Introduction
Mechanical stress is important for functional
development and maintenance of animal tissues. In various cases,
organs and tissues adapt their morphology and function in response
to acute or chronic mechanical stress, for example, pressure
overload leads to cardiovascular hypertrophy (1) and excess mechanical stress may alter
cartilaginous composition and metabolism, resulting in
osteoarthritis (2). Certain
epidemiological investigations have demonstrated that long-lasting
mechanical stress due to a history of vaginal delivery, pregnancy
and high abdominal pressure may be the most common risk factor of
pelvic organ prolapse (POP) (3,4).
Oxidative stress is a harmful imbalance between the production and
the removal of free radicals, including reactive oxygen species
(ROS) and lipid peroxidation end products (5). Currently, there appears to be
evidence that the occurrence of POP may be associated with the
imbalance of the oxidation-reduction equilibrium in vivo
(6,7) and remodeling of the extracellular
matrix (ECM), but this has not been not clearly demonstrated. When
fibroblasts react to changes in mechanical stress, components of
the ECM are also altered, particularly when a certain degree of
force is reached, which may induce oxidative damage and
subsequently activate the antioxidation system (8).
Transforming growth factor-β1 (TGF-β1) has been
reported to be a profibrogenic cytokine that contributes to
multiple forms of fibrosis, including cardiac fibrosis associated
with heart failure (9,10). Certain evidence suggests that
mechanical stress may induce the release of TGF-β1 (11). TGF-β1 may alter the balance between
ECM synthesis and degradation in numerous processes, including
tissue injury (12) and pulmonary
fibrosis (13). Previous studies
have demonstrated that remodeling of connective tissues may
contribute to the pathogenesis of POP (14,15).
The present study hypothesized that the TGF-β1
pathway, along with oxidative stress, is associated with remodeling
of the ECM induced by mechanical stretch. Human uterosacral
ligament fibroblasts (hUSLFs) treated with mechanical stress and
hydrogen peroxide were cultured in order to elucidate the
association between mechanical stress and the TGF-β1 signaling
pathway and to define the role of oxidative stress in the
remodeling of the ECM.
Materials and methods
Ethics statement
Human samples used in the present study were
obtained according to the principles expressed in the Declaration
of Helsinki, and were approved by the Institutional Review Boards
of the Renmin Hospital of Wuhan University (Wuhan, China). Written
informed consent was obtained from the patients.
Clinical specimens
A cohort of 15 patients who had undergone a total
vaginal hysterectomy due to benign uterine disease at Renmin
Hospital of Wuhan University had samples of the uterosacral
ligament tissues collected. Inclusion criteria were as follows: i)
Patients who had not taken any oral estrogen for at least 3 months;
ii) patients who were not suffering from estrogen-responsive
diseases, including endometriosis; and iii) patients without
diabetes and cardiovascular disease.
Cell culture
A modified enzyme digestion method was used to
obtain hUSLFs. The tissues (0.5×0.5×0.2 cm) were placed into
Dulbecco's modified Eagle's medium (DMEM; Genom Hangzhou, China)
immediately following separation during surgery, and were then
taken to the laboratory for exposure to a 4°C environment within 30
min. The tissues were washed with phosphate-buffered saline (PBS;
Genom) containing 100 U/ml penicillin G and 100 mg/ml streptomycin
(Genom), and were then cut into small pieces. The tissues were then
digested with 1% collagenase I (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 3 h at 37°C in 5%
CO2, followed by further digestion with 0.25% trypsin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 5 min.
Fetal bovine serum (FBS; Hyclone, GE Healthcare, Logan, UT, USA)
was used to terminate the digestion. DMEM containing 15% FBS was
then slowly added to the culture flask. The medium was replaced
every two days and the primary hUSLFs cultures were grown to
confluence for passage. Fibroblasts at passage 2–3 were used.
Mechanical stress
The fibroblasts were loaded with mechanical stress
by a four-point bending device (Chengdu Miracle Chemicals Co.,
Ltd., Chengdu, China) composed of three sections, including a
mechanical system, main engine and strain loading plate. A cell
suspension containing fibroblasts was produced with DMEM containing
10% FBS, following digestion by trypsin and EDTA (Sigma-Aldrich;
Merck Millipore). Cell suspension (2 ml) was added onto a plate
that had been preprocessed using rat tail collagen and placed into
an 11 mm culture dish, which was incubated at a temperature of 37°C
with 5% CO2 for a minimum of 24 h. Following cell
adherence and subconfluence, the cell plate was put into the strain
loading plate under a loading strain of 0, 1,333 (1 mm) or 5,333 µ
(4 mm) at a frequency of 0.1 Hz, for 4 h.
Hydrogen peroxide treatment
Fibroblasts at passage 4–6 were manipulated to cell
suspension with DMEM containing 10% FBS, following digestion by
trypsin. Cells were plated in 6-well plates and grown overnight at
37°C to 80–90% confluence. Fibroblasts were incubated with
H2O2 at a concentration of 0, 0.2, 0.4 and
0.8 mmol/l for 4 h at 37°C under 5% CO2.
Cell proliferation detection
Following exposure to mechanical strain and hydrogen
peroxide, a Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Shanghai, China) was used to count the cells and
detect their magnitude of proliferation. Coverslips and culture
plates were then washed three times with PBS. The cells then
underwent digestion with 0.25% trypsin and EDTA, followed by the
addition of DMEM containing 15% FBS to the cell precipitation. The
cells were subsequently centrifuged at 125 × g for 5 min at
37°C. Cell concentration was adjusted to 2 million/ml and 100 µl
cell suspension was added to a 96-well plate. After 12–24 h of
incubation, CCK-8 solution (10 µl/well) was added to each well and
incubated for 2 h. The optical density at a wavelength of 450 nm
was detected using an enzyme-labeled instrument.
