Introduction
Gliomas are the most common primary malignant tumor
in the central nervous system and account for >70% of all brain
tumors (1). The 2007 World Health
Organization (WHO) classification criteria distinguishes gliomas
into four pathological grades (WHO I to IV) (2). Among them, glioblastoma multiforme
(GBM, WHO IV) accounts for approximately half of all gliomas and
carries the worst prognosis (3).
Despite the rapid development of multimodal
therapeutic strategies over recent years, including surgical
resection, local radiotherapy and systemic chemotherapy, the
prognosis of gliomas remains dismal. For example, temozolomide
(TMZ) is a new generation alkylating agent that has been
demonstrated to be one of the most effective chemotherapeutic
agents for treating glioma. However, TMZ only extends the overall
survival from 12 to 15 months (4),
due to intrinsic or acquired chemoresistance to alkylating agents
(5). However, the majority of
patients with GBM are currently treated with a uniform standardized
regimen, regardless of the individual molecular characteristics of
each tumor, which may be important for the clinical outcome.
Indeed, recent large-scale genomic analyses have demonstrated that
remarkable molecular heterogeneity exists in GBMs (6). The molecular information in each
tumor tissue is extremely valuable for establishing individualized
treatment for gliomas. Thus, the identification of a key molecule,
not only for clarifying glioma mechanisms, but also to predict
prognosis and the response to treatment in human gliomas has become
an increasingly urgent issue.
Differentiated embryo chondrocyte expressed gene 1
(Dec1), also termed split and hairy related protein 2, basic
helix-loop-helix binding protein 2 (BHLHB2) or stimulated with
retinoic acid 13 (Stra13), is a member of the basic
helix-loop-helix (bHLH) family of transcriptional factors. Dec1 has
critical functions in various cellular events, including cell
differentiation and proliferation (7–9),
cell-cycle arrest (10,11), inhibition of apoptosis (12–15),
immunoregulation (16–18), cellular metabolism (19–21)
and circadian rhythms (22–24).
Accumulating evidence has identified overexpression of Dec1 in a
variety of human tumors and highlighted its contribution to
oncogenesis. For example, Stra13 expression was significantly
increased during the progression from normal to in situ and
invasive breast carcinoma, and positively correlated with the tumor
grade (25). Zheng et al
(26) identified that Dec1
expression was significantly increased during the tumor progression
from well-differentiated to moderately-differentiated and
poorly-differentiated gastric cancer tissues (26). Additionally, Stra13 was reported to
be abundantly expressed in colon cancers, but not in adjacent
normal tissues (12). In renal
carcinoma, Stra13 acted as a target of von Hippel-Lindau tumor
suppressor protein (pVHL), and its downregulated expression by pVHL
indicated its effect in renal carcinogenesis (27). There was a close association
between Dec1 overexpression and hypoxia inducible factor 1-α, and
carbonic anhydrase-9 in non-small cell lung cancer (28). Together this evidence suggests
crucial roles for Dec1 in malignancy progression. However, the
profile of Dec1 expression, or its prognostic and therapeutic
significance in human gliomas has not been systematically
analyzed.
The present study investigated Dec1 expression by
immunohistochemistry (IHC) and further analyzed the correlations
between Dec1 expression and clinical variables, prognosis, response
to TMZ chemotherapy and TMZ-induced apoptosis in human glioma
specimens. Furthermore, the contribution of Dec1 overexpression to
the antiglioma effect of TMZ in vivo was evaluated.
Materials and methods
Patients and specimens
A total of 157 resected glioma specimens obtained
from the Department of Neurosurgery, Xi'Jing Hospital, Fourth
Military Medical University (Xi'an, China) from 2007 to 2010, were
analyzed in this study. Slides were carefully evaluated and
categorized by histological subtype and pathological grade
(according to the WHO classifications in 2007) by two pathologists
as follows: 45 cases of low-grade glioma (LGG), including grade I
(10 pilocytic astrocytomas and 4 myxopapillary ependymomas) and
grade II (16 diffuse astrocytomas, 9 oligoastrocytomas and 6
oligodendrogliomas); 112 cases of high-grade glioma (HGG),
including grade III (7 anaplastic astrocytomas, 8 anaplastic
oligodendrogliomas and 7 anaplastic oligoastrocytomas) and grade IV
(90 GBMs). All patients were histologically diagnosed with gliomas,
which were disassociated tissues during the initial surgery and did
not receive radio- or chemotherapy prior to surgery. Among the
newly diagnosed gliomas, 86 patients with HGG were treated with
oral TMZ following surgery. The clinical information is presented
in Table I. Patients were
followed-up for 5 years by telephone or questionnaire letters.
 | Table I.Correlations between Dec1 expression
and the clinical variables in glioma patients. |
Table I.
Correlations between Dec1 expression
and the clinical variables in glioma patients.
| Variable | Description | n | Dec1-low
expression | Dec1-high
expression | P-value |
|---|
| Age (years) | <50 | 76 | 36 | 40 | 0.239a |
|
| ≥50 | 81 | 46 | 35 |
|
| Gender | Male | 75 | 45 | 30 | 0.063a |
|
| Female | 82 | 37 | 45 |
|
| KPS | <80 | 56 | 22 | 34 | 0.016a |
|
| ≥80 | 101 | 60 | 41 |
|
| Grade | I | 14 | 12 | 2 | 0.023b |
|
| II | 31 | 16 | 15 |
|
|
| III | 22 | 14 | 8 |
|
|
| IV | 90 | 40 | 50 |
|
| Tumor location | Supratentorial | 94 | 50 | 44 | 0.769a |
|
| Infratentorial | 63 | 32 | 31 |
|
| Tumor size | <3 cm | 78 | 38 | 40 | 0.383a |
|
| ≥3 cm | 79 | 44 | 35 |
|
| Surgery
resectionc | Total | 91 | 48 | 43 | 0.879a |
|
| Subtotal | 66 | 34 | 32 |
|
| TMZ
chemotherapyd | Resistant | 46 | 16 | 30 | 0.020a |
|
| Sensitive | 40 | 24 | 16 |
|
Progression-free survival (PFS) was defined as the
time from the date of the initial surgery to the first recurrence
(confirmed by MRI) or death. Overall survival (OS) was calculated
from the date of the initial surgery until death or the last
follow-up. TMZ resistance was standardized as PFS of >1 year.
Patients who succumbed to diseases not directly associated with
glioma or due to unexpected events were excluded from the study.
The use of samples in the current study was approved by the
Research Ethics Committee of Neurosurgery Department, Xi'Jing
Hospital, Fourth Military Medical University (Xi'an, China).
Informed consent was obtained from all patients.
IHC and evaluation of staining
IHC assays were performed by the polymer-peroxidase
method as follows. Briefly, the 3-µm thick slides were
deparaffinized and rehydrated, then the sections were rinsed in
distilled water for 1 min. Antigen retrieval was performed by
boiling in 0.01 M citrate buffer (pH 6.0) in a pressure cooker for
86 sec. Following cooling, 0.3% hydrogen peroxide was used to block
nonspecific sites for 30 min, and sections were then incubated with
Triton-X for 20 min. Following a wash with PBS and incubation with
goat serum (1:20 dilution; OriGene Technologies, Inc.) at room
temperature for 1 h, sections were incubated with rabbit polyclonal
anti-Dec1 antibody (1:250 dilution; cat. no. ab90594; Abcam,
Cambridge, MA, USA) or rabbit monoclonal Ki-67 antibody
(1:500-1,000 dilution; cat. no. ab16667; Abcam) at 4°C overnight.
The specimens were rinsed briefly in PBS and incubated at room
temperature first with horseradish peroxidase-conjugated
anti-rabbit antibody (cat. no. TA130023; OriGene Technologies,
Inc., Beijing, China) for 30 min, and then avidin-biotin peroxidase
(OriGene Technologies, Inc.) and 20 min. The slides were stained
with diaminobenzidine (OriGene Technologies, Inc.) and water was
added to terminate the staining. Slides were counterstained with
Meyer's hematoxylin (Baso Diagnostics, Inc., Zhuhai, China).
