Introduction
Lung cancer is the leading cause of
cancer-associated mortality in men and women, and its incidence is
increasing worldwide (1,2). It was estimated that there would be
221,200 new cases of lung cancer, and 158,040 patients would
succumb to the disease, in the USA in 2015 (3). Based on histology, lung cancer may be
classified into small-cell lung cancer and non-small-cell lung
cancer (NSCLC) (4). NSCLC, which
is responsible for >85% of lung cancer cases, includes squamous
cell carcinoma, adenocarcinoma, bronchioloalveolar carcinoma and
large-cell carcinoma (5).
Currently, the primary therapeutic treatments for NSCLC are
comprehensive, including surgical resection, chemotherapy and
radiotherapy (6). Despite advances
in treatment, the 5-year survival rate for NSCLC has remained at
15–20%, with a median survival of 8–12 months (7). The majority of NSCLC patients are
diagnosed at a late or advanced stage of the disease, when their
tumors and metastatic lesions have become intractable to current
standard therapies (8). It is
therefore necessary to elucidate the underlying molecular
mechanisms that regulate NSCLC growth and metastasis, and
investigate novel therapeutic strategies for patients with
NSCLC.
Numerous studies have demonstrated that microRNAs
(miRNAs) contribute to carcinogenesis, development and metastasis
in various human cancers (9–11).
miRNAs are non-coding, endogenous, single-stranded RNA molecules of
19–22 nucleotides in length (12).
miRNAs negatively regulate the expression of target messenger RNAs
(mRNAs) through binding to the 3′ untranslated region (UTR) of
target mRNAs in a complementary base-pairing manner, resulting in
translational repression or mRNA degradation (13). miRNAs are involved in a number of
physiological and pathological processes, including cell
proliferation, differentiation, morphogenesis, cell cycle
progression, apoptosis, metastasis, and glucose and lipid
metabolism (14–16). It is now well established that
miRNAs may act as oncogenes or tumor suppressors in the initiation
and progression of human cancers, depending on their target mRNAs
(17,18). These studies indicated that miRNAs
are important regulators of tumorigenesis and may be investigated
as potential targeted therapy for the treatment of cancers.
miR-361 has been studied in multiple human cancers.
However, the role of miR-361 in NSCLC is still unclear. The
objective of the present study was, therefore, to elucidate the
expression and biological roles of miR-361 in NSCLC, and to
investigate its underlying molecular mechanisms.
Materials and methods
Patients and clinical specimens
The present study was approved by the Ethics
Committee of Tianjin Baodi Hospital (Tianjin, China), and written
informed consent was obtained from all subjects. NSCLC tissue and
matched normal adjacent tissue (NAT) samples were obtained from 34
NSCLC patients who underwent surgery resection at Tianjin Hospital.
None of the patients received any therapeutic treatment prior to
participation in the present study. Tissues were immediately
snap-frozen in liquid nitrogen and stored at −80°C until
analysis.
Cell culture and transfection
H23 and A549 human NSCLC cell lines, BEAS-2B human
non-tumorigenic bronchial epithelial cells and HEK293T human
embryonic kidney cells were purchased from the Chinese Academy of
Sciences (Shanghai, China). BEAS-2B cells were maintained in LHC-9
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). H23, A549 and HEK293T cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% FBS, 100 U/ml penicillin (Gibco; Thermo Fisher Scientific,
Inc.) and 100 mg/ml streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). All cell lines were incubated in a humidified 5% CO2 cell
incubator at 37°C.
miR-361 mimic, negative control (NC) and the
luciferase reporter plasmids, PGL3-WT1-3′UTR wild-type (Wt) and
PGL3-WT1-3′UTR mutant (Mut), were purchased from GeneChem Co., Ltd.
(Shanghai, China). WT1 small interfering (si)RNA and control siRNA
were obtained from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Cell transfection and cotransfection assays were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reverse transcribed using PrimeScript RT reagent kit
(Takara Bio, Inc., Otsu, Japan). qPCR was performed to detect mRNA
expression using SYBR® Premix Ex Taq (Takara Bio, Inc.),
with actin serving as an internal control. The cycling conditions
for qRCR were as follows: 95°C for 30 sec; 40 cycles of 95°C for 5
sec and 60°C for 30 sec. TaqMan® microRNA assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to measure
miRNA expression, with U6 serving as an internal control. The
primer sequences used for qPCR were: miR-361, forward
5′-GATTCTTTCTTGGGACTCGGAAGCT-3′ and reverse
5′-GGGATAAGATGCTAATGAATGTGCT-3′; U6 snRNA, forward
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse
5′-ACGCTTCACGAATTTGCGTGTC-3′; WT1, forward
5′-GGGGTAAGGAGTTCAAGGCA-3′ and reverse 5′-TGCAGCAAGAGGAAGTCCAG-3′;
β-actin, forward 5′-CTCCATGGCCTCGCTGT-3′ and reverse
5′-GCTGTCACCTTCACCGTTCC-3′. All reactions were performed in
triplicate. The relative expression levels were calculated using
the 2−ΔΔCq method (19).
Cell proliferation assay
Cell proliferation was evaluated using the MTT assay
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). In brief,
3,000 cells were seeded into each well of a 96-well plate.