Detection of intracellular ROS
Cellular ROS production was determined
fluorometrically using dichlorofluorescein (DCF) diacetate as a
fluorescent probe. Following administration of specific treatments,
the cells were incubated with the probe for 60 min at 37°C in the
dark, and they were then washed and resuspended in
phosphate-buffered saline. The fluorescence emitted at a wavelength
of 488 nm was measured with a fluorescence microscope. The values
were expressed as a percentage of the fluorescence measured in the
control, and ROS levels were expressed as a percentage of that in
the control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells following
treatment, using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. For
mRNA analysis, cDNA was amplified from 2.0 µg total RNA in a final
volume of 20 µl using Revert Aid™ First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.). Human GAPDH was
amplified as an internal control. The RT-qPCR reaction was
performed using Takara SYBR Premix ExTaq system (Takara Bio, Inc.,
Otsu, Japan). qPCR was performed as follows: 30 sec at 95°C; 40
cycles of 5 sec at 95°C, and 34 sec at 60°C; 15 sec at 95°C, 1 min
at 60°C, 15 sec at 95°C and 15 sec at 60°C. The ABI 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used. The data was processed using 2−ΔΔCq
method relative to GAPDH (16).
Primer sequence information is listed in Table I.
 | Table I.Primer sequences used for polymerase
chain reaction. |
Table I.
Primer sequences used for polymerase
chain reaction.
| Gene | Sequence
(5′-3′) |
|---|
| COL1A1 | F:
CAAGACGAAGACATCCCACCAATC |
|
| R:
ACAGATCACGTCATCGCACAACA |
| COL3A1 | F:
TCGCTCTGCTTCATCCCACTAT |
|
| R:
CTTCCAGACATCTCTATCCGCAT |
| Elastin | F:
TGTCCATCCTCCACCCCTCT |
|
| R:
CGGTCGTAGTCCTCAGTGGT |
| MMP-2 | F:
AGTTTCCATTCCGCTTCCAG |
|
| R:
CGGTCGTAGTCCTCAGTGGT |
| TIMP-2 | F:
TCTGGAAACGACATTTATGG |
|
| R:
GTTGGAGGCCTGCTTATGGG |
| TGF-β1 | F:
TATTGAGCACCTTGGGCACT |
|
| R:
ACCTCTCTGGGCTTGTTTCC |
| GAPDH | F:
GAAGGTGAAGGTCGGAGTC |
|
| R:
GAAGATGGTGATGGGATTTC |
Western blot analysis
Following washing in cold PBS, the harvested cells
were lysed on ice for 30 min in 100 mmol/l lysis buffer. Extracts
were centrifuged at 16,099 × g at 37°C for 15 min. The
supernatant was collected as the total cellular protein extract.
The protein concentrations were determined using a bicinchoninic
acid protein assay kit. For western blot analysis, equal quantities
of protein (35 µg) were run in each lane on a Tris-glycine gel
using 10% SDS-PAGE. Following electrophoresis, the proteins were
transferred to a polyvinylidene difluoride membrane and then the
membranes were probed with the following primary antibodies
overnight at 4°C: Anti-collagen, type 1 α1 chain (COL1A1; 1:400;
cat. no. sc-8784; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-collagen type 3 α1 chain (COL3A1; 1:400; cat. no. sc-28888;
Santa Cruz Biotechnology, Inc.), anti-matrix metalloproteinase-2
(MMP-2; 1:400; cat. no. sc-10736; Santa Cruz Biotechnology, Inc.),
anti-TIMP metallopeptidase inhibitor 2 (TIMP-2; 1:500; cat. no.
sc-5539; Santa Cruz Biotechnology, Inc.), anti-TGF-β1 (1:1,000;
cat. no. ab92486; Abcam, Cambridge, UK), anti-mothers against
decapentaplegic homolog 2 (Smad2; 1:500; cat. no. ab63576; Abcam),
anti-phosphorylated (p)-Smad2 (1:300; cat. no. ab53100; Abcam) and
anti-GAPDH (1:1,000; cat. no. sc-20357; Santa Cruz Biotechnology,
Inc.). Subsequent to washing in TBST, the membrane was incubated
with diluted horseradish peroxidase-conjugated secondary antibodies
IRDye 800CW goat anti-rabbit and goat anti-mouse secondary
antibodies (diluted 1/10,000, cat. nos. P/N 925–32211 and P/N
925-32210, LI-COR Biosciences, Ltd., Lincoln, NE, USA) at 37°C for
1 h. The Odyssey Imaging system (LI-COR Biosciences, Ltd.) was used
for quantification of proteins. Experiments were performed three
times obtaining similar results.
Statistical analysis
Statistical analyses were performed with SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). Data are presented as the
mean ± standard deviation, and depict the average of at least 3
independent experiments. All experiments were analyzed with
independent samples t-test and 95% confidence interval was used.
Dunnett's T3 test was used for the unequal variances. P<0.05 was
considered to indicate a statistically significant difference and
P<0.01 was considered highly significant.