Dec1 immunostaining was evaluated independently and
blindly by two pathologists. The results were compared and
re-examined until a consensus was reached for each score. The
immunoreactivity score (IRS) of Dec1 was evaluated according to the
percentage of positive cells and the staining intensity. IRS was
determined by multiplying the two factors: i) Percentage of
positive cells (<5%, scored 0; 6–25%, scored 1; 26–50%, scored
2; 51–75%, scored 3; >75%, scored 4); and ii) staining intensity
(negative staining, 0; weak staining, 1; moderate staining, 2;
strong staining, 3). The proliferative index was calculated by
determining the percentage of KI-67-positive cells.
TdT-mediated dUTP nick ending-labeling
(TUNEL) assay
To investigate TMZ-induced apoptosis of tumor cells
in glioma patients, an additional 63 patients with recurrent GBM
were selected from those that had been histologically documented
with malignant glioma and suffered a relapse following the
postoperative TMZ chemotherapy. Specimens of these recurrent GBM
patients were isolated from the second surgery and used for the
TUNEL assay with a DeadEnd™ Fluorometric TUNEL system (Promega
Corporation, Madison, WI, USA), which was performed according the
manufacturer's instructions. Tissues were counterstained with DAPI.
Images were captured using a fluorescent microscope (Olympus BX51;
Olympus Corporation, Tokyo, Japan) using an RGB filter. To evaluate
the percentage of apoptotic cells (apoptotic index) in human glioma
specimens, TIFF (RGB) images were transferred into the RGB channel
in Adobe Photoshop CS5 software (Adobe System Inc., San Jose, CA,
USA). Identical adjustment of contrast and brightness was performed
within for all images presented.
Cell culture, plasmid construction and
stable gene transfection
The U87 GBM cell line (American Type Culture
Collection, Manassas, VA, USA) was maintained as an adherent
monolayer culture in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) in a humidified incubator containing 5% CO2 at 37°C.
Plasmids containing full-length Dec1 were generated
by reverse transcription-polymerase chain reaction and subcloned
into the pENTR™3C vector (Invitrogen; Thermo Fisher Scientific,
Inc.). Primers for Dec1 were as follows: 5′-gga agg agt tcg aac cat
gga gcg gat ccc cag cgc gca-3′ (forward), and 5′-tgc ggc cgc act
cga gct agt ctt tgg ttt cta agt tta aag −3′ (reverse). Dec1
pLenti-6.3 expression vectors were obtained using the Gateway™
technology (Invitrogen; Thermo Fisher Scientific, Inc.). The
pLenti-6.3-cherry vector was used as a negative control. The
recombinant expression vector and control vector were packaged in
293T cells (American Type Culture Collection) with the plasmids
psPAX2 and pMD2.G (Invitrogen; Thermo Fisher Scientific, Inc.)
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. U87 stable cell
lines overexpressing Dec1 and cherry were selected with 8 mg/ml
blasticidin (Invitrogen; Thermo Fisher Scientific, Inc.) and
maintained in medium containing 4 mg/ml blasticidin.
Tumor generation and drug treatment in
a mouse model
Nude female BALB/c mice (18–20 g) used for all in
vivo studies were obtained from the Laboratory Animal Center of
the Fourth Military Medical University (Xi'an, China), and
experiments were performed in accordance with guidelines of the
Fourth Military Medical University Animal Care and Use Committee.
The mice were maintained under pathogen-free conditions in an air
flow cabinet at 23°C, with 12 h/12 h day/night cycle, and water and
food ad libitum. When mice were six weeks old, tumor
generation was initiated by subcutaneous injection of
1×106 stable U87 cells overexpressing Dec1 or cherry
(n=5 per group) in each flank of a nude mouse. Palpable tumors were
generated ~5–7days postinoculation, and TMZ was added to the diet
at 50 mg/kg for 5 consecutive days. The tumor size was measured
with a slide caliper and the tumor volume was recorded using the
following formula: Volume = a × b2 / 2 (a = the larger
dimension; b = the smaller dimension). Following 30 days of
inoculation, the mice were sacrificed by decapitation. Tumors were
removed, weighed and paraffin-embedded. Pathological sections
(3-µm) were prepared for TUNEL and Ki-67 immunostaining
analysis.
Statistical analysis
All statistical analyses were performed using the
SPSS 13.0 package (SPSS Inc., Chicago, IL, USA). Values are
presented as the mean ± standard deviation. Relationships between
Dec1 expression levels and clinical variables were analyzed by the
Mann-Whitney test or the Kruskal-Wallis test. The Kaplan-Meier
method was used to plot survival curves and the prognostic
differences in groups with different Dec1 expression were tested by
log-rank analysis. Hazard ratios (HR) of different prognostic
factors were evaluated by Cox multivariate analysis. Differences of
Dec1 IRS and the apoptotic index (AI) between the low Dec1
expression group and the high Dec1 expression group or between the
TMZ-resistant group and the TMZ-sensitive group were compared using
the Student's t-test. The nonparametric Spearman's rank correlation
coefficient was performed to assess the correlation between AI and
Dec1 IRS. P<0.05 was considered to indicate a statistically
significant difference.
Results
Correlation between Dec1 expression
with clinical variables in glioma patients
Correlations between Dec1 expression levels with
clinical variables were analyzed in 157 cases of newly diagnosed
gliomas (Table I). Dec1 expression
levels were evaluated by IHC, and patients were stratified as low
Dec1 expression (IRS <8) or high Dec1 expression (IRS ≥8),
according to the mean IRS of Dec1 (6.44±3.73). Among the total 157
cases, 82 patients exhibited low Dec1 expression, whereas 75 cases
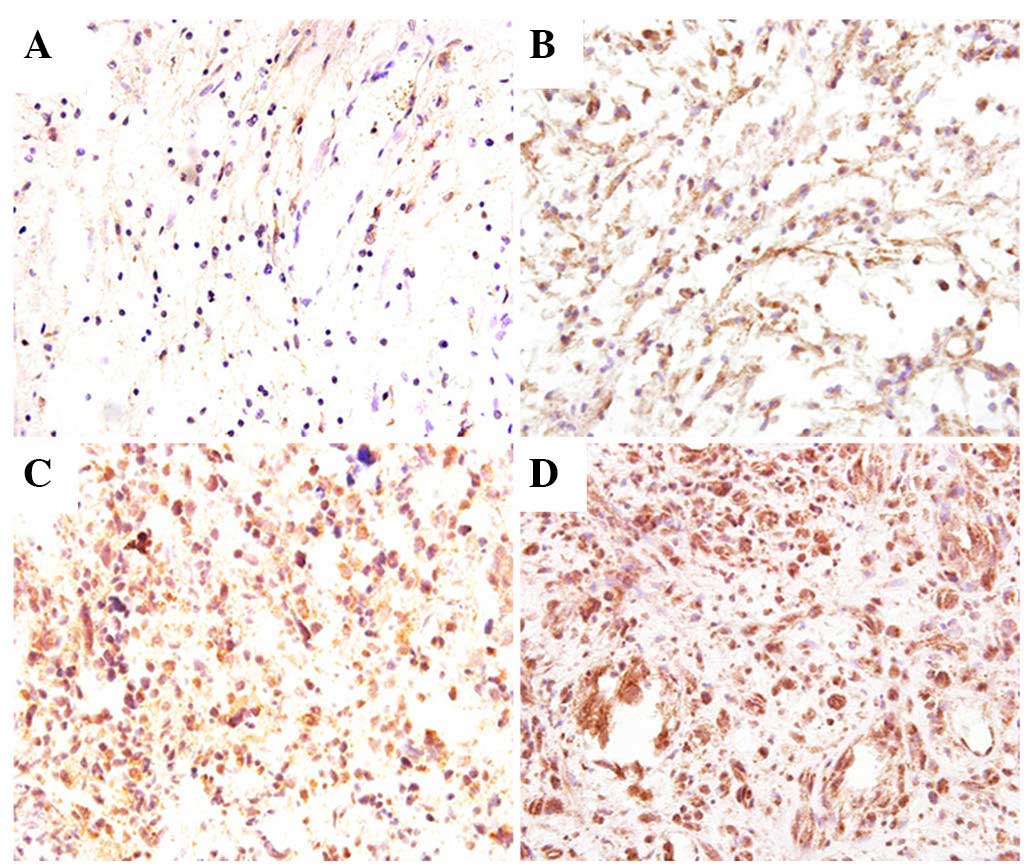
exhibited high Dec1 expression. Representative staining images are
presented in Fig. 1. In LGG, a
lower number of Dec1 positive cells was observed exhibiting a weak
staining intensity, whereas in HGG, nearly all tumor cells were
strongly stained. Dec1 expression increased with the progression of
pathologic grades (P=0.023). In addition, Dec1 expression was also
significantly correlated with the Karnofsky performance status
(KPS; P=0.016). No statistically significance was detected between
the expression levels of Dec1 and age, gender, tumor location,
tumor size or surgical resection.
