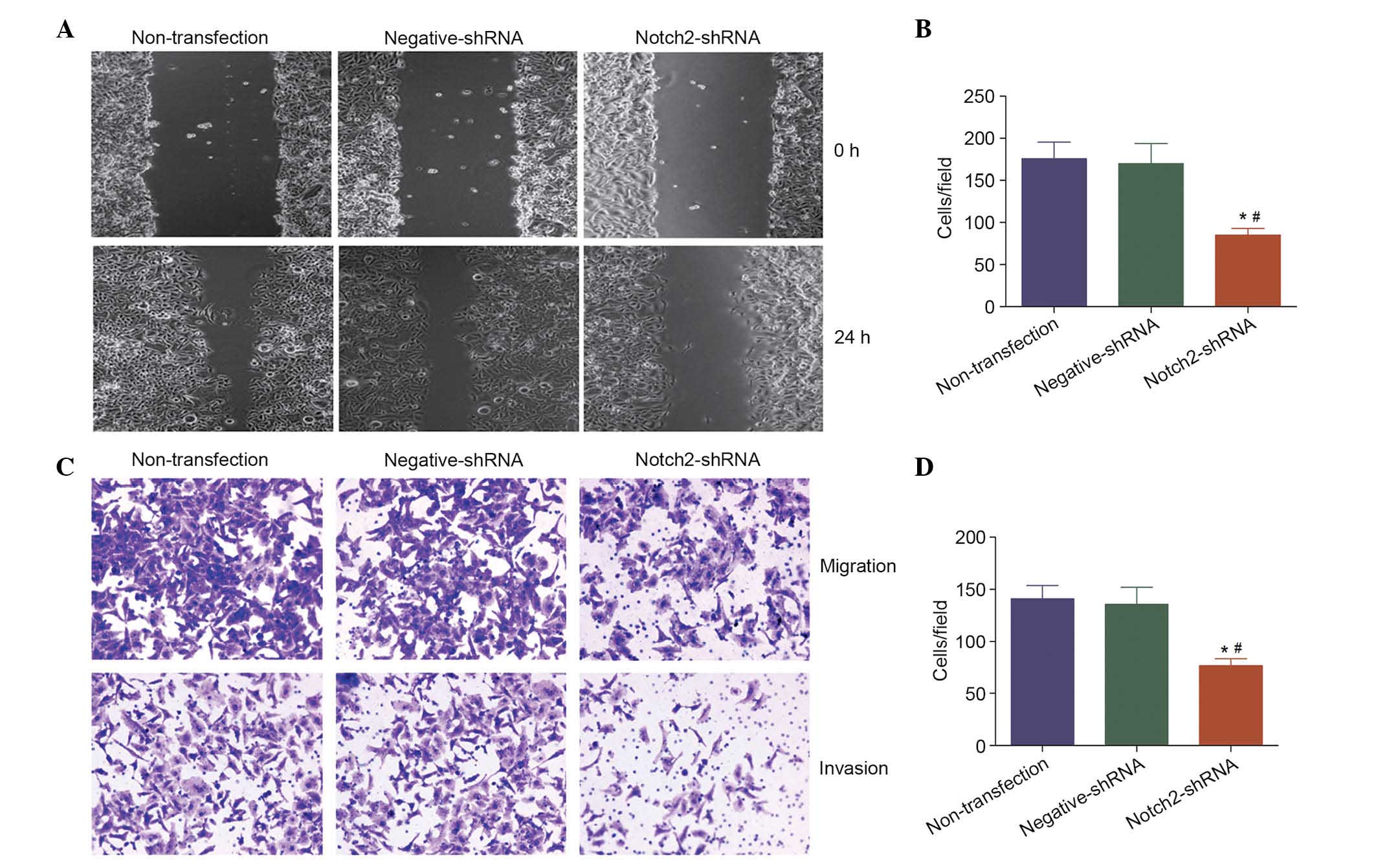
Notch 2 increases cell migration and
invasion in vitro
The present study additionally investigated the
effect of Notch 2 on the migration and invasion abilities of LSCC.
Compared with negative-shRNA and non-transfected Hep-2 cells,
knockdown of NOTCH2 mRNA significantly inhibited cell migration
(P<0.05; Fig. 4A and B) and
invasion (P<0.05; Fig. 4C and
D). These results support the theory that NOTCH2 is an oncogene
that may contribute to the migration and invasion of LSCC.