Results
Effect of mechanical stress on
oxidation-antioxidation balance of hUSLFs
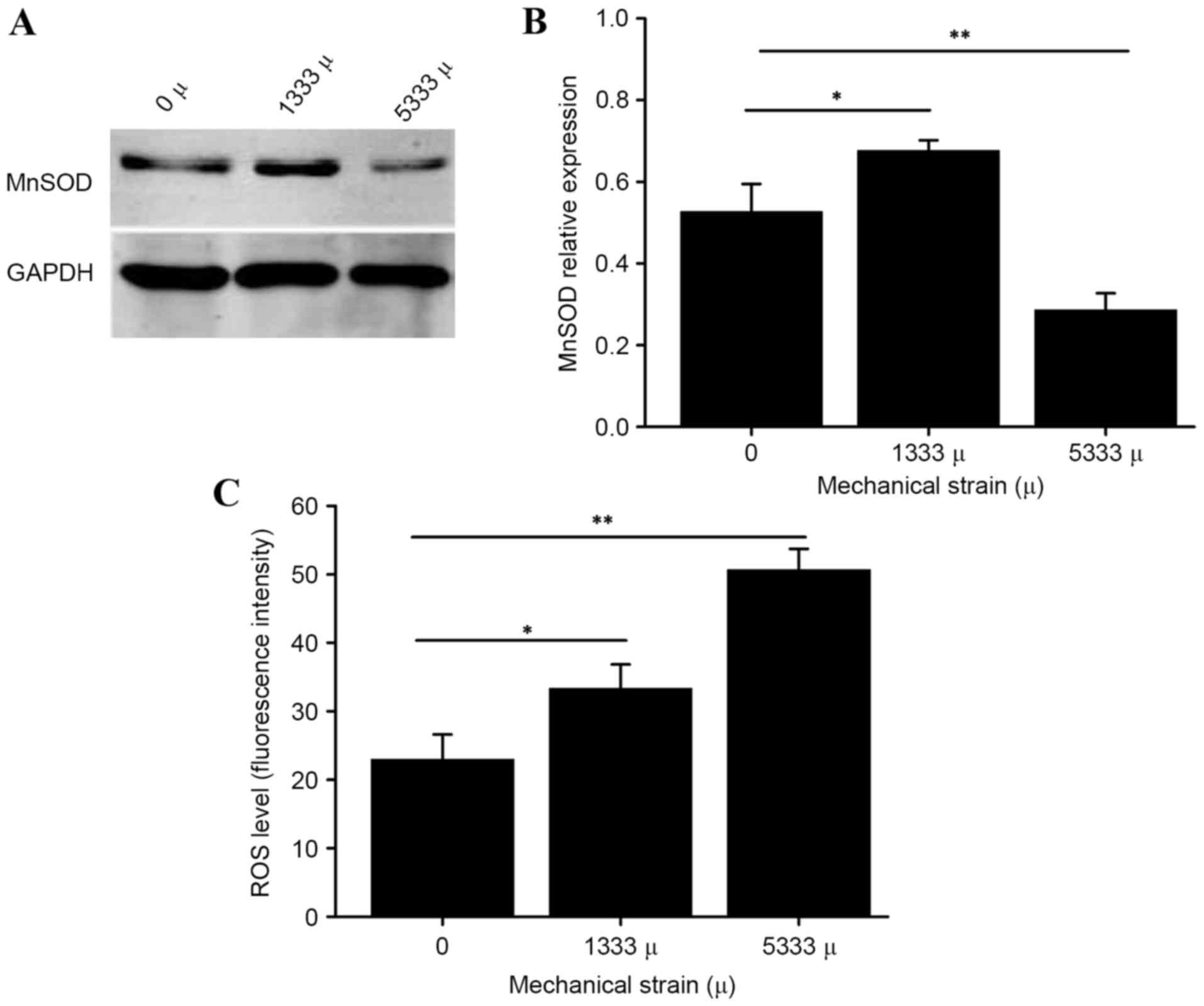
As presented in Fig.
1, using DCF fluorescence labeling, the present study observed
an increase in intracellular ROS triggered by mechanical stress.
Furthermore, following loading of 5,333 µ mechanical stress, the
manganese superoxide dismutase (MnSOD) protein expression levels in
hUSLFs were lower than those of the control group, (P<0.01)
while increased with the strain of 1,333 µ (P<0.05), suggesting
that mechanical stress has an effect on oxidation-antioxidation
products in parametrial ligament fibroblasts. hUSLFs were then
incubated with different concentrations of hydrogen peroxide in the
following experiments to investigate potential associations.
Effect of mechanical stress and
hydrogen peroxide on proliferation of hUSLFs
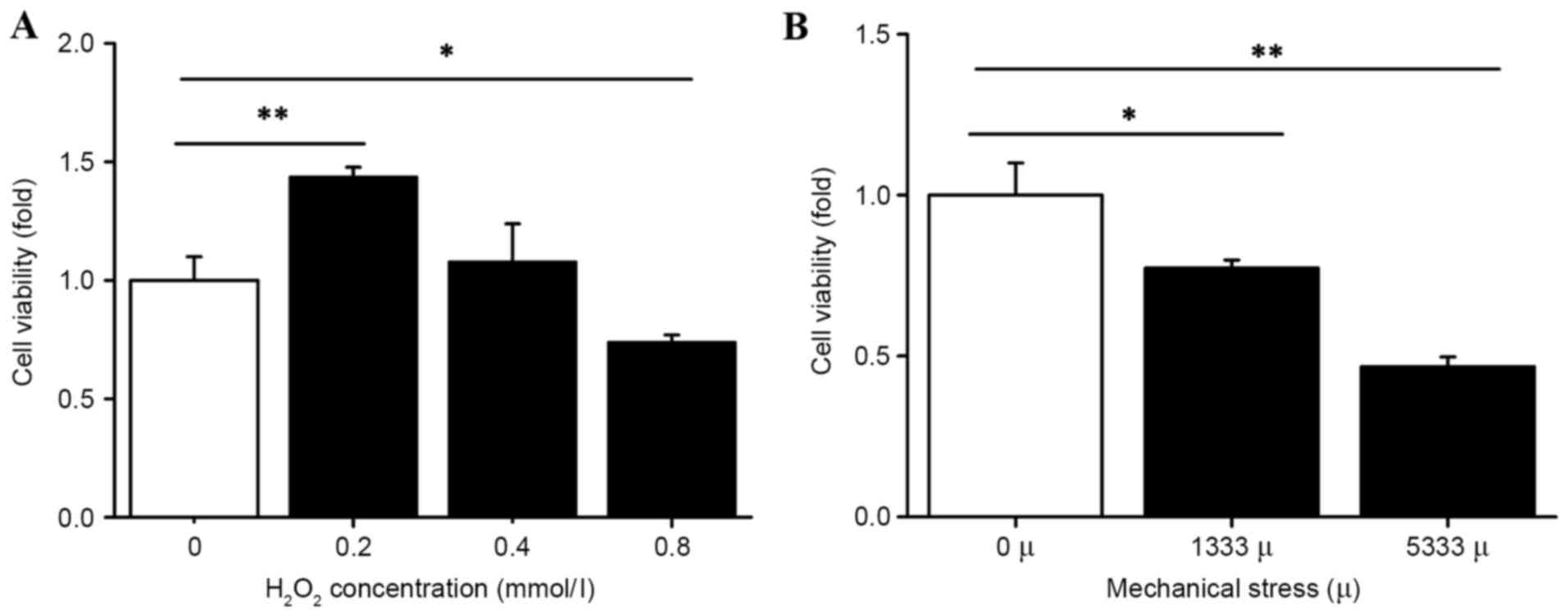
The present study assessed the effect of mechanical
stress and H2O2 on hUSLFs by measuring cell
viability with a CCK-8 assay. As presented in Fig. 2, H2O2
treatment was administered at a range of 0.2 to 0.8 mmol/l. Cell
proliferation was significantly inhibited at the high concentration
of 0.8 mmol/l (P<0.05) and concentrations of 0.2 mmol/l
(P<0.01) and 0.4 mmol/l promoted cell proliferation.