High Dec1 expression is associated
with poor prognosis in patients with glioma
The Kaplan-Meier analyses for PFS and OS were
performed to evaluate the prognostic differences between Dec1
expression groups in patients with glioma. The postoperative mean
PFS and OS of all eligible patients with glioma were 18.1 and 23.1
months, respectively. The postoperative mean PFS and OS of patients
with low Dec1 expression were 22 and 27 months, respectively,
whereas those of patients with high Dec1 expression were 13.8 and
18.8 months, respectively. Patients with low Dec1 expression
exhibited longer PFS and OS compared to those with high Dec1
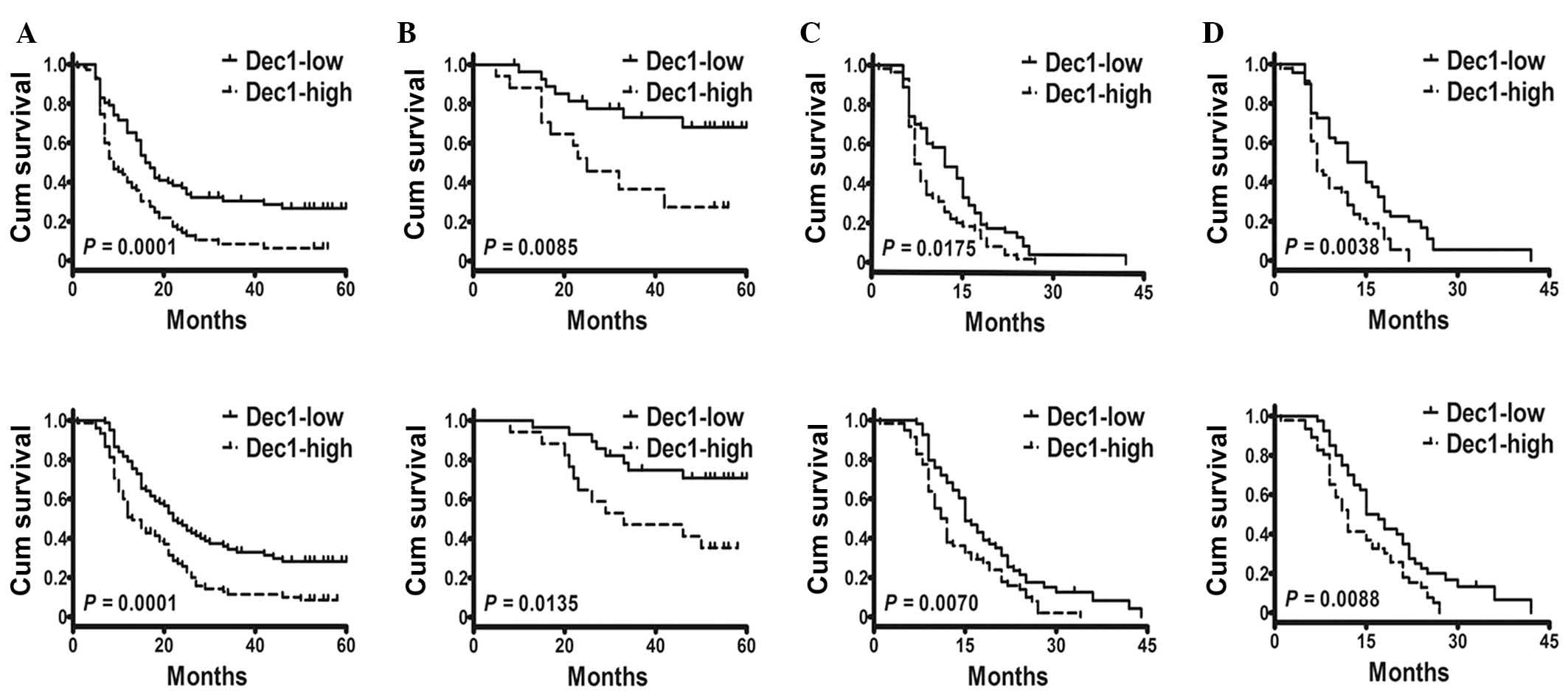
expression (log-rank test, P=0.0001 for both; Fig. 2A). In addition, the considered
variables, including age, KPS and WHO grade, also exhibited
statistically significance at log-rank test in all cases of glioma
for PFS and OS (data not shown). However, gender, tumor location,
tumor size or surgery resection had no prognostic value for PFS and
OS in the present cohort (data not shown).
Kaplan-Meier analyses were also performed in
different subgroups stratified according to the malignant degree.
As high pathological grade of gliomas is considered a marker of
high risk, the patients were classified into two subgroups: LGG and
HGG. In LGG, the postoperative mean PFS and OS were 34.5 and 42
months, respectively. The postoperative mean PFS and OS of patients
with low Dec1 expression were 39.2 and 45.3 months, respectively,
whereas those of patients with high Dec1 expression were 26.8 and
36.6 months, respectively. High Dec1 expression was significantly
correlated with shorter PFS and OS in LGG (log-rank test, P=0.0085
and P=0.0135, respectively; Fig.
2B). Similarly in HGG, the postoperative mean PFS and OS were
11.5 and 15.5 months, respectively. The postoperative mean PFS and
OS of patients with low Dec1 expression were 13.1 and 17.6 months,
respectively, while those of patients with high Dec1 expression
were 10 and 13.6 months, respectively. Dec1 expression in HGG also
proved to be a prognostic factor for both PFS and OS (log-rank
test, P=0.0175 and P=0.0070, respectively; Fig. 2C). In addition, age and KPS had
prognostic value for PFS and OS in LGG, while radiotherapy
postsurgery did not contribute to long-term benefits in the current
cohort (data not shown). In HGG, age, KPS and standard treatment
also significantly predicted PFS and OS (data not shown).
Using the Cox proportional hazards model, univariate
and multivariate analyses were performed in patients with glioma
(Tables II and III). In LGG, low Dec1 expression was
identified as a favorable prognostic indicator for PFS (P=0.009;
HR, 0.226) and OS (P=0.010; HR, 0.246). Similarly, age and KPS were
also identified as independent prognostic factors for PFS and OS.
However, for PFS and OS, no statistically significance was found
among other variables, including gender, tumor location, tumor
size, surgery resection or radiotherapy (Table II). The Cox proportional hazards
model in HGG also revealed low Dec1 expression as a favorable
predictor of PFS (P=0.015; HR, 0.581) and OS (P=0.009; HR, 0.569).
In addition, KPS and standard treatment were also demonstrated to
be independent prognostic factors for PFS and OS in HGG, whereas
other variables, including age, gender, tumor location, tumor size
and surgery resection were not prognostic factors (Table III).
 | Table II.Univariate and multivariate analyses
of progression-free survival and overall survival in low-grade
glioma. |
Table II.
