Introduction
Endomorphins (EMs) are a type of opioid peptide,
which were initially identified in 1997 by Zadina et al
(1); their identification prompted
research into endogenous opioid peptides. EMs have important roles
in the body with regards to analgesia (2), feeding behavior (3), gastrointestinal movement (4) and inflammation (5). In recent years, EMs have been
reported to have an important role in cardiovascular biology
(6), and have been shown to
protect endothelial cells by slowing down the process of apoptosis
and promoting proliferation under physiological concentrations
(7,8).
In recent years, the incidence of acute myocardial
infarction has increased, and myocardial ischemia/reperfusion
injury (MIRI) is considered a complex problem. Due to the time
limit of ischemic preconditioning (IPC) and the invasive operation
associated with ischemic postconditioning (IPO), pharmacological
postconditioning (9) is considered
a more feasible measure to reduce MIRI. Previous studies have
suggested that exogenous opioids, including morphine, fentanyl and
remifentanil, can resist MIRI and exert myocardial protection;
however, the long-standing or excessive use of exogenous drugs may
produce toxicity to the organism (10,11).
Therefore, the use of EMs, which are endogenous opioids, may be
considered a promising novel therapeutic strategy. Numerous studies
have confirmed that MIRI can induce oxidative stress, mitochondrial
dysfunction and inflammation (12,13).
Furthermore, previous studies have reported that EMs have an
important role in clearing oxygen free radicals (14) and inhibiting mitochondrial
dysfunction (15). It has also
been demonstrated that EMs may participate in chronic hypoxia in
the protection of rat MIRI (16).
There are two types of EM: EM-1 and EM-2. EM-1 is widely
distributed in the brain (17) and
may have a certain correlation with cardiovascular regulatory
function.
Cell apoptosis has a substantial role in MIRI; it is
an important process in the occurrence and development of MIRI
(18), and a relevant mechanism
underlying myocardial damage and myocardial cell loss (19–21).
Therefore, the present study aimed to determine the effects of EM-1
postconditioning on MIRI and myocardial cell apoptosis in a rat
model, and to analyze the underlying mechanisms.
Materials and methods
Animals
Male clean-grade Sprague Dawley rats (weight,
250–350 g; age, 3 months) were obtained from the Animal Center of
Bengbu Medical College (Bengbu, China). The rats were fed a normal
diet and had ad libitum access to water. All rats were
housed in cages at 25±1°C with a fixed 12-h light/dark cycle. All
animal procedures were conducted in accordance with the United
States National Institutes of Health Guide, and were approved by
the Animal Use and Care Committee of Bengbu Medical College.
Materials and primary reagents
EM-1 was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Lactate dehydrogenase (LDH),
malondialdehyde (MDA) and superoxide dismutase (SOD) assay kits
were purchased from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). The creatine kinase MB (CK-MB) isoenzyme
enzyme-linked immunosorbent assay (ELISA) kit, interleukin-6 (IL-6)
ELISA kit and tumor necrosis factor-α (TNF-α) ELISA kit were
purchased from Biocalvin Co., Ltd. (Jiangsu, China). cDNA (#K1622)
and polymerase chain reaction (PCR) kits were purchased from
Fermentas (Thermo Fisher Scientific, Inc., Waltham, MA, USA
(#K0171). B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein
(Bax) and β-actin primers were synthesized by Sangon Biotech Co.,
Ltd. (Shanghai China). Mouse β-actin antibody was purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Experimental method
MIRI rat model generation in vivo
All rats were fasted for 12 h and were given ad
libitum access to water prior to the trial. The rats were
injected with 4% chloral hydrate (1 ml/100 g) intra-peritoneally
prior to the operation. Anaesthetized rats were fixed on the
operating table in a supine position. A tracheal cannula was
inserted into the rats, which was connected to a breathing machine
(tidal volume, 2–3 ml/100 g; respiratory rate, 70–80 times/min).
The right carotid artery of each rat was separated and an arterial
cannula was inserted, which was connected to the Med-Lab system
(Nanjing Medease Science and Technology Co., Ltd., Nanjing China),
in order to record changes to heart rate (HR) and mean arterial
pressure (MAP). The chest of each rat was sheared and an incision
was made along the left sternal border, separating the pericardium
and exposing the heart. Subsequently, 5–0 fine silk was threaded
through the bottom of the left anterior descending coronary artery
(LAD). Following LAD ligation, ST-T elevation shown in the
electrocardiogram indicated the success of ischemia. The LAD was
ligated for 30 min (ischemia) followed by 120 min reperfusion in
vivo, following 20 min stabilization. The MIRI model was thus
accomplished (22).
Animal experimental groups
Male Sprague Dawley rats (n=48) were randomly
divided into four groups (n=12/group): Sham group,
ischemia/reperfusion group (IR), IPO group and EM-1
postconditioning (50 μg/kg) group (EM50). The groups
underwent the following procedures: i) Sham group, LAD ligation
with no other intervention for 150 min; ii) IR group: LAD was
ligated for 30 min (ischemia), and was reperfused for l20 min in
vivo; iii) IPO group, after 30 min ischemia, three cycles of
LAD clamping for 15 sec and declamping for 15 sec were performed
prior to reperfusion; iv) EM50 group: EM-1 (50 μg/kg) was
administered intravenously following LAD ligation for 25 min,
subsequently the LAD was reperfused for l20 min in vivo.
Determination of hemodynamic
characteristics
MAP and HR were continuously monitored and recorded
using the Med-Lab hemodynamic system throughout the whole process
of the experiment. Rate pressure product (RPP) was calculated using
the following equation: RPP = MAP × HR.
Plasma IL-6, TNF-α and MDA content,
and LDH, CK-MB and SOD activity
Following reperfusion, arterial blood samples were
collected and placed in heparinized centrifuge tubes, and were
centrifuged at 1,509 × g for 20 min at 4°C. The supernatant was
collected and stored at −80°C. MDA content, and LDH and SOD
activities were measured using colorimetric assays. IL-6 and TNF-α
content, and CK-MB activity were measured by enzyme-linked
immunosorbent assay using commercially available kits according to
the manufacturers' protocols.
