Introduction
The World Health Organisation (WHO) defines
osteoporosis as a systemic skeletal disease characterized by low
bone density and micro-architectural deterioration of bone tissue,
which renders bone susceptible to fractures (1). Osteoporosis has become a primary
public health concern worldwide, as its incidence increases with
the ageing population (2). As a
dynamic system which continuously undergoes reconstruction, the
bone system maintains a balance between osteoblastic bone formation
and osteoclastic bone resorption (3). Increased osteoclastogenesis or
osteoclastic activity may cause an imbalance in bone remodeling and
accelerate bone loss (4), as
demonstrated in various skeletal disorders, including osteoporosis,
rheumatoid arthritis and Paget's disease of bone (5). Thus, osteoclasts are potential
targets for anti-resorptive agents.
Osteoclasts are multinucleate cells derived from
hematopoietic cells (6). Receptor
activator of nuclear factor κB ligand (RANKL) is important in
osteoclastogenesis and activation of mature osteoclasts (7). Binding of RANKL to its receptor RANK
leads to the recruitment of tumor necrosis factor receptor
associated factor-6 (TRAF-6) (8),
thus activating downstream signaling molecules, including
mitogen-activated protein kinases (MAPK), Src and the subsequent
transcription factors, nuclear factor of activated T cell-c1
(NFAT-c1) and c-Fos (9). This
ultimately leads to the expression of genes involved in osteoclast
differentiation and bone resorption, including tartrate-resistant
acid phosphatase (TRAP), cathepsin K (CTSK) and matrix
metalloproteinase-9 (MMP-9) (9,10).
This suggests that agents that inhibit RANKL signaling may provide
therapeutic potential in the treatment of diseases where bone loss
is a symptom (11,12).
In recent years, traditional Chinese medicines have
become increasingly investigated in the treatment and prevention of
osteoporosis (13). Numerous
studies have revealed that traditional Chinese medicines have
beneficial effects in vivo, in animal models of osteoporosis
and in vitro, in models of osteoclast differentiation
(14–16). In contrast to long-term hormone
replacement therapy, which may cause estrogen-like side-effects
(17), traditional Chinese
medicines are prepared from natural plants and are considered to
have fewer side-effects (18),
thus providing patients with treatment alternatives.
Among these natural herbal medicines, Fructus
ligustri Lucidi (FLL), the fruit of Ligustrum lucidum
Ait, is commonly used to tonify kidney and strengthen bone
(19). Certain studies have
revealed the positive effects of FLL in the improvement of bone
properties, modulation of bone turnover, improvement of calcium
balance in rats (20) and
promotion of osteogenesis of mesenchymal stem cells (21). Previous studies conducted by our
laboratory demonstrated that an ethanol extract of FLL may increase
bone mineral density and improve bone mechanical properties in
growing female rats (22), and
upregulate calcium absorption-associated gene expression in the
kidney and duodenum (23). In
additfion, it has been revealed to downregulate the gene expression
ratio of RANKL/osteoprotegerin in MC3T3-E1 cells (an
osteoblast-like cell line) (24).
However, the effect of FLL on osteoclast differentiation and bone
resorption, and its underlying mechanism, remain to be elucidated,
and the active compounds present in FLL have not yet been
identified. Oleanolic acid (OA) and ursolic acid (UA) are two
primary active components in FLL extract. Certain studies have
indicated that OA (or its derivative) may inhibit
osteoclastogenesis and mRNA expression levels of bone-associated
genes in vitro (24,25);
however, the effect of UA on osteoclast differentiation and bone
resorption, to the best of our knowledge, remains to be
elucidated.
The present study aims to elucidate the effect of
FLL on osteoclasts, and its underlying mechanism, and to identify
the active ingredients. RANKL-induced RAW264.7 murine
monocyte/macrophage cells underwent osteoclastic differentiation
in vitro. The inhibitory effect on osteoclastogenesis was
assessed by measuring the expression levels of
osteoclast-associated genes.
Materials and methods
FLL extract
FLL extract was prepared by Layn Natural Ingredients
Corporation (Guilin, China). Briefly, dried and powdered crude FLL
seeds were extracted twice with 70% ethanol. They were subsequently
filtered and concentrated under reduced pressure and lyophilized
into powder at a yield of 20% by weight of the starting materials.
Two primary compounds of FLL extract, OA and UA, were detected and
quantified with ultra-performance liquid chromatography (UPLC). A
photo-diode array (PDA) detector with 210 nm wavelength and a BEH
C18 column (2.1×100 mm; 1.7 µm) were used. The separating
conditions were as follows: Methanol, water and formic acid were
selected as the mobile phase at a volume ratio of 80:20:0.1 and the
flow rate was 0.4 ml/min. Finally, they were confirmed by retention
time compared with standard solutions.
Cell culture
The RAW264.7 murine monocyte/macrophage cell line
was obtained from the Cell Resource Center, Peking Union Medical
College (Beijing, China). The cells were maintained in high glucose
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), penicillin
(100 U/ml) and streptomycin (100 µg/ml; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany). Incubations were performed at 37°C
in 5% CO2. The medium was replaced every day and the cells were
subcultured every 3 days. FLL, OA and UA were dissolved in 0.1%
dimethyl sulfoxide (DMSO; Amresco, LLC, Solon, OH, USA) and further
diluted with cell culture medium to an appropriate concentration.
In addition, 0.1% DMSO was added to untreated cells and control
groups and demonstrated no effect.
Assesment of cytotoxicity
To evaluate the cytotoxicity of the FLL extract
along with OA and UA, cell viability assays on RAW264.7 cells were
conducted via conventional MTT (Amresco, LLC) assays. Briefly,
RAW264.7 cells were seeded in 96-well plates (1×103 cells per well)
and cultured overnight. Following this, the medium was replaced
with media containing various concentrations of FLL, OA or UA.
Following a 24-h incubation period, 5 mg/ml MTT (Amresco, LLC)
solution was added and the plates were incubated for a further 4 h.
At the end of the incubation period, the medium was replaced with
150 µl DMSO per well for solubilization of formazan crystals and
the optical density values were measured at a wavelength of 540 nm,
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Osteoclast differentiation of RAW264.7
cells
For the differentiation of RAW264.7 cells into
multinucleate osteoclasts, the cells were seeded into a 96-well
plate at a density of 3×103 cells per well and incubated overnight.
