Introduction
As one of the major life-threatening diseases,
end-stage renal disease (ESRD) is increasing in developed and the
developing countries, and imposes a major social and economic
burden on the majority of countries (1,2).
Patients with ESRD have three principal choices for renal
replacement therapy, including hemodialysis (HD), peritoneal
dialysis (PD) and kidney transplantation (3). PD and HD are dialysis options for
patients with ESRD for whom preemptive kidney transplantation is
not an option. There is limited evidence that one form of dialysis
is more successful than the other, although patients on PD have
significantly improved quality of life in physical and
psychological aspects, and have significantly lower mortality
rates, compared with patients on HD (4). At present, an increasing number of
patients are opting for PD. According to statistics, the number of
patients treated with PD increased worldwide between 1997 and 2008,
with a 2.5-fold increase in the prevalence of patients on PD in
developing countries (5). However,
peritoneal fibrosis remains a serious complication of long-term PD,
which leads to the failure of peritoneal function and ending of
dialysis. A variety of injury-inducing factors contribute to the
occurrence of peritoneal fibrosis, including bioincompatible
dialysate components, uremic toxins, refractory or recurrent
infectious peritonitis and chronic inflammation (6). Chronic inflammation is a major cause
of peritoneal fibrosis and dysfunction.
Epithelial-mesenchymal transition (EMT), which has
previously been described in chronic inflammatory and fibrogenic
diseases, is a conserved process in which polarized, immobile
epithelial cells lose tight junctions and associated adherence, and
become migratory mesenchymal cells (7). Emerging evidence shows that the EMT
of peritoneal mesothelial cells induced by chronic inflammation may
be an important process in peritoneal fibrosis (8). LPS is a critical factor, which can
induce EMT and the production of extracellular matrix in several
tissues and organs (9), therefore,
reducing the frequency of peritonitis in patients remains a
challenge.
Further investigations are required to identify
mechanisms, which can delay or minimize the occurrence of
LPS-induced EMT during PD. As an antioxidant, melatonin has
antifibrotic properties, although there are limited data providing
support for this. Thus, melatonin may be a novel option for
inhibiting LPS-induced EMT and assist in reducing the risk of
peritoneal fibrosis. The present study aimed to find novel
treatment procedures for renal fibrosis using melatonin.
Materials and methods
Reagents
Ultra-pure LPSs from Escherichia coli
(O111:B4) were obtained from Invivogen; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Melatonin, RNase-free DNaseI, DMSO and
Triton X-100 were purchased from Sigma-Aldrich; Thermo Fisher
Scientific, Inc.). SP600125 and BAY 11–7082 were purchased from
Beyotime Institute of Biotechnology (Shanghai, China). Dulbecco's
modified Eagle's medium/F12 (DMEM/F12) and fetal bovine serum (FBS)
were purchased from Gibco; Thermo Fisher Scientific, Inc.
Anti-GAPDH, anti-total (t)-c-Jun N-terminal kinase (JNK),
anti-phosphorylated (p)-JNK, anti-vimentin, anti-E-cadherin,
anti-α-smooth muscle actin (SMA) and anti-Snail antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The ECL kit was purchased Pierce; Thermo Fisher Scientific,
Inc.). All water used was glass distilled.
Cell culture
The human peritoneal mesothelial cell line (HMrSV5)
was purchased from the Cell Culture Centre, Chinese Academy of
Medical Sciences (Beijing, China). The HMrSV5 cells were cultured
in DMEM/F12 medium containing 10% FBS in a humidified atmosphere
consisting of 95% O2 and 5% CO2 at 37°C. The cells were subcultured
every 3 days using 0.2% trypsin and 0.02% EDTA. For experiments,
the cells were seeded at a density of 3×104
cells/cm2 and cultured for 24 h to obtain monolayers in
3 ml DMEM/F12 with 10% FBS. At 80% confluence, the cells were
exposed to the following conditions: i) control groups, the cells
were treated with fresh serum-free DMEM/F12 only; ii) experimental
groups, the cells were subjected to pretreatment with 10 µg/ml LPS
for 24, 48 and 72 h in the absence or presence of 1 µM melatonin at
37°C in a humidified atmosphere of 5% CO2. To further
elucidate into this signaling pathway leading to the protection of
EMT by melatonin in HMrSV5 cells, 10 µmol/l SP600125 or BAY 11
77082 (10 mM) were used. Cells were incubated with or without 10 µM
of SP600125 or 10 mM BAY 11 77082 for 1 h, and then exposed to 10
µg/ml LPS for 48 h with or without 1 µM melatonin pretreatment. For
experiments, the cells were cultured for 24 h to obtain monolayers
in 3 ml DMEM/F12 with 10% FBS. Following rinsing of the cells with
phosphate-buffered saline (PBS), the medium was replaced and the
cells were cultured further.
Immunofluorescence staining
The HMrSV5 cells were seeded into six-well plates
for 24 h. Following fixing with ice-cold methanol for 5 min, the
cells were permeabilized with 0.1% Triton-X100 for 5 min, blocked
with 1% bovine serum albumin (Sangon Biotech Co., Ltd., Shanghai,
China) for 10 min, and incubated with mouse monoclonal
anti-E-cadherin antibody (cat. no. sc-21791; 1:400) or
anti-vimentin antibody (cat. no. sc-373717; 1:400) for 2 h at 37°C.