Following overnight incubation, cells were transfected with miR-361
mimic, NC miRNA, WT1 siRNA or siRNA ctrl. At various time points
following transfection, MTT assays were conducted by adding 20 µl 5
mg/ml MTT assay reagent into each well and incubating plates at
37°C for 4 h. The medium was carefully removed and 200 µl dimethyl
sulfoxide (Sigma-Aldrich; Merck Millipore) was added to solubilize
the formazan precipitates. Optical density was measured at a
wavelength of 490 nm using a spectrophotometer. Each sample was
analyzed in triplicate.
Transwell assay
Transwell assays were performed to analyze cell
migration and invasion abilities using transwell chambers with a
pore size of 8-µm (Corning Incorporated, Corning, NY, USA). For
invasion assays, transwell chambers were coated with Matrigel (BD
Biosciences, San Jose, CA, USA), according to the manufacturer's
instructions. Cells were harvested 48 h following transfection and
resuspended in culture medium without FBS. Transfected cells
(5×104) in 200 µl serum-free culture medium were seeded
into the upper chamber of the transwell, and 500 µl culture medium
supplemented with 20% FBS was added into the lower chamber.
Following a 24-h incubation, membranes were stained with 0.5%
crystal violet (Beyotime Institute of Biotechnology, Nantong,
China) for 20 min. Cells that had not migrated or invaded to the
basal side of the membranes were removed carefully with cotton
wool. The protocol of migration assays was the same with invasion
assay, but without Matrigel coating. The migration and invasion
abilities were evaluated by counting five fields per membrane under
an inverted microscope (Olympus Corporation, Tokyo, Japan). Each
assay was repeated three times.
Western blotting
Total protein was extracted from cells using
radioimmunoprecipitation assay buffer containing protease
inhibitors (Thermo Fisher Scientific, Inc.). Equal quantities of
proteins (20 µg per lane) were loaded onto 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels. Following
electrophoresis, proteins were transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked with 5% non-fat milk powder in phosphate-buffered
saline with 0.05% Tween 20 (PBST) at room temperature for 2 h and
incubated with the primary antibodies: Mouse anti-human monoclonal
WT1 antibody (1:500; catalog no. sc-7385; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and mouse anti-human monoclonal actin
antibody (1:500; catalog no. sc-8432; Santa Cruz Biotechnology,
Inc.). Following incubation at 4°C overnight, the membranes were
washed three times with PBST, probed with goat anti-mouse
HRP-conjugated secondary antibody (1:2,000 dilution; sc-2005; Santa
Cruz Biotechnology, Inc.) at room temperature for 1 h and developed
with Enhanced Chemiluminescence solution (Pierce Biotechnology,
Inc., Rockford, IL, USA). Actin served as an internal control. The
protein intensities were quantified using AlphaEase FC software
(version 4.0.1; ProteinSimple, San Jose, CA, USA).
Dual-Luciferase reporter assay
HEK293T cells were seeded into 24-well plates at a
density of 30–40% confluence. Cells were cotransfected with miR-361
mimic or NC and PGL3-WT1-3′UTR Wt or PGL3-WT1-3′UTR Mut using
Lipofectamine 2000 when confluence reached 70–80%. At 48 h
following transfection, firefly and renilla luciferase activities
were measured using a Dual-Luciferase® Reporter Assay
system (Promega Corporation, Madison, WI, USA). Firefly luciferase
activity served as an internal control. Each sample was analyzed in
triplicate and the assay was repeated three times.
Statistical analysis
Data are presented as mean ± standard deviation. All
analyses were performed in SPSS software version 17.0 (SPSS, Inc.,
Chicago, IL, USA) using Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-361 is downregulated in NSCLC
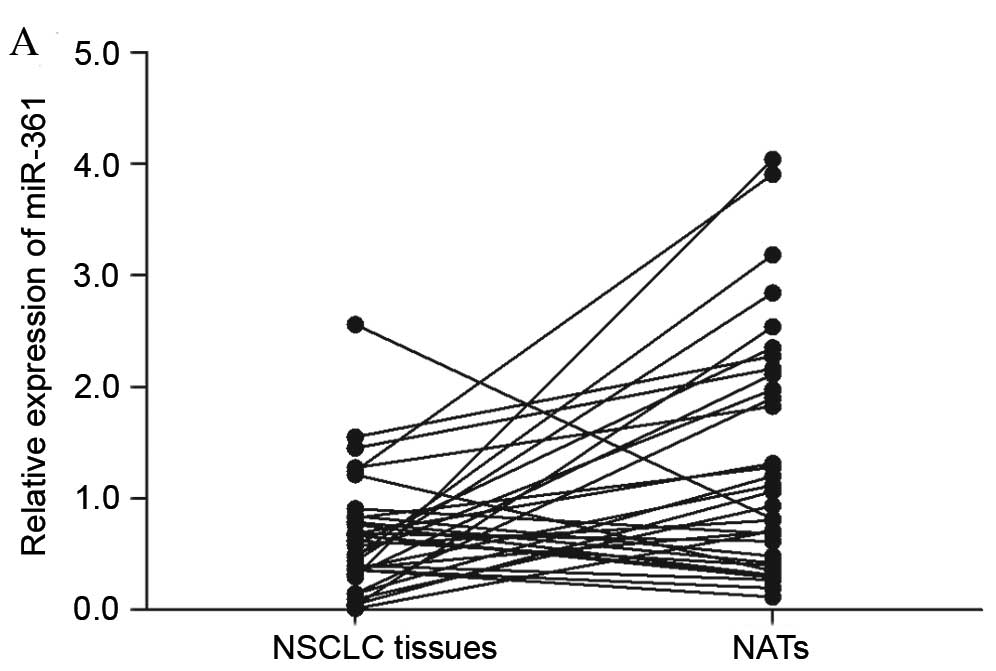
In the present study, RT-qPCR was performed to
measure the miR-361 expression levels in patients with NSCLC.
miR-361 expression was significantly downregulated in NSCLC tissues
compared with matched NATs (P=0.015; Fig. 1A and B). In addition, the
expression level of miR-361 was measured in NSCLC cell lines.