Univariate and multivariate analyses
of progression-free survival and overall survival in low-grade
glioma.
| Clinical
variables | Unadjusted
HRa(95% CI) | P-value | Adjusted
HRb (95% CI) | P-value |
|---|
| Progression-free
survival |
|
|
|
|
|
Age |
|
|
|
|
|
<50 vs.
≥50 | 0.363
(0.141–0.930) | 0.035 | 0.273
(0.089–0.836) | 0.023 |
|
Gender |
|
|
|
|
|
Male vs.
female | 1.329
(0.534–3.307) | 0.540 | 1.350
(0.490–3.720) | 0.562 |
|
KPS |
|
|
|
|
|
≥80 vs.
<80 | 0.198
(0.076–0.513) | 0.001 | 0.207
(0.059–0.725) | 0.014 |
| Dec1
expression |
|
|
|
|
|
Low vs. high | 0.312
(0.124–0.786) | 0.013 | 0.226
(0.074–0.693) | 0.009 |
| Tumor
location |
|
|
|
|
|
Sup. vs. inf. | 1.451
(0.550–3.830) | 0.452 | 0.720
(0.237–2.186) | 0.562 |
| Tumor
size |
|
|
|
|
|
≥3 cm vs. <3
cm | 2.814
(1.105–7.165) | 0.030 | 1.409
(0.423–4.691) | 0.576 |
| Surgery
resection |
|
|
|
|
|
Total vs.
subtotal | 0.820
(0.311–2.159) | 0.688 | 0.435
(0.101–1.874) | 0.264 |
|
Radiotherapy |
|
|
|
|
|
No vs. yes | 1.213
(0.353–4.165) | 0.759 | 2.418
(0.388–15.06) | 0.344 |
| Overall
survival |
|
|
|
|
|
Age |
|
|
|
|
|
<50 vs.
≥50 | 0.362
(0.141–0.930) | 0.035 | 0.283
(0.092–0.873) | 0.028 |
|
Gender |
|
|
|
|
|
Male vs.
female | 1.425
(0.570–3.559) | 0.448 | 1.055
(0.385–2.889) | 0.917 |
|
KPS |
|
|
|
|
|
≥80 vs.
<80 | 0.242
(0.095–0.618) | 0.003 | 0.257
(0.080–0.828) | 0.023 |
| Dec1
expression |
|
|
|
|
|
Low vs. high | 0.337
(0.135–0.840) | 0.020 | 0.246
(0.085–0.710) | 0.010 |
| Tumor
location |
|
|
|
|
|
Sup. vs. inf. | 1.318
(0.501–3.468) | 0.576 | 0.585
(0.196–1.748) | 0.337 |
| Tumor
size |
|
|
|
|
|
≥3 cm vs. <3
cm | 3.007
(1.178–7.678) | 0.021 | 1.772
(0.554–5.664) | 0.334 |
| Surgery
resection |
|
|
|
|
|
Total vs.
subtotal | 0.890
(0.337–2.350) | 0.814 | 0.521
(0.120–2.266) | 0.385 |
|
Radiotherapy |
|
|
|
|
|
No vs. yes | 1.309
(0.381–4.501) | 0.669 | 1.996
(0.312–12.77) | 0.466 |
 | Table III.Univariate and multivariate analyses
of progression-free survival and overall survival in high-grade
glioma. |
Table III.
Univariate and multivariate analyses
of progression-free survival and overall survival in high-grade
glioma.
| Clinical
variables | Unadjusted
HRa(95% CI) | P-value | Adjusted
HRb(95% CI) | P-value |
|---|
| Progression-free
survival |
|
|
|
|
|
|
Age |
|
|
|
|
|
|
<50 vs.
≥50 | 0.628
(0.420–0.938) | 0.023 | 0.768
(0.493–1.196) | 0.243 |
|
Gender |
|
|
|
|
|
|
Male vs.
female | 0.860
(0.584–1.267) | 0.445 | 0.945
(0.620–1.440) | 0.793 |
|
KPS |
|
|
|
|
|
|
≥80 vs.
<80 | 0.363
(0.234–0.562) | 0.000 | 0.570
(0.351–0.925) | 0.023 |
| Dec1
expression |
|
|
|
|
|
|
Low vs. high | 0.643
(0.434–0.954) | 0.028 | 0.581
(0.375–0.900) | 0.015 |
| Tumor
location |
|
|
|
|
|
|
Sup. vs. inf. | 0.941
(0.631–1.401) | 0.763 | 1.052
(0.697–1.587) | 0.809 |
| Tumor
size |
|
|
|
|
|
|
≥3 cm vs. <3
cm | 0.879
(0.594–1.299) | 0.516 | 0.994
(0.643–1.537) | 0.978 |
| Surgery
resection |
|
|
|
|
|
|
Total vs.
subtotal | 1.055
(0.711–1.565) | 0.790 | 0.849
(0.564–1.277) | 0.432 |
|
Standard
treatmentc |
|
|
|
|
|
|
No vs. yes | 3.081
(2.031–4.673) | 0.000 | 2.823
(1.736–4.591) | 0.000 |
| Overall
survival |
|
|
|
|
|
|
Age |
|
|
|
|
|
|
<50 vs.
≥50 | 0.641
(0.429–0.956) | 0.029 | 0.861
(0.554–1.339) | 0.506 |
|
Gender |
|
|
|
|
|
|
Male vs.
female | 0.827
(0.561–1.218) | 0.336 | 0.877
(0.577–1.334) | 0.541 |
|
KPS |
|
|
|
|
|
|
≥80 vs.
<80 | 0.347
(0.226–0.535) | 0.000 | 0.549
(0.340–0.887) | 0.014 |
| Dec1
expression |
|
|
|
|
|
|
Low vs. high | 0.598
(0.402–0.889) | 0.011 | 0.569
(0.374–0.866) | 0.009 |
| Tumor
location |
|
|
|
|
|
|
Sup. vs. inf. | 0.849
(0.570–1.266) | 0.422 | 0.761
(0.501–1.157) | 0.202 |
| Tumor
size |
|
|
|
|
|
|
≥3 cm vs. <3
cm | 0.815
(0.552–1.205) | 0.306 | 0.978
(0.634–1.509) | 0.920 |
| Surgery
resection |
|
|
|
|
|
|
Total vs.
subtotal | 1.169
(0.788–1.734) | 0.437 | 0.974
(0.651–1.455) | 0.896 |
|
Standard
treatmentc |
|
|
|
|
|
|
No vs. yes | 3.531
(2.340–5.329) | 0.000 | 3.124
(1.941–5.027) | 0.000 |
High Dec1 expression predicts poor
responses to TMZ chemotherapy in HGG patients
Among the patients with HGG, 86 cases were treated
with TMZ following surgery. The mean Dec1 IRS in all TMZ-treated
patients with HGG was 7.14±3.87, and the mean Dec1 IRS in the
TMZ-resistant group (8.39±3.44) was significantly higher than that
of the TMZ-sensitive group (5.7±3.86, t=3.389; P=0.001). In
addition, 30 out of 46 (65.2%) patients with high Dec1 expression
exhibited resistance to TMZ chemotherapy, compared with 16 out of
40 (40%) patients with low Dec1 expression. A statistically
significant correlation was observed between Dec1 expression level
and the response to TMZ chemotherapy (P=0.020; Table I). The mean postoperative PFS and
OS of all TMZ-treated patients with HGG were 11.9 and 15.9 months,
respectively. The mean PFS and OS of patients with low Dec1
expression were 14.3 and 18.3 months, respectively, whereas those
of patients with high Dec1 expression were 9.7 and 13.7 months,
respectively. HGG patients with low Dec1 expression exhibited
better survival than those with high Dec1 expression when they
received TMZ chemotherapy (log-rank test, P=0.0038 and P=0.0088,
respectively; Fig. 2D).