Following this, 50 ng/ml RANKL (PeproTech, Inc., Rocky Hill, NJ,
USA) was added into the medium, which was replaced every 3 days.
After 5 days, the multinucleate cells had differentiated into
mature osteoclasts.
TRAP-positive multinucleate cell
staining and TRAP activity assay
In the present study, TRAP was used as a marker of
osteoclasts. The RAW264.7 cells were seeded into a 96-well plate
and incubated overnight. Following this, 50 ng/ml RANKL and various
concentrations of FLL (10, 25, 50 and 100 µg/ml), OA (0.36, 0.89,
1.78 and 3.56 µg/ml) and UA (0.10, 0.26, 0.52 and 1.03 µg/ml) were
added into the medium. Concentrations of OA and UA were determined
based on concentrations of FLL. Every concentration of UA or OA was
consistent with its proportion in the FLL extract (demonstrated by
UPLC results). After 5 days of culture, the medium in the 96-well
plate was transferred to a new plate for the TRAP activity assay.
The cells were washed twice with pre-warmed PBS. For the TRAP
activity assay, an Acid Phosphatase assay kit (BioVision, Inc.,
Milpitas, CA, USA) was used. Briefly, p-nitrophenyl was used as a
phosphatase substrate that becomes yellow (λmax=405 nm) when
dephosphorylated by acid phosphatase. The TRAP staining method
involved cells fixed in 4% paraformaldehyde for 20 min, followed by
staining for TRAP using a Leukocyte Acid Phosphatase kit
(Sigma-Aldrich, Merck Millipore) according to the manufacturer's
protocol. The TRAP-positive cells appeared dark red and those
containing ≥3 nuclei were defined as osteoclasts. The number of
osteoclasts per well was counted and imaged using a light
microscope (Olympus Corporation, Tokyo, Japan).
Apoptosis assay
To examine the effect of FLL, OA and UA on the
apoptosis of osteoclasts, the RAW 264.7 cells were seeded into
60-mm cell culture dishes at a density of 1×105 cells per dish,
stimulated with RANKL and cultured for 5 days. Following this,
various concentrations of FLL, OA and UA were added into the medium
for a further 2 days. Subsequently, the cells were treated with
trypsin without EDTA, harvested and stained with an Annexin
V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) Apoptosis
Detection kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Finally, the cells were analyzed using a FACSCalibur flow cytometer
(BD Biosciences, San Jose, CA, USA) and the data were analyzed by
CellQuest Pro version 5.1 (BD Biosciences).
RNA extraction
Total RNA was isolated from cells treated with 50
ng/ml RANKL and FLL, OA and UA using TRIzol® reagent
(Beijing Biotides Biotechnology Co., Ltd., Beijing, China)
according to the manufacturer's protocol. The quality and quantity
of the extracted mRNA were assessed by spectrophotometry, with the
ratios of absorbance at 260 and 280 nm ranging from 1.9 to 2.0.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
cDNA was synthesized with a RT system kit (Promega
Biotech Co., Ltd., Beijing, China) and stored at −20°C until
further processing. Expression of selected genes was measured by
qPCR using a Bio-Rad CFX9600 system (Bio-Rad Laboratories, Inc.)
with the Maxima SYBR® Green qPCR Master Mix (2X;
Invitrogen; Thermo Fisher Scientific, Inc.). Primer sequences were
as follows: For TRAP, forward 5′-GAACCGTGCAGACGATGG-3′ and reverse
5′-GGAAGTTCCAGCGCTTGG-3′; for CTSK, forward
5′-CTGCCCATAACCTGGAGG-3′ and reverse 5′-GCCCTGGTTCTTGACTGG-3′; for
MMP-9, forward 5′-GGTCTAGGCCCAGAGGTA-3′ and reverse
5′-GGTCGTAGGTCACGTAGC-3′; for TRAF-6, forward
5′-ATTCTCGACCAGTCTGAAG-3′ and reverse 5′-ATGAAGGTTCCCTGTCT-3′; for
NFAT-c1, forward 5′-TCATCCTGTCCAACACCAAA-3′ and reverse
5′-TCACCCTGGTGTTCTTCCTC-3′; for c-Fos, forward
5′-CGAAGGGAACGGAATAAGAT-3′ and reverse 5′-GCAACGCAGACTTCTCATC-3′;
for Src, forward 5′-TCGTGAGGGAGAGTGAGAC-3′ and reverse
5′-GCGGGAGGTGATGTAGAAAC-3′; for GAPDH, forward
5′-AACTTTGGCATTGTGGAAGG-3′ and reverse 5′-ACACATTGGGGGTAGGAACA-3′.
For each primer pair, the analysis was performed in triplicate with
a total volume of 25 µl reaction mixture. The cycling conditions
for PCR were as follows: An initial denaturation step of 10 min at
95°C, followed by 45 cycles of denaturation at 94°C for 10 sec,
annealing at 55°C for 30 sec and extension at 72°C for 20 sec.
Melting curves were assesed to ensure the amplification was
specific. Relative gene expression was determined by employing the
formula 2-ΔΔCq (26). mRNA
expression levels in all experimental groups were compared with
those of the control group.
Statistical analysis
All data are expressed as the mean ± standard
deviation. At least 3 independent experiments were conducted in
duplicate. Statistical analysis was performed using SPSS software
version 13.0 for Windows (SPSS, Inc., Chicago, IL, USA). One-way
analyses of variance were used to identify differences between
values of experimental and control groups. All identified
differences were tested for homogeneity of variances, if equal
variance was indicated, followed by the least significant
difference test (LSD), or Tamhane's T2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Analysis of the FLL extract
Peaks of OA and UA appeared at 24.582 and 26.093
min, respectively, in HPLC, compared with the retention times of
the standard solutions, 24.5 min for OA and 26.0 min for UA. Using
the external standard method, the contents of OA and UA in FLL were
quantified as 3.56 and 1.03%, respectively. The present study
selected the concentrations of 10, 25 50 and 100 µg/ml FLL on the
basis of a previous study about the effect of FLL ethanol extract
on osteoblast-like cell line (22). Based on the quantity of OA and UA
in FLL, the concentrations of 0.36, 0.89, 1.78 and 3.56 µg/ml OA,
and 0.10, 0.26, 0.52 and 1.03 µg/ml UA were selected.