Determination of myocardial infarct
size
The rats were injected with 4% chloral hydrate (1
ml/100 g) intraperitoneally and sacrificed by beheading prior to
heart removal. The heart was removed alongside the LAD at the end
of reperfusion, and 1% Evans blue was injected into the heart
through the aorta. The heart was subsequently cut into 2 mm
sections vertical to the longitudinal axis after freezing, and the
sections were dyed with 1% triphenyl tetrazolium chloride in a 37°C
water bath for 10–15 min. Subsequently, the sections were fixed in
10% formalin buffer and were divided into various regions according
to color; the blue zone is considered the non-infarcted zone, the
red zone is the area at risk (AAR), and the white zone is used to
determine infarct size (IS). The relative area was measured using
Image-Pro Plus software 6.0 (Media Cybernetics, Inc., Rockford, MD,
USA) following image acquisition. The myocardial IS was expressed
as a percentage of the AAR.
Detection of Bcl-2 and Bax mRNA levels
by reverse transcription (RT)-PCR
Following the operation, left anterior myocardial
tissues (0.1 g) were collected from each group and homogenized, and
total RNA was extracted using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Total RNA (2 μg) was reverse transcribed to cDNA
in a 25 μl PCR reaction volume, containing 1 μl
forward primer, 1 μl reverse primer, 9 μl
nuclease-free water, 12.5 μl PCR Master mix and 1.5
μl cDNA (all reagents were obtained from Fermentas; Thermo
Fisher Scientific, Inc.) Subsequently RNA concentration was
detected, and RT and PCR amplification were conducted. The primer
sequences are shown in Table I.
The PCR cycling conditions were as follows: Initial denaturation at
95°C for 3 min; followed by 30 cycles of denaturation at 95°C for
50 sec, annealing at 59.4°C (β-actin), 64.5°C (Bax), 61.5°C (Bcl-2)
for 50 sec, and extension at 72°C for 60 sec; and final extension
at 72°C for 10 min; PCR products were then maintained at 4°C. The
PCR products were analyzed by 1% agarose gel electrophoresis and
were stained with ethidium bromide. The densitometric results for
Bcl-2 and Bax were compared with corresponding β-actin levels to
account for loading differences. Tanon Dots 3. 1. 2 software (Tanon
Science & Technology Co., Ltd., Shanghai, China) was used for
analysis.
 | Table IPolymerase chain reaction primers for
Bax, Bcl-2 and β-actin. |
Table I
Polymerase chain reaction primers for
Bax, Bcl-2 and β-actin.
| Gene | Primer | Sequence | Product (bp) |
|---|
| Bax | Forward | 5′-GGA TCG AGC AGA
GAG GAT GG-3′ | 464 |
| Reverse | 5′-TGG TGA GTG AGG
CAG TGA GG-3′ | |
| Bcl-2 | Forward | 5′-CTG GTG GAC AAC
ATC GCT CTG-3′ | 228 |
| Reverse | 5′-GGT CTG CTG ACC
TCA CTT GTG-3′ | |
| β-actin | Forward | 5′-GAT GGT GGG TAT
GGG TCA GAA GGA C-3′ | 632 |
| Reverse | 5′-GCT CAT TGC CGA
TAG TGA TGA CT-3′ | |
Detection of cleaved caspase-3 protein
by western blotting
Following the operation, rat ventricular tissues
(0.1 g) were collected from each group and were homogenized in 1 ml
protein extraction buffer (10 μl phenylmethylsulfonyl
fluoride, 990 μl lysis buffer). The supernatant was
collected following centrifugation (12,000 x g for 30 min at 4°C)
and protein content was determined using the bicinchoninic acid
method. The quantity of the total protein samples was 20 μg,
which was diluted in sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) loading buffer. Following denaturation,
the samples were separated by 12% SDS-PAGE and were transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% skimmed milk at 37°C for 120 min. The membranes were then
incubated at 4°C overnight with rabbit cleaved caspase-3 antibody
(1:1,000 cat. no. #9664) and mouse β-actin antibody (1:500; cat.
no. #BM0627). Subsequently, membranes were incubated for 60 min at
37°C with horseradish peroxidase (HRP)-conjugated anti-mouse
immunoglobulin (Ig)G (1:10,000; cat. no. #BA1050)) or
HRP-conjugated anti-rabbit IgG (1:10,000; cat. no. #BA1054)
secondary antibodies. The membranes were visualized using a
chemiluminescent HRP substrate. The band densities were determined
and analyzed with a automatic digital gel image analysis system
Tanon 3500 (Tanon Science & Technology Co., Ltd., Shanghai,
China).
Statistical analysis
Data are presented as the mean ± standard deviation
(n=12). One-way analysis of variance followed by least significance
difference test was used for multiple comparisons. All data were
analyzed using GraphPad Prism version 4.0 software (GraphPad
Software, Inc., San Diego, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Alterations to hemodynamic
characteristics
Basal HR, MAP and RPP were not identified to be
significantly different between the groups at baseline (P>0.05).
During the period of ischemia, HR, MAP and RPP were significantly
decreased in the IR group (P<0.01) compared with in the sham
group. Compared with in the IR group, HR, MAP and RPP were
increased in the IPO and EM50 groups (P<0.05, P<0.01). During
the period of reperfusion, HR, MAP and RPP were significantly
decreased in the IR group (P<0.01) compared with the sham group.
Compared with in the IR group, HR, MAP and RPP were increased in
the IPO and EM50 groups (P<0.05, P<0.01) (Table II).
 | Table IIHemodynamic characteristics of the
rats in each group. |
Table II
Hemodynamic characteristics of the
rats in each group.