Similar to the expression pattern in NSCLC samples, miR-361
expression levels were decreased in H23 and A549 NSCLC cell lines
compared with the non-cancerous BEAS-2B cell line (P=0.020 for H23
and P=0.004 for A549; Fig.
1C).
miR-361 is upregulated in NSCLC cells
following transfection with miR-361 mimics
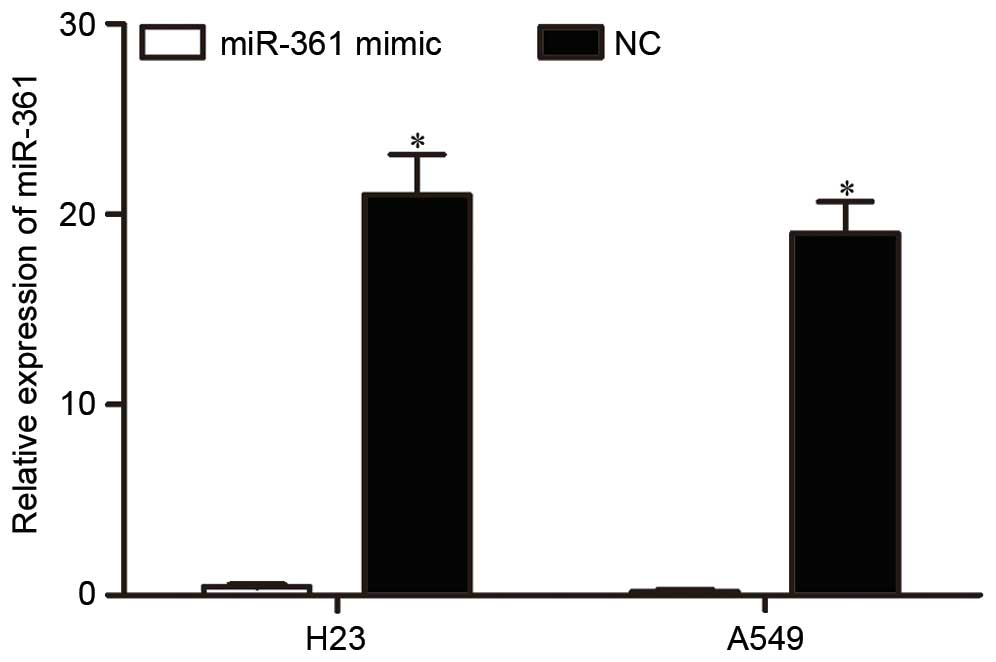
To investigate the function of miR-361 in NSCLC, an
miR-361 mimic or NC was transfected into H23 and A549 cells.
Following transfection, RT-qPCR was performed to detect miR-361
expression. As presented in Fig.
2, miR-361 was significantly upregulated in H23 and A549 cells
following transfection with an miR-361 mimic (P<0.001 for H23
and P<0.001 for A549).
miR-361 suppresses proliferation of
NSCLC cells
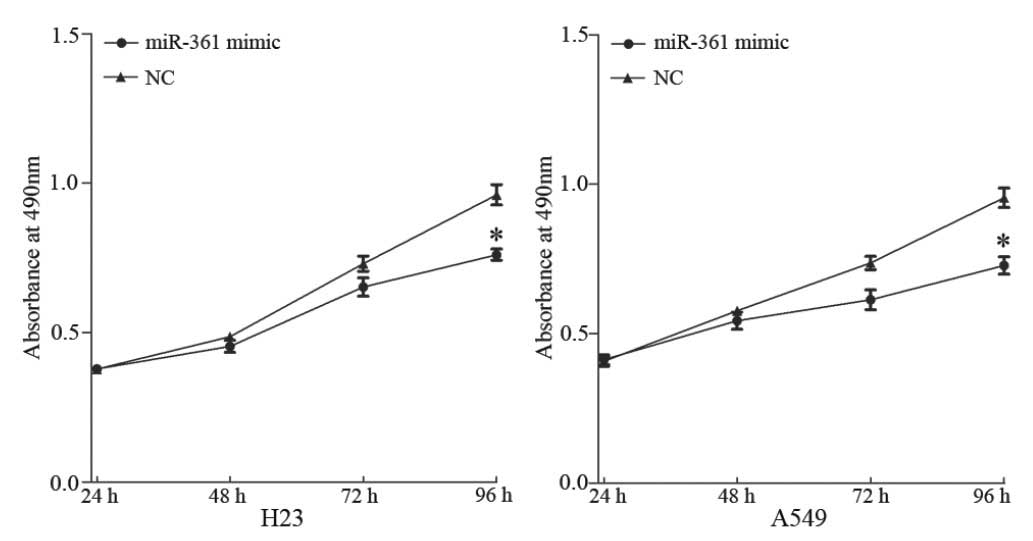
To investigate the effect of miR-361 in the
regulation of NSCLC cell growth, a proliferation assay was
performed. Overexpression of miR-361 inhibited the proliferation of
H23 and A549 cells compared with cells transfected with NC
(P<0.05; Fig. 3).