Correlation of Dec1 expression with
apoptosis in TMZ-treated recurrent GBMs
All 63 cases of TMZ-treated recurrent GBM specimens
were examined by Dec1 IHC and the TUNEL assay, and representative
images of both are presented in Fig.
3A. TMZ-induced apoptotic cells were detected in all these
specimens. The AI was expressed as the percentage of the apoptotic
cells and ranged from 0.31–5.81% (mean, 2.18±1.35%). The AI in low
Dec1 expression group (2.70±1.42%) was significantly higher
compared with the high Dec1 expression group (1.76±1.15%; t=2.822,
P=0.007; Fig. 3B). Furthermore,
the increase of Dec1 IRS in the recurrent GBM specimens was
significantly correlated with a decrease of AI (r=−0.341, P=0.006;
Fig. 3C). In addition, a
significant difference in AI was observed between the TMZ-resistant
group and TMZ-sensitive group (1.81±1.20% and 2.67±1.40%,
respectively; t=−2.539, P=0.014; Fig.
3D).
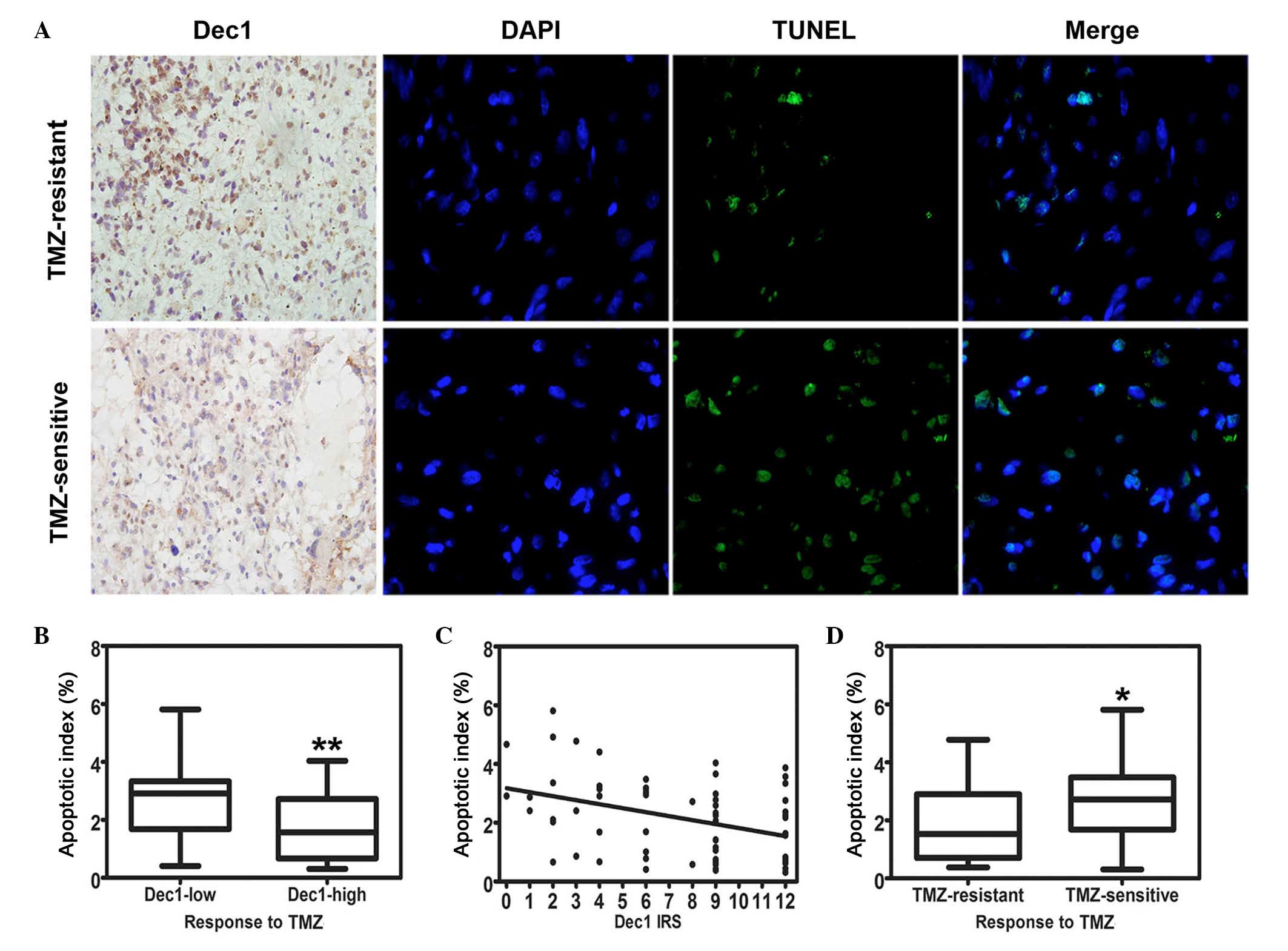 | Figure 3.Correlation of Dec1 expression with
TMZ-induced apoptosis in TMZ-treated patients with recurrent GBMs.
(A) Representative photographs of Dec1 immunostaining
(magnification, ×400) and apoptosis detection by TUNEL assay
(magnification, ×1,000). Upper row: recurrent GBM patients
resistant to TMZ chemotherapy exhibited high expression of Dec1 and
fewer apoptotic tumor cells. Lower row: recurrent GBM patients
sensitive to TMZ chemotherapy exhibited low expression of Dec1 and
more apoptotic tumor cells. Arrowheads indicate apoptotic cells in
recurrent GBM tissues. (B) Box-whisker plot presenting the
correlation of Dec1 expression levels with apoptotic index
(**P=0.006 vs. Dec-low). (C) Scatterplot of the correlation of Dec1
IRS with apoptotic index. With the increase in Dec1 IRS, the
apoptotic index decreased markedly (r=−0.341). By linear
regression, a trend line representing the best fit indicated on the
scatterplot. (D) Box-whisker plot presenting the correlation of the
response to TMZ chemotherapy with apoptotic index (*P<0.05 vs.
TMZ-resistant). The values represent the mean ± standard deviation
of 63 fields. GBM, glioblastoma multiforme; Dec1, differentiated
embryo chondrocyte expressed gene 1; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick end labeling; TMZ,
temozolomide; IRS, immunoreactivity score. |
Dec1 overexpression significantly
promotes the tumorigenicity of U87 cells xenografts in TMZ-treated
nude mice
To confirm the correlation between overexpression of
Dec1 and TMZ resistance in patients with GBM, the effects of Dec1
overexpression on TMZ resistance were investigated in vivo.
