Introduction
Since the discovery of inhalation anesthetics and
their clinical application, researchers have gained an improved
understanding of ‘inhalation anesthetics and inhalation anesthesia’
(1). The advantages and
disadvantages of inhalation anesthetics are continuously being
elucidated, and inhalation anesthetics with obvious deficiencies
subsequently fall into disuse (2).
Recently, inhalation anesthesia has become the primary method for
general anesthesia (3). Inhalation
anesthetics are metabolized and decompose in organisms (4). The majority of inhalation anesthetics
can discharge through the lungs in their primary forms. Therefore,
they are safe and effective, and have a higher controllability
(5). However, researchers are
continuously trying to identify safer inhalation anesthetics
(6). Isoflurane is a type of
halocarbon inhalation anesthetic first identified in the 1970s,
which has a high efficiency and controllability, and serves an
important role in maintaining the effects of general anesthesia
(7,8).
Recent studies investigating the mechanisms of
neuron damage and protection in the central nervous system have
made significant progress (8,9).
These studies have provided information about how calcium channels,
the cell membrane potential and various transmitters influence
neuron damage and protection (10). Thus, the principles of general
inhalation anesthesia may be further elucidated, including the
clinical anesthesia phenomenon, and an increased understanding of
the unwanted effects of these may help to avoid their side
effects.
The state of consciousness is dependent on the
electrophysiological characteristics of the central nervous system
(11). The mechanisms of general
anesthesia by isoflurane involve the hyperpolarization of nerve
cells (12). However, a previous
study demonstrated that isoflurane can induce virulence in
different nerve cells (12). By
contrast, the pre-processing of isoflurane by nerve cells has also
been shown to exhibit neuroprotective effects, however, the
mechanisms involved in this process are currently unclear (13). Ca2+ serves an essential
role as a signaling molecule in nerve cells. An imbalance can
result in excess Ca2+, which may be caused by neurocyte
injury (14).
The transient receptor potential cation channel
subfamily V member 1 (TRPV1) receptor is universally expressed
across the central nervous system, including the hippocampus,
cerebral cortex and thalamus (15). During isoflurane-induced
neurotoxicity, cellular edema resulting from disrupted energy
metabolism may activate the TRPV1 receptor by altering the tension
of the cytomembrane (16). In
addition, lipid dysbolism of the cytomembrane occurs when the brain
is ischemic, which increases the levels of free arachidonic acid
(17). Oxygen and glucose
deprivation, due to pharmacon-mediated TRPV1 receptor inhibition,
leads to neuronal loss in region 1 of the hippocampus proper due to
cellular edema, which has protective functions (18).
Glutamate excitotoxity is considered to be the
primary mechanism underlying neuron injury induced by cerebral
ischemia (19). When
isoflurane-induced neurotoxicity occurs, a large quantity of
excitatory neurotransmitters are released from presynaptic
membranes due to disruption of metabolic cellular energy and the
depolarization of cytomembranes (20). This leads to the elimination of
glutamic acid, which accumulates between synaptic clefts. Glutamic
acid binds to and activates postsynaptic membranes, resulting in
the opening of N-methyl-D-aspartate (NMDA) ion channels, and an
excess influx of Ca2+ (21).
The rosaceous hawthorn plant is used as a digestion
aid in traditional Chinese medicine (22). Modern pharmacological methods have
discovered that flavonoid compounds extracted from Chinese hawthorn
leaves can regulate the lipid profile of blood, reduce blood
pressure, enhance the outflow volume of the extracorporeal coronary
artery, resist oxidation and protect the ischemic myocardium
(23). Vitexin is an active
compound extracted from hawthorn leaves, which has protective
functions during myocardial ischemia (24). A previous study demonstrated that
Chinese hawthorn leaves may possess cardiotonic, antianginal,
antiarrhythmic and antioxidative properties, and mitigate the
effects of acute myocardial ischemia (25). Therefore, the aim of the present
study was to investigate whether vitexin may also protect against
isoflurane-induced neurotoxicity.
Materials and methods
Rat details
A total of 30 male Sprague Dawley rats (250–300 g)
were obtained from the Experimental Animal Center of the Central
Hospital of Cangzhou (Hebei, China). The rats were maintained in 12
h dark/light cycles at 23±2°C with 55±5% humidity, and provided
with food and water ad libitum. The animal procedures used
in this study were approved by the Standing Committee on Animals at
The Central Hospital of Cangzhou.
Isoflurane and vitexin treatment of
rats and visualization of neuron cells
A total of 30 Sprague Dawley rats were separated at
random into the following five equally-sized treatment groups: i)
Control; ii) isoflurane-treated; and iii) 1 mg/kg; iv) 3 mg/kg; and
v) 10 mg/kg vitexin-treated groups, respectively. The isoflurane
and vitexin-treated groups were exposed to 1.4% isoflurane
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) in a 100%
oxygen environment for 2 h. Following isoflurane treatment, the
vitexin-treated group additionally received 1, 3 and 10 mg/kg
vitexin (Sigma-Aldrich; Merck Millipore) for 30 min. The rats were
euthanized using decollation under anesthesia. Samples of rat brain
tissue slices were fixed in 10% formalin buffer overnight and then
dehydrated using 90% ethanol for 1 h and 100% ethanol for 2 h. They
were subsequently cleared with xylene for 2 h and then embedded in
paraffin at 60°C.
Cell lines
Human PC12 pheochromocytoma neurosecretory cells
were cultured in high-glucose Dulbecco's modified Eagle's medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing
9% heat-inactivated fetal calf serum (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA), 100 U/ml penicillin, 100
µg/ml streptomycin, and 2 mM L-glutamine (Thermo Fisher Scientific,
Inc.), and were maintained in an incubator at 37°C in 5%
CO2 and with 95% humidity.
Cell treatment and viability
analysis
PC12 cells were seeded at a density of
1×104 cells/well in 96-well plates before they were
exposed to 2% isoflurane for 12 h and then cultured with 1, 10 and
100 µM vitexin for 24 h. MTT solution (Beyotime Institute of
Biotechnology, Haimen, China) was added into each well at a final
concentration of 0.5 mg/ml and cells were subsequently incubated at
37°C for 4 h. Dimethyl sulfoxide solution (98%; 150 µl; Sangon
Biotech Co., Ltd., Shanghai, China) was then added to each well.
The optical density (OD) was read at 570 nm using the Universal
Microplate Reader (Elx800; BioTek instruments, Inc., Winooki, VT,
USA).
Enzyme-linked immunosorbent assay
(ELISA)
PC12 cells were seeded at a density of
1×104 cells/well in 96-well plates before they were
exposed to 2% isoflurane for 12 h and then treated with 1, 10 and
100 µM vitexin for 24 h. PC12 cells were immediately collected and
centrifuged at 4,000 × g for 10 min. ELISA kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) were used to
measure serum tumor necrosis factor-α (TNF-α; cat. no. R019),
interleukin-6 (IL-6; cat. no. R016), glutathione synthetase (GSH;
cat. no. A005) and superoxide dismutase (SOD; cat. no. A001-1)
concentrations.