| Variable | Baseline | Ischemia 30
min | Reperfusion
|
|---|
| 30 min | 60 min | 120 min |
|---|
| HR (beats/min) |
| Sham | 399.33±23.77 | 394.50±24.44 | 396.83±23.46 | 383.83±21.15 | 367.50±28.66 |
| IR | 390.50±20.42 |
273.83±49.14a |
299.47±48.19a |
281.26±44.60a |
284.01±34.99a |
| IPO | 412.84±28.11 |
320.63±25.77a,b |
345.82±25.48b,c |
340.60±38.48d |
341.14±20.44d |
| EM50 | 404.55±11.26 |
347.20±22.27b,d |
364.23±22.76d |
352.16±14.46d |
339.94±26.65d |
| MAP (mmHg) |
| Sham | 106.14±7.23 | 99.67±7.91 | 94.00±6.40 | 92.56±7.19 | 92.67±6.89 |
| IR | 117.51±10.62 | 55.89±21.63a | 70.85±18.04a | 66.38±15.30a | 55.83±20.98a |
| IPO | 115.29±7.68 | 77.08±9.65b,c | 88.77±7.99b | 84.70±10.19b | 80.10±22.31b |
| EM50 | 110.98±13.24 | 93.98±22.79d | 93.36±12.41d | 85.52±10.72b | 75.02±14.45 |
| RPP
(mmHg/min/10−3) |
| Sham | 42.52±5.30 | 39.42±5.06 | 37.39±4.30 | 35.60±4.20 | 34.10±4.15 |
| IR | 45.80±3.63 | 15.79±7.93a | 20.77±4.74a | 18.73±5.64a | 16.18±7.00a |
| IPO | 47.61±4.83 | 24.88±5.02a,b | 30.83±4.89d | 28.72±3.88d | 27.22±7.09d |
| EM50 | 44.99±6.22 | 32.90±9.37d | 34.20±6.42d | 30.22±4.87b | 25.66±6.13b,c |
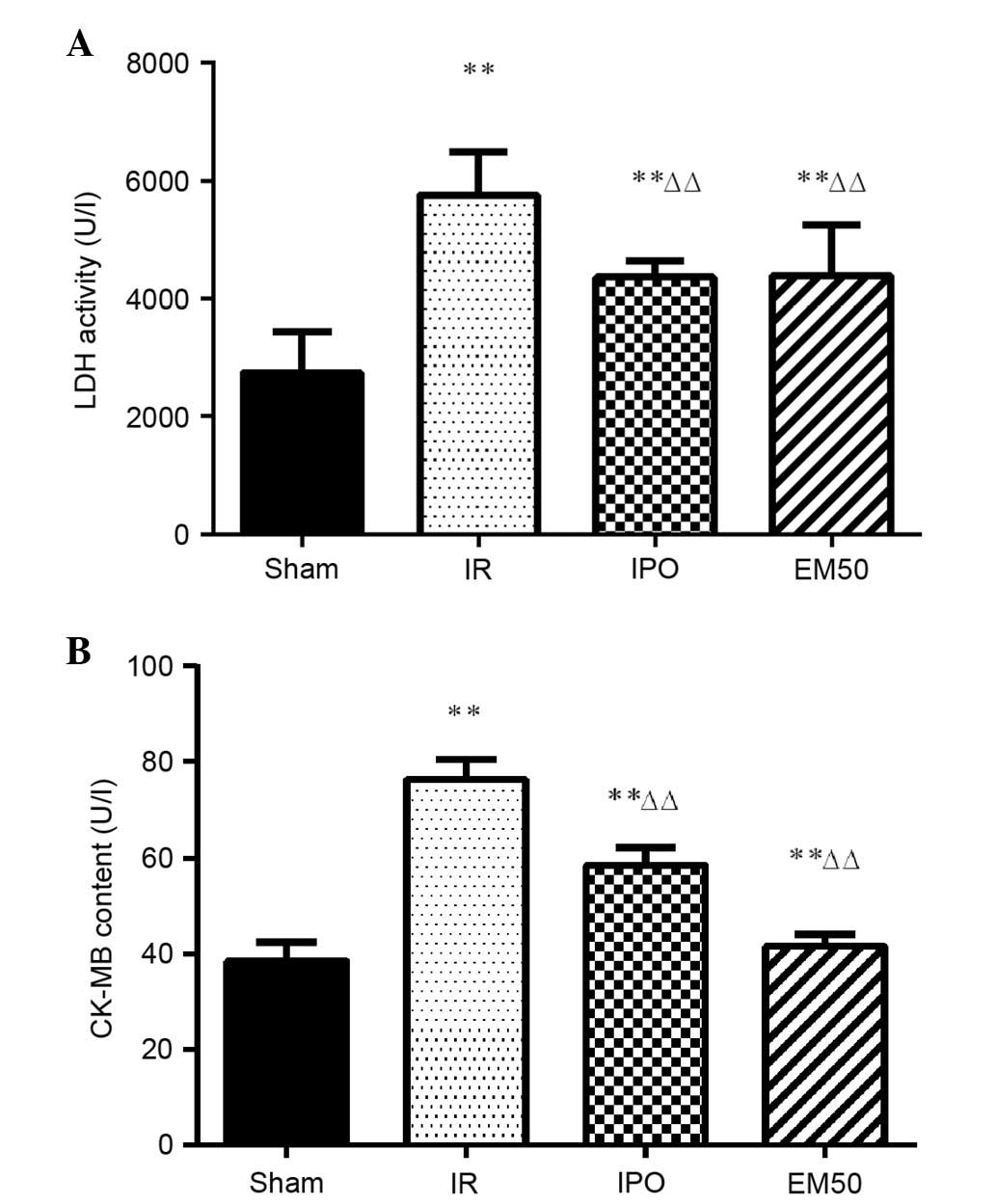
Alterations in LDH and CK-MB plasma
activities
In the IR, IPO and EM50 groups, LDH and CK-MB
activities were significantly higher compared with in the sham
group (P<0.01). Compared with in the IR group, LDH and CK-MB
activities were significantly decreased in the IPO and EM50 groups
(Fig. 1; P<0.01).