Following three washes in PBS, the cells were incubated with
tetramethylrhodamine isothiocyanate- and fluorescein
isothiocyanate-conjugated goat anti-mouse IgG (1:100 in PBS) for
0.5 h at room temperature. Subsequently, Hoechst 33342 was added to
the cells for 15 min. Following washing three times with PBS, all
the samples were examined using optical microscopy (Olympus
Corporation, Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from the HMrSV5 cells using
TRIzol (Tiangen Biotech Co., Ltd., Beijing, China). The RT-PCR kit
was purchased from (Takara Bio, Inc., Otsu, Japan). First-strand
cDNA was synthesized from a 1 µg aliquot of the total RNA samples
using oligo-dT primers and reverse transcriptase. The following
primers were used: GAPDH, forward 5-CGGAGTCAACGGATTTGGTCGTAT-3 and
reverse 5-AGCCTTCTCCATGGTGGTGAAGAC-3; E-cadherin, forward
5-TTGCTCACATTTCCCAACTCCTC-3 and reverse
5-CACCTTCAGCCATCCTGTTTCTC-3; Vimentin, forward
5-GCTGAATGACCGCTTCGCCAACT-3 and reverse
5-AGCTCCCGCATCTCCTCCTCGTA-3; α-SMA, forward
5-AAGAGGAAGACAGCACAGCTC-3 and reverse 5-TTACAGAGCCCAGAGCCATT-3;
Toll-like receptor (TLR)-4, forward 5-TGTCTGAACTCCCTCCAGGT-3 and
reverse 5-CACACTGAGGACCGACACAC-3; nuclear factor (NF)-κB, forward
5-TGGTGAAGACCTTGCTGCTAAATGC-3 and reverse
5-ACTGGGTGAGGTTGTCTGTCGGTA-3. RT-PCR was performed for 30 cycles.
The reactions were performed with a Gene Amp PCR system 9700
(PerkinElmer, Inc., Waltham, MA, USA). The amplified products were
separated by electrophoresis on a 2% agarose gel and visualized by
ethidium bromide staining. Each product was visualized following
separation and using GAPDH as an internal control. The image
density was quantified using a FluoroImager SI (Amersham Pharmacia
Biotech, Amersham, UK).
Western blot analysis
The HMrSV5 cells were washed with cold PBS three
times and lysed in RIPA buffer (Beyotime Institute of
Biotechnology). The cell lysates were harvested by centrifugation
at 12,000 g for 10 min at 4°C, and the protein concentration was
determined using a Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The samples, containing 10 µg of proteins, were
electrophoresed on a 10% SDS-PAGE gel and then transferred onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were incubated at 4°C overnight with the
following primary antibodies: Anti-α-SMA (cat. no. sc-324317;
1:400), anti-E-cadherin (1:400), anti-vimentin (1:400), anti-t-JNK
(cat. no. sc-137018; 1:400), anti-p-JNK (cat. no. sc-6254; 1:400),
anti-Snail (cat. no. sc-10432; 1:400) and anti-GAPDH (cat. no.
sc-365062; 1:1,000). Subsequently, the membranes were incubated
with the appropriate peroxidase-conjugated secondary antibodies
(cat. no. sc-3795) for 2 h at room temperature, and detection was
performed using an enhanced chemiluminescence kit (Pierce; Thermo
Fisher Scientific, Inc.). The relative expression levels of the
proteins were analyzed, and the results were quantified using
QuantityOne software (Bio-Rad Laboratories, Inc.
Statistical analysis
The data were analyzed statistically using SPSS
software 18.0 (IBM SPSS, Armonk, NY, USA) and presented as the mean
± standard deviation. Analysis was performed using one-way analysis
of variance followed by Dunnett's post-hoc test. P<0.05 was
considered indicative of a statistically significant
difference.