Western blot analysis
PC12 cells were seeded at a density of
2×106 cells/well in 6-well plates before they were
exposed to 2% isoflurane for 12 h and then treated with 1, 10 and
100 µM vitexin for 24 h. PC12 cells were subsequently harvested in
RIPA Lysis Buffer with protease inhibitors (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and total protein was
extracted by centrifuging at 12,000 × g for 10 min at 4°C,
and according to the manufacturer's instructions. Protein samples
(50 µg) were separated using 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked with 5% non-fat milk diluted in
tris-phosphate buffer containing 0.05% Tween 20 for 1 h, and
incubated overnight at 4°C with the following primary antibodies:
Polyclonal caspase-3 (cat. no. sc-56052; dilution, 1:1,000; Santa
Cruz Biotechnology, Inc.); β-secretase 1 (BACE; cat. no. sc-365948;
dilution, 1:1,000; Santa Cruz Biotechnology, Inc.); transient
receptor potential cation channel subfamily V member 1 (TRPV1; cat.
no. PAB27817; dilution, 1:1,000; Santa Cruz Biotechnology, Inc.);
glutamate ionotropic receptor NMDA type subunit 2B (NR2B; cat. no.
14544; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA); and GAPDH (cat. no. sc-365062; dilution, 1:10,000; Santa
Cruz Biotechnology, Inc.). The membranes were incubated with the
anti-mouse or anti-rabbit IgG-horseradish peroxidase-conjugated
secondary antibody, (cat. nos. SN133 and SN134, respectively;
dilution, 1:5,000; Sunshine Biotechnology Co., Ltd., Nanjing,
China), and bands were visualized using an enhanced
chemiluminescence method.
Reactive oxygen species (ROS)
measurement
PC12 cells were seeded at a density of
1×104 cells/well in 96-well plates before they were
exposed to 2% isoflurane for 12 h and treated with 1, 10 and 100 µM
vitexin for 24 h. PC12 cells were cultured with
2′,7′-dichlorofluorescein diacetate for 6 h, then incubated with
cell lysis buffer (OxiSelect ROS assay kit; Cell Biolabs, Inc., San
Diego, CA, USA) for 5 min at room temperature. The OD was read at
