Introduction
Osteosarcoma is the most common bone malignancy
diagnosed in children and young adults (1–3), and
the overall five-year survival rate of patients with osteosarcoma
is ~61.6% (1). Prior to the
1970's, when the standard treatment primarily consisted of surgical
resection, the prognosis of patients with osteosarcoma was poor,
with five-year survival rates of <20% (2,4).
Over the last few decades, the combined use of multi-agent
chemotherapy and surgery has improved the five-year survival rates
of patients with osteosarcoma to 60–70% (1,4).
Although the use of effective adjuvant chemotherapeutic agents has
improved the survival rates dramatically, the prognosis for
patients with osteosarcoma remains unsatisfactory (5,6).
Therefore, the identification of novel chemotherapeutic agents, and
the molecular mechanisms by which they function, are important for
improving the outcome of osteosarcoma treatment.
Vitamin K (VK) is a group of fat-soluble vitamins
that serve important roles in blood coagulation and bone metabolism
(7). VK exist as natural and
synthetic forms. VK1 (phylloquinone) and VK2 (menaquinone) are
naturally occurring vitamins, which are produced by plants and
bacteria, respectively (7–14). VK3 and VK4 (menadiones) are
synthetic derivatives of VK1 and VK2 (14). In recent years, an increasing
number of studies have investigated the antitumor effects of VKs
(7–14). These studies have demonstrated that
VK inhibits the growth and induces apoptosis in multiple cancer
cell types, including leukemia, hepatocellular carcinoma, and lung,
breast, oral, bladder and prostate cancers (7–14).
The anticancer activity of VK4 in prostate cancer cells was
demonstrated by Jiang et al (14). However, the molecular mechanisms
underlying VK4-induced apoptosis in these cells remain largely
unknown. Therefore, the aim of the present study was to investigate
whether VK4 exerts anticancer effects in osteosarcoma cells, and to
identify the potential molecular mechanisms involved.
Materials and methods
Chemicals and antibodies
All chemicals were purchased from Sigma-Aldrich
(Merck Millipore, Darmstadt, Germany) unless otherwise stated. VK4
was purchased from Shanghai Tauto Biotech Co., Ltd. (Shanghai,
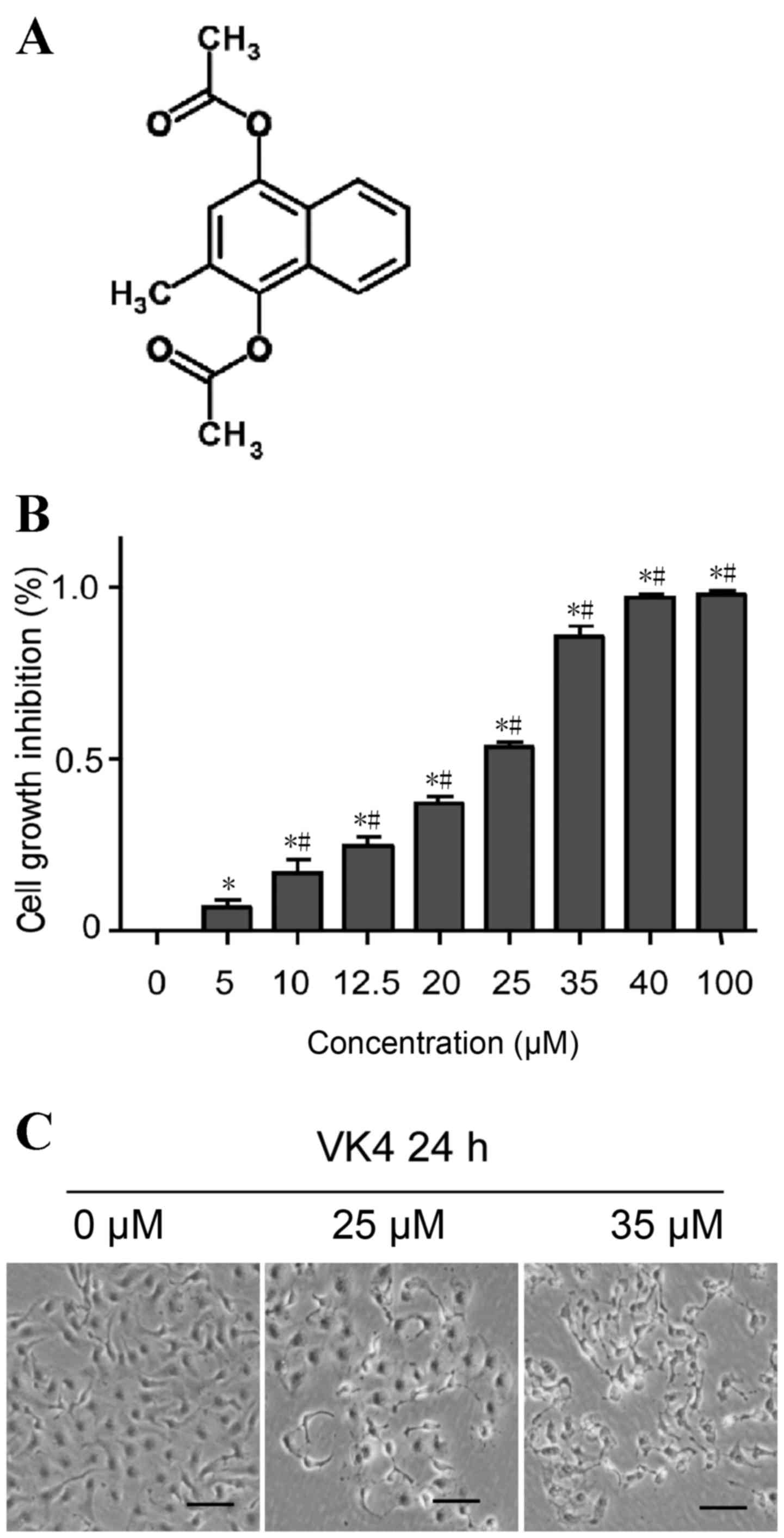
China), and the chemical structure of VK4 is shown in Fig. 1A. Fetal bovine serum (FBS) was
purchased from Hangzhou Sijiqing Biological Engineering Materials
Co., Ltd. (Hangzhou, China). The Annexin V-FITC Apoptosis Detection
kit, Reactive Oxygen Species assay kit and primary antibodies
against Bax, Bcl-2 and caspase-3 were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). The β-actin primary
antibody and the horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG or goat anti-mouse IgG secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Cell culture and treatments