Notch 2 affects the expression of cell
proliferation- and apoptosis-associated proteins
To investigate the effect of Notch pathway proteins
on the apoptosis of cancer cells, the expression levels of proteins
associated with the Notch pathway were evaluated. Following
knockdown of Notch 2 protein expression, the protein expression
levels of phospho-mitogen-activated protein kinase 1 (p-ERK)
(P=0.003 and P=0.016), c-Myc (P=0.011 and P=0.048) and Bcl2
(P=0.003 and P=0.012) were also downregulated (P<0.05), whereas
the expression level of Bcl2-associated X protein (Bax) was
upregulated (P<0.05) compared with negative-shRNA and
non-transfected Hep-2 cells (Fig.
5). Notch 2 knockdown demonstrated no effect on the protein
expression levels of total-ERK (t-ERK), phospho-v-akt murine
thymoma viral oncogene homolog 1 (p-Akt) or total-Akt (t-Akt)
(P>0.05; Fig. 5).
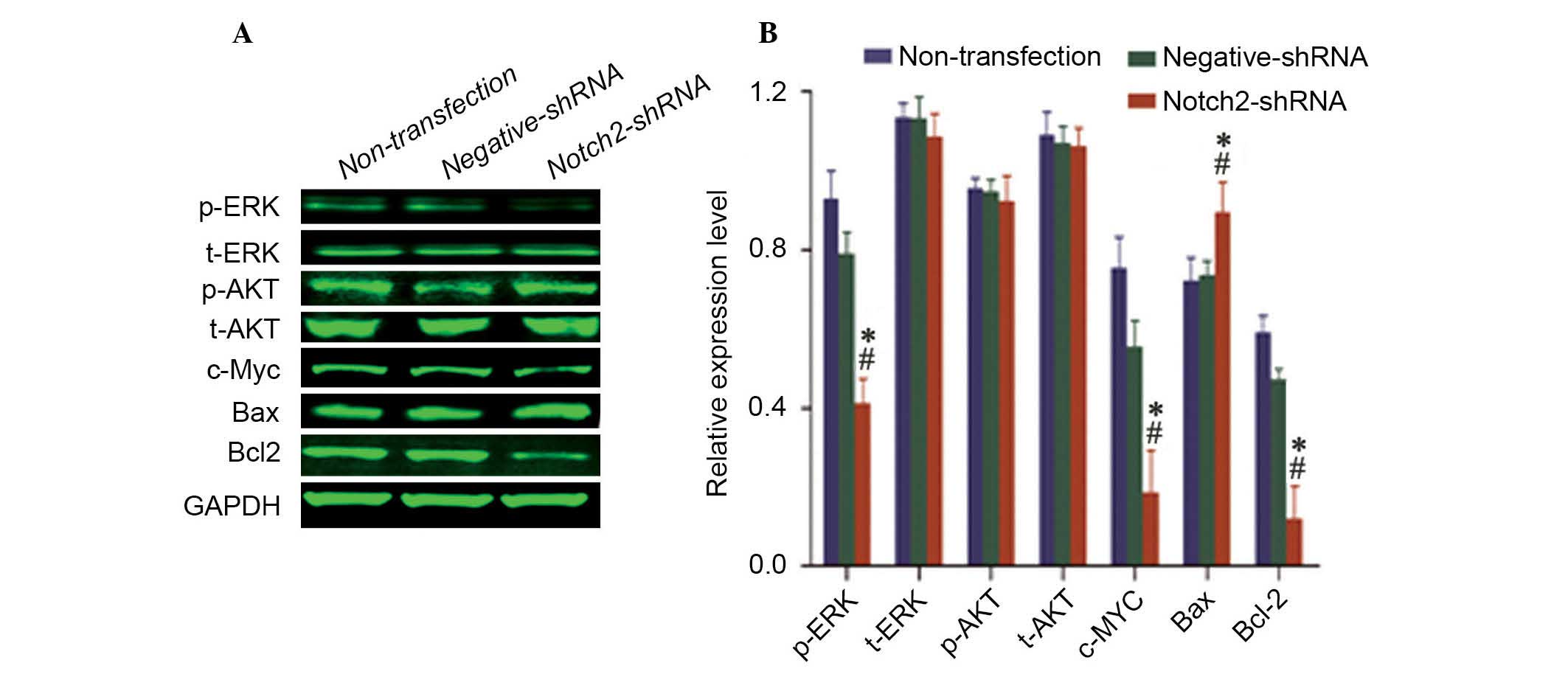 | Figure 5Knockdown of NOTCH2 affects the
expression of Notch 2 signaling pathway target genes in Hep-2
cells. (A) Western blot analysis demonstrating that the expression
levels of p-ERK, c-Myc and Bcl-2 were downregulated, and the
expression of Bax was upregulated. No differences were observed in
the expression of t-ERK, p-Akt and t-Akt. (B) Quantification of the
protein bands by densitometry. The results of three independent
experiments are presented. All the histograms present the
GAPDH-normalized mean ± standard deviation of the band density from
the three experiments. *P<0.05 vs. non-transfected
cells, #P<0.05 vs. negative shRNA. shRNA, short
hairpin RNA; p, phospho; t, total; ERK, mitogen-activated protein
kinase; AKT, v-akt murine thymoma viral oncogene homolog 1;
c-Myc, v-myc avian myelocytomatosis viral oncogene homolog;
Bax, BCL2-associated X protein; Bcl2, B-cell CLL/lymphoma 2. |
Discussion
The Notch signaling pathway has been associated with
the initiation and development of various types of human cancer,
including breast, brain, cervix, lung, colon, head and neck,
kidney, bone marrow, lymph nodes and stomach cancer (5,21–25).
Therefore, Notch signaling has become an attractive anticancer drug
target (26). However, the
molecular alterations of the Notch signaling pathways in LSCC are
less well defined and the precise mechanisms of Notch 2-mediated
tumor proliferation and anti-apoptotic effects remain unclear. The
present study aimed to investigate the importance of the Notch 2