480/530 nm using the aforementioned microplate reader (Bio-Tek
instruments, Inc.).
Analysis of cytosolic calcium
levels
The levels of cytosolic calcium were determined as
described previously (26). PC12
cells were treated with isoflurane and vitexin using the
aforementioned procedures, before they were treated with Fura-2
(Invitrogen; Thermo Fisher Scientific, Inc.) and perfused with
Tyrode's buffer. The levels of cytosolic calcium were recorded
using a spectrofluoroscopy system (IonOptix, Westwood, MA, USA) at
340/380 nm.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Experiments for each treatment group was conducted in triplicate.
Statistical analyses were performed using the Student's t-test and
P<0.05 was considered to indicate a statistically significant
difference.
Results
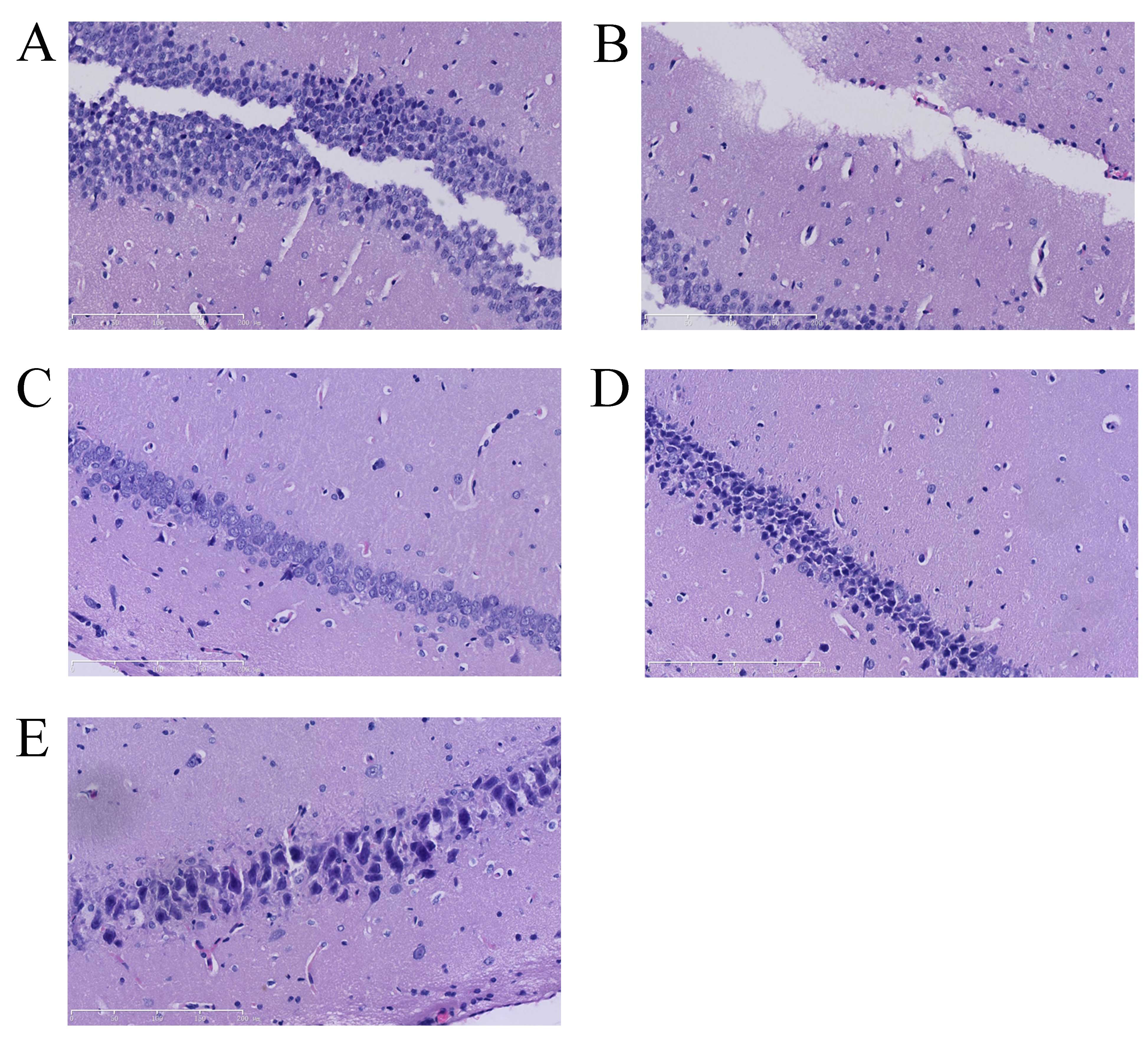
Vitexin protects against
isoflurane-induced neurotoxicity in rat brain tissue slices
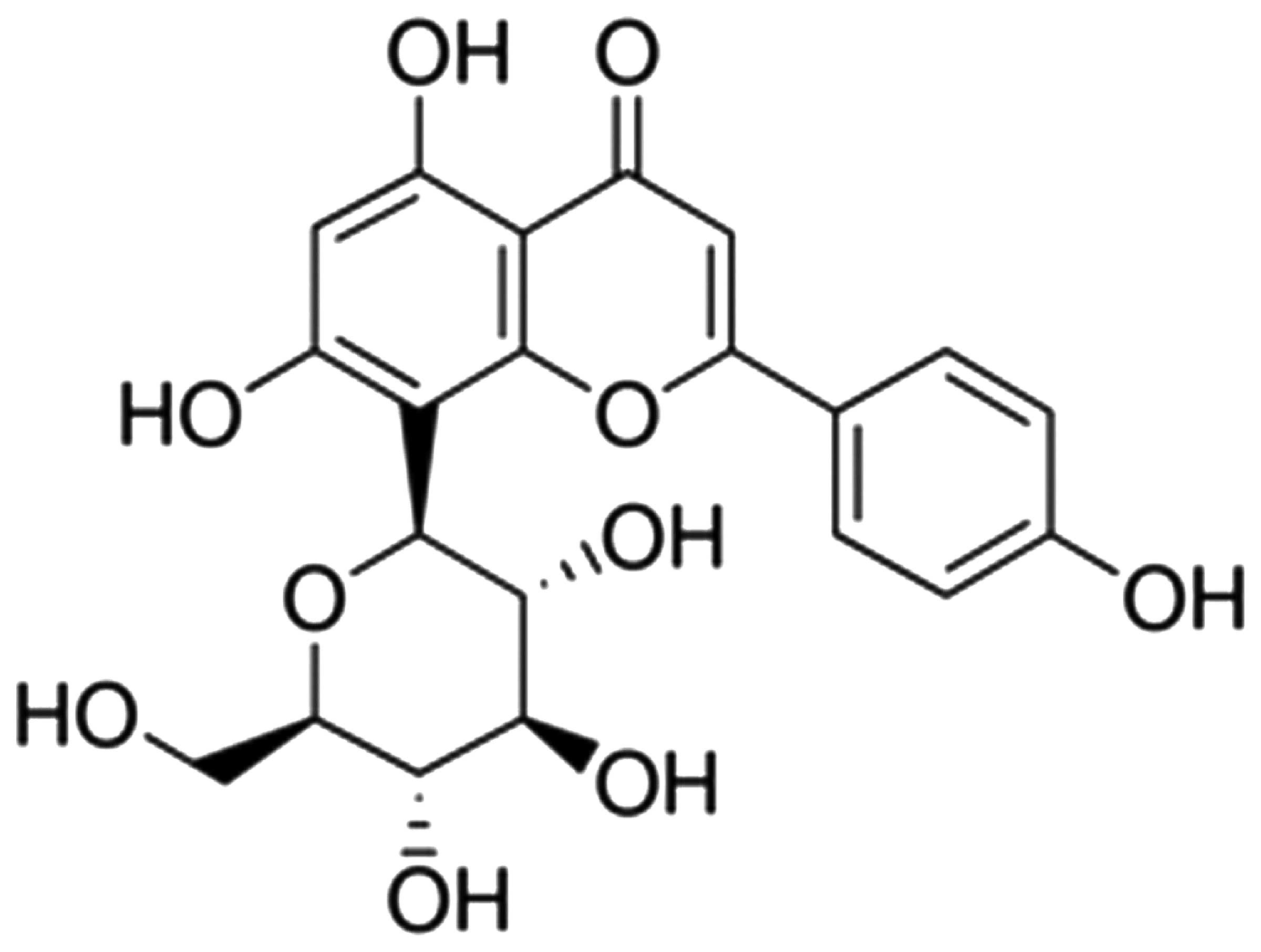
The chemical structure of vitexin is shown in
Fig. 1. Since isoflurane treatment
of rats results in neurotoxicity, the initial aim of this study was
to examine the potential neuroprotective effects of vitexin in
isoflurane-treated rats. The number of neuron cells in the control
group was markedly higher than that of the isoflurane-induced group
(Fig. 2A and B). A notable
increase in neuron cells was observed in the isoflurane plus
vitexin (10 mg/kg)-treated group compared with the
isoflurane-treated group (Fig. 2B and
E).
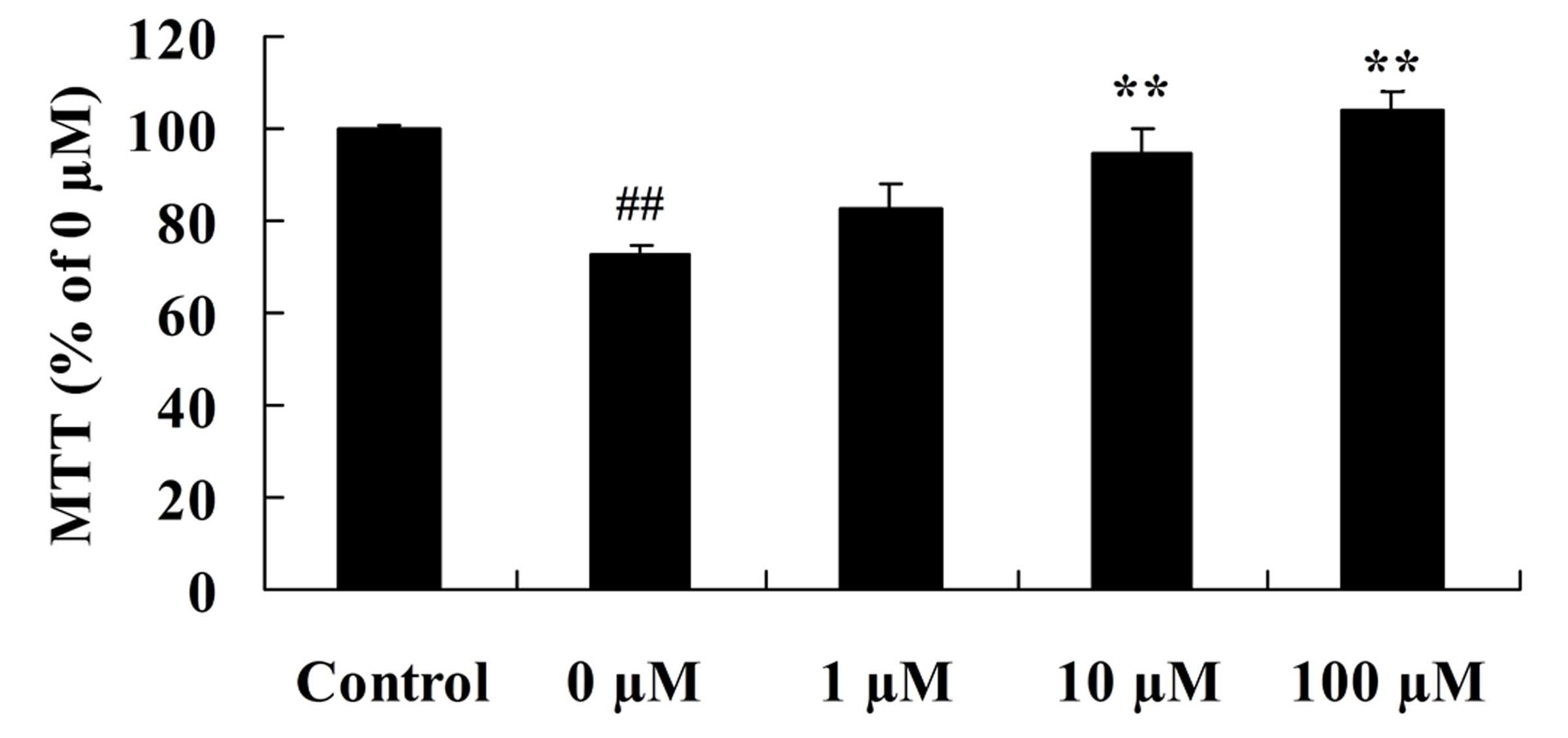
Vitexin increases the growth of
isoflurane-treated PC12 cells
To determine whether the neuroprotective effects of
vitexin following isoflurane treatment involves cell growth, the
growth of human PC12 pheochromocytoma neurosecretory cells
following treatment with isoflurane and vitexin was investigated.
As shown in Fig. 3, a significant
increase in the growth of PC12 cells was observed following
isoflurane plus 10 or 100 µM vitexin treatment, compared with
isoflurane treatment alone (P=0.0079 and 0.0021, respectively).