Results
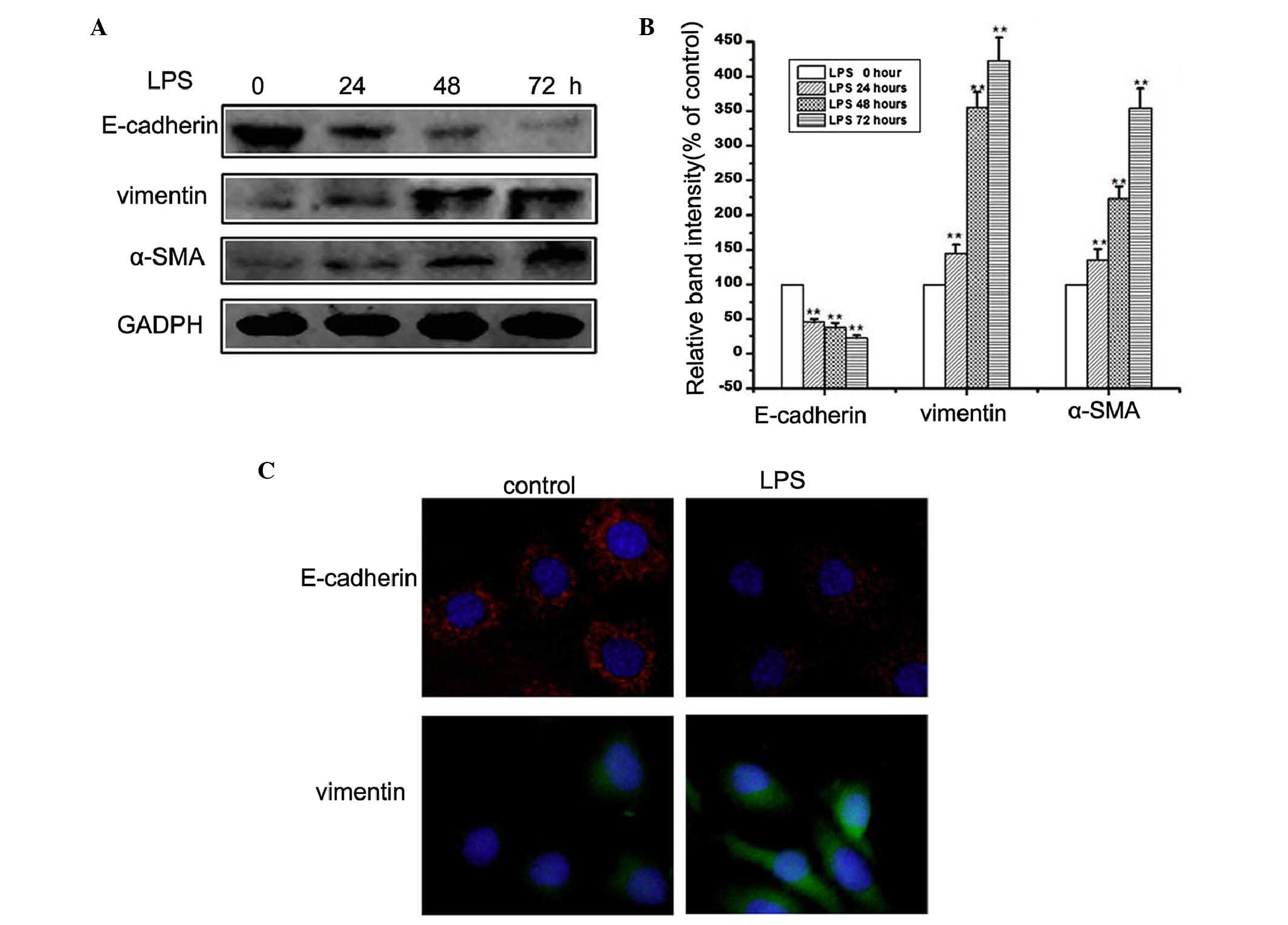
LPS induces EMT in HMrSV5 cells
LPS is released during the lysis of bacteria. It has
been reported that LPS can induce cytokines from immune cells and
induce cell apoptosis (10),
however, whether LPS induces EMT in HMrSV5 cells remains to be
elucidated. To investigate LPS-induced EMT, in the present study,
HMrSV5 cells were treated with 10 µg/ml LPS for 24, 48 and 72 h,
and the expression levels of E-cadherin, vimentin and α-SMA were
detected using western blot analysis and immunofluorescence. The
concentration of LPS was selected based on a previous study
(11). As shown in Fig. 1A and B, the results of the western
blot analysis showed that LPS treatment downregulated the level of
E-cadherin, and upregulated the expression levels of vimentin and
α-SMA. Similarly, immunofluorescence confirmed the that the protein
expression of vimentin was increased, and that of E-cadherin was
decreased following culture with LPS (Fig. 1C). Collectively, these observations
suggested that the HMrSV5 cells had undergone EMT following
treatment with LPS.