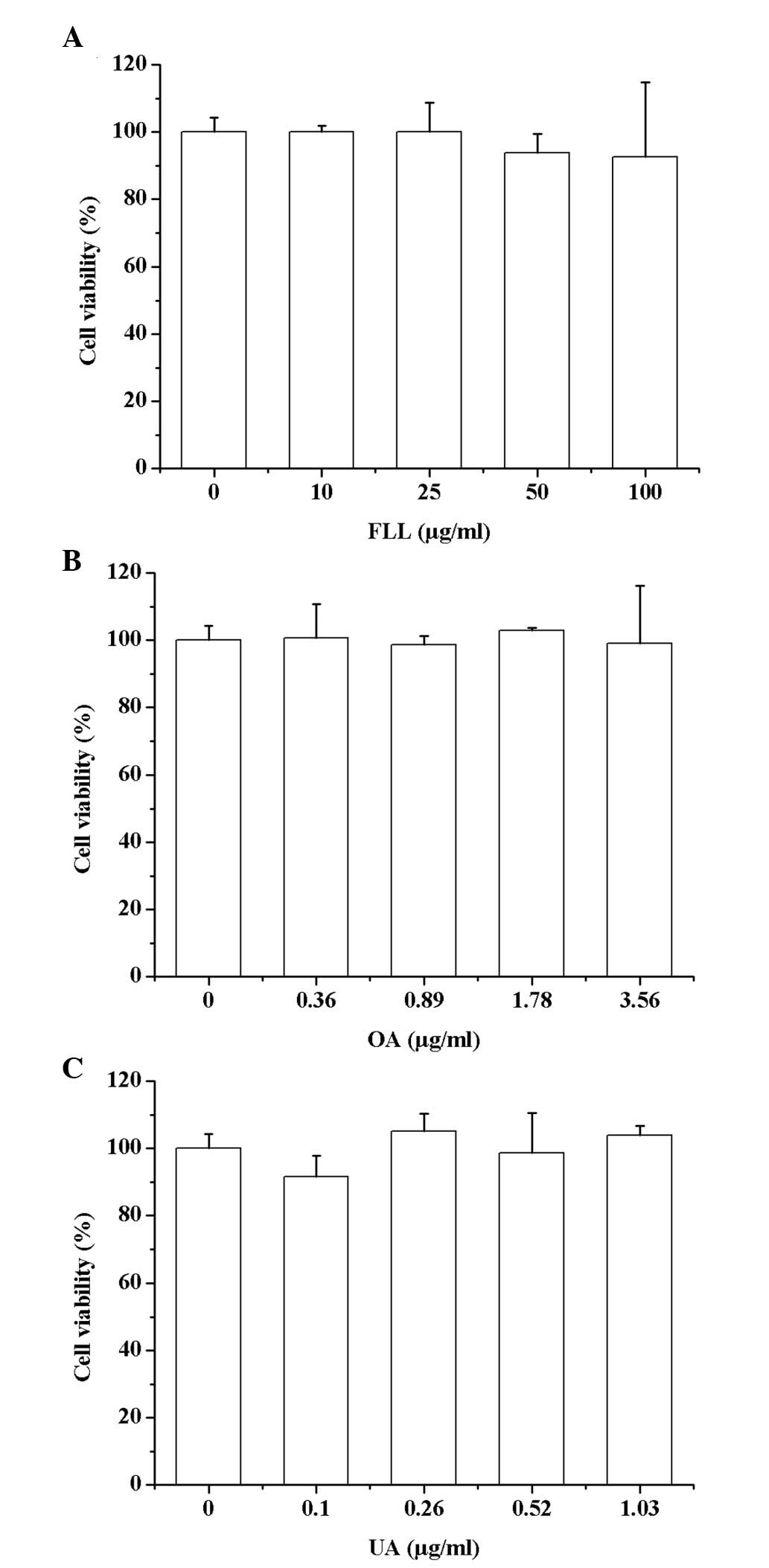
FLL, OA and UA are not cytotoxic to
RAW264.7 cells
As presented in Fig.
1, no concentration of the FLL extract (10, 25, 50 and 100
µg/ml), OA (0.36, 0.89, 1.78 and 3.56 µg/ml) or UA (0.10, 0.26,
0.52 and 1.03 µg/ml) induced cytotoxicity in RAW264.7 cells
compared with the control group.