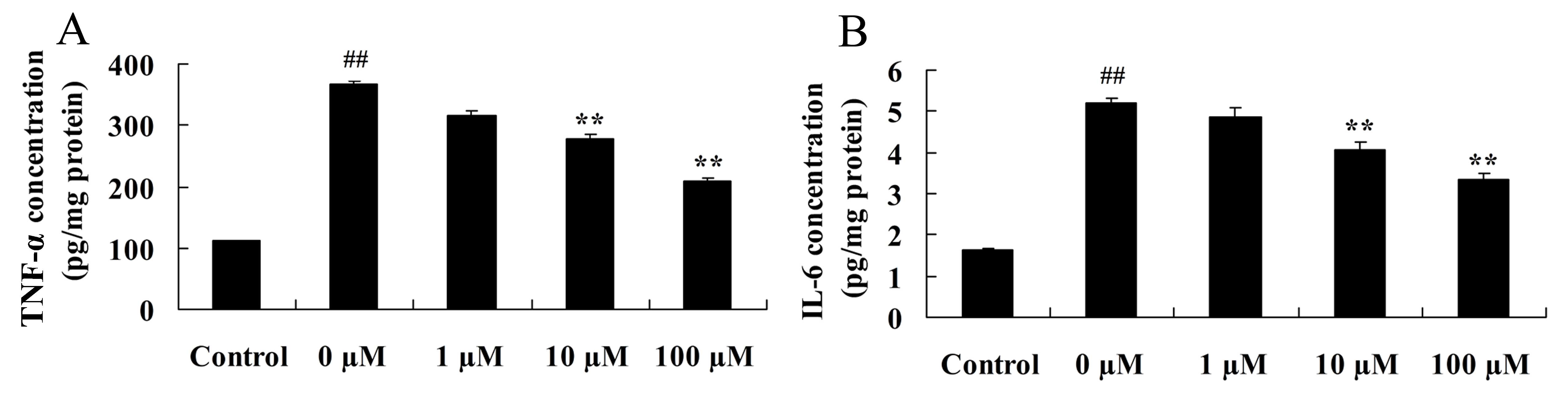
Vitexin prevents the activation of
isoflurane-induced inflammatory signaling pathways
Considering the key role of induced
neuroinflammation in aged rodents (27), the neuroprotective effects of
vitexin in inhibiting the level of pro-inflammatory cytokines,
TNF-α and IL-6, in isoflurane-treated PC12 cells was investigated
using ELISA. As shown in Fig. 4,
treatment of PC12 cells with isoflurane plus 10 or 100 µM vitexin,
significantly reduced TNF-α (P=0.0088 and 0.0038, respectively) and
IL-6 (P=0.0066 and 0.0049, respectively) protein levels compared
with isoflurane treatment alone.
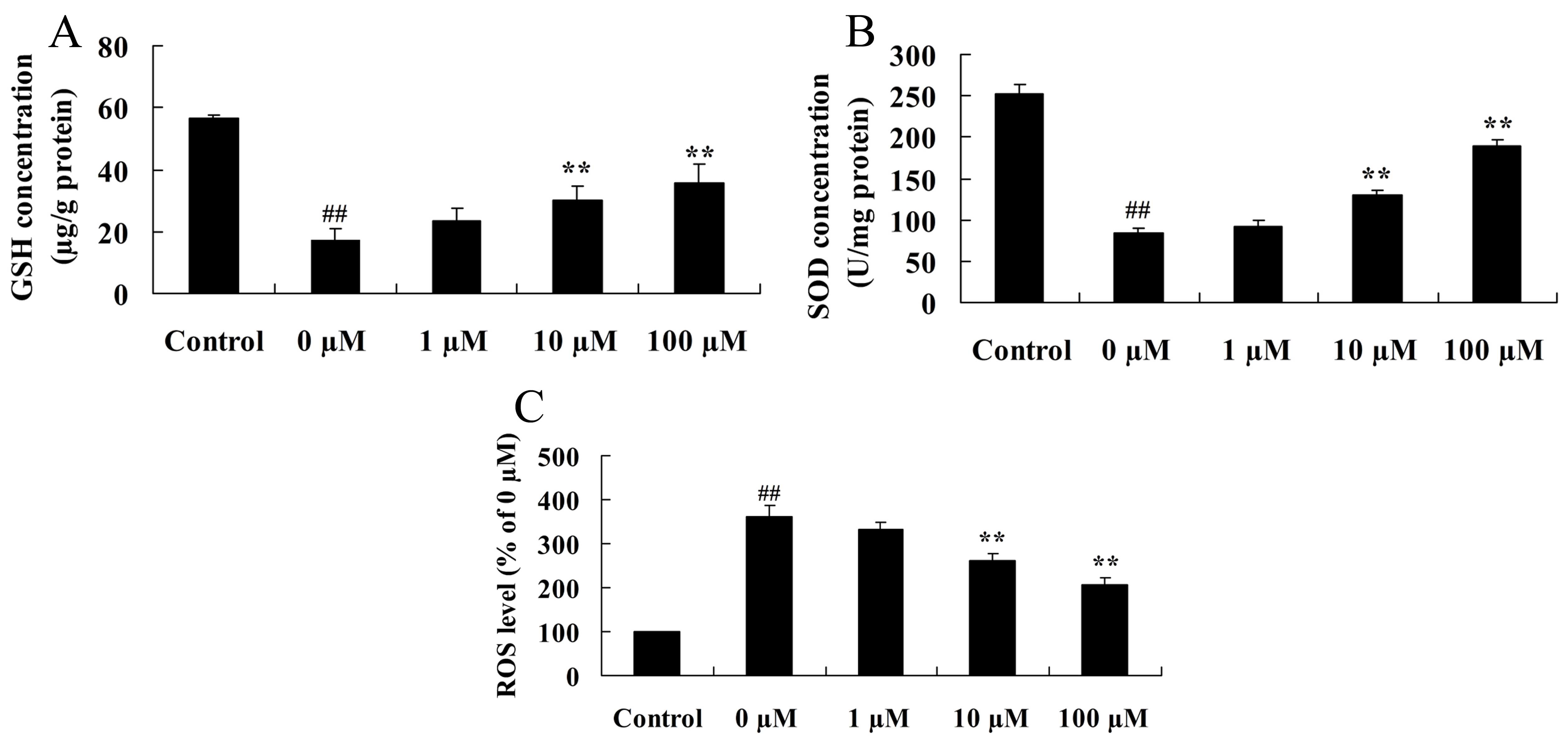
Vitexin protects against the
isoflurane-induced increase in oxidative stress
Considering the key role of oxidative stress in
isoflurane-induced PC12 cells, the neuroprotective effects of
vitexin against isoflurane-induced GSH and SOD protein expression
levels were investigated. As shown in Fig. 5A and B, isoflurane-induced GSH and
SOD concentrations in PC12 cells were significantly increased
following pretreatment of cells with 10 or 100 µM vitexin (GSH,
P=0.0069 and 0.0033; SOD, P=0.0059 and 0.0025 for 10 and 100 µM
vitexin, respectively). In addition, as shown in Fig. 5C, PC12 cells treated with
isoflurane plus 10 and 100 µM vitexin, demonstrated a significant
reduction in ROS levels compared with isoflurane-treated PC12 cells
(P=0.0041 and 0.0018, respectively).