U87 GBM cells stably expressing Dec1 or cherry (control) were
established and transplanted into nude mice to generate tumor
xenograft models. At ~30 days post-implantation, tumors were
removed. A significant TMZ-resistant effect was observed in the
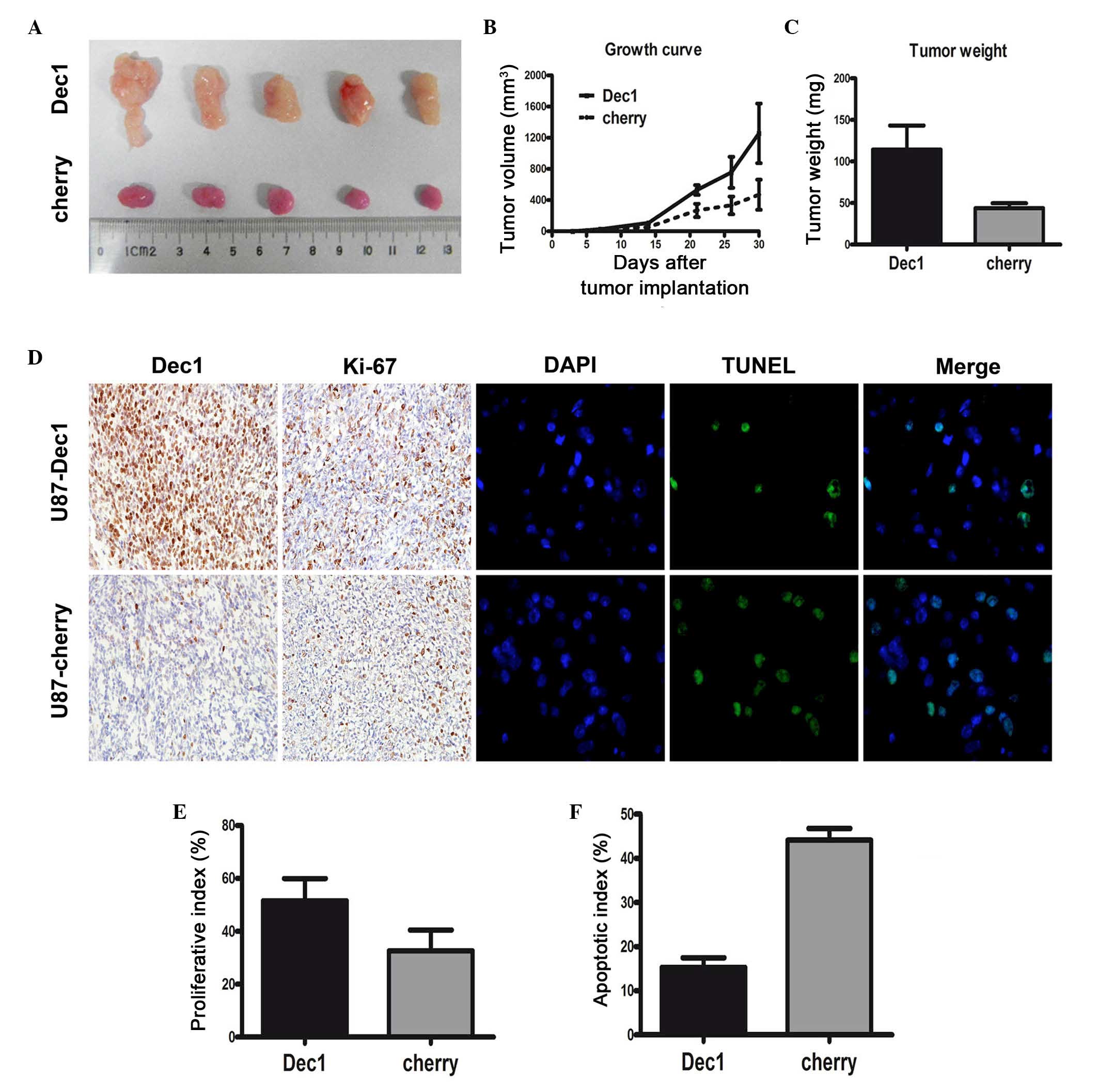
U87-Dec1 tumors compared with the U87-cherry tumors (Fig. 4A). Following treatment with TMZ,
the mean tumor volume and weight in U87-Dec1 tumors (1257±382.7
mm3 and 114±12.99 mg, respectively) was significantly
higher compared with the U87-cherry tumors (468.8±193.4
mm3 and 44±2.63 mg, respectively; P<0.001 for both;
Fig. 4B and C). In U87-Dec1 and
U87-cherry tumors treated with TMZ, Dec1 levels were assessed by
IHC and proliferation and apoptosis was detected by Ki-67
immunostaining and TUNEL, respectively (Fig. 4D). The proliferative index in
U87-Dec1 tumors (51.6±3.74%) was significantly higher compared with
the U87-cherry tumors (38.20±3.55%; t=2.600, P=0.032; Fig. 4E), and the AI was significantly
lower in U87-Dec1 tumors (15.26±2.20%) compared with U87-cherry
tumors (44.10±2.69%; t=8.296, P<0.0001; Fig. 4F).
Discussion
The present study investigated the expression status
of Dec1 in 157 cases of newly diagnosed glioma and 63 cases of
TMZ-treated recurrent GBM. High Dec1 expression, which was closely
associated with pathological grade, KPS and poor response to TMZ
chemotherapy, was a significant risk factor for PFS and OS in the
patients with newly diagnosed glioma. In addition, a negative
correlation between Dec1 expression with the AI was detected in the
TMZ-treated recurrent GBMs. In in vivo experiments, Dec1
overexpression promoted the tumorigenicity of U87 xenografts in
nude mice treated with TMZ and significantly attenuated the
antiglioma effect of TMZ. Together, the results demonstrated that
Dec1 may be a valuable prognostic indicator for clinical outcome,
and notably, a predictive factor for the response to TMZ
chemotherapy in patients with glioma.
Various studies have investigated Dec1 to clarify
its role in oncogenesis in multiple types of human tumor (12,14,25–30).
However, its value as a prognostic marker has not been previously
determined. In the current study, in LGG and HGG, high Dec1
expression was correlated with unfavorable outcomes and was
identified as an independent prognostic factor (Fig. 2; Tables II and III). In gliomas, complete resection is
nearly impossible for the primary tumor due to its infiltrative
nature. Therefore, radiotherapy- or chemotherapy-induced apoptosis
of the residual glioma cells will dramatically impact the clinical
outcome (Fig. 3D). The
anti-apoptotic effect of Dec1 has been previously confirmed by
other studies (12–14,31–33).
Indeed the results of the present study indicated that Dec1
overexpression significantly decreased apoptosis in vivo
(Figs. 3 and 4). Based on this evidence, we speculate
that the correlation between high Dec1 expression and the poor
outcome may be explained by the ability of Dec1 to confer partial
resistance to chemotherapy-induced apoptosis. In addition, HGGs
were either poorly differentiated or undifferentiated, and
consequently carried a dismal prognosis. The current study observed
that Dec1 expression was increased with increasing WHO grade, which
was consistent with its capacity to antagonize differentiation in
various types of human tumor (25,26).
Furthermore, several previous studies provided evidence of Dec1
involvement in multiple cellular pathways, including p53,
VHL/hypoxia and Janus kinase/Signal Transducer and Activator of
Transcription signaling, which are crucial for tumor malignancy
(27,31,32,34–36).
Poor differentiation and high malignancy were closely associated
with unfavorable clinical outcome. Other reasons for the poor
prognosis of gliomas may be the functions of Dec1 in cell
differentiation and malignancy. Therefore, inhibition of Dec1
expression may contribute to the long-term benefits for patients
with glioma, suggesting that Dec1 might be a novel potential
molecular target for glioma biological therapy.
By contrast with the role of Dec1 as a significant
risk factor for the poor outcome of gliomas described above, an
opposing prognostic effect of Dec1 had been previously reported
(37), demonstrating that low
BHLHB2 (Dec1) expression predicted shorter survival in pancreatic
ductal adenocarcinoma. Notably, the correlation of Dec1 expression
levels with differentiation or malignant progression in various
types of human cancer is not unique. In a previous study, Dec1
expression was increased during the progression from well
differentiated to poorly differentiated tumor tissues in gastric
cancer, which was consistent with findings in breast cancer and the
present study (25,26). Shi et al (30) reported that low Dec1 expression was
associated with poor histological differentiation and malignant
progression in hepatocellular carcinoma. These findings indicate
that the expression of Dec1 might be a context-dependent prognostic
factor. Similar to Dec1, Kruppel like factor 5 (KLF5) has also been
previously reported to exert opposing prognostic roles in different
types of cancer. For instance, patients with non-small cell lung
cancer with high levels of KLF5 expression had a significantly
better disease-specific survival compared with intermediate to no
KLF5 expression (38). Patients
with nuclear KLF5 staining had a significantly lower disease-free
survival rate compared with patients with negative nuclear staining
in gastric cancer (39).
Clarifying the different prognostic effects of Dec1 in various
types of human tumor warrants further investigation.
To date, the most important breakthrough and most
commonly used strategy in HGG chemotherapy was the introduction of
the alkylating agent, TMZ (40).