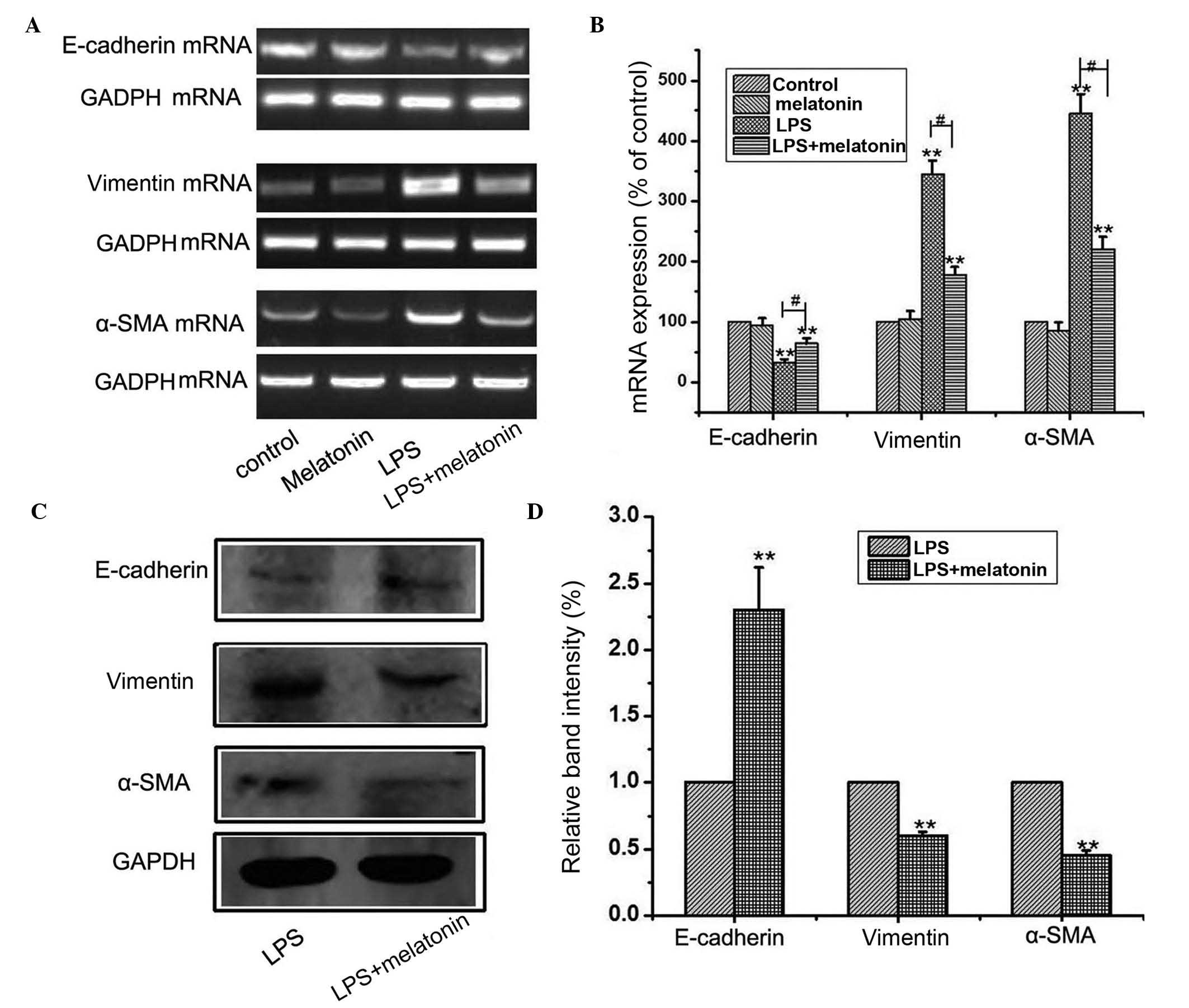
Melatonin suppresses LPS-induced
EMT
Previous reports have shown that melatonin has an
anti-inflammatory effect (12).
The present study investigated the effects of melatonin on
LPS-induced EMT in HMrSV5 cells. The HMrSV5 cells were subjected to
10 µg/ml LPS for 48 h in the absence or presence of 1 µM melatonin.
The concentration of melatonin was selected based on a previous
study (13), and had no effect on
proliferation or apoptosis. The effects of melatonin on LPS-induced
EMT of HMrSV5 cells were assessed using western blot and RT-PCR
analyses. The EMT induced by LPS was attenuated by co-treating the
cells with 1 µM melatonin, which was evidenced by the reduced
upregulation of α-SMA and vimentin, and amelioration of the
inhibited expression of E-cadherin at the mRNA (Fig. 2A) and protein (Fig. 2B) levels. These results showed that
melatonin supplementation reversed LPS-induced EMT in the HMrSV5
cells.
Melatonin inhibits the LPS-induced
activation of TLR4/JNK signaling
TLRs recognize a variety of microbial structural
components, termed pathogen-associated molecular patterns. TLR4/JNK
signaling is involved in tumor invasion and EMT (14). The present study examined whether
melatonin mediated its effects on EMT in HMrSV5 cells through this
pathway. The HMrSV5 cells were cultured with 10 µg/ml LPS, with or
without 1 µM melatonin for 48 h, and the levels of TLR-4, t-JNK and
p-JNK were detected using RT-PCR and western blot analyses. The
results revealed that LPS upregulated the level of TLR-4 and the
phosphorylation of JNK. However, no significant change was observed
in the expression of t-JNK (P>0.05; Fig. 3A and B). These results led to the
hypothesis that melatonin may suppress EMT in HMrSV5 cells through
inhibiting the TLR4/JNK pathway. The present study further
investigated the effect of melatonin on the TLR4/JNK pathway with
10 µmol/l SP600125, an inhibitor of JNK. The concentration of
SP600125 was selected based on a previous study (15). From the results of Fig. 3C, The inhibitor of JNK effectively
eliminated the protective effect of melatonin, causing a decrease
in the level of E-cadherin, and increases in the levels of vimentin
and α-SMA. Taken together, these findings suggested that the
inactivation of the TLR4/JNK pathway was a critical event in the
melatonin-induced anti-EMT effect in HMrSV5 cells.