signaling pathway in LSCC tissues and Hep-2 laryngeal cancer
cells.
The current study demonstrated that Notch 2
expression was upregulated in LSCC tissues compared with tissues
from normal vocal polyps. This upregulation was increased further
in tissues with lymph node metastasis compared with LSCC tissues
without lymph node metastasis, indicating that Notch 2 may be
oncogenic during the tumorigenesis and metastasis of LSCC.
The present study additionally demonstrated that
shRNA-mediated downregulation of Notch 2 expression in Hep-2
laryngeal cancer cells inhibited cell proliferation and induced
cell apoptosis. It was observed that NOTCH2 mRNA knockdown
decreased Hep-2 cell proliferation compared with controls. In
addition, the decreased expression of Notch 2 resulted in an
increased proportion of apoptotic cells, as measured by flow
cytometry. Again, this result suggests that NOTCH2 is an oncogene
that may contribute to the proliferation and evasion of apoptosis
in LSCC.
Previous studies have demonstrated that the Notch
receptors regulate cell proliferation and apoptosis through
multiple downstream pathways (2,27,28).
The protein expression of Notch 2, in particular, has been
demonstrated to affect downstream signaling pathways in different
carcinoma cells (2,29,30).
The current study examined the expression levels of the
conventional Notch target genes in Hep-2 cells. It was observed
that the silencing of Notch 2 resulted in decreased phosphorylation
levels of ERK, and decreased expression levels of c-Myc and Bcl2,
whereas the expression of the pro-apoptotic protein Bax was
upregulated. However, no changes were observed in the expression of
t-ERK, p-Akt and t-Akt following NOTCH 2 knockdown. The biological
effects of ERK are induced via p-ERK1/2 (31). Zhou et al (32) demonstrated that the Notch
inhibitor, DAPT, reduced ERK1/2 activity and decreased the protein
expression levels of matrix metalloproteinase (MMP)-2, MMP-9 and
vascular endothelial growth factor, resulting in the suppression of
hepatocellular carcinoma invasion. Asnaghi et al (33) reported that Notch blockade reduced
ERK activity in uveal melanoma cells. They implied that the Notch
signaling pathway may modulate ERK activity to regulate cell
proliferation and apoptosis. The results of the current study
demonstrated that NOTCH 2 mRNA knockdown decreased the
phosphorylation level of ERK, which indicates reduced ERK activity.