miR-361 suppresses migration and
invasion of NSCLC cells
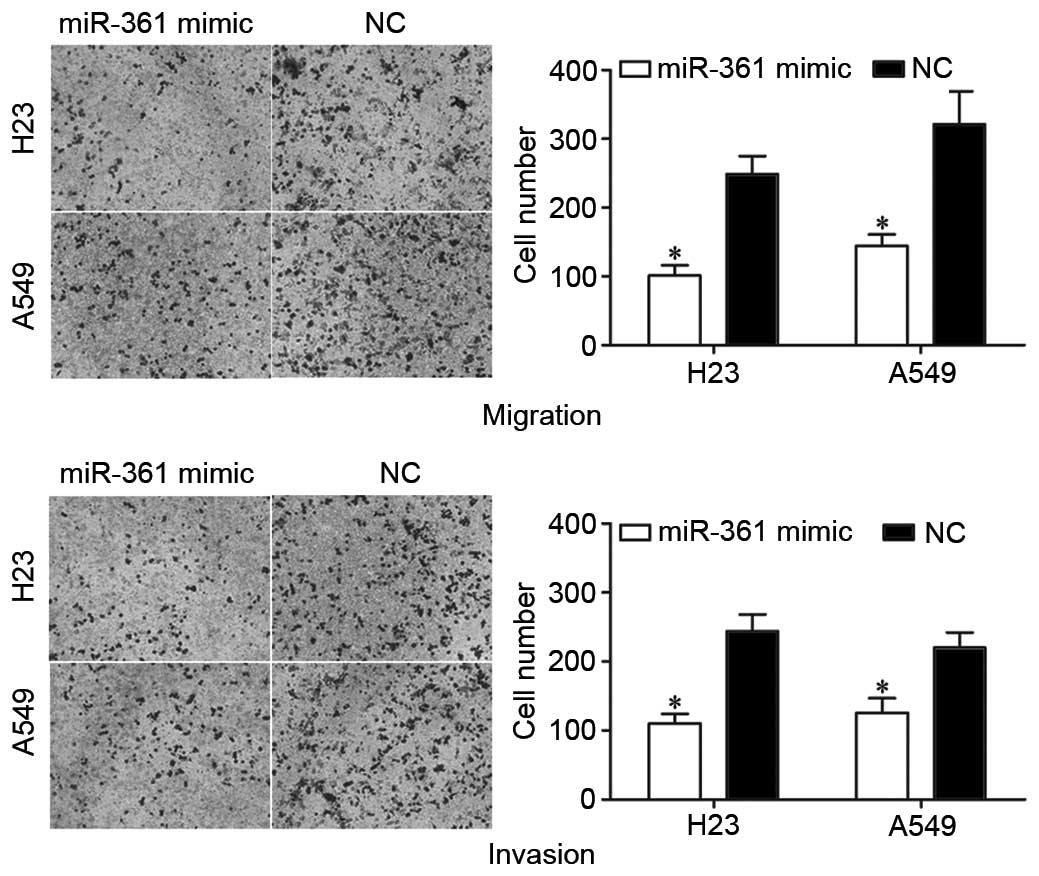
To investigate the potential effect of miR-361 on
NSCLC metastasis, Transwell assays were performed. As presented in
Fig. 4, miR-361 overexpression
significantly suppressed the migration (P=0.026 for H23 and P=0.022
for A549) and invasion (P=0.028 for H23 and P=0.035 for A549) of
H23 and A549 cells compared with NC-transfected cells.
WT1 is a target of miR-361 in
vitro
The online bioinformatics tools, TargetScan
(www.targetscan.org/vert_71/) and
miRanda (www.microrna.org/microrna/home.do) were used to
identify the direct target of miR-361. Based on bioinformatic
analysis, 239 potential targets were predicted. Among these
putative targets, WT1 was selected for further investigation as it
was upregulated and identified as an oncogene in lung cancer
(20), with the predicted 3′UTR
binding site presented in Fig. 5A.
Dual-Luciferase reporter assays were performed to determine whether
miR-361 directly targeted the 3′UTR of WT1. miR-361 decreased
PGL3-WT1-3′UTR Wt luciferase activity in HEK293T cells compared
with NC (P=0.019; Fig. 5B), but
not PGL3-WT1-3′UTR Mut luciferase activity compared with NC
(P>0.05; Fig. 5B). These
findings suggested that WT1 is a direct target gene of miR-361.