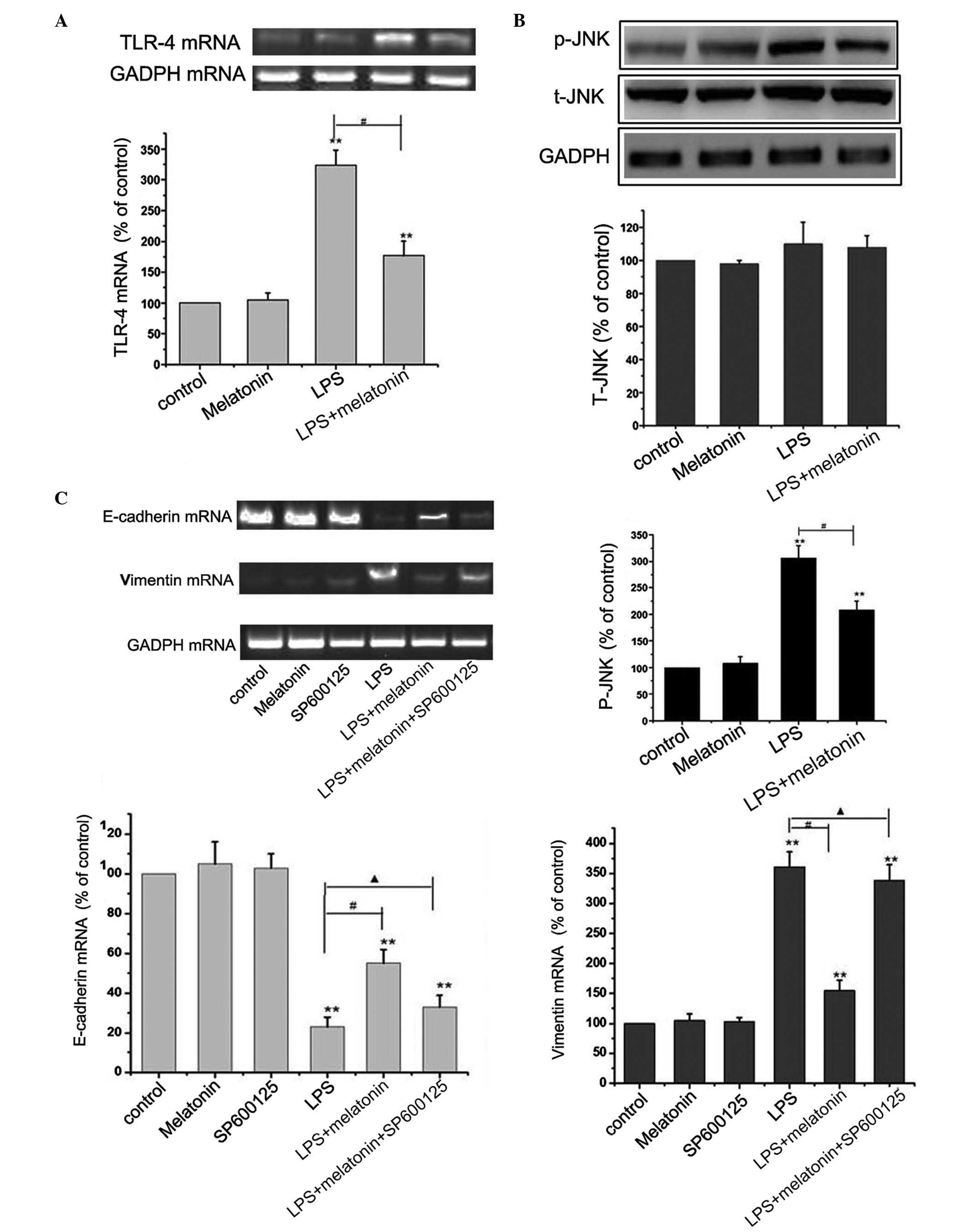 | Figure 3.Melatonin inhibits LPS-induced
epithelial-mesenchymal transition through the TLR4/JNK signaling
pathway. (A) HMrSV5 cells were cultured with 10 µg/ml LPS, with or
without 1 µM melatonin, for 48 h, and the mRNA level of TLR-4 was
detected using RT-PCR analysis. GAPDH was used as an internal
control (**P<0.01, vs. control; #P<0.01 LPS, vs.
LPS+melatonin). (B) Expression levels of t-JNK and p-JNK were
detected using western blot analysis. GAPDH was used as an internal
control (**P<0.01, vs. control; #P<0.01 LPS, vs.
LPS+melatonin). (C) Cells were incubated with or without 10 µM of
SP600125 for 1 h, and then exposed to 10 µg/ml LPS for 48 h with or
without 1 µM melatonin pretreatment. The mRNA expression levels of
vimentin, E-cadherin and α-SMA were detected using RT-PCR analyses.