Therefore, the downregulation of Notch 2 inhibited the
proliferation of Hep-2 cells by altering the activity and
expression of cell proliferation and apoptosis-associated
proteins.
Furthermore, the present study demonstrated that
NOTCH2 knockdown inhibits the in vitro migration and
invasion ability of Hep-2 cells, however, the underlying molecular
mechanisms involved remain unclear (34). Further studies are required to
precisely determine the importance of Notch 2 signaling pathways in
the invasion and lymph node metastasis of LSCC.
In summary, the results of the current study
indicate that Notch 2 is highly expressed in LSCC tissues and is
associated with increased tumorigenesis and metastasis.
Additionally, knockdown of NOTCH2 inhibits cell proliferation,
induces apoptosis, and decreases cell migration and invasion of
Hep-2 cancer cells in vitro. Therefore, the results of the
present study suggest that the Notch 2 signaling pathway is
important in the cell growth, apoptosis regulation and metastasis
of LSCC. Together, these results suggest that the development of
Notch 2 inhibitors to decrease cancer cell growth and metastasis
may be a promising therapeutic strategy for the treatment of
patients with LSCC.
Acknowledgments
The present work was supported by the National
Natural Science Foundation of China (grant nos. 81172569 and
81372880).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramdass B, Maliekal TT, Lakshmi S, Rehman
M, Rema P, Nair P, Mukherjee G, Reddy BK, Krishna S and
Radhakrishna Pillai M: Coexpression of Notch1 and NF kappaB
signaling pathway components in human cervical cancer progression.
Gynecol Oncol. 104:352–361. 2007. View Article : Google Scholar
|
|
3
|
Liu M, Wu H, Liu T, Li Y, Wang F, Wan H,
Li X and Tang H: Regulation of the cell cycle gene, BTG2, by miR-21
in human laryngeal carcinoma. Cell Res. 19:828–837. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li JL, Sainson RC, Oon CE, Turley H, Leek
R, Sheldon H, Bridges E, Shi W, Snell C, Bowden ET, et al:
DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy
in vivo. Cancer Res. 71:6073–6083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capaccione KM and Pine SR: The Notch
signaling pathway as a mediator of tumor survival. Carcinogenesis.
34:1420–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ntziachristos P, Lim JS, Sage J and
Aifantis I: From fly wings to targeted cancer therapies: A
centennial for notch signaling. Cancer Cell. 25:318–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bin Hafeez B, Adhami VM, Asim M, Siddiqui
IA, Bhat KM, Zhong W, Saleem M, Din M, Setaluri V and Mukhtar H:
Targeted knockdown of Notch1 inhibits invasion of human prostate
cancer cells concomitant with inhibition of matrix
metalloproteinase-9 and urokinase plasminogen activator. Clin
Cancer Res. 15:452–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Li Y, Banerjee S, Kong D, Ahmad A,
Nogueira V, Hay N and Sarkar FH: Down-regulation of Notch-1 and
Jagged-1 inhibits prostate cancer cell growth, migration and
invasion, and induces apoptosis via inactivation of Akt, mTOR, and
NF-kappaB signaling pathways. J Cell Biochem. 109:726–736.
2010.PubMed/NCBI
|
|
9
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiaramonte R, Basile A, Tassi E,
Calzavara E, Cecchinato V, Rossi V, Biondi A and Comi P: A wide
role for NOTCH1 signaling in acute leukemia. Cancer Lett.
219:113–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mirandola L, Comi P, Cobos E, Kast WM,
Chiriva-Internati M and Chiaramonte R: Notching from T-cell to
B-cell lymphoid malignancies. Cancer Lett. 308:1–13. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weng AP, Ferrando AA, Lee W, Morris JP IV,
Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT and Aster
JC: Activating mutations of NOTCH1 in human T cell acute
lymphoblastic leukemia. Science. 306:269–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiaramonte R: Still puzzling Notch
signaling in B-cell malignancies. Leuk Res. 30:1331–1332. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hubmann R, Schwarzmeier JD, Shehata M,
Hilgarth M, Duechler M, Dettke M and Berger R: Notch2 is involved
in the overexpression of CD23 in B-cell chronic lymphocytic
leukemia. Blood. 99:3742–3747. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trøen G, Wlodarska I, Warsame A, Hernández
Llodrà S, De Wolf-Peeters C and Delabie J: NOTCH2 mutations in
marginal zone lymphoma. Haematologica. 93:1107–1109. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Egloff AM and Grandis JR: Molecular
pathways: Context-dependent approaches to Notch targeting as cancer
therapy. Clin Cancer Res. 8:5188–5195. 2012. View Article : Google Scholar
|
|
17
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar
|
|
18
|
Yin L, Velazquez OC and Liu ZJ: Notch
signaling: Emerging molecular targets for cancer therapy. Biochem
Pharmacol. 80:690–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu D, Li Y, Wang W, Zhao Q, Li J, Lu Y,
Li M, Dong G, Zhang H, Xie H and Ji G: High level of Notch1 protein
is associated with poor overall survival in colorectal cancer. Ann
Surg Oncol. 17:1337–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–8. 2001.