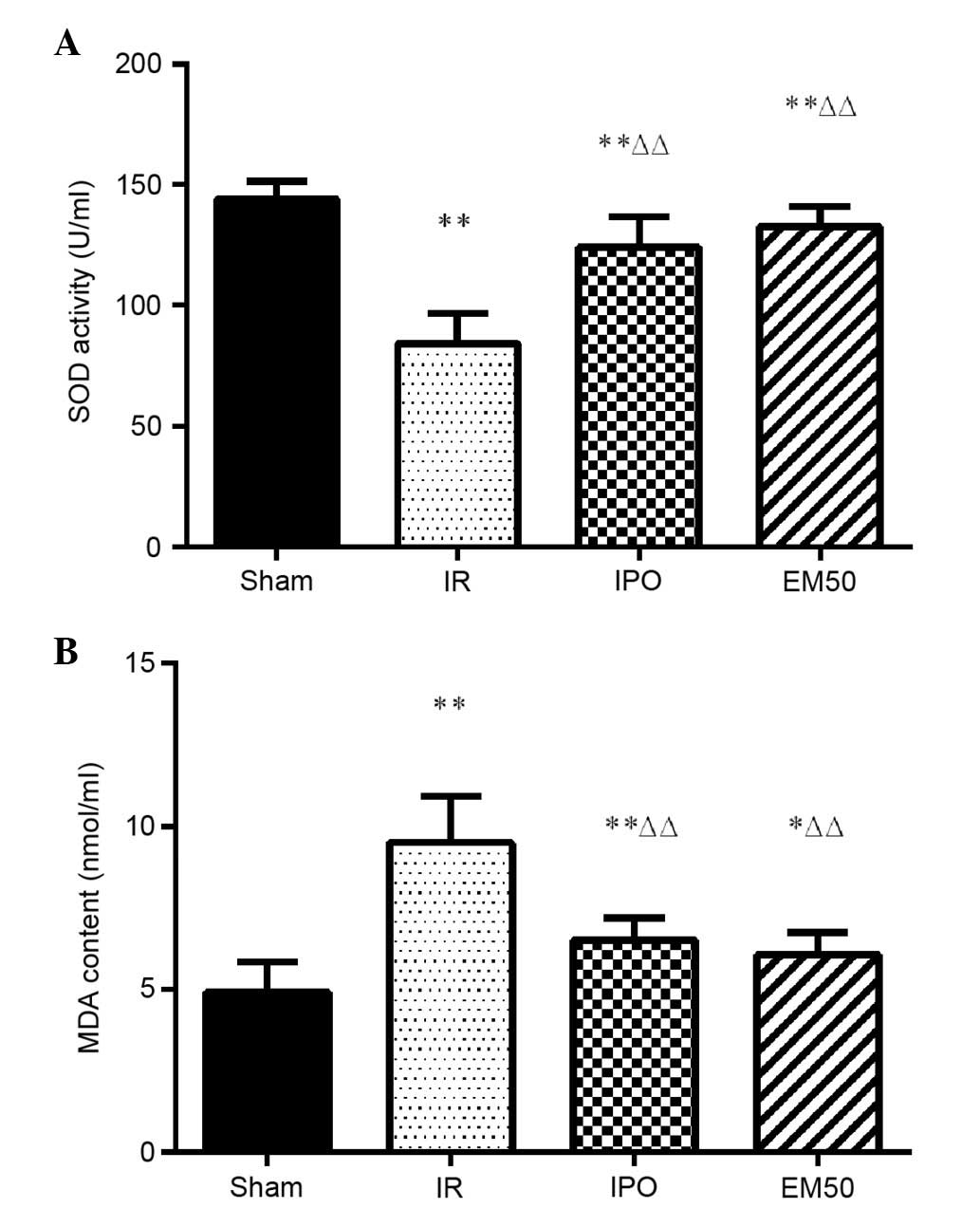
Alterations in MDA content and SOD
activity in the plasma
Compared with Sham group, in IR, IPO, and EM50
groups, SOD activity was significantly reduced and MDA content was
increased. Compared with in the IR group, SOD activity was
significantly increased and MDA content was significantly decreased
in the IPO and EM50 groups (Fig.
2; P<0.01).
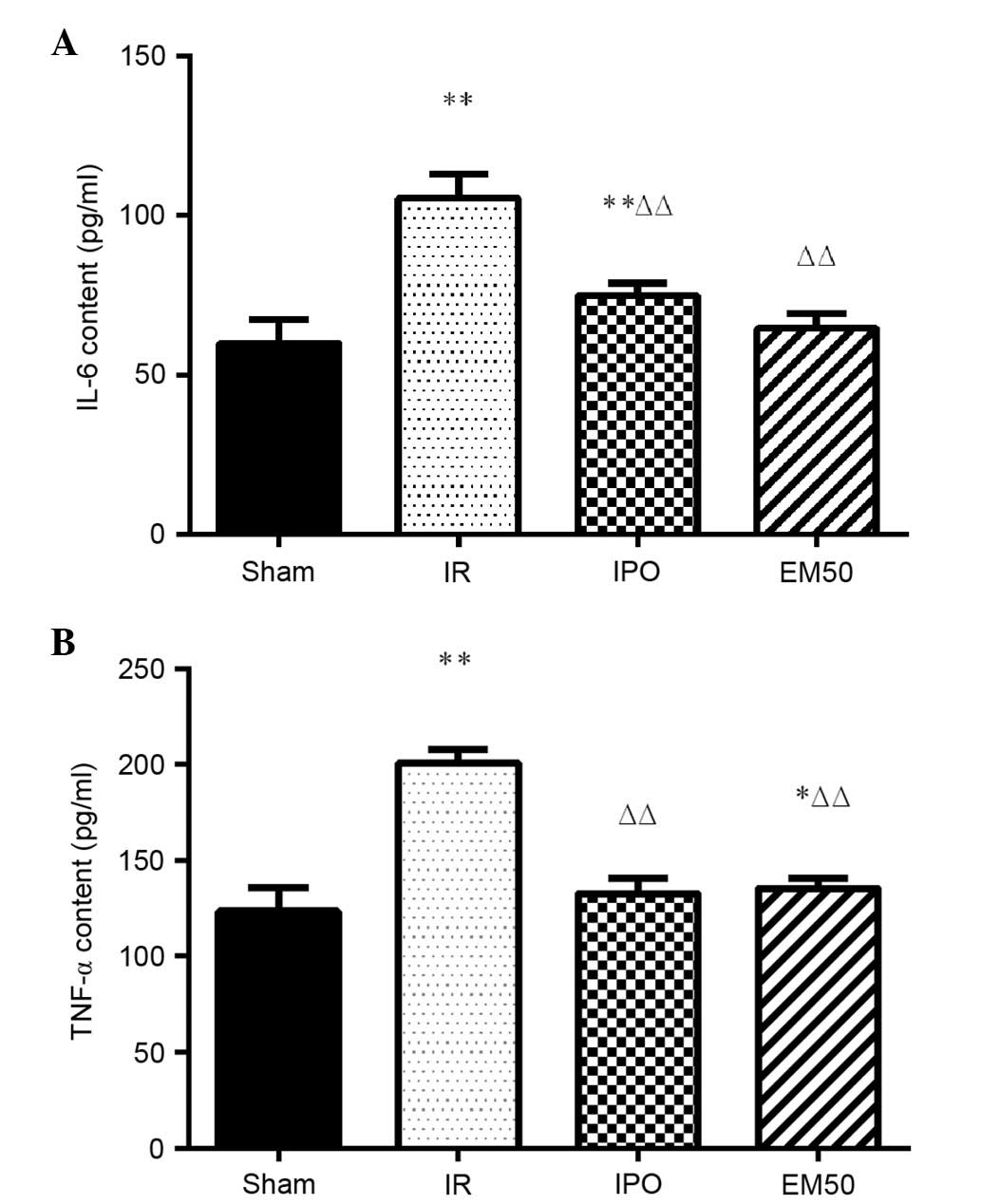
Alterations in IL-6 and TNF-α plasma
content
In the IR group, IL-6 and TNF-α levels were
significantly increased compared with in the sham group
(P<0.01). Compared with in the IR group, IL-6 and TNF-α levels
were significantly decreased in the IPO and EM50 groups (P<0.01;
Fig. 3).