Thus, the response to TMZ chemotherapy significantly affects the
prognosis of gliomas. The present study provided additional
evidence that high Dec1 expression negatively affected the response
to TMZ chemotherapy in patients with HGG, which indicated that Dec1
was a prognostic factor and predictive factor for the response to
TMZ chemotherapy in glioma patients. Given this evidence, high Dec1
expression may be correlated with poor prognosis in patients with
glioma.
Traditionally, prognostic factors intend to
objectively predict patient clinical outcome independent of
treatment, while predictive factors aim to foretell the response of
a patient to a specific therapeutic intervention and are associated
with tumor sensitivity or resistance to the therapy. However,
various factors exhibit both prognostic and predictive
significance. For instance, human epidermal growth factor receptor
2 (HER2) has been validated as a prognostic factor and predictor of
response to HER2-targeting therapy (41).
The original aim of the current study was to
investigate the correlation of Dec1 expression with tumor
malignancy and determine whether Dec1 expression may predict the
outcome in 157 cases of newly diagnosed glioma. Surprisingly, the
86 cases of the TMZ-treated glioma patients were classified into
resistant- and sensitive-groups according to the duration of PFS, a
statistical significance between Dec1 expression levels and the
response to TMZ chemotherapy was detected (P=0.020; Table I). Furthermore, the low Dec1
expression group exhibited longer PFS and OS compared with the high
Dec1 expression group in the TMZ-treated patients with HGG
(Fig. 2D). Together this evidence
revealed that, similar to HER2, Dec1 displayed a strong prognostic
effect, and also appeared to predict a poor response to TMZ
treatment.
Standard therapeutic strategies of maximal tumor
resection and postoperative radiation combined with TMZ
chemotherapy are almost never curative for GBMs, due to the
infiltrative nature of GBMs, and the intrinsic or acquired
resistance to radiation and chemotherapy (42). Therefore, identifying mechanisms of
resistance to TMZ chemotherapy in GBMs may provide useful
information for further individualized therapeutic strategies. The
results of the current study indicated that Dec1 overexpression
significantly attenuated the antiglioma effect of TMZ in
vivo (Fig. 4). In addition,
several previous studies provide evidence of the involvement of
Dec1 in TMZ resistance. One report observed that Dec1 and Dec2
decreased mutL homolog 1 (MLH1) expression via binding to the E-box
motifs in the MLH1 promoter region (43). MLH1 is considered to be an
important factor in the DNA mismatch repair (MMR) pathway, and
defects in this pathway are critical for mediating the tolerance of
the cytotoxic effect of alkylating agents (44). Furthermore, the cytotoxicity of TMZ
was low in colon cancer cells lines with hMLH1 mutation, and all
five cell lines in the National Cancer Institute tumor panel that
were deficient in hMLH1 activity were resistant to TMZ (45,46).
Therefore, as a MLH1 repressor, Dec1 overexpression may
consequently decrease the cytotoxicity of TMZ. Additionally, it is
well established that via the MMR system, TMZ induces double-strand
breaks and triggers p53-dependent cell cycle arrest and apoptosis
(47). As a novel target gene of
the p53 family, Dec1 inhibited DNA damage-induced cell death by
attenuating p53 induction of macrophage inhibitory cytokine-1.
Furthermore, as a hypoxia-regulated transcription factor, Dec1 is
upregulated by hypoxia in cancer, and the hypoxia-adaptation
involves decreased leakage of reactive oxygen species (ROS)
(48,49). TMZ-induced DNA damage caused
enhanced ROS generation and decreased ROS production-induced
acquisition of chemoresistance (50). Generally, chemoresistance in
gliomas may be influenced by the dysregulation of
apoptosis-regulating genes and proteins. The results of the present
study demonstrated an antagonizing role of high Dec1 expression in
TMZ-induced apoptosis of the tumor cells in TMZ-treated recurrent
GBMs, which was consistent with previous studies indicating that
Dec1 is an anti-apoptotic transcription factor (12–15,31–33).
Together this evidence suggests that Dec1 is involved in
TMZ-resistance and suggests that inhibition of Dec1 expression may
enhance the cytotoxicity of TMZ. The underlying mechanisms involved
require further investigation.
In conclusion, the data of the present study
provided evidence that Dec1 expression is closely associated with
the malignant grade and TMZ-induced apoptosis of human glioma. It
was additionally demonstrated that Dec1 is a prognostic indicator
to evaluate the outcome of patients with glioma. Furthermore, Dec1
expression negatively affected the response to TMZ chemotherapy in
patients with HGG. This valuable prognostic indicator for clinical
outcome and predictive factor of the response to TMZ chemotherapy
may be a potential novel molecular target for glioma biological
therapy, particularly in patients with TMZ-resistance.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81001123 and 81572469) and
the Natural Science Foundation of Shaanxi Province of China (grant
no. S2012jc7293).
References
|
1
|
Ohgaki H: Epidemiology of brain tumors.
Methods Mol Biol. 472:323–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneider T, Mawrin C, Scherlach C, Skalej
M and Firsching R: Gliomas in adults. Dtsch Arztebl Int.
107:799–807; quiz 808. 2010.PubMed/NCBI
|
|
4
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chamberlain MC: Temozolomide: Therapeutic
limitations in the treatment of adult high-grade gliomas. Expert
Rev Neurother. 10:1537–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen M, Kawamoto T, Yan W, Nakamasu K,
Tamagami M, Koyano Y, Noshiro M and Kato Y: Molecular
characterization of the novel basic helix-loop-helix protein DEC1
expressed in differentiated human embryo chondrocytes. Biochem
Biophys Res Commun. 236:294–298. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boudjelal M, Taneja R, Matsubara S,
Bouillet P, Dolle P and Chambon P: Overexpression of Stra13, a
novel retinoic acid-inducible gene of the basic helix-loop-helix
family, inhibits mesodermal and promotes neuronal differentiation
of P19 cells. Genes Dev. 11:2052–2065. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossner MJ, Dörr J, Gass P, Schwab MH and
Nave KA: SHARPs: Mammalian enhancer-of-split- and hairy-related
proteins coupled to neuronal stimulation. Mol Cell Neurosci.
10:460–475. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhawal UK, Sato F, Arakawa Y, Fujimoto K,
Kawamoto T, Tanimoto K, Ito Y, Sasahira T, Sakurai T, Kobayashi M,
et al: Basic helix-loop-helix transcription factor DEC1 negatively
regulates cyclin D1. J Pathol. 224:420–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun H and Taneja R: Stra13 expression is
associated with growth arrest and represses transcription through
histone deacetylase (HDAC)-dependent and HDAC-independent
mechanisms. Proc Natl Acad Sci USA. 97:4058–4063. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhang H, Xie M, Hu M, Ge S, Yang D,
Wan Y and Yan B: Abundant expression of DEC1/stra13/sharp2 in colon
carcinoma: Its antagonizing role in serum deprivation-induced
apoptosis and selective inhibition of procaspase activation.
Biochem J. 367:413–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thin TH, Li L, Chung TK, Sun H and Taneja
R: Stra13 is induced by genotoxic stress and regulates
ionizing-radiation-induced apoptosis. Embo Rep. 8:401–407. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ehata S, Hanyu A, Hayashi M, Aburatani H,
Kato Y, Fujime M, Saitoh M, Miyazawa K, Imamura T and Miyazono K:
Transforming growth factor-beta promotes survival of mammary
carcinoma cells through induction of antiapoptotic transcription
factor DEC1. Cancer Res. 67:9694–9703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y
and Yan B: The expression of antiapoptotic protein survivin is
transcriptionally upregulated by DEC1 primarily through multiple
sp1 binding sites in the proximal promoter. Oncogene. 25:3296–3306.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seimiya M, Bahar R, Wang Y, Kawamura K,
Tada Y, Okada S, Hatano M, Tokuhisa T, Saisho H, Watanabe T, et al:
Clast5/Stra13 is a negative regulator of B lymphocyte activation.