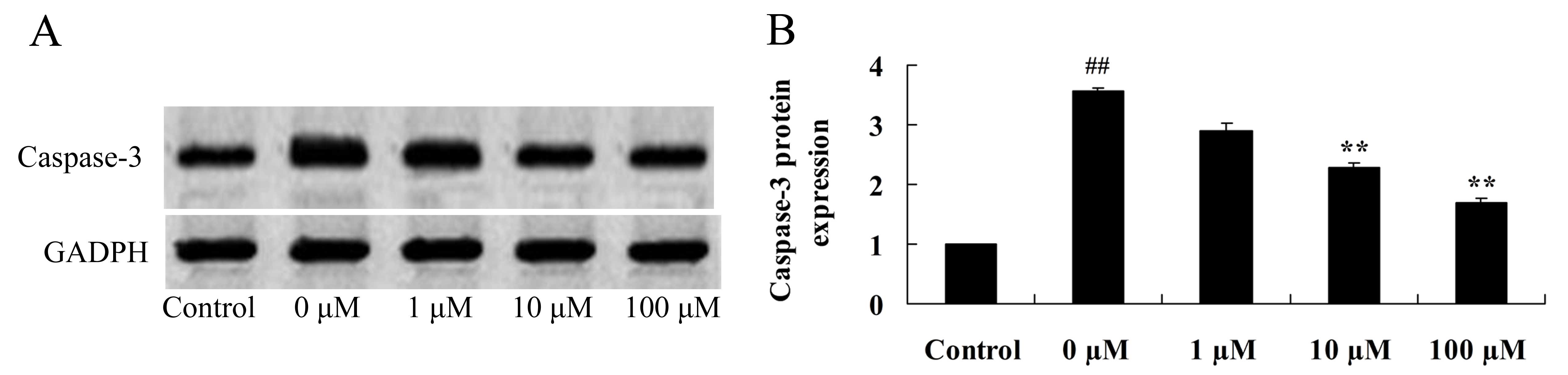
Vitexin protects against
isoflurane-induced caspase-3 activation
In order to investigate the neuroprotective effects
of vitexin against isoflurane-induced caspase-3 activation, the
protein expression levels of caspase-3 in isoflurane and
vitexin-treated PC12 cells were determined using western blot
analysis. Caspase-3 protein expression levels in isoflurane-treated
PC12 cells were significantly reduced following treatment with 10
and 100 µM vitexin (P=0.0069 and 0.0033, respectively; Fig. 6).
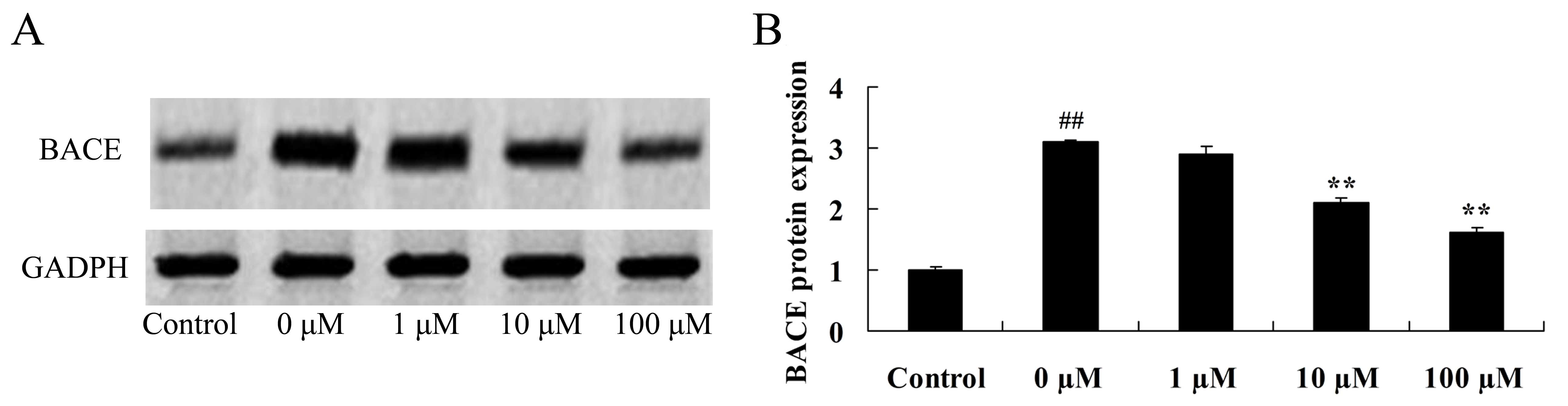
Vitexin protects against the
isoflurane-induced increase in BACE protein expression
A previous study demonstrated that BACE promotes
amyloid beta peptide production and affects the inhibition of
hypomnesis (28). Therefore, the
neuroprotective effect of vitexin against isoflurane-induced BACE
levels was investigated by evaluating BACE protein expression
levels in isoflurane plus vitexin-treated PC12cellsusing western
blot analysis. As demonstrated in Fig.
7, treatment of PC12 cells with 10 or 100 µM vitexin following
exposure to isoflurane, was associated with a significant reduction
in BACE protein expression levels (P=0.0042 and 0.0018,
respectively).
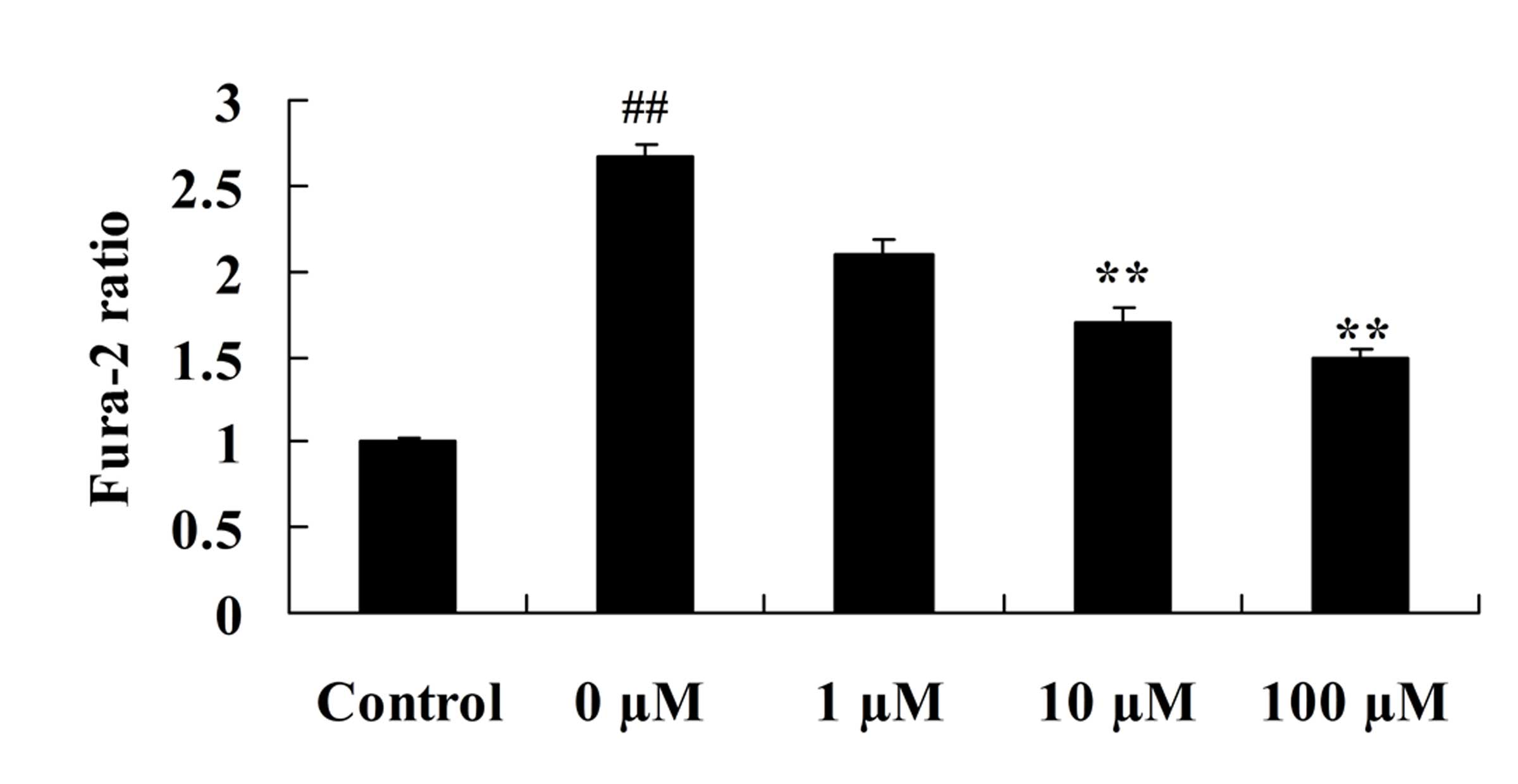
Vitexin protects against the
isoflurane-induced increase in cytosolic calcium levels
To investigate the role of vitexin in preventing the
isoflurane-induced increase in cytosolic calcium levels, the levels
of cytosolic calcium in isoflurane-induced PC12 cells following
vitexin treatment were investigated. As demonstrated in Fig. 8, cytosolic calcium levels in
isoflurane-induced PC12 cells were significantly reduced following
treatment with 10 and 100 µM vitexin (P=0.0031 and 0.0009,
respectively).