View Article : Google Scholar
|
|
21
|
Wang J, Wang C, Meng Q, Li S, Sun X, Bo Y
and Yao W: siRNA targeting Notch 1 decreases glioma stem cell
proliferation and tumor growth. Mol Biol Rep. 39:2497–2503. 2012.
View Article : Google Scholar
|
|
22
|
Ristorcelli E and Lombardo D: Targeting
Notch signaling in pancreatic cancer. Expert Opin Ther Targets.
14:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jundt F, Anagnostopoulos I, Förster R,
Mathas S, Stein H and Dörken B: Activated Notch1 signaling promotes
tumor cell proliferation and survival in Hodgkin and anaplastic
large cell lymphoma. Blood. 99:3398–3403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu ZJ, Xiao M, Balint K, Smalley KS,
Brafford P, Qiu R, Pinnix CC, Li X and Herlyn M: Notch1 signaling
promotes primary melanoma progression by activating
mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt
pathways and up-regulating N-cadherin expression. Cancer Res.
66:4182–4190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Espinoza I and Miele L: Notch inhibitors
for cancer treatment. Pharmacol Ther. 139:95–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin R, Nakada M, Teng L, Furuta T, Sabit
H, Hayashi Y, Demuth T, Hirao A, Sato H, Zhao G and Hamada J:
Combination therapy using Notch and Akt inhibitors is effective for
suppressing invasion but not proliferation in glioma cells.
Neurosci Lett. 534:316–321. 2013. View Article : Google Scholar
|
|
28
|
Sivasankaran B, Degen M, Ghaffari A, Hegi
ME, Hamou F, Ionescu MC, Zweifel C, Tolnay M, Wasner M,
Mergenthaler S, et al: Tenascin C is a novel RBPJkappa induced
target gene for Notch signaling in gliomas. Cancer Res. 69:458–465.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo DH, Zhou Q, Hu SK, Xia YQ, Xu CC, Lin
TS, Pan YT, Wu JS and Jin R: Differential expression of Notch1
intracellular domain and p21 proteins, and their clinical
significance in gastric cancer. Oncol Lett. 7:471–478.
2014.PubMed/NCBI
|
|
30
|
Ramakrishnan V, Ansell S, Haug J, Grote D,
Kimlinger T, Stenson M, Timm M, Wellik L, Halling T, Rajkumar SV
and Kumar S: MRK003, a γ-secretase inhibitor exhibits promising in
vitro pre-clinical activity in multiple myeloma and non-Hodgkin's
lymphoma. Leukemia. 26:340–348. 2012. View Article : Google Scholar
|
|
31
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: Conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
32
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and -9 and vascular endothelial growth factor.
Oncology Reports. 28:874–882. 2012.PubMed/NCBI
|
|
33
|
Asnaghi L, Ebrahimi KB, Schreck KC, Bar
EE, Coonfield ML, Bell WR, Handa J, Merbs SL, Harbour JW and
Eberhart CG: Notch signaling promotes growth and invasion in uveal
melanoma. Clin Cancer Res. 18:654–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pickering CR, Zhou JH, Lee JJ, Drummond
JA, Peng SA, Saade RE, Tsai KY, Curry JL, Tetzlaff MT, Lai SY, et
al: Mutational landscape of aggressive cutaneous squamous cell
carcinoma. Clin Cancer Res. 20:6582–6592. 2014. View Article : Google Scholar : PubMed/NCBI
|