Data in all graphs are presented as the mean ± standard deviation
(n=6). GAPDH was used as an internal control (**P<0.01, vs.
control; #P<0.01 LPS, vs. LPS+melatonin;
▲P>0.05 LPS+melatonin+SP600125, vs. LPS). LPS,
lipopolysaccharide; TLR4, Toll-like receptor 4; JNK, c-Jun
N-terminal kinase; T-, total; P-, phosphorylated; α-SMA, α-smooth
muscle actin; RT-PCR, reverse transcription-polymerase chain
reaction. |
Melatonin inhibits the LPS-induced
activation of TLR4/NF-κB-Snail signaling pathway
Several studies have reported that TLR4/NF-κB
inactivation can protect EMT (16). To further elucidate into this
signaling pathway leading to the protection of EMT by melatonin in
HMrSV5 cells, a specific NF-κB inhibitor, BAY 11–77082 (10 mM) was
used. The concentration of BAY 11–7082 was selected based on a
previous study (17). As shown in
Fig. 4A-C, melatonin significantly
downregulated the increased expression levels of TLR-4, NF-κB and
Snail, determined using RT-PCR and western blot analyses. However,
the inhibitor of NF-κB effectively eliminated the protective effect
of melatonin, causing a decrease in the level of E-cadherin, and
increases in the levels of vimentin and α-SMA. Taken together,
these findings suggested that inactivation of the TLR4/NF-κB-Snail
pathway was a critical event in the melatonin-induced anti-EMT
effect in HMrSV5 cells.
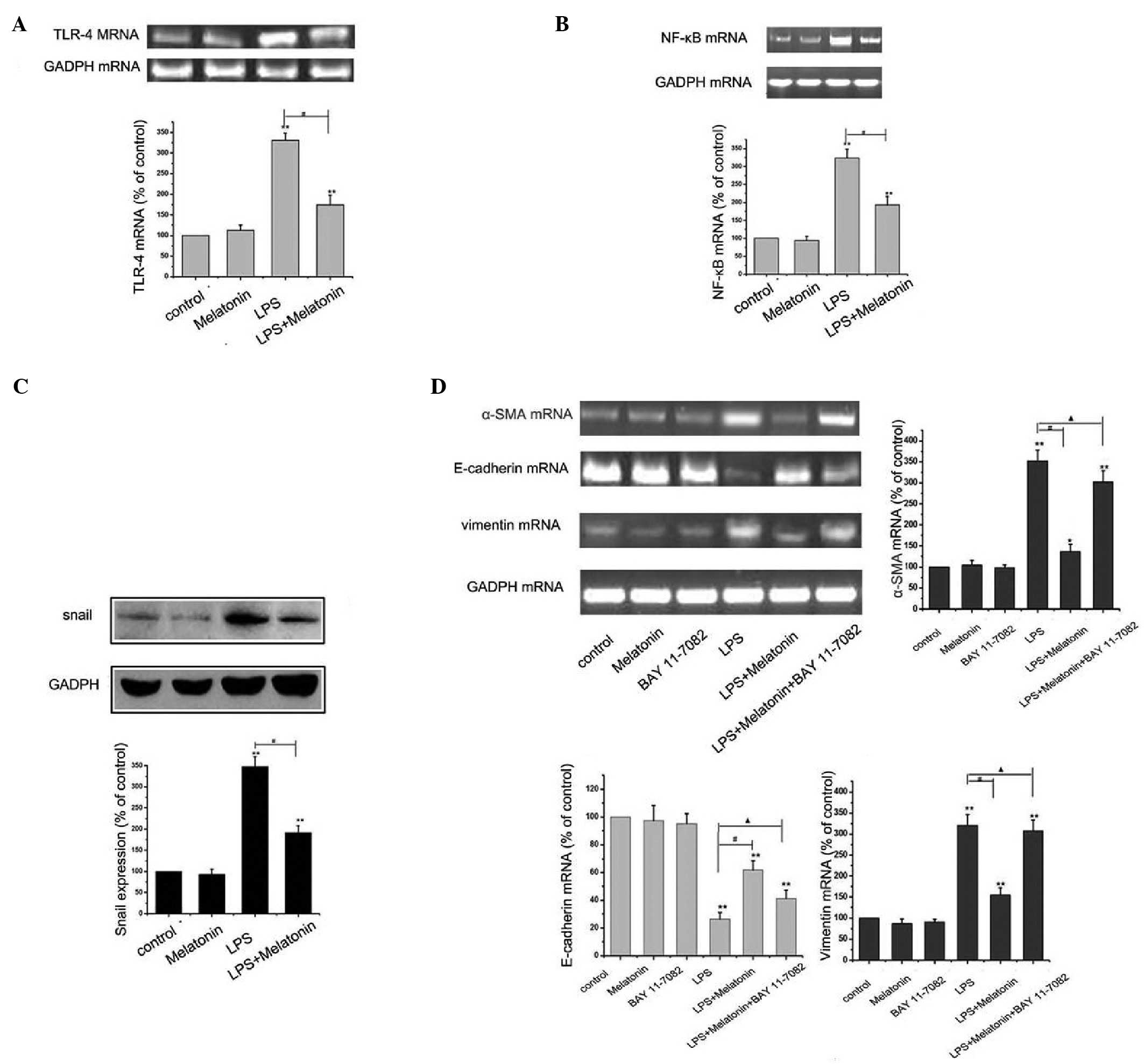 | Figure 4.Melatonin inhibits LPS-induced
epithelial-mesenchymal transition through the TLR4/NF-κB-Snail
signaling pathway. (A) HMrSV5 cells were cultured in 10 µg/ml LPS
with or without 1 µM melatonin for 48 h, and the mRNA expression of
TLR4 was detected using RT-PCR analysis. GAPDH was used as an
internal control (**P<0.01, vs. control; #P<0.01
LPS, vs. LPS+melatonin). (B) Cells were treated, as described above
and the expression of NF-κB was detected using RT-PCR analysis.