The human osteosarcoma U2OS cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C and 5% CO2 in a humidified atmosphere. VK4 was
dissolved in dimethyl sulfoxide (DMSO). Cells were treated with 0,
25 or 35 µM VK4 dissolved in DMSO, where the final concentration of
DMSO was <1%. DMSO-only treated cells were used as a
control.
Determination of cell viability
The effect of VK4 on cell viability was determined
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
assay as described previously (9).
Briefly, 1×104 U2OS cells/well were seeded in a 96-well
plate and treated with 0, 5, 10, 12.5, 20, 25, 35, 40, and 100 µM
VK4 for 24 h. Following treatment, MTT reagent (500 µg/ml) was
added and cells were incubated at 37°C for a further 4 h. DMSO (150
µl) was subsequently added to dissolve the formazan crystals and
the absorbance was read at 570 nm using a microplate reader (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Data were expressed as
the percentage viability, assuming that the absorbance of untreated
control cells was 100%. The percentage cell viability was
calculated using the following formula: Cell viability (%) =
[(A570, sample - A570, blank) / (A570,
control - A570, blank)] ×100.
Analysis of morphological alterations
of cells by light microscopy
U2OS cells were treated with 0, 25 or 35 µM VK4 for
24 h. Shrinkage and detachment of the VK4 treated cells were
observed by phase contrast microscopy (Olympus IX71 microscope;
Olympus Corporation, Tokyo, Japan) (n=3).
Analysis of apoptosis by annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
staining
A total of 4×106 U2OS cells were treated
with 0, 25 or 35 µM VK4 in the presence or absence of the
pan-caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-(O-methyl)
-fluoromethylketone (Z-VAD-FMK; 50 µM). Following treatment, cells
were harvested, washed with PBS, and resuspended in 200 µl binding
buffer containing 5 µl annexin V before they were incubated in the
dark for 10 min at room temperature, according to the
manufacturer's instructions (Beyotime Institute of Biotechnology).
Cells were subsequently labeled with 10 µl PI and the samples were
immediately analyzed by the Multicycle AV software using the
Beckman Coulter Epics XL Flow Cytometer (Beckman Coulter, Inc.,
Brea, CA, USA).
Cell cycle analysis
Cell cycle analysis was performed as described
previously (10). Briefly,
4×106 U2OS cells were treated with 0, 25 or 35 µM VK4
for 24 h. The cells were then washed with PBS and fixed with 70%
ice cold ethanol at 4°C overnight. After washing twice with PBS,
cells were stained with a PBS solution containing 50 µg/ml of PI
and 100 µg/ml RNase A for 30 min in the dark at room temperature.
The stained cells were analyzed for cell cycle phase distribution
using Beckman Coulter Epics XL (Beckman Coulter, Inc.).