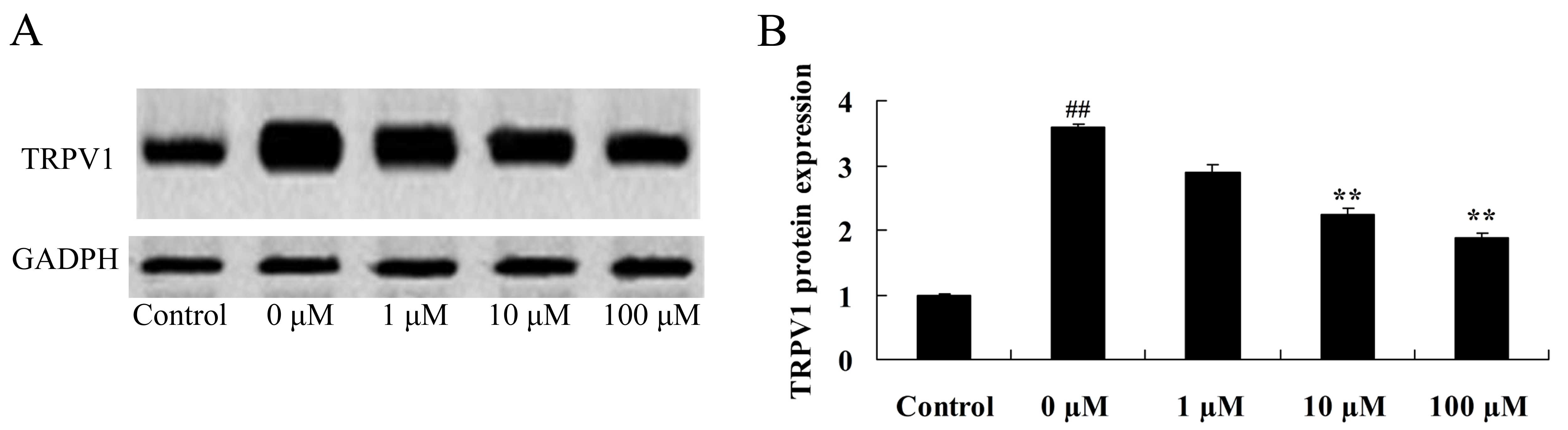
Vitexin protects against the
isoflurane-induced increase in TRPV1 expression levels
In order to further investigate the neuroprotective
role of vitexin in isoflurane-treated PC12 cells, TRPV1 protein
expression levels were examined in isoflurane-treated PC12 cells
following treatment with vitexin using western blot analysis. As
shown in Fig. 9, treatment with 10
and 100 µM vitexin significantly suppressedTRPV1 protein expression
in isoflurane-treated PC12cells (P=0.0023 and 0.0005,
respectively).
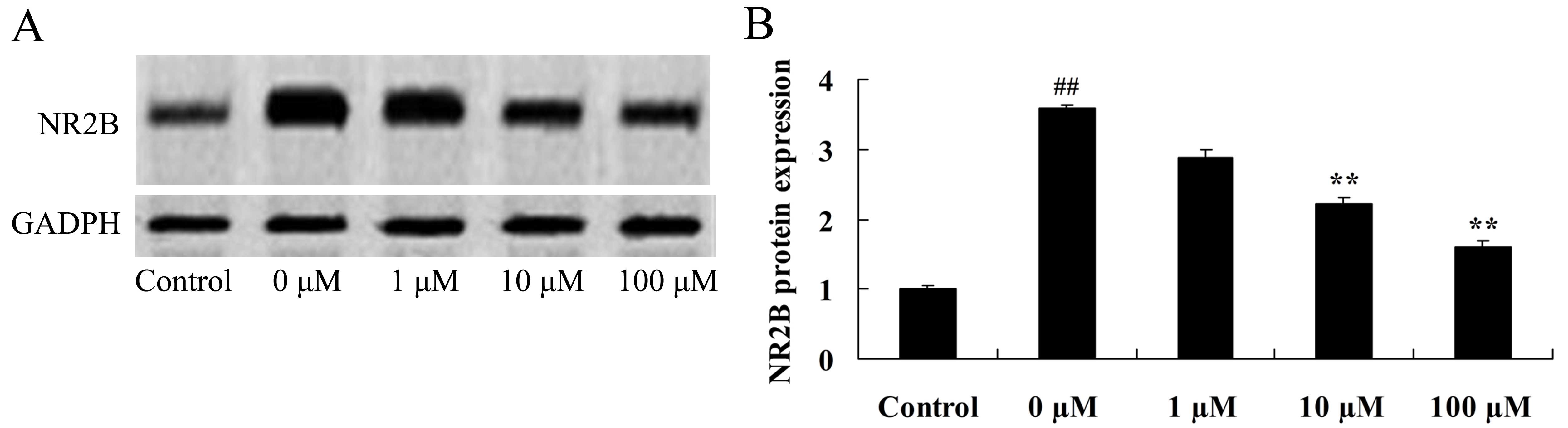
Vitexin protects against the
isoflurane-induced increase in NR2B expression levels
The NR2B subunit is a fundamental regulatory subunit
of the NMDA receptor and serves an important role in its structure
and function (29). Therefore, the
final aim of the study was to determine whether NR2B protein
expression is involved in mediating the neuroprotective effects of
vitexin in isoflurane-treated PC12 cells. As shown in Fig. 10, treatment of isoflurane-induced
PC12 cells with 10 and 100 µM vitexin significantly suppressed
isoflurane-induced NR2B protein expression levels (P=0.0045 and
0.0012, respectively).
Discussion
Consistent with other inhaled anesthetics of the
halogen family, isoflurane-mediated hyperpolarization of neurocytes
decreases the excitability of the neural network (30). Inhalation aesthetics at low
concentrations can noticeably inhibit the function of nicotinic
receptors (31). In the present
study, vitexin visibly increased the number of neuron cells in
isoflurane-treated rats. In addition, vitexin increased the growth
and reduced isoflurane-induced TNF-α, IL-6, GSH and SOD levels in
isoflurane-treated PC12 cells. Using a rat pup model, Min et
al (22) demonstrated that
vitexin reduces hypoxia-ischemia neonatal brain injury. Consistent
with these observations, Dong et al (32) demonstrated that vitexin protects
against myocardial ischemia/reperfusion injury through attenuating
the inflammatory response. Furthermore, Borghi et al
(33) observed that vitexin
inhibits inflammation-associated pain through TRPV1 and oxidative
stress.