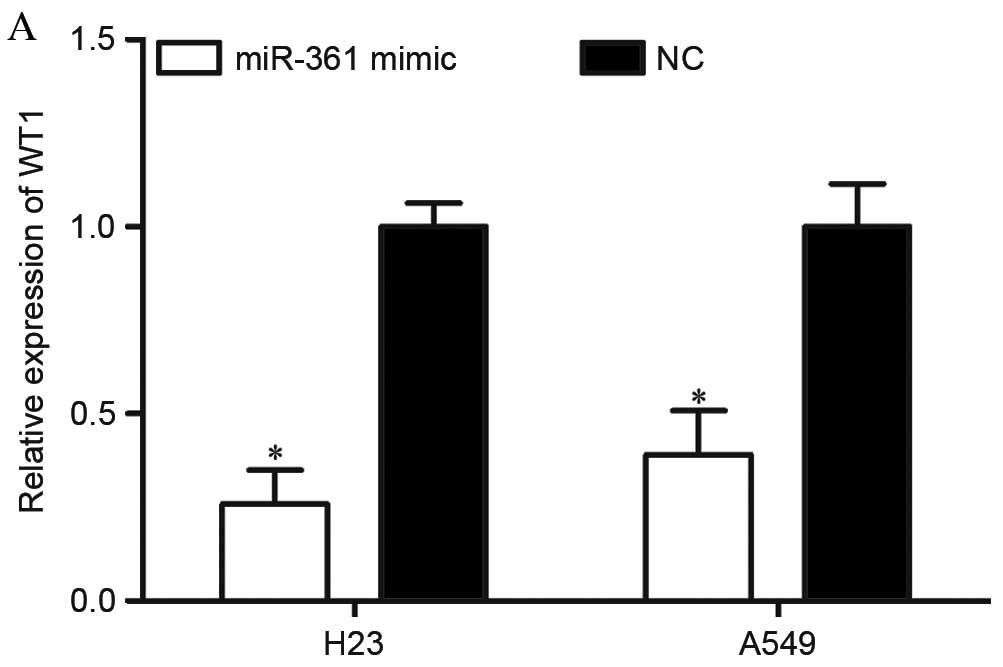
miR-361 decreases WT1 expression in
NSCLC cells
To further investigate whether WT1 was regulated by
miR-361 in NSCLC cells, RT-qPCR and western blotting were
performed. A total of 72 h following transfection, miR-361
overexpression decreased WT1 mRNA expression levels in H23 and A549
cells compared with NC-transfected cells (P=0.017 for H23 and
P=0.031 for A549; Fig. 6A).
Western blotting revealed that WT1 protein expression levels were
downregulated by miR-361 overexpression in H23 and A549 cells,
compared with NC-transfected controls (P=0.012 for H23 and P=0.026
for A549; Fig. 6B).
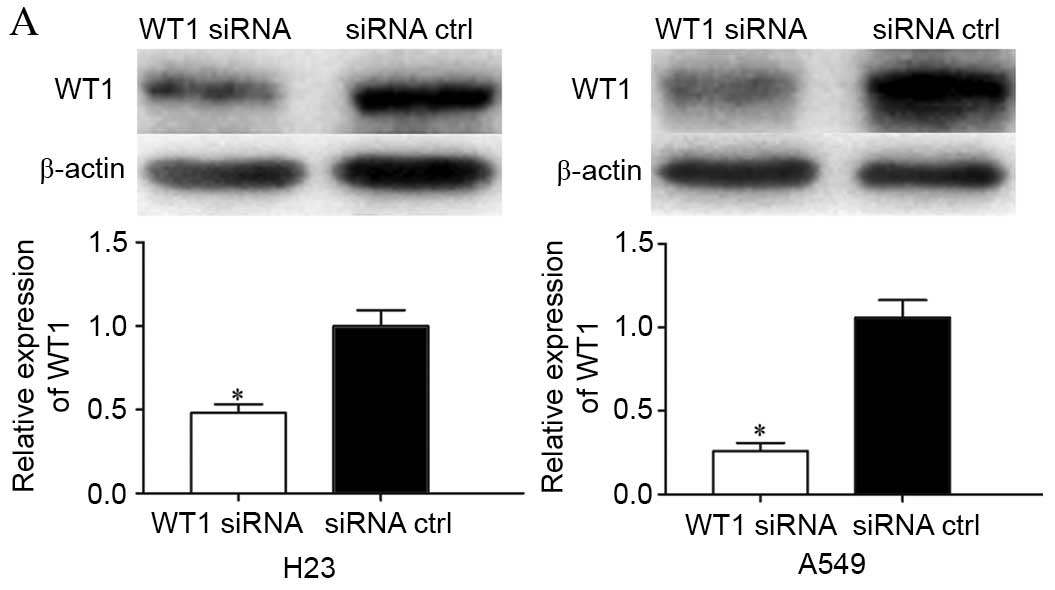
Knockdown of WT1 has similar effects
to miR-361 overexpression in NSCLC cells
To investigate the functional effects of WT1 in
NSCLC, WT1 or control siRNA was transfected into H23 and A549
cells. Following transfection, western blotting was performed to
detect WT1 expression. As presented in Fig. 7A, WT1 was significantly
downregulated in WT1 siRNA-transfected H23 and A549 cells compared
with cells transfected with control siRNA (P=0.034 for H23 and
P=0.018 for A549).
Proliferation assays and Transwell assays revealed
that knockdown of WT1 significantly suppressed the growth (P=0.015
for H23 and P=0.010 for A549; Fig.
7B), migration (P=0.039 for H23 and P=0.024 for A549; Fig. 7C) and invasion (P=0.019 for H23 and
P=0.023 for A549; Fig. 7C) of H23
and A549 cells (P<0.05). These findings demonstrated that the
effects of WT1 siRNA were similar to the effects exerted by miR-361
in NSCLC cells, indicating that WT1 is a functional target of
miR-361 in NSCLC.