GAPDH was used as an internal control (**P<0.01. vs. control;
#P<0.01 LPS vs, LPS+melatonin). (C) Cells were
treated, as described above, and the expression of Snail was
detected using western blot analysis. GAPDH was used as an internal
control (**P<0.01, vs. control; #P<0.01 LPS, vs.
LPS+melatonin). (D) Cells were incubated with or without 10 µM of
BAY 11–7082 for 1 h, and then exposed to 10 µg/ml LPS for 48 h in
the presence or absence of 1 µM melatonin pretreatment. The mRNA
expression levels of vimentin, E-cadherin and α-SMA were detected
using RT-PCR analysis. (**P<0.01, vs. control;
#P<0.01 LPS, vs. LPS+melatonin; ▲P>0.05
LPS+melatonin+BAY 11–7082, vs. LPS). All data are presented as the
mean ± standard deviation (n=6). GAPDH was used as an internal
control EMT, epithelial-mesenchymal transition; LPS,
lipopolysaccharide; TLR4, Toll-like receptor 4; α-SMA, α-smooth
muscle actin; NF-κB, nuclear factor-κB; RT-PCR, reverse
transcription-polymerase chain reaction. |
Discussion
The neurohormone melatonin is secreted rhythmically
by a circadian pacemaker located in the suprachiasmatic nucleus
(18). Melatonin regulates several
physiological functions, including sleep and circadian rhythms,
immune function, body weight and energy balance, and has anticancer
effects (19,20). In addition, as a potent
antioxidant, melatonin has been found to be effective in a variety
of disorders linked to oxidative stress and inflammation (21). Several studies have demonstrated
that melatonin alleviates the LPS-induced expression of
inflammatory cytokines/chemokines. Melatonin has been found to
regulate LPS-induced proinflammatory and anti-inflammatory
cytokines in the serum, fluid, liver and brain (22). Melatonin inhibits the production of
NO and protects neural stem cells against LPS-induced inflammatory
stress (23). Melatonin also
reduces the expression of pro-inflammatory mediators and enhances
the expression of heme oxygenase 1 via the NF-κB, p38
mitogen-activated protein kinase and nuclear factor erythroid 2
related factor 2 cascade signaling pathways in murine macrophages
(24). In the present study, it
was confirmed that melatonin was associated with anti-inflammation
effects and has a protective role in LPS-induced EMT in HMrSV5
cells, reducing the upregulation of α-SMA and vimentin, and
ameliorating the expression of E-cadherin (Fig. 2).
LPS-induced inflammation and LPS-induced EMT are key
in peritoneal fibrosis in PD. A previous study found that exposure
to LPS in a rat model led to peritoneal fibrosis and
neoangiogenesis (25). TLR4 is
responsible for the immediate response to Gram-negative bacteria
and is recruited to initiate a signaling cascade leading to
production of pro-inflammatory cytokines. The presents study
investigated the expression of TLR4 during LPS stimulation. A shown
in Fig. 3, LPS significantly
upregulated the level of TLR4, determined using RT-PCR. A previous
study confirmed this result, in which LPS/TLR4 signaling led to the
activation of the transcription factors, NF-κB, IL-6 and TNF-α
(26). In the present study, it
was confirmed that melatonin was associated with TLR4/JNK and
TLR4/NF-κB-Snail inactivation in LPS-treated HMrSV5 cells (Figs. 3 and 4). Therefore, it is possible that
melatonin suppresses LPS-induced EMT via inhibiting the TLR4/JNK
and TLR4/NF-κB-Snail pathways. Therefore, targeting inflammation,
EMT or associated cell signaling pathways may inhibit peritoneal
fibrosis in PD. The findings of the present study indicated that
melatonin offers potential in the prevention and therapy of
peritoneal fibrosis.
References
|
1
|
Rastogi A, Linden A and Nissenson AR:
Disease management in chronic kidney disease. Adv Chronic Kidney
Dis. 15:19–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zelmer JL: The economic burden of
end-stage renal disease in Canada. Kidney Int. 72:1122–1129. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knoll GA and Nichol G: Dialysis, kidney
transplantation, or pancreas transplantation for patients with
diabetes mellitus and renal failure: A decision analysis of
treatment options. J Am Soc Nephrol. 14:500–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mousavi SS Beladi, Hayati F, Valavi E,
Rekabi F and Mousavi MB: Comparison of survival in patients with
end-stage renal disease receiving hemodialysis versus peritoneal
dialysis. Saudi J Kidney Dis Transpl. 26:392–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain AK, Blake P, Cordy P and Garg AX:
Global trends in rates of peritoneal dialysis. J Am Soc Nephrol.