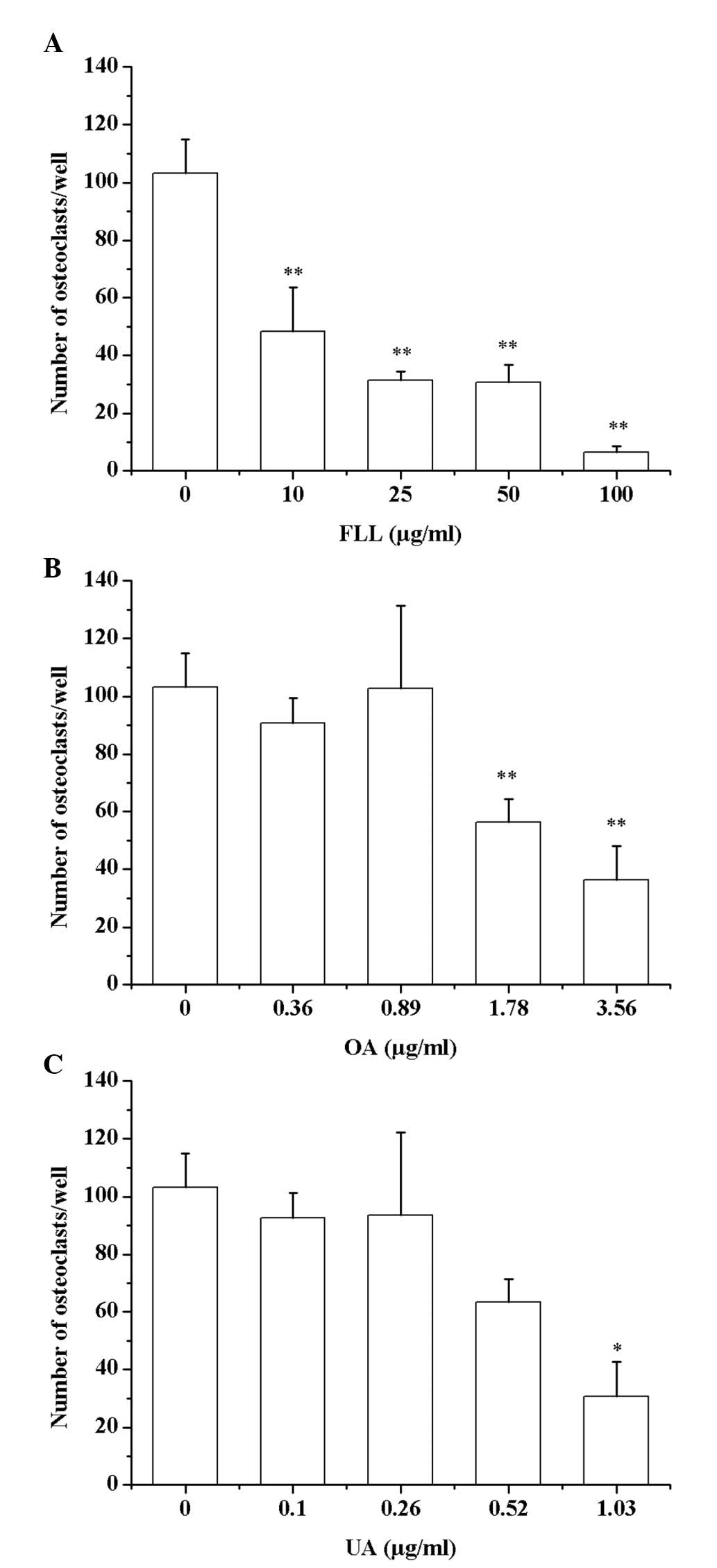
FLL, OA and UA inhibit RANKL-induced
osteoclastogenesis of RAW264.7 cells
To examine the effect of FLL, OA and UA on
osteoclast differentiation, the RANKL-treated cells were stained
for TRAP and TRAP activity was assessed. In the TRAP activity assay
(Table I), significant differences
were observed in all the FLL, OA and UA treated groups except in
the lowest concentration groups, but not in a dose-dependent
manner. Regarding TRAP staining, FLL significantly inhibited the
osteoclastogenesis of RAW264.7 cells in a dose-dependent manner.
The number of osteoclasts per well declined with increasing FLL
concentration, and significant differences were identified between
all treated groups and the control group (Fig. 2A; P<0.01). However, in OA- and
UA-treated groups significant differences were identified only in
the high concentration groups (1.78 µg/ml OA vs. Control group:
P=0.007; 3.56 µg/ml OA vs. Control group: P=0.001; and 1.03 µg/ml
UA vs. Control group: P=0.016 compared with the control group
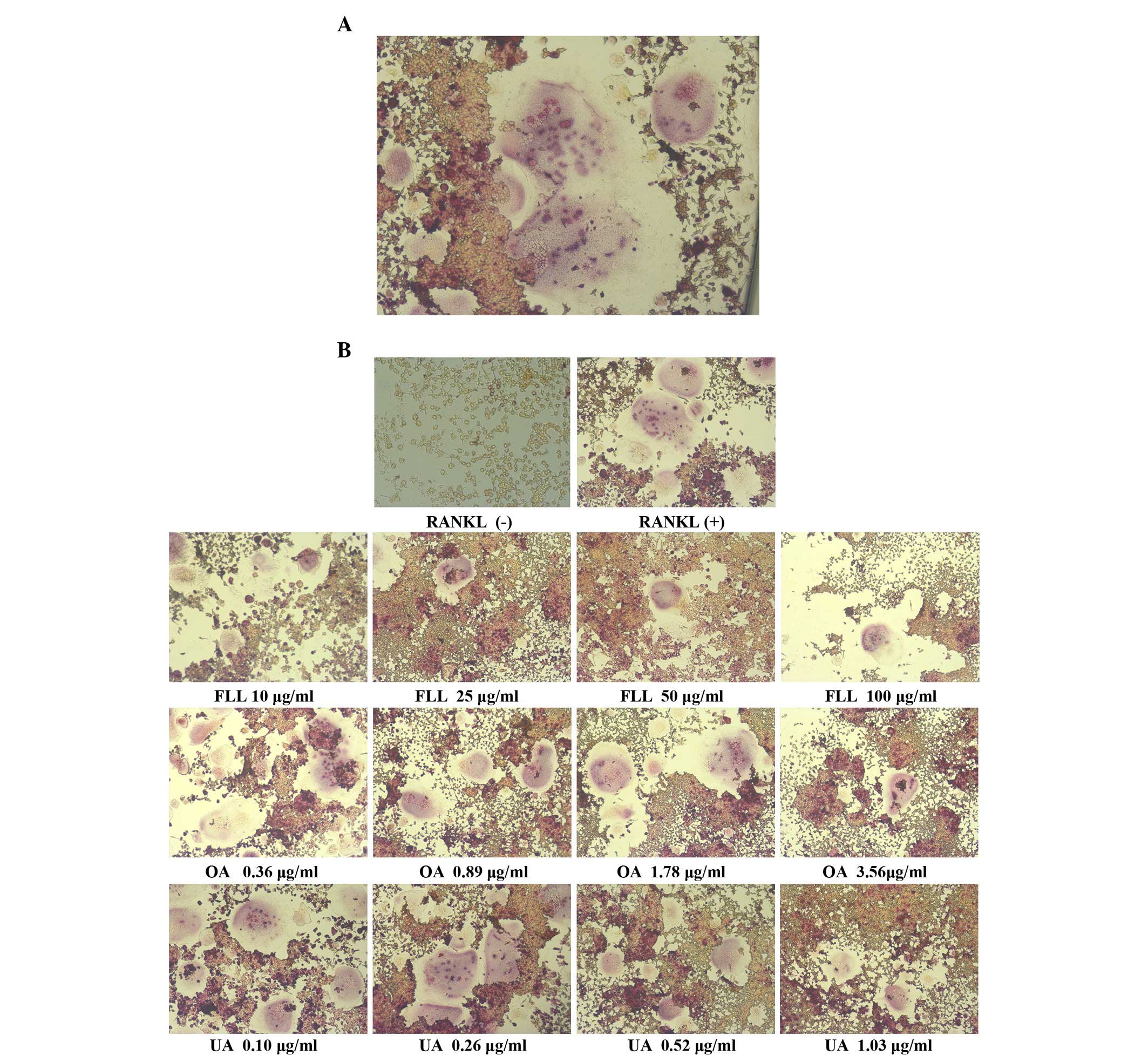
(Fig. 2B and C). Fig. 3 presented the images of TRAP
stained cells. The TRAP-positive multinucleate cells appeared dark
red and contained ≥3 nuclei, whereas the RAW264.7 cells without
RANKL treatment appeared brown. The number of multinucleate
osteoclasts observed decreased with increasing concentrations of
FLL, OA and UA.