Furthermore, the cell viability decreased under the
influence of mechanical force. The group with strains of 1,333 and
5,333 µ indicated lower cell viability compared with the control
group, (P<0.05 and P<0.01, respectively; Fig. 2) demonstrating that the cell
viability gradually decreased with the increase in mechanical
force.
Effects of mechanical stress and
hydrogen peroxide on protein and mRNA expression levels of ECM
components
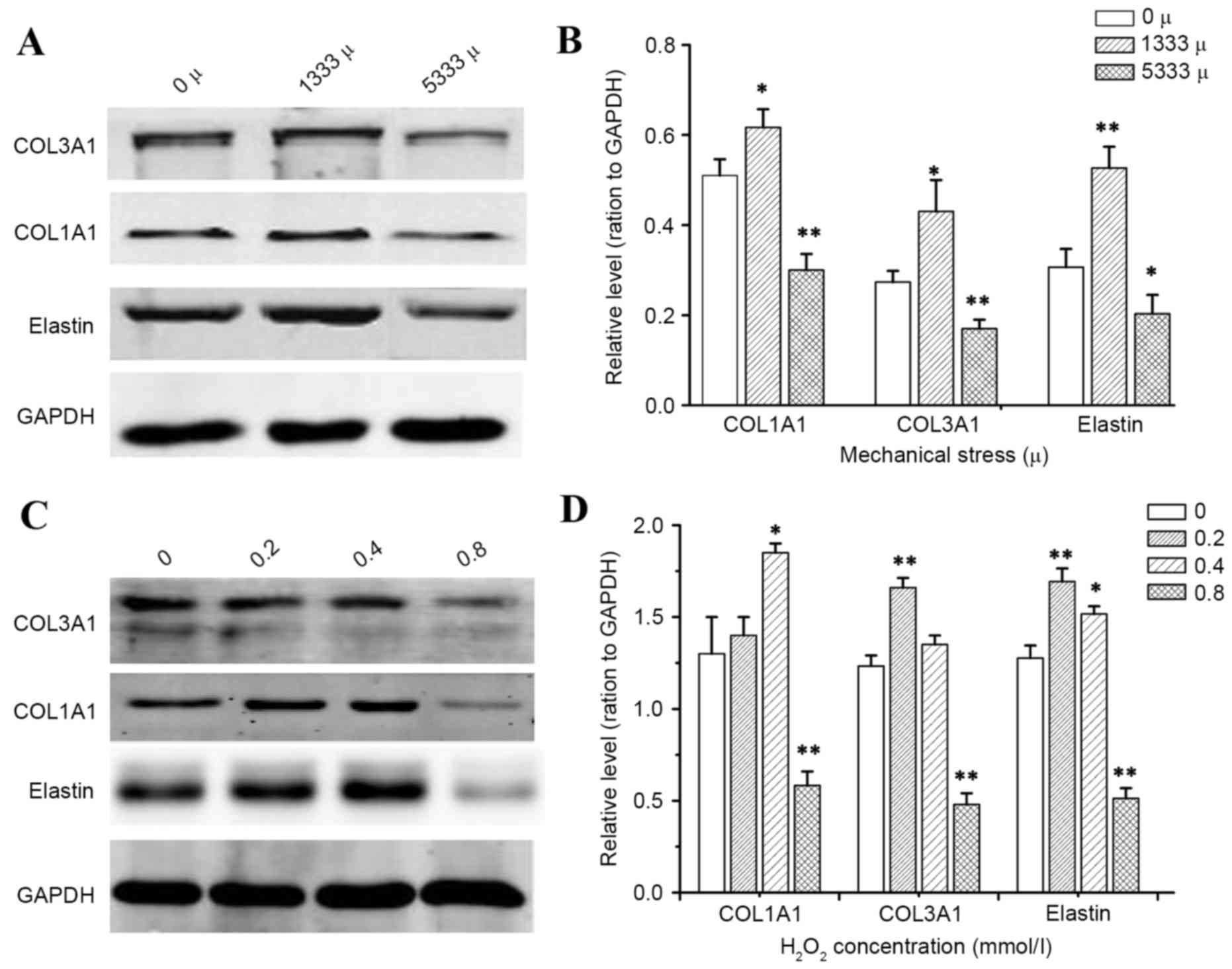
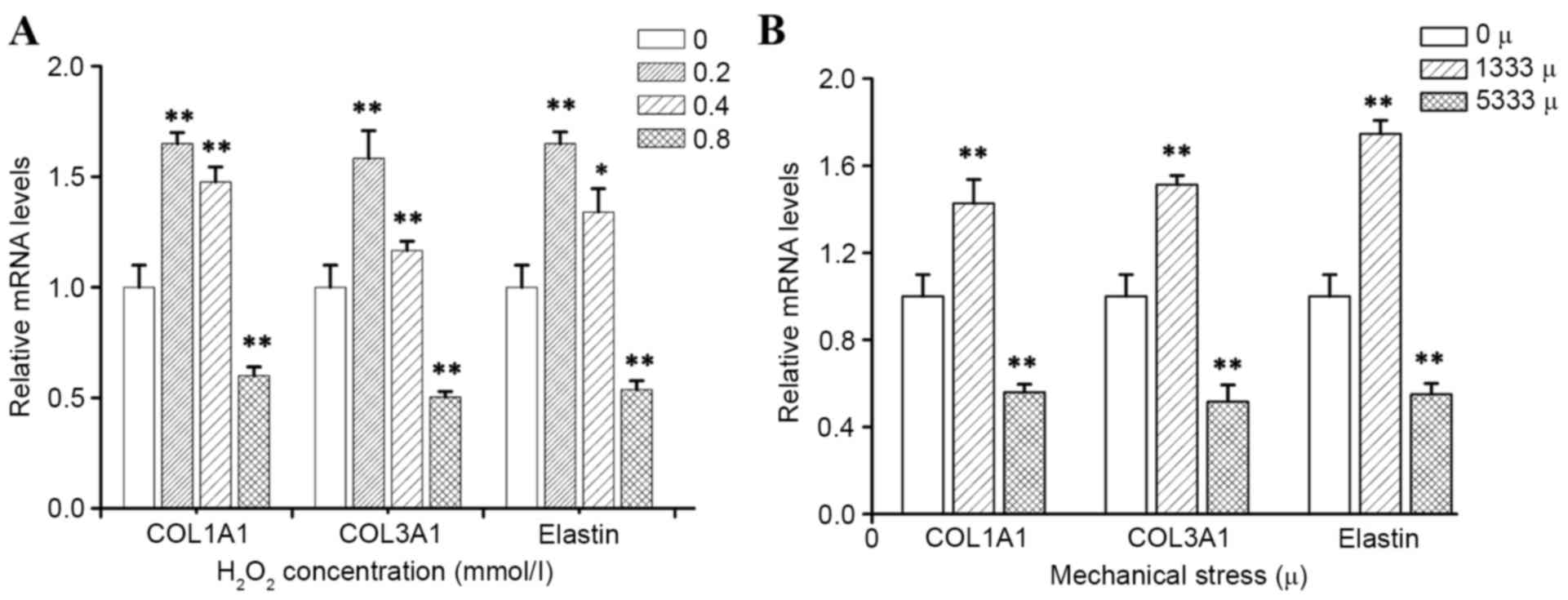
To determine how mechanical stress and
H2O2 alter the production of individual ECM
components, which are known to be key in the pathological process
of POP, the present study detected mRNA and protein expression
levels of precursor COL1A1, precursor COL3A1 and elastin by RT-qPCR
and western blotting, respectively. As presented in Figs. 3 and 4, the results demonstrated that
incubation of hUSLFs with H2O2 resulted in a
significant inhibitory effect on the expression of COL1A1, COL3A1
and elastin at the high concentration of 0.8 mmol/l (P<0.01),
while H2O2 at a concentration of 0.2 and 0.4
mmol/l promoted the expression of mRNA and protein. Furthermore,
the expression of mRNA and protein levels significantly decreased
with high strains of 5,333 µ, (P<0.01), however, the levels
increased with lower strains of 1,333 µ.
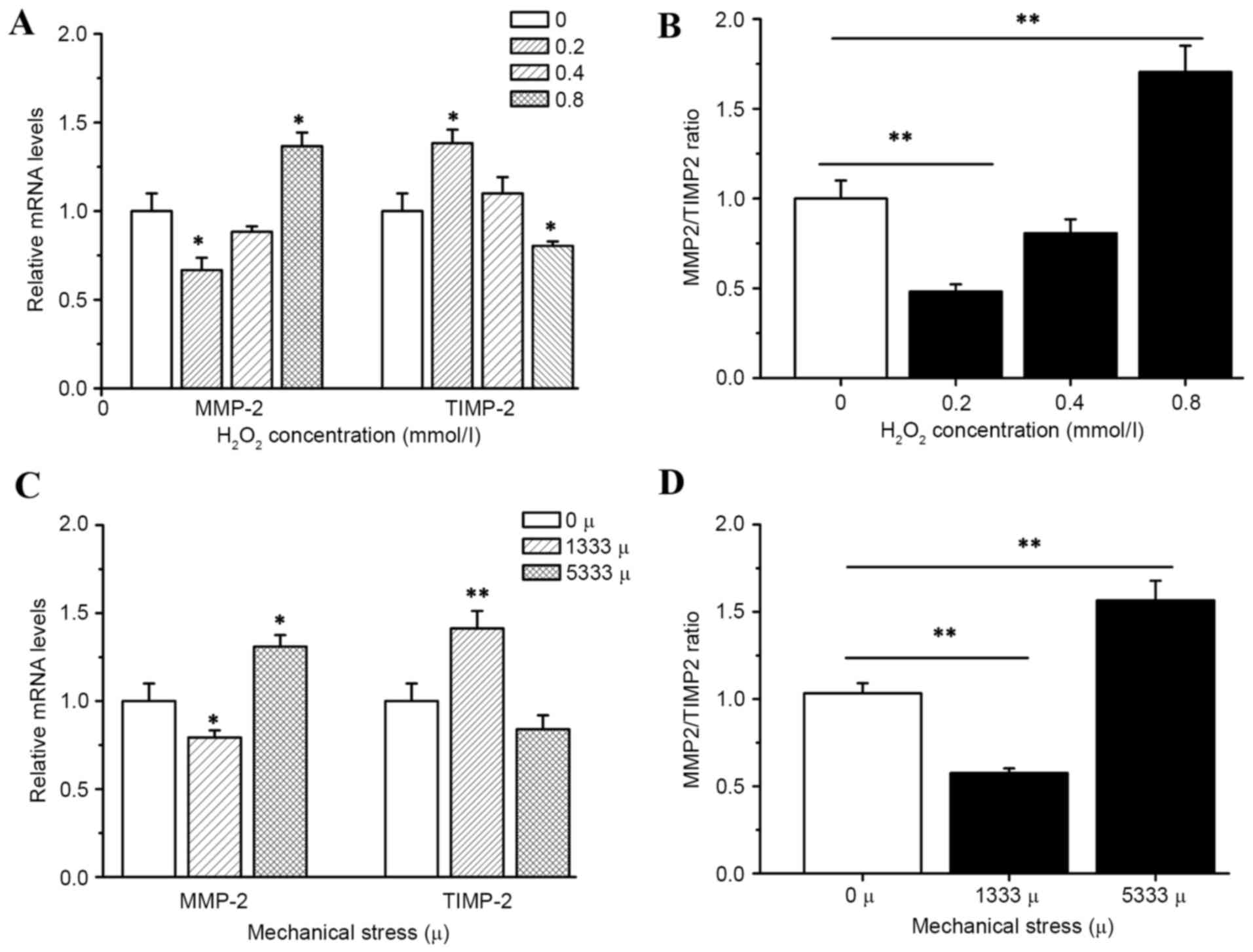
Effects of mechanical stress and
hydrogen peroxide on mRNA expression of matrix MMP-2 and
TIMP-2
Incubation of hUSLFs with H2O2
resulted in significant inhibition of MMP-2 expression in
transcription levels at low concentration of 0.2 mmol/l
(P<0.05). As the activity of MMP-2 is primarily regulated via
the antiprotease TIMP-2, the present study then investigated how