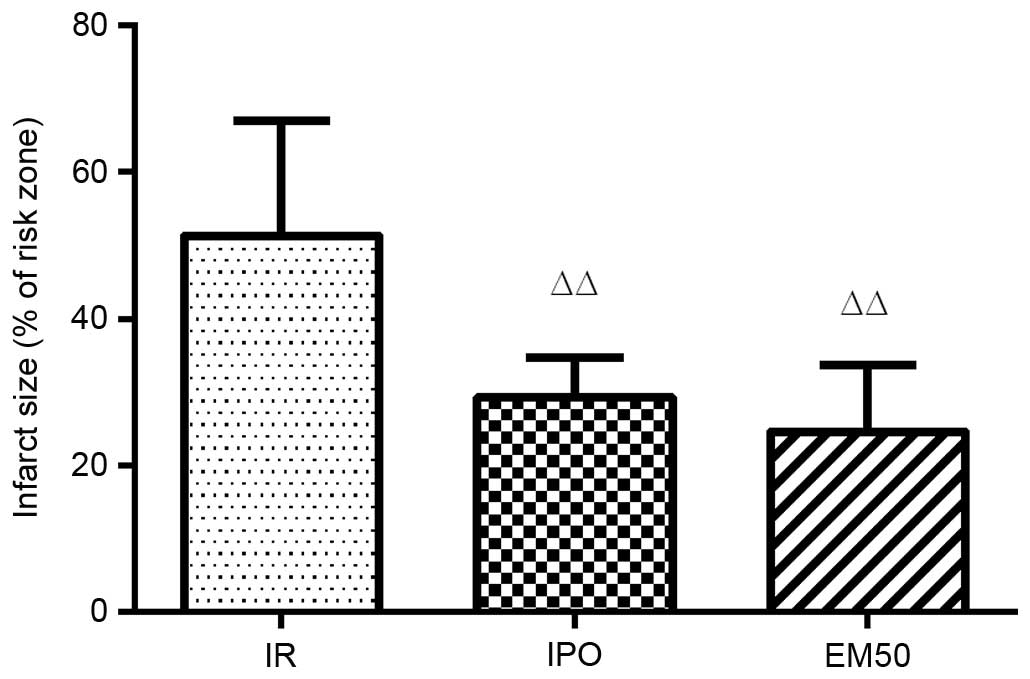
Alterations to myocardial infarct size in
rats
Myocardial infarct size (% IS/AAR) was significantly
decreased in the IPO and EM50 groups (P<0.01) compared with in
the IR group (Fig. 4).
Alterations in the mRNA expression levels
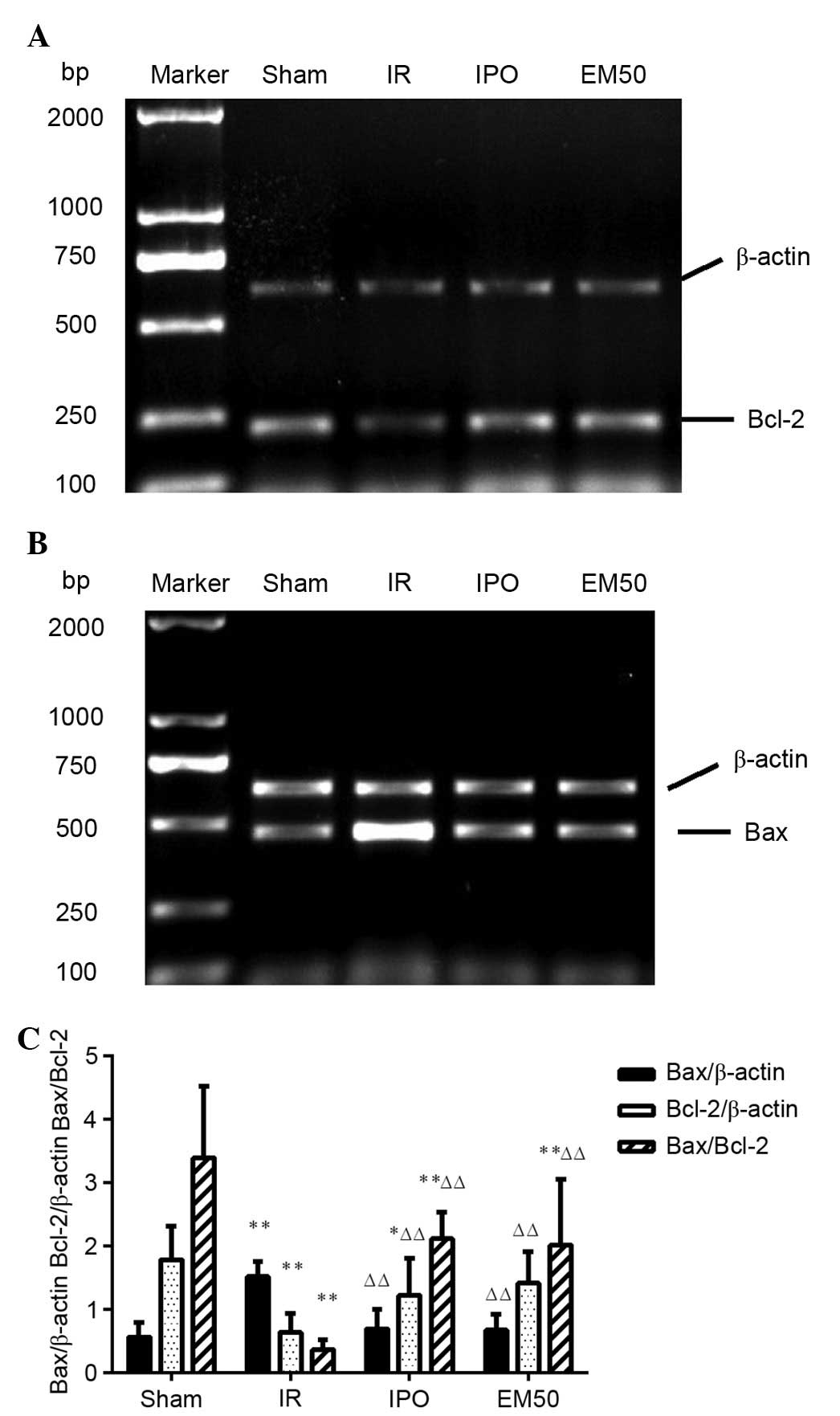
of myocardial Bcl-2 and Bax
The results of the RT-PCR revealed that, compared
with the sham group, the mRNA expression levels of Bcl-2 and the
ratio of Bcl-2/Bax were significantly reduced (P<0.01), whereas
the expression levels of Bax were significantly increased
(P<0.01) in the IR group. Compared with in the IR group, the
mRNA expression levels of Bcl-2 and the ratio of Bcl-2/Bax were
significantly increased (P<0.01), whereas the mRNA expression
levels of Bax were significantly reduced (P<0.01) in the IPO and
EM50 groups (Fig. 5).