 | Table I.Effect of FLL, OA and UA on TRAP
activity in RANKL-induced RAW264.7 cells. |
Table I.
Effect of FLL, OA and UA on TRAP
activity in RANKL-induced RAW264.7 cells.
| Treatment | Concentration
(µg/ml) | TRAP activity
(%) | P-value |
|---|
| FLL | 0 |
100.0±10.4 | / |
| FLL | 10 |
71.2±8.1 | 1.000 |
| FLL | 25 |
66.6±1.6 | 0.006 |
| FLL | 50 |
70.5±4.4 | 0.007 |
| FLL | 100 |
76.1±6.1 | 0.008 |
| OA | 0 |
100.0±10.4 | / |
| OA | 0.36 |
51.0±5.3 | 0.939 |
| OA | 0.89 |
48.4±5.3 |
4.086×10−8 |
| OA | 1.78 |
55.7±8.9 |
1.616×10−8 |
| OA | 3.56 |
60.8±15.0 |
5.527×10−8 |
| UA | 0 |
100.0±10.4 | / |
| UA | 0.10 |
54.2±16.2 | 1.000 |
| UA | 0.26 |
44.0±3.3 | 0.014 |
| UA | 0.52 |
41.3±4.7 |
2.743×10−4 |
| UA | 1.03 |
45.4±5.9 |
1.395×10−4 |
FLL, OA and UA demonstrated no effect
on apoptosis of osteoclasts
As presented in Table
II, FLL (10, 25, 50 and 100 µg/ml), OA (0.36, 0.89, 1.78 and
3.56 µg/ml) and UA (0.10, 0.26, 0.52 and 1.03 µg/ml) demonstrated
no effect on apoptosis of osteoclasts at the early (annexin
V-FITC+/PI-) or late (annexin V-FITC+/PI+) stages compared with the
control group.
 | Table II.Effect of FLL, OA and UA on
osteoclast apoptosis. |
Table II.
Effect of FLL, OA and UA on
osteoclast apoptosis.
|
|
| Apoptosis rate
(%) |
|---|
|
|
|
|
|---|
| Treatment | Concentration
(µg/ml) | Early stage | Late stage |
|---|
| FLL | 0 | 5.4±3.3 | 2.1±1.4 |
| FLL | 10 | 5.5±2.0 | 2.1±1.3 |
| FLL | 25 | 6.6±4.5 | 2.5±1.2 |
| FLL | 50 | 5.6±3.0 | 2.8±1.8 |
| FLL | 100 | 6.8±3.8 | 3.1±1.7 |
| OA | 0 | 5.4±3.3 | 2.1±1.4 |
| OA | 0.36 | 6.6±3.5 | 2.9±1.2 |
| OA | 0.89 | 4.8±1.9 | 2.6±1.2 |
| OA | 1.78 | 6.4±3.2 | 2.6±1.2 |
| OA | 3.56 | 5.0±2.0 | 2.8±1.5 |
| UA | 0 | 5.4±3.3 | 2.1±1.4 |
| UA | 0.10 | 6.0±2.1 | 2.3±1.5 |
| UA | 0.26 | 6.0±3.7 | 2.6±1.6 |
| UA | 0.52 | 4.9±4.3 | 2.2±1.8 |
| UA | 1.03 | 4.7±2.5 | 2.1±0.7 |
RANKL-induced mRNA expression levels
of osteoclast-associated genes were suppressed by FLL, OA and
UA
To further examine the mechanisms underlying the
effect of FLL, OA and UA on osteoclast differentiation, the present
study investigated the mRNA expression levels of various
osteoclast-associated genes. As presented in Fig. 4, FLL, OA and UA decreased the mRNA
expression levels of osteoclast markers including TRAP, CTSK and
MMP-9 (Fig. 4A). In addition,
RANKL-induced expression of TRAF-6 (Fig. 4B), which is recruited following
RANKL binding to RANK and induces downstream signaling pathways,
was downregulated. Furthermore, RANKL-induced expression of key
transcription factors, including c-Fos and NFAT, was inhibited by
FLL, OA and UA (Fig. 4B); however,
no effect was observed on RANKL-induced expression of Src (Fig. 4B).