Biochem Biophys Res Commun. 292:121–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun H, Lu B, Li RQ, Flavell RA and Taneja
R: Defective T cell activation and autoimmune disorder in
Stra13-deficient mice. Nat Immunol. 2:1040–1047. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyazaki K, Miyazaki M, Guo Y, Yamasaki N,
Kanno M, Honda Z, Oda H, Kawamoto H and Honda H: The role of the
basic helix-loop-helix transcription factor Dec1 in the regulatory
T cells. J Immunol. 185:7330–7339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yun Z, Maecker HL, Johnson RS and Giaccia
AJ: Inhibition of PPAR gamma 2 gene expression by the
HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of
adipogenesis by hypoxia. Dev Cell. 2:331–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iizuka K and Horikawa Y: Regulation of
lipogenesis via BHLHB2/DEC1 and ChREBP feedback looping. Biochem
Biophys Res Commun. 374:95–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada K, Ogata-Kawata H, Matsuura K and
Miyamoto K: SHARP-2/Stra13/DEC1 as a potential repressor of
phosphoenolpyruvate carboxykinase gene expression. FEBS Lett.
579:1509–1514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakashima A, Kawamoto T, Honda KK, Ueshima
T, Noshiro M, Iwata T, Fujimoto K, Kubo H, Honma S, Yorioka N, et
al: DEC1 modulates the circadian phase of clock gene expression.
Mol Cell Biol. 28:4080–4092. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossner MJ, Oster H, Wichert SP, Reinecke
L, Wehr MC, Reinecke J, Eichele G, Taneja R and Nave KA: Disturbed
clockwork resetting in Sharp-1 and Sharp-2 single and double mutant
mice. PLoS One. 3:e27622008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Honma S, Kawamoto T, Takagi Y, Fujimoto K,
Sato F, Noshiro M, Kato Y and Honma K: Dec1 and Dec2 are regulators
of the mammalian molecular clock. Nature. 419:841–844. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakrabarti J, Turley H, Campo L, Han C,
Harris AL, Gatter KC and Fox SB: The transcription factor DEC1
(stra13, SHARP2) is associated with the hypoxic response and high
tumour grade in human breast cancers. Br J Cancer. 91:954–958.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng Y, Jia Y, Wang Y, Wang M, Li B, Shi
X, Ma X, Xiao D and Sun Y: The hypoxia-regulated transcription
factor DEC1 (Stra13, SHARP-2) and its expression in gastric cancer.
OMICS. 13:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ivanova AV, Ivanov SV,
Danilkovitch-Miagkova A and Lerman MI: Regulation of STRA13 by the
von Hippel-Lindau tumor suppressor protein, hypoxia, and the
UBC9/ubiquitin proteasome degradation pathway. J Biol Chem.
276:15306–15315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Turley H, Wykoff CC, Gatter KC and Harris AL: DEC1 (STRA13)
protein expression relates to hypoxia-inducible factor 1-alpha and
carbonic anhydrase-9 overexpression in non-small cell lung cancer.
J Pathol. 200:222–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L and Li QQ: Embryo-chondrocyte
expressed gene 1, downregulating hypoxia-inducible factor 1alpha,
is another marker of lung tumor hypoxia. Acta Pharmacol Sin.
28:549–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi XH, Zheng Y, Sun Q, Cui J, Liu QH, Qü
F and Wang YS: DEC1 nuclear expression: A marker of differentiation
grade in hepatocellular carcinoma. World J Gastroenterol.
17:2037–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qian Y, Zhang J, Yan B and Chen X: DEC1, a
basic helix-loop-helix transcription factor and a novel target gene
of the p53 family, mediates p53-dependent premature senescence. J
Biol Chem. 283:2896–2905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qian Y, Jung YS and Chen X: Differentiated
embryo-chondrocyte expressed gene 1 regulates p53-dependent cell
survival versus cell death through macrophage inhibitory
cytokine-1. Proc Natl Acad Sci USA. 109:11300–11305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sato F, Bhawal UK, Kawamoto T, Fujimoto K,
Imaizumi T, Imanaka T, Kondo J, Koyanagi S, Noshiro M, Yoshida H,
et al: Basic-helix-loop-helix (bHLH) transcription factor DEC2
negatively regulates vascular endothelial growth factor expression.
Genes Cells. 13:131–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ivanov SV, Salnikow K, Ivanova AV, Bai L
and Lerman MI: Hypoxic repression of STAT1 and its downstream genes
by a pVHL/HIF-1 target DEC1/STRA13. Oncogene. 26:802–812. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wykoff CC, Pugh CW, Maxwell PH, Harris AL
and Ratcliffe PJ: Identification of novel hypoxia dependent and
independent target genes of the von Hippel-Lindau (VHL) tumour
suppressor by mRNA differential expression profiling. Oncogene.
19:6297–6305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ivanova AV, Ivanov SV, Zhang X, Ivanov VN,
Timofeeva OA and Lerman MI: STRA13 interacts with STAT3 and
modulates transcription of STAT3-dependent targets. J Mol Biol.
340:641–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Reiser-Erkan C, Michalski CW,
Raggi MC, Quan L, Yupei Z, Friess H, Erkan M and Kleeff J: Hypoxia
inducible BHLHB2 is a novel and independent prognostic marker in
pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun.
401:422–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meyer SE, Hasenstein JR, Baktula A, Velu
CS, Xu Y, Wan H, Whitsett JA, Gilks CB and Grimes HL: Kruppel-like
factor 5 is not required for K-RasG12D lung tumorigenesis, but
represses ABCG2 expression and is associated with better
disease-specific survival. Am J Pathol. 177:1503–1513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Soon MS, Hsu LS, Chen CJ, Chu PY, Liou JH,
Lin SH, Hsu JD and Yeh KT: Expression of Krűppel-like factor 5 in
gastric cancer and its clinical correlation in Taiwan. Virchows
Arch. 459:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pazo CR and Antón A: Advanced
HER2-positive gastric cancer: Current and future targeted
therapies. Crit Rev Oncol Hematol. 85:350–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakamura H, Tanimoto K, Hiyama K, Yunokawa
M, Kawamoto T, Kato Y, Yoshiga K, Poellinger L, Hiyama E and
Nishiyama M: Human mismatch repair gene, MLH1, is transcriptionally
repressed by the hypoxia-inducible transcription factors, DEC1 and
DEC2. Oncogene. 27:4200–4209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sarkaria JN, Kitange GJ, James CD, Plummer
R, Calvert H, Weller M and Wick W: Mechanisms of chemoresistance to
alkylating agents in malignant glioma. Clin Cancer Res.
14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Taverna P, Liu L, Hanson AJ, Monks A and
Gerson SL: Characterization of MLH1 and MSH2 DNA mismatch repair
proteins in cell lines of the NCI anticancer drug screen. Cancer
Chemother Pharmacol. 46:507–516. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu L, Markowitz S and Gerson SL: Mismatch
repair mutations override alkyltransferase in conferring resistance
to temozolomide but not to 1,3-bis (2-chloroethyl) nitrosourea.
Cancer Res. 56:5375–5379. 1996.PubMed/NCBI
|
|
47
|
Hickman MJ and Samson LD: Role of DNA
mismatch repair and p53 in signaling induction of apoptosis by
alkylating agents. Proc Natl Acad Sci USA. 96:10764–10769. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Turley H, Wykoff CC, Troup S, Watson PH,
Gatter KC and Harris AL: The hypoxia-regulated transcription factor
DEC1 (Stra13, SHARP-2) and its expression in human tissues and
tumours. J Pathol. 203:808–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ali SS, Hsiao M, Zhao HW, Dugan LL, Haddad
GG and Zhou D: Hypoxia-adaptation involves mitochondrial metabolic
depression and decreased ROS leakage. PLoS One. 7:e368012012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oliva CR, Moellering DR, Gillespie GY and
Griguer CE: Acquisition of chemoresistance in gliomas is associated
with increased mitochondrial coupling and decreased ROS production.
PLoS One. 6:e246652011. View Article : Google Scholar : PubMed/NCBI
|