Discussion
Lung cancer is a malignant tumor that represents a
serious challenge to human health. NSCLC accounts for >85% of
total lung cancer cases and has a poor prognosis (5). Therefore, it is critical to
understand the molecular mechanisms underlying NSCLC, and
investigate novel therapeutic strategies for the treatment of this
disease. It has been well documented that deregulated miRNA
expression contributes to NSCLC carcinogenesis and progression
(21,22). Identification of tumor-associated
miRNAs and the determination of their roles and target mRNAs is
essential to develop novel therapeutic targets using this strategy
(17). In the present study, it
was demonstrated that miR-361 was downregulated in NSCLC tissues
and cell lines. However, in a few patients, miR-361 was
upregulated, which may be due to the tissue specificity of miR-361
expression. In addition, miR-361 significantly inhibited the
proliferation, migration and invasion of NSCLC cells. Furthermore,
WT1 was identified as a direct target of miR-361. These results
suggested that miR-361 may function as a tumor suppressor in NSCLC
and may be investigated as a potential therapy for the treatment of
NSCLC.
miR-361 is located on Xq21.2, in an intron between
exons 9 and 10 of the choroideremia gene (23). miR-361 is downregulated in numerous
human cancers, including gastric cancer (24), colorectal cancer (24), prostate cancer (25) and cutaneous squamous cell carcinoma
(23). In these cancers, miR-361
functions as a tumor suppressor. For example, in colorectal cancer,
the expression level of miR-361 was negatively correlated with lung
metastasis and disease progression. Furthermore, ectopic expression
of miR-361 suppressed cell proliferation, migration and invasion
via staphylococcal nuclease domain containing-1 (24). Liu et al (25) reported that miR-361 inhibited
prostate cancer growth and enhanced apoptosis in vitro and
in vivo by directly targeting the signal transducer and
activator of transcription 6/B-cell lymphoma-extra large signaling
pathway. However, in cervical cancer, miR-361 has been reported to
be upregulated, acting as an oncogene. Enforced miR-361 expression
significantly increased cervical cancer cell growth, migration and
invasion through mediation of epithelial-to-mesenchymal transition
(26). These conflicting findings
indicated that the expression and functions of miR-361 in tumors
are diverse and tissue-specific.
Identification of miR-361 target mRNAs is important
for understanding its role in NSCLC carcinogenesis and progression.
In the present study, WT1 was identified as a direct target of
miR-361 in NSCLC. Bioinformatics analysis revealed that WT1 mRNA
contained a miR-361 seven-nucleotide seed match at positions
1,128–1,135 of the WT1 3′UTR. Dual-Luciferase reporter assays
confirmed that miR-361 directly targeted the 3′UTR of WT1. In
addition, RT-qPCR and western blotting demonstrated that miR-361
negatively regulated WT1 expression. Furthermore, knockdown of WT1
had similar effects to miR-361 overexpression in NSCLC cells. These
results verified that miR-361 directly targeted WT1 to decrease
NSCLC cell growth, migration and invasion. miR-361 may therefore be
investigated as a potential targeted therapy to inhibit the rapid
growth and metastasis of NSCLC.
WT1, located on chromosome 11p13, was first
identified in 1990 in the childhood kidney cancer Wilms' tumor
(27). It encodes a zinc-finger
transcription factor consisting of four zinc finger domains at the
C terminus and a glutamine and proline-rich domain at the N
terminus (28,29). Previous studies have revealed that
WT1 is upregulated in a large number of human cancers, including
breast cancer, ovarian cancer, glioblastoma and soft tissue sarcoma
(30–33). In functional studies, WT1 was
demonstrated to be a tumor suppressor, which is inactivated in
Wilms' tumor (27). However,
studies have identified WT1 as an oncogene in other human tumors
(34–37). For example, in ovarian cancer, high
WT1 expression was correlated with the stage of the disease,
ascites production and metastasis. In addition, WT1 promoted
ovarian cancer invasion (38,39).
In breast cancer, WT1 expression level was associated with
basal-like and receptor tyrosine-protein kinase erbB-2 molecular
subtypes and poor prognosis (40).
In addition, it was demonstrated to enhance growth and inhibit
apoptosis of breast cancer cells (41,42).
Furthermore, WT1 promoted migration, metastasis and angiogenesis,
and induced drug resistance of cancer cells (43).
In NSCLC, WT1 was upregulated in cancer specimens
compared with NATs (44).
Functional studies revealed that WT1 enhanced cell proliferation,
invasion, cisplatin-resistance and decreased apoptosis (44–47).
The present study, in accordance with previous findings,
demonstrated that knockdown of WT1 inhibited growth, migration and
invasion of NSCLC cells. These findings indicated that it may be
beneficial to investigate novel therapies targeting WT1. miR-361
may be investigated as a potential therapy to target WT1 and
therefore inhibit the rapid growth and metastasis of NSCLC.
In conclusion, the results of the present study
revealed that miR-361 was downregulated in NSCLC tissues and cell
lines. Ectopic expression of miR-361 suppressed the proliferation,
migration and invasion of NSCLC cells via the direct targeting of
WT1. The present study provided novel insights into the molecular
mechanism underlying the rapid growth and metastasis of NSCLC, and
identified the association between miR-361 and WT1 as a potential
therapeutic target for the treatment of NSCLC.