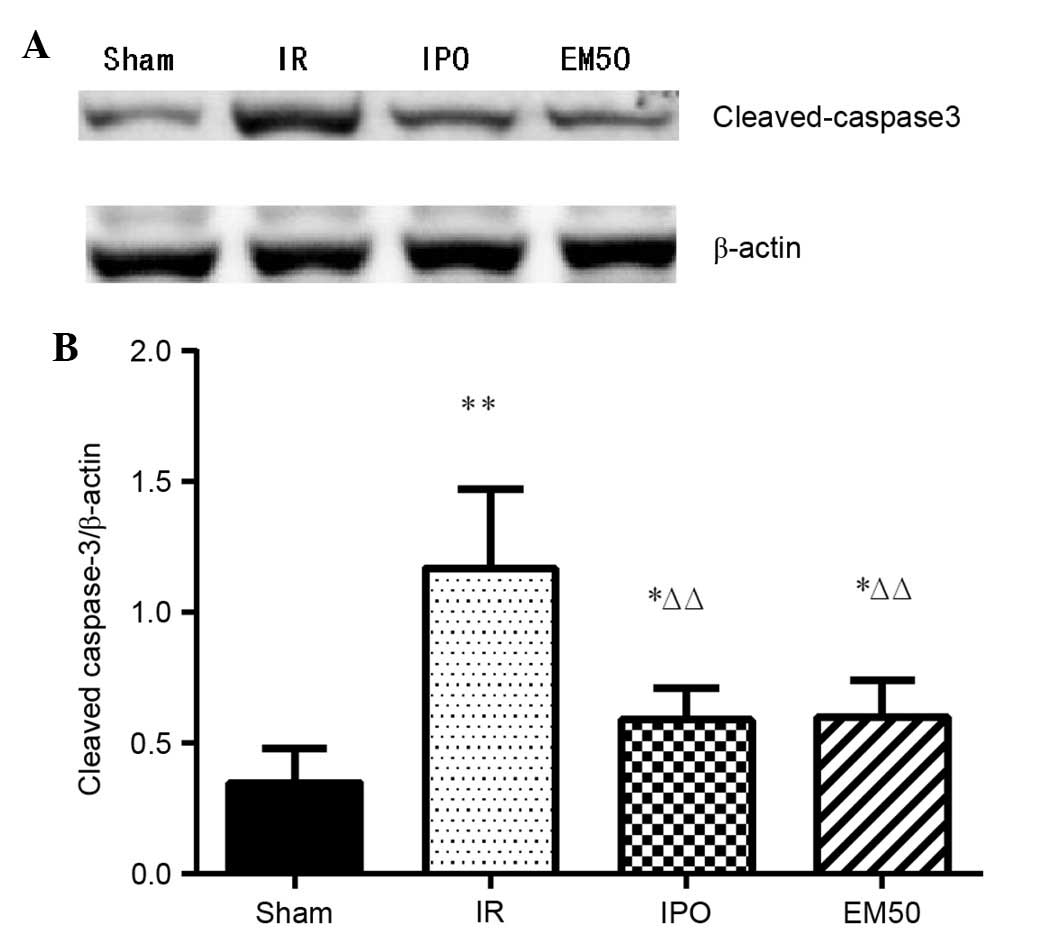
Alterations in the protein expression
levels of cleaved caspase-3
The protein expression levels of cleaved caspase-3
were higher in the IR, IPO and EM50 groups compared with in the
sham group (P<0.05, P<0.01). Compared with in the IR group,
the ratio of cleaved caspase-3/β-actin was significantly reduced in
the IPO and EM50 groups (P<0.01; Fig. 6).
Discussion
The myocardial restoration of blood flow following
ischemia may induce significant pathological and physiological
reperfusion-associated alterations to myocardial cells and the
local vascular network, further aggravating myocardial damage; this
process is known as MIRI. In the present study, a successful rat
model of MIRI was generated in vivo. Hemodynamic indexes,
myocardial enzymes and myocardial infarct size all reflect the
degree of I/R damage. Previous studies have demonstrated that the
primary mechanisms underlying MIRI may include excessive production
of free radicals, infiltration of inflammatory cells, mitochondrial
dysfunction and apoptosis of myocardial cells (19,23–25).
Therefore, reducing the generation of free radicals (26), inhibiting the inflammatory response
and suppressing apoptosis (27)
may markedly reduce I/R injury.