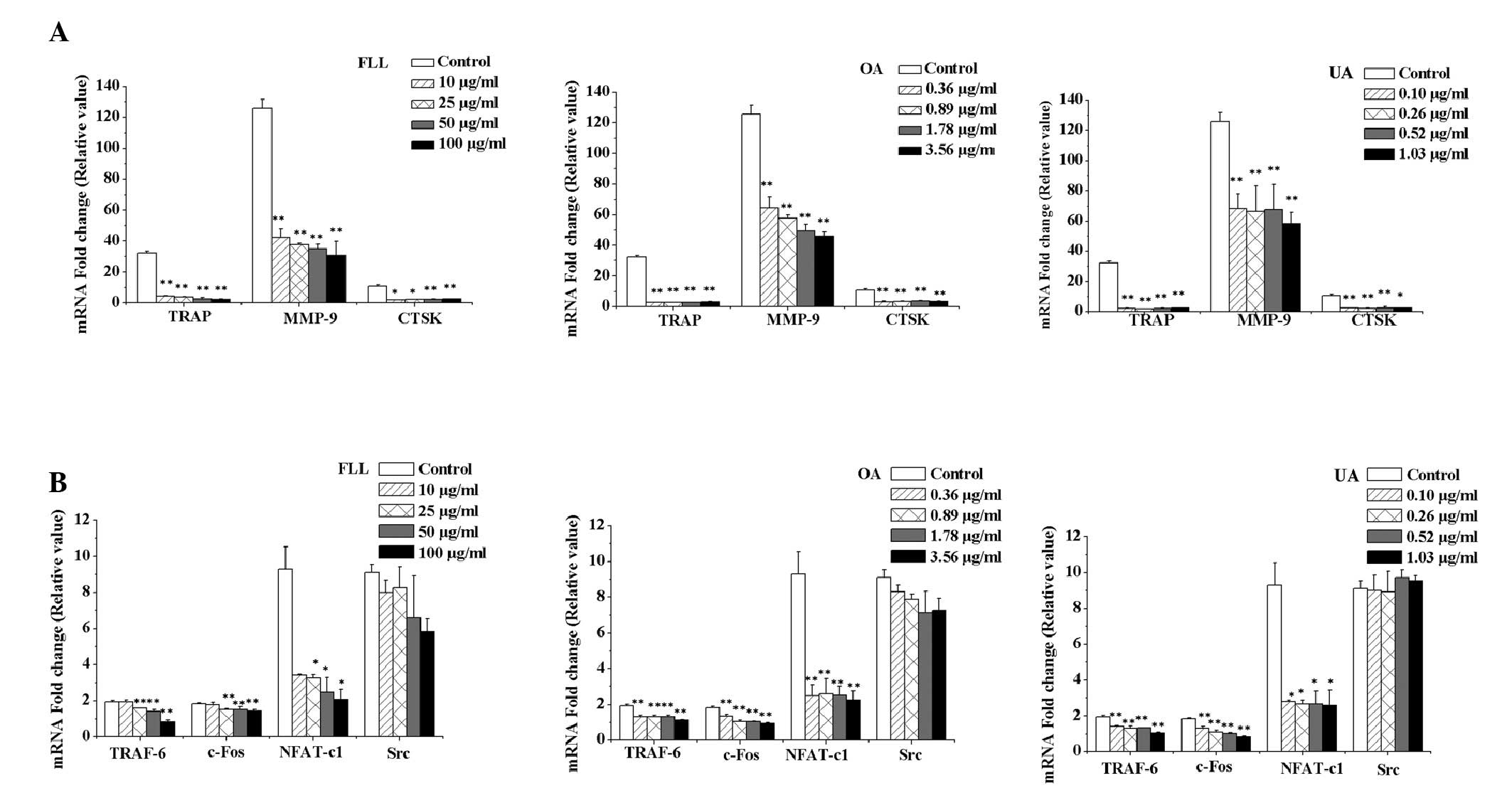 | Figure 4.mRNA expression levels of
osteoclast-associated genes. Reverse transcription-quantitative
polymerase chain reaction was performed to determine the effect of
FLL, OA and UA on the mRNA expression levels of
osteoclast-associated genes. (A) Effect of FLL, OA and UA on the
mRNA expression levels of the osteoclast markers, TRAP, MMP-9 and
CTSK in RAW264.7 cells stimulated with RANKL. All concentrations of
FLL, OA and UA reduced the mRNA expression levels of all three
genes. (B) Effect of FLL, OA and UA on the mRNA expression levels
of the signaling-associated genes, TRAF-6, c-Fos, NFAT-c1 and Src
in RAW264.7 cells stimulated with RANKL. FLL, OA and UA reduced the
mRNA expression levels of TRAF-6, c-Fos and NFAT-c1; however, no
effect on Src was observed. The mRNA fold change value of TRAP,
MMP-9, CTSK, TRAF-6, c-Fos, NFAT-c1 and Src in RAW264.7 cells were
all set as 1. Control cells were cultured with RANKL alone. All
data were presented as the mean ± standard deviation (n=3).
*P<0.05 and **P<0.01 vs. the control group. FLL, Fructus
ligustri Lucidi; OA, oleanolic acid; UA, ursolic acid; RANKL,
receptor activator of nuclear factor κB ligand; TRAP, tartrate
resistant acid phosphatase; CTSK, cathepsin K; MMP-9, matrix
metalloproteinase-9; TRAF-6, tumor necrosis factor receptor
associated factor-6; NFAT-c1, nuclear factor of activated T
cell-c1. |
Discussion
Increased bone resorption by osteoclasts has a
marked association with the occurrence of a large proportion of
osteoporosis cases (27).
Therefore, agents inhibiting osteoclast formation may make a
notable therapeutic contribution to the treatment of osteoporosis
(28). The present study was
designed to clarify the effects of FLL on osteoclastogenesis in
vitro, and the mechanisms underlying its effects. The results
demonstrated that ethanol extract of FLL may inhibit RANKL-induced
osteoclastogenesis in RAW264.7 cells without inducing cytotoxicity.
In addition, the results suggested that OA and UA in FLL together
account for its bioactive effects.
The results of the present study suggested that
ethanol extract of FLL is an efficient but mild inhibitor of
osteoclastogenesis. FLL extract and its two primary components, OA
and UA, markedly suppressed RANKL-induced multinucleate osteoclast
formation and TRAP activity simultaneously, without leading to
cytotoxicity or affecting osteoclast apoptosis. Furthermore,
RANKL-induced mRNA expression levels of TRAP, MMP-9 and CTSK, which
are regarded as osteoclast markers, were all efficiently
suppressed. The expression of these marker genes is associated with
the terminal differentiation of monocyte-macrophage lineage cells
to osteoclasts (29,30), and allowed them to resorb bone
matrix (31). TRAP and CTSK are
lysosomal enzymes that contribute to osteoclast maturation
(32) and bone matrix degradation
(33), whereas MMP-9 is the most
abundant MMP family member and is required for osteoclast migration
(34).