H2O2 affects TIMP-2. The results indicated
that the expression of TIMP-2 was significantly decreased by
H2O2 at the high concentration of 0.8 mmol/l
(P<0.05), while MMP-2 levels demonstrated a decrease at 0.2
mmol/l and an increase at 0.8 mmol/l as presented in Fig. 5. The ratios of enzyme/inhibitor
mRNA expression were calculated, in order to provide a more
comprehensive method to analyze the proteolytic balance. In cells
treated with 0.8 mmol/l H2O2 a significant
increase in the ratio of enzyme/inhibitor mRNA expression levels
(P<0.01) was observed, which may facilitate the degradation of
ECM.
In addition, the cells with strain of 1,333 µ
demonstrated significantly higher mRNA expression of TIMP-2
(P<0.01), and lower mRNA expression of MMP-2 compared with the
control group (P<0.05), but a high strain of 5,333 µ indicated
an opposite result (P<0.01 of MMP-2/TIMP-2.
Effect of excess mechanical stress and
hydrogen peroxide on the TGF-β1/Smad2 pathway
TGF-β1 initiates cellular activity by binding to and
activating TGF-β receptor II (TRβII) and is mediated by Smad
transcription factors. In patients with POP, the expression of ECM
proteins were decreased, therefore, the present study only selected
the mechanical stress level of 5,333 µ and
H2O2 concentration of 0.8 mmol/l to
investigate the activity of the TGF-β1/Smad2 signaling pathway. The
western blot analysis revealed that expression of TGF-β1 was
significantly decreased by excess mechanical stress of 5,333 µ and
H2O2 at concentration of 0.8 mmol/l (Fig. 6; P<0.01). The Smad family is the
most important mediator for TGF-β1 signaling, which resulted in
examination of the post-receptor regulation of TGF-β1. As presented