The myocardial restoration of blood flow following
ischemia may produce a large number of free radicals, which can
cause oxidative stress, lipid peroxidation and lead to further
injury of the myocardial tissue. SOD is one of the most important
radical scavenging enzymes, whereas MDA is a metabolite of lipid
peroxidation; therefore, measuring plasma SOD activity and MDA
content can reflect the production of oxygen free radicals and the
degree of myocardial cell damage (28). The results of the present study
indicated that SOD activity was increased and MDA content was
reduced in the plasma from IPO and EM50 groups compared with in the
IR group. These results suggested that EM-1 postconditioning may
alleviate MIRI by reducing the production of free radicals.
Inflammation is an important mechanism that is
closely associated with the occurrence and development of MIRI
(29). MIRI is characterized by a
local or systemic inflammatory response, the development of which
is complex. TNF-α is a cytokine that is predominantly produced by
macrophages, which is involved in the formation and development of
MIRI. It has previously been reported that excessive activation of
TNF-α can significantly damage myocardial function and prompt
myocardial cell apoptosis, thus increasing myocardial damage
(30). IL-6 is considered a marker
of inflammation, which is responsible for inflammatory regulation
and is also closely associated with the occurrence and development
of MIRI (31). The present study
indicated that in the IPO and EM50 groups the plasma levels of IL-6
and TNF-α were reduced compared with in the IR group. These
findings suggested that EM-1 postconditioning may alleviate MIRI by
inhibiting the inflammatory response.
Cell apoptosis is the primary mechanism underlying
MIRI. Cell apoptosis is controlled by several genes and enzymatic
reactions, the molecular regulatory mechanism of which is complex.
The Bcl-2 family has an important role in cell apoptosis, and the
Bcl-2 gene is the most representative anti-apoptotic gene of the
Bcl-2 family. The Bax gene shares 21% amino acid sequence homology
with the Bcl-2 gene, and is able to suppress the anti-apoptotic
function of Bcl-2; the Bcl-2/Bax ratio (32,33)
determines the occurrence of cell apoptosis. The cysteine aspartic
acid specific protease, or caspase, family also has an important
role in mediating apoptosis. Caspase-3 is an important caspase
family enzyme that induces the execution of apoptosis, and is
involved in the process of cell apoptosis after activation by
enzyme digestion. In addition, caspase-3 is an important proteinase
of the caspase enzyme cascade reaction; therefore, caspase-3 is
considered an enzymatic marker of apoptosis. The present study
demonstrated that in the IPO and EM50 groups the Bcl-2/Bax ratio
was increased, whereas the protein expression levels of cleaved
caspase-3 were reduced compared with the IR group; therefore, EM-1
postconditioning may produce anti-apoptotic effects in MIRI.
There are several mechanisms underlying MIRI and
their relationship is complex. Causal relationships may exist
between the mechanisms, and they may interact with each other
leading to myocardial injury. The number of neutrophils is markedly
increased during reperfusion, and neutrophils produce an excess of
oxygen free radicals, which can in turn induce oxidative stress,
lipid peroxidation and inflammation, thus promoting cell apoptosis
leading to further injury of myocardial tissue. It has previously
been reported that the production of oxygen free radicals,
accumulation of neutrophils and complement activation may be
associated with the inflammatory response (34). Furthermore, the excessive
activation of TNF-α can prompt myocardial cell apoptosis and
increase myocardial damage (30).
The present study demonstrated that EMs may produce anti-apoptotic
effects by resisting oxidative stress, lipid peroxidation and
inhibiting the inflammatory response; it may be hypothesized that
EMs also produce an anti-apoptotic effect via certain signaling
pathways, such as the phopshoinositide 3-kinase/Akt and
extracellular signal-regulated kinases /2 signaling pathways; the
related mechanisms require further study.
In conclusion, the present study demonstrated that
EM-1 postconditioning may exert myocardial protection and
anti-apoptotic effects by resisting oxidative stress, lipid
peroxidation and inhibiting the inflammatory response. Although EMs
only have four amino acid residues, they contain abundant
information. Therefore, in the future, EMs may have broad
application prospects, not only in analgesia, but also in the
treatment of cardiovascular conditions.
Acknowledgments
The present study was supported by research grants
from the Chinese National Natural Science Foundation (grant no.
81472656) and the Anhui Province Natural Science Foundation (grant
no. 1508085MH170).
References
|
1
|
Zadina JE, Hackler L, Ge LJ and Kastin AJ:
A potent and selective endogenous agonist for the mu-opiate
receptor. Nature. 386:499–502. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizoguchi H, Takagi H, Watanabe C,
Yonezawa A, Sato T, Sakurada T and Sakurada S: Involvement of
multiple μ-opioid receptor subtypes on the presynaptic or
postsynaptic inhibition of spinal pain transmission. Peptides.
51:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brunetti L, Ferrante C, Orlando G,
Recinella L, Leone S, Chiavaroli A, Di Nisio C, Shohreh R, Manippa
F, Ricciuti A, et al: Orexigenic effects of endomorphin-2 (EM-2)
related to decreased CRH gene expression and increased dopamine and
norepinephrine activity in the hypothalamus. Peptides. 48:83–88.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang CL, Zhou Y, Guo C, Zhang Y and Wang
R: In vivo characterization of intestinal effects of endomorphin-1
and endomorphin-2 in type 1 diabetic mice. Eur J Pharmacol.
698:499–504. 2013. View Article : Google Scholar
|
|
5
|
Block L, Björklund U, Westerlund A,
Jörneberg P, Biber B and Hansson E: A new concept affecting
restoration of inflammation-reactive astrocytes. Neuroscience.