23:533–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ueno T, Nakashima A, Doi S, Kawamoto T,
Honda K, Yokoyama Y, Doi T, Higashi Y, Yorioka N, Kato Y, et al:
Mesenchymal stem cells ameliorate experimental peritoneal fibrosis
by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney
Int. 84:297–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strippoli R, Moreno-Vicente R, Battistelli
C, Battistelli C, Cicchini C, Noce V, Amicone L, Marchetti A, Del
Pozo MA and Tripodi M: Molecular mechanisms underlying peritoneal
EMT and fibrosis. Stem Cells Int. 35436782016.PubMed/NCBI
|
|
9
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang C, Zheng H, He W, Lu G, Li X, Deng Y
and Zeng M: Ghrelin ameliorates the human alveolar epithelial A549
cell apoptosis induced by lipopolysaccharide. Biochem Biophys Res
Commun. 474:83–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jing YY, Han ZP, Sun K, Zhang SS, Hou J,
Liu Y, Li R, Gao L, Zhao X, Zhao QD, et al: Toll-like receptor 4
signaling promotes epithelial-mesenchymal transition in human
hepatocellular carcinoma induced by lipopolysaccharide. BMC Med.
10:982012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esposito E and Cuzzocrea S:
Antiinflammatory activity of melatonin in central nervous system.
Curr Neuropharmacol. 8:228–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Obana-Koshino A, Ono H, Miura J, Sakai M,
Uchida H, Nakamura W, Nohara K, Maruyama Y, Hattori A and Sakai T:
Melatonin inhibits embryonic salivary gland branching morphogenesis
by regulating both epithelial cell adhesion and morphology. PLoS
One. 10:e01199602015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pirianov G, Torsney E, Howe F and
Cockerill GW: Rosiglitazone negatively regulates c-Jun N-terminal
kinase and toll-like receptor 4 proinflammatory signalling during
initiation of experimental aortic aneurysms. Atherosclerosis.
225:69–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong XX, Liu JM, Qiu XY, Pan F, Yu SB and
Chen XQ: Piperlongumine induces apoptotic and autophagic death of
the primary myeloid leukemia cells from patients via activation of
ROS-p38/JNK pathways. Acta Pharmacol Sin. 36:362–374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naugler WE and Karin M: NF-kappaB and
cancer-identifying targets and mechanisms. Curr Opin Genet Dev.
18:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S
and Yu Y: Interleukin-17-induced EMT promotes lung cancer cell
migration and invasion via NF-κB/ZEB1 signal pathway. Am J Cancer
Res. 5:1169–1179. 2015.PubMed/NCBI
|
|
18
|
Houdek P, Polidarová L, Nováková M, Matějů
K, Kubík Š and Sumová A: Melatonin administered during the fetal
stage affects circadian clock in the suprachiasmatic nucleus but
not in the liver. Dev Neurobiol. 75:131–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Pedro N, Martínez-Alvarez RM and
Delgado MJ: Melatonin reduces body weight in goldfish (Carassius
auratus): Effects on metabolic resources and some feeding
regulators. J Pineal Res. 45:32–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Plaimee P, Weerapreeyakul N, Barusrux S
and Johns NP: Melatonin potentiates cisplatin-induced apoptosis and
cell cycle arrest in human lung adenocarcinoma cells. Cell Prolif.
48:67–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carrasco C, Marchena AM, Holguín-Arévalo
MS, Martín-Partido G, Rodríguez AB, Paredes SD and Pariente JA:
Anti-inflammatory effects of melatonin in a rat model of
caerulein-induced acute pancreatitis. Cell Biochem Funct.
31:585–590. 2013.PubMed/NCBI
|
|
22
|
Zhao H, Wu QQ, Cao LF, Qing HY, Zhang C,
Chen YH, Wang H, Liu RY and Xu DX: Melatonin inhibits endoplasmic
reticulum stress and epithelial-mesenchymal transition during
bleomycin-induced pulmonary fibrosis in mice. PLoS One.
9:e972662014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song J, Kang SM, Lee KM and Lee JE: The
protective effect of melatonin on neural stem cell against
LPS-induced inflammation. Biomed Res Int. 2015:8543592015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aparicio-Soto M, Alarcón-de-la-Lastra C,
Cárdeno A, Sánchez-Fidalgo S and Sanchez-Hidalgo M: Melatonin
modulates microsomal PGE synthase 1 and NF-E2-related
factor-2-regulated antioxidant enzyme expression in LPS-induced
murine peritoneal macrophages. Br J Pharmacol. 171:134–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Margetts PJ, Kolb M, Yu L, Hoff CM and
Gauldie J: A chronic inflammatory infusion model of peritoneal
dialysis in rats. Perit Dial Int. 21:(Suppl 3). S368–S372.
2001.PubMed/NCBI
|
|
26
|
Nijland R, Hofland T and van Strijp JA:
Recognition of LPS by TLR4: Potential for anti-inflammatory
therapies. Mar Drugs. 12:4260–4273. 2014. View Article : Google Scholar : PubMed/NCBI
|