in Fig. 6, excess mechanical
stress and H2O2 treatment significantly
suppressed the levels of p-Smad2 (P<0.01). These results suggest
that excess mechanical stress and H2O2 may
lead to the suppression of TGF-β1/Smad2 signaling.
Discussion
Numerous pathophysiological processes in the human
body result in cells withstanding varying levels of mechanical
stress. Cells that can be affected include cardiac myocytes and
fibroblasts (17), vascular
endothelial and smooth muscle cells (18), tendon and ligament fibroblasts
(19,20) and fibroblasts and myofibroblasts
during wound healing. The present study observed an increase in
intracellular ROS and abnormal expression of MnSOD, an antioxidant
enzyme, triggered by mechanical stress (Fig. 1), demonstrating that mechanical
stress has an effect on oxidation-antioxidation products in hUSLFs.
Thus, hUSLFs were incubated with different concentrations of
H2O2 in the subsequent experiments to
investigate the potential associations.
The activity and proliferation ability of cells is
reduced when the cells are subjected to external injury and this
acts as an important indicator in measuring cell state (21). The present study provided data to
support that excessive mechanical stress and
H2O2 suppress hUSLFs growth.
The balance between ECM protein production and
degradation is essential for the onset of POP. The ECM is a major
component of connective tissues, which provide a framework for the
pelvic support system. The largest class of fibrous ECM molecules
is the collagen family, which includes at least 16 different types
of collagen (22). Fibroblasts can
synthesize precursors of collagen, including COL1A1 and COL3A1, and
elastin, which are secreted into the ECM and assemble into fibrils.