Inhalation anesthetics used at concentrations higher
than the clinical range may induce the following effects:
Inhibition of the voltage susceptibility of Na+,
K+ and Ca2+, thereby reducing the
transmission of harmful ostensive stimuli; promote the
hyperpolarization of the cell membranes; relieve the overload of
Ca2+; analgesia; anesthesia; and protective cerebral
functions (34). Whether
neurocytes can be protected against neurotoxicity, and the
potential molecular and physiological mechanisms involved, has
drawn substantial attention (35).
A previous study demonstrated that isoflurane can induce
cytotoxicity in different neurocytes at different concentrations
and exposure times (36). The
results of the present study demonstrated that vitexin
significantly downregulated caspase-3 and BACE protein expression
levels, and reduced ROS and cytosolic calcium levels in
isoflurane-induced PC12 cells. Yang et al (23) provided evidence to suggest that
vitexin protected the PC12 cells against 20 h of
reoxygenation-induced injury, through a reduction in ROS production
and caspase 3/7 activities.
Ca2+ influx, mediated by the
voltage-gated calcium channel, is an important mechanism for
activating the presynaptic membrane (37). The TRPV4 receptor is a type of
Ca2+channel (24). A
previous study reported that isoflurane-induced neurotoxicity in
rats was associated with increased TRPV4 protein expression, which
may have led to the over-activation of the TRPV4 receptor (38). Furthermore, a TRPV4-mediated
Ca2+ influx was observed. Treatment with a TRPV4
receptor agonist was associated with increased Ca2+ and
enhanced excitability of cells. An increase in the cellular
excitability and the extension of depolarization may be responsible
for increasing Ca2+ influx further (39). Through the influx of
Ca2+, the TRPV4 receptor activity may promote the
release of presynaptic glutamic acid. Moreover, additional
receptors, such as the metabotropic glutamate receptor and nicotine
acetylcholine receptor, also participate in regulating the release
of glutamic acid from the presynaptic membrane (40). In the present study, vitexin
significantly suppressed the expression levels of TRPV1 protein in
isoflurane-induced PC12 cells. In addition, Borghi et al
(33) demonstrated that vitexin
inhibits inflammatory pain by targeting TRPV1 and oxidative stress
in mice.
In the nervous tissues of mammals, the functional
N-methyl-D-aspartate (NMDA) receptor consists of NR1 and NR2
subunits (41). The NR2B subunit
is a fundamental regulatory subunit of the NMDA receptor and serves
an important role in its structure and function (29). Phosphorylation of NR2B may increase
the opening rate and time of the NMDA receptor, resulting in
increased ion influx (42).
Selective NR2B subunit inhibitors prevent hypotonic-stimulated
enhancement of NMDA receptor activity whereas, selective NR2A
subunit inhibitors do not demonstrate any obvious alterations of
the NMDA receptor following hypotonic stimulation (43). This suggests that the NR2B subunit
may be an important target for the regulation of the NMDA receptor
through the TRPV4 receptor (44).
In the present study, vitexin significantly suppressed
isoflurane-induced NR2B protein expression levels in PC12 cells.
Consistent with these observations, Yang et al (45) reported that vitexin inhibited NMDA
receptor activity in cultured cortical neurons.
In conclusion, the present study demonstrated that
vitexin mediates neuroprotective effects against isoflurane-induced
neurotoxicity by targeting the TRPV1 and NR2B signaling pathways.
This suggests that vitexin may be a strong candidate as a
neurotoxicity drug. However, these results require validation in
further studies.
References
|
1
|
Qu X, Xu C, Wang H, Xu J, Liu W, Wang Y,
Jia X, Xie Z, Xu Z, Ji C, et al: Hippocampal glutamate level and
glutamate aspartate transporter (GLAST) are up-regulated in senior
rat associated with isoflurane-induced spatial learning/memory
impairment. Neurochem Res. 38:59–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka K, Kehl F, Gu W, Krolikowski JG,
Pagel PS, Warltier DC and Kersten JR: Isoflurane-induced
preconditioning is attenuated by diabetes. Am J Physiol Heart Circ
Physiol. 282:H2018–H2023. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benveniste H and Makaryus R: Are we moving
closer to noninvasive imaging and monitoring of neonatal
anesthesia-induced neurotoxicity? Anesthesiology. 125:22–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stary CM, Sun X and Giffard RG: Astrocytes
protect against isoflurane neurotoxicity by buffering
pro-brain-derived neurotrophic factor. Anesthesiology. 123:810–819.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An J, Stadnicka A, Kwok WM and Bosnjak ZJ:
Contribution of reactive oxygen species to isoflurane-induced
sensitization of cardiac sarcolemmal adenosine
triphosphate-sensitive potassium channel to pinacidil.
Anesthesiology. 100:575–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazoit JX, Roulleau P and Baujard C:
Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque
brain: Isoflurane or ischemia-reperfusion? Anesthesiology.
113:1245–1246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang J and Jiang H: Effect of the inhaled
anesthetics isoflurane, sevoflurane and desflurane on the
neuropathogenesis of Alzheimer's disease (review). Mol Med Rep.
12:3–12. 2015.PubMed/NCBI
|
|
8
|
Wei H: The role of calcium dysregulation
in anesthetic-mediated neurotoxicity. Anesth Analg. 113:972–974.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan C, Xu Z, Dong Y, Zhang Y, Zhang J,
McAuliffe S, Yue Y, Li T and Xie Z: The potential dual effects of
anesthetic isoflurane on hypoxia-induced caspase-3 activation and
increases in β-site amyloid precursor protein-cleaving enzyme
levels. Anesth Analg. 113:145–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jevtovic-Todorovic V, Kirby CO and Olney
JW: Isoflurane and propofol block neurotoxicity caused by MK-801 in
the rat posterior cingulate/retrosplenial cortex. J Cereb Blood
Flow Metab. 17:168–174. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bickler PE, Warren DE, Clark JP, Gabatto
P, Gregersen M and Brosnan H: Anesthetic protection of neurons
injured by hypothermia and rewarming: Roles of intracellular
Ca2+ and excitotoxicity. Anesthesiology. 117:280–292.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hara M, Zhou ZY and Hemmings HC Jr:
α2-adrenergic receptor and isoflurane modulation of presynaptic
Ca2+ influx and exocytosis in hippocampal neurons.
Anesthesiology. 125:535–546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bickler PE, Zhan X and Fahlman CS:
Isoflurane preconditions hippocampal neurons against oxygen-glucose
deprivation: Role of intracellular Ca2+ and
mitogen-activated protein kinase signaling. Anesthesiology.
103:532–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gemes G, Oyster KD, Pan B, Wu HE, Bangaru
ML, Tang Q and Hogan QH: Painful nerve injury increases plasma
membrane Ca2+-ATPase activity in axotomized sensory
neurons. Mol Pain. 8:462012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez JC, Lopez-Zapata DF and Wilkins
RJ: TRPV4 channels activity in bovine articular chondrocytes:
Regulation by obesity-associated mediators. Cell Calcium.