In addition, the results of the present study
suggested that nuclear factor κB (NF-κB)/mitogen-activated protein
kinases (MAPK) may be associated with the FLL-mediated inhibition
of osteoclastogenesis. mRNA expression levels of various key
molecules of these signaling pathways were observed to be
downregulated. Binding of RANKL to its receptor RANK recruits and
induces the trimerization of the adaptor molecule TRAF-6 (7), thus leading to activation of MAPKs,
phosphatidylinositol 3-kinase and NF-κB (8–10).
TRAF-6-deficient mice have marked levels of osteoporosis and
inhibited osteoclast formation (35). The results of the present study
suggested that RANKL-induced assembly of TRAF-6 may be limited by
FLL, OA and UA, and consequently suppress downstream signaling via
TRAF-6. Furthermore, it was observed that transcription factors
including c-Fos and NFAT-c1 may be involved. c-Fos is essential for
osteoclast differentiation and c-Fos-deficient mice develop
osteoporosis (36). NFAT-c1,
activated by NF-κB, is considered a key regulator of RANKL-induced
osteoclast differentiation, fusion and activation (30,37).
Furthermore, the activity of NFAT-c1 is enhanced by the
overexpression of c-Fos (38),
indicating that cooperation between c-Fos and NFAT-c1 may be
important for osteoclast formation and activation. The present
study demonstrated that FLL, OA and UA inhibited RANKL-induced
expression of c-Fos and NFAT-c1, thereby attenuating
osteoclastogenesis. Notably, the present study did not observe any
downregulation of RANKL-induced expression of Src. Src is involved
in osteoclast differentiation (39) and activation of Src family members
regulated the activation of the anti-apoptotic protein kinase B
(40). Thus expression and
activattion of Src may be associated with mortality. Osteoclasts
from Src-deficient mice failed to form ruffled borders and
resorption lacunae (41). This
evidence suggests that FLL is a mild agent, as it did not
demonstrate an effect on Src and did not promote apoptosis in the
present study.
However, interactions of OA and UA in FLL remain to
be elucidated. Concentrations of OA and UA in the present study
were selected according to their proportion in FLL, to understand
their specific roles in the bioactive effects of FLL. The results
suggested that a combination of OA and UA accounted for the effect
of FLL on osteoclastogenesis inhibition, as they inhibited
osteoclastogenesis independently. However, the effects of FLL, OA
and UA on osteoclastogenesis and mRNA expression levels were not
identical. For example, at the lowest concentration (0.36 µg/ml for
OA and 0.10 µg/ml for UA), the two components suppressed mRNA
expression levels of NFAT-c1; however, the lowest concentration of
FLL (10 µg/ml) appeared to exert no effect. These inconsistencies
suggested complex interactions between OA and UA in FLL. OA and UA
exhibit various biological activities, including anti-inflammatory,
anticancer and hepatoprotective effects (42,43).
Additionally, there are various other ingredients in FLL that may
function in this specific process, including linoleic acid,
salidroside and acetyl oleanolic acid (44). Thus, further investigation is
necessary to fully elucidate the underlying mechanisms.
In conclusion, the results of the present study
demonstrated that ethanol extract of FLL may inhibit
osteoclastogenesis in RAW264.7 cells via RANKL signaling pathways.
The results suggested that the number of RANKL-induced osteoclasts
and their bone-resorption abilities were reduced in the presence of
ethanol extract of FFL. OA and UA were demonstrated to be active
components in FLL; however, their exact underlying mechanism of
action and any potential interactions between them remain to be
elucidated. FLL is now a compound of interest for the prevention
and treatment of osteoporosis, and the results of the present study
indicated that OA and UA may be used instead of FLL for future
treatments.
Acknowledgements
The present study was supported by the special fund
for cultivation and development of Beijing Science and Technology
Innovation Base (grant no. Z151100001615035).
References
|
1
|
Prevention and management of osteoporosis,
. World Health Organ Tech Rep Ser. 921:1–164, back cover.
2003.PubMed/NCBI
|
|
2
|
Lane NE: Epidemiology, etiology, and
diagnosis of osteoporosis. Am J Obstet Gynecol. 194(2): Suppl.
S3–S11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teitelbaum SL: Bone Resorption by
Osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novack DV and Teitelbaum SL: The
osteoclast: Friend or foe? Annu Rev Pathol. 3:457–484. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pérez-Sayáns M, Somoza-Martín JM,
Barros-Angueira F, Rey JM and García-García A: RANK/RANKL/OPG role
in distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 109:679–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chambers TJ: Regulation of the
differentiation and function of osteoclasts. J Pathol. 192:4–13.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeshita S, Kaji K and Kudo A:
Identification and characterization of the new osteoclast
progenitor with macrophage phenotypes being able to differentiate
into mature osteoclasts. J Bone Miner Res. 15:1477–1488. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng X and Teitelbaum SL: Osteoclasts: New
Insights. Bone Res. 1:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasuda H: RANKL, a necessary chance for
clinical application to osteoporosis and cancer-related bone
diseases. World J Orthop. 4:207–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong J and O'Brien CA: Osteocyte RANKL:
New insights into the control of bone remodeling. J Bone Miner Res.
27:499–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung PC and Siu WS: Herbal treatment for
osteoporosis: A current review. J Tradit Complement Med. 3:82–87.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Putnam SE, Scutt AM, Bicknell K, Priestley
CM and Williamson EM: Natural products as alternative treatments
for metabolic bone disorders and for maintenance of bone health.
Phytother Res. 21:99–112. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong RW and Rabie AB: Traditional Chinese
medicines and bone formation-a review. J Oral Maxillofac Surg.
64:828–837. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li F, Yang Y, Zhu P, Chen W, Qi D, Shi X,
Zhang C, Yang Z and Li P: Echinacoside promotes bone regeneration
by increasing OPG/RANKL ratio in MC3T3-E1 cells. Fitoterapia.
83:1443–1450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adami S, Idolazzi L, Fracassi E, Gatti D
and Rossini M: Osteoporosis Treatment: When to discontinue and when
to re-start. Bone Res. 4:323–335. 2013. View Article : Google Scholar
|
|
18
|
Rufus P, Mohamed N and Shuid AN:
Beneficial effects of traditional Chinese medicine on the treatment
of osteoporosis on ovariectomised rat models. Curr Drug Targets.