Collagen and elastin are the predominant components of connective
tissue in uterine ligaments. Type I collagen contributes strength
to connective tissues, whereas type III collagen and elastin confer
flexibility (23). The ECM is a
physical barrier with important structural functions and ECM
signaling is received by integrins. Integrin clustering occurs
following binding to the ECM, generating intracellular signaling.
The present study investigated the change in expression of ECM
proteins in hUSLFs upon exposure to mechanical stress and hydrogen
peroxide treatment. The present study demonstrated that mechanical
stress or hydrogen peroxide could significantly inhibit COL1A1,
COL3A1 and elastin expression, however this only occurred when the
mechanical stress level reached a certain magnitude and hydrogen
peroxide attained a certain concentration. Smaller forces and a
lower concentration of H2O2 could stimulate
the ECM protein expression.
Catabolic enzymes, including collagenases or MMPs,
cleave the fibrous proteins of the ECM in a process known as
catabolism. The ECM is constantly remodeled, and its homeostasis
depends on the balance between the synthesis and degradation by
MMPs further controlled by activators and inhibitors (TIMPs). MMP-2
is secreted into the extracellular space, and uses elastin,
fibronectin and type IV collagen, which are all important
components of ECM, as its substrates. TIMP-2 is important in
inhibiting MMP-2 deposition and preventing ECM degradation. The
present study revealed that the mRNA level of MMP-2 was upregulated
and TIMP-2 decreased by overexposure to mechanical force and
H2O2, and additionally the MMP-2/TIMP-2 ratio
was increased, which may account for the degradation of the ECM
components.
TGF-β1 activation enhances fibrogenesis and
remodeling of ECM. TGF-β1/Smad signaling triggers myofibroblasts to
synthesize collagen I and collagen III (24). It controls the deposition and
turnover of ECM components, including the fibrillar collagens and
fibronectin, and regulates the expression of matrix degrading
proteolytic enzymes MMPs and their specific tissue inhibitors TIMPs
(25,26). TGF-β1 initiates its cellular
actions by binding to and activating TRβII and is mediated by Smad
transcription factors. Particular cells were selected to undergo
further investigation, based on the ECM component expression
levels. The cells were treated with 5,333 µ strain and 0.8 mmol/l
H2O2 to investigate the effect on the TGF-β1
signaling pathway. The activation of TGF-β1 in hUSLFs upon
treatment was investigated by western blot analysis. Excessive
mechanical force and high concentrations of
H2O2 downregulated the expression levels of
TGF-β1 and p-Smad2, suggesting that the remodeling of ECM induced
by mechanical stress and H2O2 is associated
with the inhibition of the TGF-β1 signaling pathway. Previous
studies have indicated that the TGF-β1/Smad signaling pathway is
crucial for the activation of a number of fibrillar collagen genes
(27,28). The results of the present study
indicated that the change of expression of COL1Al, COL3A1 and
elastin, which affected TGF-β1/Smad signaling, was activated by
mechanical stress and H2O2, in order to
trigger ECM deposition or degradation in hUSLFs. Smad2 is the
classic downstream signal transducer of TGF-β1, which is a major
profibrotic factor. The data collectively suggests that the TGF-β1
pathway is important in the cellular response to mechanical stress
or H2O2. The Smad signaling pathway is
pivotal in signal transduction from the receptors of the TGF-β1
superfamily members to the nucleus. However, novel evidence
supports the hypothesis that non-Smad signaling pathways also
participate in TGF-β1 signaling (29). The phosphoinositide-3-kinase/Akt
signaling pathway is one of the important non-Smad pathways and is
involved in protein synthesis and cell growth (30). In addition to the Smad signaling
pathway, TGF-β1 may activate the Ras/mitogen activated protein
kinase (MAPK) kinase/extracellular-regulated kinase signaling
pathway (31). It has been
reported that the p38 MAPK signaling pathway is involved in the
induction of COL1A1 mRNA by TGF-β1 in rat glomerular mesangial
cells (5,32).
In conclusion, the results of the present study
demonstrated that the disturbance of the balance of MMP/TIMP, via
regulation of the TGF-β1 signaling pathway, is the major mechanism
via which excess mechanical stress or H2O2
affects the remodeling of ECM.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81270684 and
81471442).
References
|
1
|
Pereira AM, Tudor C, Kanger JS,
Subramaniam V and Martin-Blanco E: Integrin-dependent activation of
the JNK signaling pathway by mechanical stress. PLoS One.
6:e261822011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furumatsu T, Matsumoto E, Kanazawa T,
Fujii M, Lu Z, Kajiki R and Ozaki T: Tensile strain increases
expression of CCN2 and COL2A1 by activating TGF-β-Smad2/3 pathway
in chondrocytic cells. J Biomech. 46:1508–1515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dieter AA, Wilkins MF and Wu JM:
Epidemiological trends and future care needs for pelvic floor
disorders. Curr Opin Obstet Gynecol. 27:380–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Majkusiak W, Horosz E, Tomasik P,
Zwierzchowska A, Wielgoś M and Barcz E: Quality of life assessment
in women after cervicosacropexy with polypropylene mesh for pelvic
organ prolapse: A preliminary study. Prz Menopauzalny. 14:126–129.
2015.PubMed/NCBI
|
|
5
|
Das J, Ghosh J, Manna P and Sil PC:
Acetaminophen induced acute liver failure via oxidative stress and
JNK activation: Protective role of taurine by the suppression of
cytochrome P450 2E1. Free Radic Res. 44:340–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ewies A and Elshafie M: High isoprostane
level in cardinal ligament-derived fibroblasts and urine sample of
women with uterine prolapse. BJOG. 116:126–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li BS, Hong L, Min J, Wu DB, Hu M and Guo
WJ: The expression of glutathione peroxidase-1 and the anabolism of
collagen regulation pathway transforming growth
factor-beta1-connective tissue growth factor in women with uterine
prolapse and the clinic significance. Clin Exp Obstet Gynecol.