References
|
1
|
Ilic N, Petricevic A, Arar D, Kotarac S,
Banovic J, Ilic NF, Tripkovic A and Grandic L: Skip mediastinal
nodal metastases in the IIIa/N2 non-small cell lung cancer. J
Thorac Oncol. 2:1018–1021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ni T, Mao G, Xue Q, Liu Y, Chen B, Cui X,
Lv L, Jia L, Wang Y and Ji L: Upregulated expression of ILF2 in
non-small cell lung cancer is associated with tumor cell
proliferation and poor prognosis. J Mol Histol. 46:325–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Liu Y, Røe OD, Qian Y, Guo R, Zhu
L, Yin Y and Shu Y: Gefitinib or erlotinib as maintenance therapy
in patients with advanced stage non-small cell lung cancer: A
systematic review. PLoS One. 8:e593142013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang F, Zhou J, Zhang Y, Wang Y, Cheng L,
Bai Y and Ma H: The value of microRNA-155 as a prognostic factor
for survival in non-small cell lung cancer: A meta-analysis. PLoS
One. 10:e01368892015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: EUROCARE-4 Working
Group: Recent cancer survival in Europe: A 2000-02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakashita S, Sakashita M and Tsao M Sound:
Genes and pathology of non-small cell lung carcinoma. Semin Oncol.
41:28–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei J, Ma Z, Li Y, Zhao B, Wang D and Jin
Y and Jin Y: miR-143 inhibits cell proliferation by targeting
autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol
Med Rep. 11:571–576. 2015.PubMed/NCBI
|
|
10
|
Wu D, Pan H, Zhou Y, Zhang Z, Qu P, Zhou J
and Wang W: Upregulation of microRNA-204 inhibits cell
proliferation, migration and invasion in human renal cell carcinoma
cells by downregulating SOX4. Mol Med Rep. 12:7059–7064.
2015.PubMed/NCBI
|
|
11
|
Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin
C and Zhang W: microRNA-133 inhibits cell proliferation, migration
and invasion in prostate cancer cells by targeting the epidermal
growth factor receptor. Oncol Rep. 27:1967–1975. 2012.PubMed/NCBI
|
|
12
|
Ebrahimi A and Sadroddiny E: MicroRNAs in
lung diseases: Recent findings and their pathophysiological
implications. Pulm Pharmacol Ther. 34:55–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Abdel-Mageed AB, Mondal D and Kandil
E: MicroRNA expression profiles in differentiated thyroid cancer, a
review. Int J Clin Exp Med. 6:74–80. 2013.PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Gao P, Lin F, Long M, Weng Y,
Ouyang Y, Liu L, Wei J, Chen X, He T, et al: Wilms' tumour
suppressor gene 1 (WT1) is involved in the carcinogenesis of lung
cancer through interaction with PI3K/Akt pathway. Cancer Cell Int.
13:1142013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Q, Jiang Q, Pu Q, Zhang X, Yang W, Wang
Y, Ye S, Wu S, Zhong G, Ren J, et al: MicroRNA-143 inhibits
migration and invasion of human non-small-cell lung cancer and its
relative mechanism. Int J Biol Sci. 9:680–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Yang X, Wu H, Zhou W and Liu Z:
MicroRNA-145 inhibits migration and invasion via inhibition of
fascin 1 protein expression in non-small-cell lung cancer cells.
Mol Med Rep. 12:6193–6198. 2015.PubMed/NCBI
|
|
23
|
Kanitz A, Imig J, Dziunycz PJ, Primorac A,
Galgano A, Hofbauer GF, Gerber AP and Detmar M: The expression
levels of microRNA-361-5p and its target VEGFA are inversely
correlated in human cutaneous squamous cell carcinoma. PLoS One.
7:e495682012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma F, Song H, Guo B, Zhang Y, Zheng Y, Lin
C, Wu Y, Guan G, Sha R, Zhou Q, et al: MiR-361-5p inhibits
colorectal and gastric cancer growth and metastasis by targeting
staphylococcal nuclease domain containing-1. Oncotarget.
6:17404–17416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu D, Tao T, Xu B, Chen S, Liu C, Zhang
L, Lu K, Huang Y, Jiang L, Zhang X, et al: MiR-361-5p acts as a
tumor suppressor in prostate cancer by targeting signal transducer
and activator of transcription-6 (STAT6). Biochem Biophys Res
Commun. 445:151–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, Xi X, Yan Q, Zhang Z, Cai B, Lu W
and Wan X: MicroRNA-361-5p facilitates cervical cancer progression
through mediation of epithelial-to-mesenchymal transition. Med
Oncol. 30:7512013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Call KM, Glaser T, Ito CY, Buckler AJ,
Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, et al:
Isolation and characterization of a zinc finger polypeptide gene at
the human chromosome 11 Wilms' tumor locus. Cell. 60:509–520. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gessler M, Poustka A, Cavenee W, Neve RL,
Orkin SH and Bruns GA: Homozygous deletion in Wilms tumours of a
zinc-finger gene identified by chromosome jumping. Nature.