250:536–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen ZC, Shieh JP, Chung HH, Hung CH, Lin
HJ and Cheng JT: Activation of peripheral opioid μ-receptors in
blood vessel may lower blood pressure in spontaneously hypertensive
rats. Pharmacology. 87:257–264. 2011. View Article : Google Scholar
|
|
7
|
Dai X, Song HJ, Cui SG, Wang T, Liu Q and
Wang R: The stimulative effects of endogenous opioids on
endothelial cell proliferation, migration and angiogenesis in
vitro. Eur J Pharmacol. 628:42–50. 2010. View Article : Google Scholar
|
|
8
|
Liu J, Wei S, Tian L, Yan L, Guo Q and Ma
X: Effects of endo-morphins on human umbilical vein endothelial
cells under high glucose. Peptides. 32:86–92. 2011. View Article : Google Scholar
|
|
9
|
Burley DS and Baxter GF: Pharmacological
targets revealed by myocardial postconditioning. Curr Opin
Pharmacol. 9:177–188. 2009. View Article : Google Scholar
|
|
10
|
Tanaka K, Kersten JR and Riess ML:
Opioid-induced cardioprotection. Curr Pharm Des. 20:5696–5705.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li R, Wong GT, Wong TM, Zhang Y, Xia Z and
Irwin MG: Intrathecal morphine preconditioning induces
cardioprotection via activation of delta, kappa, and mu opioid
receptors in rats. Anesth Analg. 108:23–29. 2009. View Article : Google Scholar
|
|
12
|
Brown DA, Sabbah HN and Shaikh SR:
Mitochondrial inner membrane lipids and proteins as targets for
decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther.
140:258–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015. View Article : Google Scholar
|
|
14
|
Gong P, Chen FX, Zhao Q, Ma GF and Wang R:
The oxidation metabolites of endomorphin 1 and its fragments
induced by free radicals. J Pept Sci. 15:337–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Y, Lu Y, Lin X, Gao Y, Zhao Q, Li W
and Wang R: Endomorphins and morphine limit
anoxia-reoxygenation-induced brain mitochondrial dysfunction in the
mouse. Life Sci. 82:752–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maslov LN, Naryzhnaia NV, Tsibulnikov SY,
Kolar F, Zhang Y, Wang H, Gusakova AM and Lishmanov YB: Role of
endogenous opioid peptides in the infarct size-limiting effect of
adaptation to chronic continuous hypoxia. Life Sci. 93:373–379.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin-Schild S, Gerall AA, Kastin AJ and
Zadina JE: Differential distribution of endomorphin 1- and
endomorphin 2-like immunoreactivities in the CNS of the rodent. J
Comp Neurol. 405:450–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh SS and Kang PM: Mechanisms and
inhibitors of apoptosis in cardiovascular diseases. Curr Pharm Des.
17:1783–1793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savateev AV and Savateeva-Liubimova TN:
Apoptosis-universal mechanisms of cell death and survival in
ischemia and reperfusion: Ways to pharmacological control. Eksp
Klin Farmakol. 73:44–49. 2010.In Russian.
|
|
20
|
Eefting F, Rensing B, Wigman J, Pannekoek
WJ, Liu WM, Cramer MJ, Lips DJ and Doevendans PA: Role of apoptosis
in reperfusion injury. Cardiovasc Res. 61:414–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu-hui H, Yun F, Fen J and Xing L: Study
about cardiomyocyte apoptosis in myocardial ischemic-reperfusion
injury of rats and NF-κB p65, iNOS expression. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 26:868–870. 2010.In Chinese. PubMed/NCBI
|
|
22
|
Zhou H, Hou SZ, Luo P, Zeng B, Wang JR,
Wong YF, Jiang ZH and Liu L: Ginseng protects rodent hearts from
acute myocardial ischemia reperfusion injury through
GR/ER-activated RISK pathway in an endothelial NOS-dependent
mechanism. J Ethnopharmacol. 135:287–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mozaffari MS, Liu JY, Abebe W and Baban B:
Mechanisms of load dependency of myocardial ischemia reperfusion
injury. Am J Cardiovasc Dis. 3:180–196. 2013.PubMed/NCBI
|
|
24
|
Saleem MT, Chetty MC and Kavimani S:
Putative antioxidant property of sesame oil in an oxidative stress
model of myocardial injury. J Cardiovasc Dis Res. 4:177–181. 2013.
View Article : Google Scholar :
|
|
25
|
Gottlieb RA: Cell death pathways in acute
ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther.
16:233–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurian GA, Suryanarayanan S, Raman A and
Padikkala J: Antioxidant effects of ethyl acetate extract of
Desmodium gangeticum root on myocardial ischemia reperfusion injury
in rat hearts. Chin Med. 5:32010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi X, Cui X, Wu P, Wang S, Wang G, Yang X,
Yang F, Zheng S and Li Z: Effects of N-acetylcysteine on apoptosis
induced by myocardial ischemia reperfusion injury in rats' heart
transplantation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
27:1234–1239. 2013.In Chinese.
|
|
28
|
Gao YH, Chen L, Ma YL and He QY: Chronic
intermittent hypoxia aggravates cardiomyocyte apoptosis in rat
ovariectomized model. Chin Med J (Engl). 125:3087–3092. 2012.
|
|
29
|
Wang Y, Zhang ZZ, Wu Y, Zhan J, He XH and
Wang YL: Honokiol protects rat hearts against myocardial ischemia
reperfusion injury by reducing oxidative stress and inflammation.
Exp Ther Med. 5:315–319. 2013.
|
|
30
|
Su H, Yuan Y, Wang XM, Lau WB, Wang Y,
Wang X, Gao E, Koch WJ and Ma XL: Inhibition of CTRP9, a novel and
cardiac-abundantly expressed cell survival molecule, by
TNFα-initiated oxidative signaling contributes to exacerbated
cardiac injury in diabetic mice. Basic Res Cardiol. 108:3152013.
View Article : Google Scholar
|
|
31
|
Wang Q, Cheng Y, Xue FS, Yuan YJ, Xiong J,
Li RP, Liao X and Liu JH: Postconditioning with vagal stimulation
attenuates local and systemic inflammatory responses to myocardial
ischemia reperfusion injury in rats. Inflamm Res. 61:1273–1282.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin S and Dai CL: Attenuation of
reperfusion-induced hepatocyte apoptosis is associated with
reversed bcl-2/bax ratio in hemi-hepatic artery-preserved portal
occlusion. J Surg Res. 174:298–304. 2012. View Article : Google Scholar
|
|
33
|
Yao Y, Huang C, Li ZF, Wang AY, Liu LY,
Zhao XG, Luo Y, Ni L, Zhang WG and Song TS: Exogenous
phosphatidylethanolamine induces apoptosis of human hepatoma HepG2
cells via the bcl-2/Bax pathway. World J Gastroenterol.
15:1751–1758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seelinger G, Merfort I and Schempp CM:
Anti-oxidant, anti-inflammatory and anti-allergic activities of
luteolin. Planta Med. 74:1667–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|