40:586–590. 2013.PubMed/NCBI
|
|
8
|
Kamodyová N, Bañasová L, Janšáková K,
Koborová I, Tóthová L, Stanko P and Celec P: Blood contamination in
Saliva: Impact on the measurement of Salivary oxidative stress
markers. Dis Markers. 2015:4792512015.PubMed/NCBI
|
|
9
|
Bujak M and Frangogiannis NG: The role of
TGF-beta signaling in myocardial infarction and cardiac remodeling.
Cardiovasc Res. 74:184–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang XR, Chung AC, Yang F, Yue W, Deng C,
Lau CP, Tse HF and Lan HY: Smad3 mediates cardiac inflammation and
fibrosis in angiotensin II-induced hypertensive cardiac remodeling.
Hypertension. 55:1165–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Froese AR, Shimbori C, Bellaye PS, Inman
M, Obex S, Fatima S, Jenkins G, Gauldie J, Ask K and Kolb M:
Stretch-induced activation of transforming growth factor-β1 in
pulmonary fibrosis. Am J Respir Crit Care Med. 194:84–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wells RG: Fibrogenesis. V. TGF-beta
signaling pathways. Am J Physiol Gastrointest Liver Physiol.
279:G845–G850. 2000.PubMed/NCBI
|
|
13
|
Li FZ, Cai PC, Song LJ, Zhou LL, Zhang Q,
Rao SS, Xia Y, Xiang F, Xin JB, Greer PA, et al: Crosstalk between
calpain activation and TGF-β1 augments collagen-I synthesis in
pulmonary fibrosis. Biochim Biophys Acta. 1852:1796–1804. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Landsheere L, Munaut C, Nusgens B,
Maillard C, Rubod C, Nisolle M, Cosson M and Foidart JM: Histology
of the vaginal wall in women with pelvic organ prolapse: A
literature review. Int Urogynecol J. 24:2011–2020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen B and Yeh J: Alterations in
connective tissue metabolism in stress incontinence and prolapse. J
Urol. 186:1768–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Butt RP, Laurent GJ and Bishop JE:
Mechanical load and polypeptide growth factors stimulate cardiac
fibroblast activity. Ann N Y Acad Sci. 752:387–393. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kona S, Chellamuthu P, Xu H, Hills SR and
Nguyen KT: Effects of cyclic strain and growth factors on vascular
smooth muscle cell responses. Open Biomed Eng J. 3:28–38. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang G, Crawford RC and Wang JH:
Proliferation and collagen production of human patellar tendon
fibroblasts in response to cyclic uniaxial stretching in serum-free
conditions. J Biomech. 37:1543–1550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaneko D, Sasazaki Y, Kikuchi T, Ono T,
Nemoto K, Matsumoto H and Toyama Y: Temporal effects of cyclic
stretching on distribution and gene expression of integrin and
cytoskeleton by ligament fibroblasts in vitro. Connect Tissue Res.
50:263–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu M, Gong X, Lu Y, Guo J, Wang C and Pan
Y: Molecular cloning and functional characterization of a
cell-permeable superoxide dismutase targeted to lung adenocarcinoma
cells. Inhibition cell proliferation through the Akt/p27kip1
pathway. J Biol Chem. 281:13620–13627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niu Y, Xie T, Ge K, Lin Y and Lu S:
Effects of extracellular matrix glycosylation on proliferation and
apoptosis of human dermal fibroblasts via the receptor for advanced
glycosylated end products. Am J Dermatopathol. 30:344–351. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goh JT: Biomechanical and biochemical
assessments for pelvic organ prolapse. Curr Opin Obstet Gynecol.
15:391–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gressner AM and Weiskirchen R: Modern
pathogenetic concepts of liver fibrosis suggest stellate cells and
TGF-beta as major players and therapeutic targets. J Cell Mol Med.
10:76–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blavier L, Lazaryev A, Groffen J,
Heisterkamp N, DeClerck YA and Kaartinen V: TGF-beta3-induced
palatogenesis requires matrix metalloproteinases. Mol Biol Cell.
12:1457–1466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schilling TF, Nie Q and Lander AD:
Dynamics and precision in retinoic acid morphogen gradients. Curr
Opin Genet Dev. 22:562–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verrecchia F, Chu ML and Mauviel A:
Identification of novel TGF-beta/Smad gene targets in dermal
fibroblasts using a combined cDNA microarray/promoter
transactivation approach. J Biol Chem. 276:17058–17062. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai G, Yan G, Wang G, Wan P and Zhang R:
Anti-hepatic fibrosis effects of a novel turtle shell decoction by
inhibiting hepatic stellate cell proliferation and blocking
TGF-β1/Smad signaling pathway in rats. Oncol Rep. 36:2902–2910.
2016.PubMed/NCBI
|
|
29
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trager N, Smith A, Iv G Wallace, Azuma M,
Inoue J, Beeson C, Haque A and Banik NL: Effects of a novel orally
administered calpain inhibitor SNJ-1945 on immunomodulation and
neurodegeneration in a murine model of multiple sclerosis. J
Neurochem. 130:268–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Secker GA, Shortt AJ, Sampson E, Schwarz
QP, Schultz GS and Daniels JT: TGFbeta stimulated
re-epithelialisation is regulated by CTGF and Ras/MEK/ERK
signalling. Exp Cell Res. 314:131–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xi L, Xiao C, Bandsma RH, Naples M, Adeli
K and Lewis GF: C-reactive protein impairs hepatic insulin
sensitivity and insulin signaling in rats: Role of
mitogen-activated protein kinases. Hepatology. 53:127–135. 2011.
View Article : Google Scholar : PubMed/NCBI
|