56:493–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jang Y, Jung J, Kim H, Oh J, Jeon JH, Jung
S, Kim KT, Cho H, Yang DJ, Kim SM, et al: Axonal
neuropathy-associated TRPV4 regulates neurotrophic factor-derived
axonal growth. J Biol Chem. 287:6014–6024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ciurtin C, Majeed Y, Naylor J, Sukumar P,
English AA, Emery P and Beech DJ: TRPM3 channel stimulated by
pregnenolone sulphate in synovial fibroblasts and negatively
coupled to hyaluronan. BMC Musculoskelet Disord. 11:1112010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heckel E, Boselli F, Roth S, Krudewig A,
Belting HG, Charvin G and Vermot J: Oscillatory flow modulates
mechanosensitive klf2a expression through trpv4 and trpp2 during
heart valve development. Curr Biol. 25:1354–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nemethova M, Talian I, Danielisova V,
Tkacikova S, Bonova P, Bober P, Matiasova M, Sabo J and Burda J:
Delayed bradykinin postconditioning modulates intrinsic
neuroprotective enzyme expression in the rat CA1 region after
cerebral ischemia: A proteomic study. Metab Brain Dis. Jul
8–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu W, Guo Q, Hu X, Peng L and Zhou B:
Induction of DJ-1 protects neuronal cells from isoflurane induced
neurotoxicity. Metab Brain Dis. 30:703–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang HC, Chang P, Lu SY, Zheng BW and
Jiang ZF: Protection of curcumin against amyloid-β-induced cell
damage and death involves the prevention from NMDA
receptor-mediated intracellular Ca elevation. J Recept Signal
Transduct Res. 35:450–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min JW, Hu JJ, He M, Sanchez RM, Huang WX,
Liu YQ, Bsoul NB, Han S, Yin J, Liu WH, et al: Vitexin reduces
hypoxia-ischemia neonatal brain injury by the inhibition of
HIF-1alpha in a rat pup model. Neuropharmacology. 99:38–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang ZB, Tan B, Li TB, Lou Z, Jiang JL,
Zhou YJ, Yang J, Luo XJ and Peng J: Protective effect of vitexin
compound B-1 against hypoxia/reoxygenation-induced injury in
differentiated PC12 cells via NADPH oxidase inhibition. Naunyn
Schmiedebergs Arch Pharmacol. 387:861–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edwards JE, Brown PN, Talent N, Dickinson
TA and Shipley PR: A review of the chemistry of the genus
Crataegus. Phytochemistry. 79:5–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhen Y, Wu X, Jiang Q, Li X, Chen
Z, Zhang G and Dong L: Vitexin protects brain against
ischemia/reperfusion injury via modulating mitogen-activated
protein kinase and apoptosis signaling in mice. Phytomedicine.
22:379–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Dong Y, Zhang G, Moir RD, Xia W,
Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE and Xie Z: The
inhalation anesthetic desflurane induces caspase activation and
increases amyloid beta-protein levels under hypoxic conditions. J
Biol Chem. 283:11866–11875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo X, Yang L, Chen X and Li S: Tau
hyperphosphorylation: A downstream effector of isoflurane-induced
neuroinflammation in aged rodents. Med Hypotheses. 82:94–96. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beckmann N, Doelemeyer A, Zurbruegg S,
Bigot K, Theil D, Frieauff W, Kolly C, Moulin P, Neddermann D,
Kreutzer R, et al: Longitudinal noninvasive magnetic resonance
imaging of brain microhemorrhages in BACE inhibitor-treated APP
transgenic mice. Neurobiol Aging. 45:50–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li C, Zhang N, Hu Y and Wang H: NR2B
overexpression leads to the enhancement of specific protein
phosphorylation in the brain. Brain Res. 1588:127–134. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su JY and Vo AC: Role of PKC in
isoflurane-induced biphasic contraction in skinned pulmonary
arterial strips. Anesthesiology. 96:155–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vahle-Hinz C, Detsch O, Siemers M and
Kochs E: Contributions of GABAergic and glutamatergic mechanisms to
isoflurane-induced suppression of thalamic somatosensory
information transfer. Exp Brain Res. 176:159–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong L, Fan Y, Shao X and Chen Z: Vitexin
protects against myocardial ischemia/reperfusion injury in
Langendorff-perfused rat hearts by attenuating inflammatory
response and apoptosis. Food Chem Toxicol. 49:3211–3216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Borghi SM, Carvalho TT, Staurengo-Ferrari
L, Hohmann MS, Pinge-Filho P, Casagrande R and Verri WA Jr: Vitexin
inhibits inflammatory pain in mice by targeting TRPV1, oxidative
stress and cytokines. J Nat Prod. 76:1141–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding W, Li Z, Shen X, Martin J, King SB,
Sivakumaran V, Paolocci N and Gao WD: Reversal of
isoflurane-induced depression of myocardial contraction by nitroxyl
via myofilament sensitization to Ca2+. J Pharmacol Exp
Ther. 339:825–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Q, Li K and Yao S: Effect of
inhalational anesthetics on cytotoxicity and intracellular calcium
differently in rat pheochromocytoma cells (PC12). J Huazhong Univ
Sci Technolog Med Sci. 28:104–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie Z, Dong Y, Maeda U, Moir R, Inouye SK,
Culley DJ, Crosby G and Tanzi RE: Isoflurane-induced apoptosis: A
potential pathogenic link between delirium and dementia. J Gerontol
A Biol Sci Med Sci. 61:1300–1306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harraz OF and Altier C: STIM1-mediated
bidirectional regulation of Ca(2+) entry through voltage-gated
calcium channels (VGCC) and calcium-release activated channels
(CRAC). Front Cell Neurosci. 8:432014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shan H, Messi ML, Zheng Z, Wang ZM and
Delbono O: Preservation of motor neuron Ca2+ channel
sensitivity to insulin-like growth factor-1 in brain motor cortex
from senescent rat. J Physiol. 553:49–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sturek M: Ca2+ regulatory
mechanisms of exercise protection against coronary artery disease
in metabolic syndrome and diabetes. J Appl Physiol (1985).
111:573–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng Y, Wang B, Du F, Li H, Wang S, Hu C,
Zhu C and Yu X: The involvement of PI3K-mediated and L-VGCC-gated
transient Ca2+ influx in 17β-estradiol-mediated
protection of retinal cells from H2O2-induced apoptosis with
Ca2+ overload. PLoS One. 8:e772182013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hogan-Cann AD and Anderson CM:
Physiological roles of non-neuronal NMDA receptors. Trends
Pharmacol Sci. 37:750–767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang XN, Li JM, Yang Q, Feng B, Liu SB,
Xu ZH, Guo YY and Zhao MG: Anti-apoptotic effects of hyperoside via
inhibition of NR2B-containing NMDA receptors. Pharmacol Rep.
62:949–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li XB, Guo HL, Shi TY, Yang L, Wang M,
Zhang K, Guo YY, Wu YM, Liu SB and Zhao MG: Neuroprotective effects
of a novel translocator protein (18 kDa) ligand, ZBD-2, against
focal cerebral ischemia and NMDA-induced neurotoxicity. Clin Exp
Pharmacol Physiol. 42:1068–1074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cho SI, Park UJ, Chung JM and Gwag BJ:
Neu2000, an NR2B-selective, moderate NMDA receptor antagonist and
potent spin trapping molecule for stroke. Drug News Perspect.
23:549–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang L, Yang ZM, Zhang N, Tian Z, Liu SB
and Zhao MG: Neuroprotective effects of vitexin by inhibition of
NMDA receptors in primary cultures of mouse cerebral cortical
neurons. Mol Cell Biochem. 386:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|