343:774–778. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hohenstein P and Hastie ND: The many
facets of the Wilms' tumour gene, WT1. Hum Mol Genet. 15:R196–R201.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oji Y, Ogawa H, Tamaki H, Oka Y, Tsuboi A,
Kim EH, Soma T, Tatekawa T, Kawakami M, Asada M, et al: Expression
of the Wilms' tumor gene WT1 in solid tumors and its involvement in
tumor cell growth. Jpn J Cancer Res. 90:194–204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menssen HD, Bertelmann E, Bartelt S,
Schmidt RA, Pecher G, Schramm K and Thiel E: Wilms' tumor gene
(WT1) expression in lung cancer, colon cancer and glioblastoma cell
lines compared to freshly isolated tumor specimens. J Cancer Res
Clin Oncol. 126:226–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakatsuka S, Oji Y, Horiuchi T, Kanda T,
Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M,
et al: Immunohistochemical detection of WT1 protein in a variety of
cancer cells. Mod Pathol. 19:804–814. 2006.PubMed/NCBI
|
|
33
|
Miyoshi Y, Ando A, Egawa C, Taguchi T,
Tamaki Y, Tamaki H, Sugiyama H and Noguchi S: High expression of
Wilms' tumor suppressor gene predicts poor prognosis in breast
cancer patients. Clin Cancer Res. 8:1167–1171. 2002.PubMed/NCBI
|
|
34
|
Mayo MW, Wang CY, Drouin SS, Madrid LV,
Marshall AF, Reed JC, Weissman BE and Baldwin AS: WT1 modulates
apoptosis by transcriptionally upregulating the bcl-2
proto-oncogene. EMBO J. 18:3990–4003. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Richard DJ, Schumacher V, Royer-Pokora B
and Roberts SG: Par4 is a coactivator for a splice isoform-specific
transcriptional activation domain in WT1. Genes Dev. 15:328–339.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ito K, Oji Y, Tatsumi N, Shimizu S, Kanai
Y, Nakazawa T, Asada M, Jomgeow T, Aoyagi S, Nakano Y, et al:
Antiapoptotic function of 17AA(+)WT1 (Wilms' tumor gene) isoforms
on the intrinsic apoptosis pathway. Oncogene. 25:4217–4229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tatsumi N, Oji Y, Tsuji N, Tsuda A,
Higashio M, Aoyagi S, Fukuda I, Ito K, Nakamura J, Takashima S, et
al: Wilms' tumor gene WT1-shRNA as a potent apoptosis-inducing
agent for solid tumors. Int J Oncol. 32:701–711. 2008.PubMed/NCBI
|
|
38
|
Barbolina MV, Adley BP, Shea LD and Stack
MS: Wilms tumor gene protein 1 is associated with ovarian cancer
metastasis and modulates cell invasion. Cancer. 112:1632–1641.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Z, Yamanouchi K, Ohtao T, Matsumura S,
Seino M, Shridhar V, Takahashi T, Takahashi K and Kurachi H: High
levels of Wilms' tumor 1 (WT1) expression were associated with
aggressive clinical features in ovarian cancer. Anticancer Res.
34:2331–2340. 2014.PubMed/NCBI
|
|
40
|
Qi XW, Zhang F, Yang XH, Fan LJ, Zhang Y,
Liang Y, Ren L, Zhong L, Chen QQ, Zhang KY, et al: High Wilms'
tumor 1 mRNA expression correlates with basal-like and ERBB2
molecular subtypes and poor prognosis of breast cancer. Oncol Rep.
28:1231–1236. 2012.PubMed/NCBI
|
|
41
|
Zapata-Benavides P, Tuna M,
Lopez-Berestein G and Tari AM: Downregulation of Wilms' tumor 1
protein inhibits breast cancer proliferation. Biochem Biophys Res
Commun. 295:784–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tuna M, Chavez-Reyes A and Tari AM:
HER2/neu increases the expression of Wilms' Tumor 1 (WT1) protein
to stimulate S-phase proliferation and inhibit apoptosis in breast
cancer cells. Oncogene. 24:1648–1652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qi XW, Zhang F, Wu H, Liu JL, Zong BG, Xu
C and Jiang J: Wilms' tumor 1 (WT1) expression and prognosis in
solid cancer patients: A systematic review and meta-analysis. Sci
Rep. 5:89242015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu C, Wu C, Xia Y, Zhong Z, Liu X, Xu J,
Cui F, Chen B, Røe OD, Li A and Chen Y: WT1 promotes cell
proliferation in non-small cell lung cancer cell lines through
up-regulating cyclin D1 and p-pRb in vitro and in vivo. PLoS One.
8:e688372013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu C, Wang S, Xu C, Tyler A, Li X,
Andersson C, Oji Y, Sugiyama H, Chen Y and Li A: WT1 enhances
proliferation and impedes apoptosis in KRAS mutant NSCLC via
targeting cMyc. Cell Physiol Biochem. 35:647–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu C, Wang Y, Xia Y, He S, Wang Z, Chen Y,
Wu C, Shu Y and Jiang J: Wilms' tumor 1 enhances
Cisplatin-resistance of advanced NSCLC. FEBS Lett. 588:4566–4572.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu C, Zhu W, Qian J, He S, Wu C, Chen Y
and Shu Y: WT1 promotes invasion of NSCLC via suppression of CDH1.
J Thorac Oncol. 8:1163–1169. 2013. View Article : Google Scholar : PubMed/NCBI
|