14:1689–1693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pang Z, Zhi-Yan Z, Wang W, Ma Y, Feng-Ju
N, Zhang X and Han C: The advances in research on the
pharmacological effects of Fructus Ligustri Lucidi. Biomed Res Int.
2015:2818732015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Leung PC, Che CT, Chow HK, Wu CF
and Wong MS: Improvement of bone properties and enhancement of
mineralization by ethanol extract of Fructus Ligustri Lucidi. Br J
Nutr. 99:494–502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li G, Zhang XA, Zhang JF, Chan CY, Yew DT,
He ML, Lin MC, Leung PC and Kung HF: Ethanol extract of Fructus
Ligustri Lucidi promotes osteogenesis of mesenchymal stem cells.
Phytother Res. 24:571–576. 2010.PubMed/NCBI
|
|
22
|
Lyu Y, Feng X, Zhao P, Wu Z, Xu H, Fang Y,
Hou Y, Denney L, Xu Y and Feng H: Fructus Ligustri Lucidi (FLL)
ethanol extract increases bone mineral density and improves bone
properties in growing female rats. J Bone Miner Metab. 32:616–626.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng X, Lyu Y, Wu Z, Fang Y, Xu H, Zhao P,
Xu Y and Feng H: Fructus ligustri lucidi ethanol extract improves
bone mineral density and properties through modulating calcium
absorption-related gene expression in kidney and duodenum of
growing rats. Calcif Tissue Int. 94:433–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bian Q, Liu SF, Huang JH, Yang Z, Tang DZ,
Zhou Q, Ning Y, Zhao YJ, Lu S, Shen ZY and Wang YJ: Oleanolic acid
exerts an osteoprotective effect in ovariectomy-induced
osteoporotic rats and stimulates the osteoblastic differentiation
of bone mesenchymal stem cells in vitro. Menopause. 19:225–233.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y, Huai Y, Jin J, Geng M and Li JX:
Quinoxaline derivative of oleanolic acid inhibits osteoclastic bone
resorption and prevents ovariectomy-induced bone loss. Menopause.
18:690–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bruyère O and Reginster JY: Monitoring of
osteoporosis therapy. Best Pract Res Clin Endocrinol Metab.
28:835–841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mizoguchi T, Muto A, Udagawa N, Arai A,
Yamashita T, Hosoya A, Ninomiya T, Nakamura H, Yamamoto Y, Kinugawa
S, et al: Identification of cell cycle-arrested quiescent
osteoclast precursors in vivo. J Cell Biol. 184:541–554. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siddiqi MH, Siddiqi MZ, Ahn S, Kang S, Kim
YJ, Sathishkumar N, Yang DU and Yang DC: Ginseng saponins and the
treatment of osteoporosis: Mini literature review. J Ginseng Res.
37:261–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rahman MM, Bhattacharya A and Fernandes G:
Conjugated linoleic acid inhibits osteoclast differentiation of
RAW264.7 cells by modulating RANKL signaling. J Lipid Res.
47:1739–1748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou GQ, Guo C, Song GH, Fang N, Fan WJ,
Chen XD, Yuan L and Wang ZQ: Lipopolysaccharide (LPS) promotes
osteoclast differentiation and activation by enhancing the MAPK
pathway and COX-2 expression in RAW264.7 cells. Int J Mol Med.
32:503–510. 2013.PubMed/NCBI
|
|
34
|
Suh KS, Rhee SY, Kim YS, Lee YS and Choi
EM: Xanthohumol modulates the expression of osteoclast-specific
genes during osteoclastogenesis in RAW264.7 cells. Food Chem
Toxicol. 62:99–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim N, Kadono Y, Takami M, Lee J, Lee SH,
Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, et al:
Osteoclast differentiation independent of the TRANCE-RANK-TRAF6
axis. J Exp Med. 202:589–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eriksen EF: Cellular mechanisms of bone
remodeling. Rev Endocr Metab Disord. 11:219–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakashima T, Hayashi M and Takayanagi H:
New insights into osteoclastogenic signaling mechanisms. Trends
Endocrinol Metab. 23:582–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ikeda F, Nishimura R, Matsubara T, Tanaka
S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, et
al: Critical roles of c-Jun signaling in regulation of NFAT family
and RANKL-regulated osteoclast differentiation. J Clin Invest.
114:475–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Winograd-Katz SE, Brunner MC, Mirlas N and
Geiger B: Analysis of the signaling pathways regulating
Src-dependent remodeling of the actin cytoskeleton. Eur J Cell
Biol. 90:143–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Recchia I, Rucci N, Funari A, Migliaccio
S, Taranta A, Longo M, Kneissel M, Susa M, Fabbro D and Teti A:
Reduction of c-Src activity by substituted 5,7-diphenyl-pyrrolo
[2,3-d]-pyrimidines induces osteoclast apoptosis in vivo and in
vitro. Bone. 34:65–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee EJ, Kim JL, Gong JH, Park SH and Kang
YH: Inhibition of osteoclast activation by phloretin through
disturbing αvβ3 integrin-c-Src pathway. Biomed Res Int.
2015:6801452015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ullevig SL, Kim HS, Nguyen HN, Hambright
WS, Robles AJ, Tavakoli S and Asmis R: Ursolic acid protects
monocytes against metabolic stress-induced priming and dysfunction
by preventing the induction of Nox4. Redox Biol. 2:259–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alvarado HL, Abrego G, Garduño-Ramirez ML,
Clares B, Calpena AC and García ML: Design and optimization of
oleanolic/ursolic acid-loaded nanoplatforms for ocular
anti-inflammatory applications. Nanomedicine. 11:521–530.
2015.PubMed/NCBI
|
|
44
|
Yao W, Dai J, Zheng C, Bao B, Cheng H,
Zhang L, Ding A and Li W: Quality assessment of Fructus Ligustri
Lucidi by the simultaneous determination of six compounds and
chemometric analysis. J Sep Sci. 38:1822–1827. 2015. View Article : Google Scholar : PubMed/NCBI
|