Flow cytometric analysis of reactive
oxygen species (ROS) generation and mitochondrial membrane
potential (MMP)
In order to determine the level of ROS generation
and the MMP, cells were stained with
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and rhodamine
123 (Rho-123), respectively, as described previously (9). Briefly, 4×106 U2OS cells
were incubated with 0, 25 or 35 µM VK4 for 24 h. The cells were
then harvested, washed with PBS and incubated with DCFH-DA (10 µM)
or Rho-123 (5 µg/ml) in the dark for 30 min at room temperature.
After washing with PBS, the samples were analyzed for the
fluorescence of DCF or Rho-123 by flow cytometry (Beckman Coulter
Epics XL; Beckman Coulter, Inc.).
Wound healing assay
Cell mobility was assessed using an in vitro
scratch wound assay. A total of 4×106 U2OS cells were
seeded in 6-well plates containing complete medium until 90%
confluent. The plate surface was then scraped with a sterile,
200-µl tip and washed twice with PBS to remove remaining cell
debris, before the cells were incubated in DMEM medium containing
1% FBS, in the presence or absence of 25 µM VK4 for 24 h. The cells
were then observed under an inverted light microscope (Olympus
Corporation) and photographed at ×100 magnification. Experiments
were performed in triplicate. The distance between the wound edges
was measured with a graduated ruler, and the relative scratch
breadth of VK4-treated samples was presented as the ratio of the
average breadth of treatment cells vs. the average breadth of
control cells.
Cell migration assay
In vitro cancer cell migration activities
were evaluated using Transwell microplates. For cell migration,
1×105 cells in 200 µl of DMEM medium containing 1% FBS
with or without 25 µM VK4, were seeded in the upper Transwell
insert chamber containing a polycarbonate filter (diameter, 6.5 mm;
pore size, 8 µm; Costar; Corning Life Sciences, NY, USA). DMEM
medium (600 µl) containing 10% FBS (chemoattractant) was added to
the lower chamber, and the plates were incubated for 24 h at 37°C
in 5% CO2. Cells that had traversed the membrane were
fixed with paraformaldehyde, stained with Coomassie Brilliant Blue
R-250 dye (cat. no. 27816; Sigma-Aldrich; Merck Millipore), and
were visualized and counted using an inverted light microscope. The
cells that did not migrate were removed from the top of the
transwell filters by scraping. Experiments were performed in
triplicate. The relative migration of cells was presented as the
ratio of average cell migration in the control group vs. the
average cell migration in the treatment group.
Western blot analysis
U2OS cells (4×106) were treated with 0,
25 or 35 µM VK4 for 24 h. Adherent and detached cells were
collected and total protein was extracted as described previously
(10). The protein concentrations
were determined using the NanoDrop 1000 Spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). Proteins (50 µg)
were separated by 8–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a polyvinylidine difluoride
membrane. After blocking with 5% (w/v) non-fat milk for 2 h and
washing with tris-buffered saline-Tween 20 solution (TBST),
membranes were incubated with Bcl-2 (dilution, 1:1,000; cat. no.
AB112), Bax (dilution, 1:300; cat. no. AB026), cleaved caspase-3
(dilution, 1:500; cat. no. AC031) and β-actin (dilution, 1:400;
cat. no. sc-134542) primary antibodies overnight at 4°C. After
washing with TBST, the blots were incubated with HRP-conjugated
goat anti-rabbit IgG or goat anti-mouse IgG secondary antibodies
(dilution, 1:5,000; cat. no. sc-45090) for 1 h at room temperature.
Blots were washed with TBST and signals were detected using the ECL
plus chemiluminescence kit (Merck Millipore) on X-ray film.
Statistical analysis
Data are expressed as the mean ± standard error.
Differences between two groups were determined using the Student's
t-test, while differences among >2 groups were determined using
one-way analysis of variance followed by Tukey's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
VK4 inhibits the growth of U2OS
osteosarcoma cells
The effect of VK4 on the viability of U2OS cells was
evaluated using an MTT assay. As shown in Fig. 1B, VK4 treatment significantly
inhibited the growth of U2OS cells in a dose-dependent manner
(P<0.05). The IC50 value of VK4 was 25 µM following
24 h treatment. The rate of growth inhibition of U2OS cells was
>70% at 35 µM when compared with untreated controls (Fig. 1B). Therefore, concentrations of 25
and 35 µM VK4 were selected for further mechanistic studies.
VK4 induces morphological alterations
in U2OS osteosarcoma cells
To examine the effect of VK4 on U2OS cell
morphology, cells were treated with increasing concentrations of
VK4 for 24 h and cell morphology was observed under a phase
contrast microscope. As shown in Fig.
1C, increasing concentrations of VK4 induced severe
morphological alterations indicative of cell death, including a
reduction in the total number of cells and an increase in the
number of detached cells in the culture medium.
VK4 induces apoptosis and S phase cell
cycle arrest in U2OS osteosarcoma cells
Apoptosis and cell cycle arrest are the two major
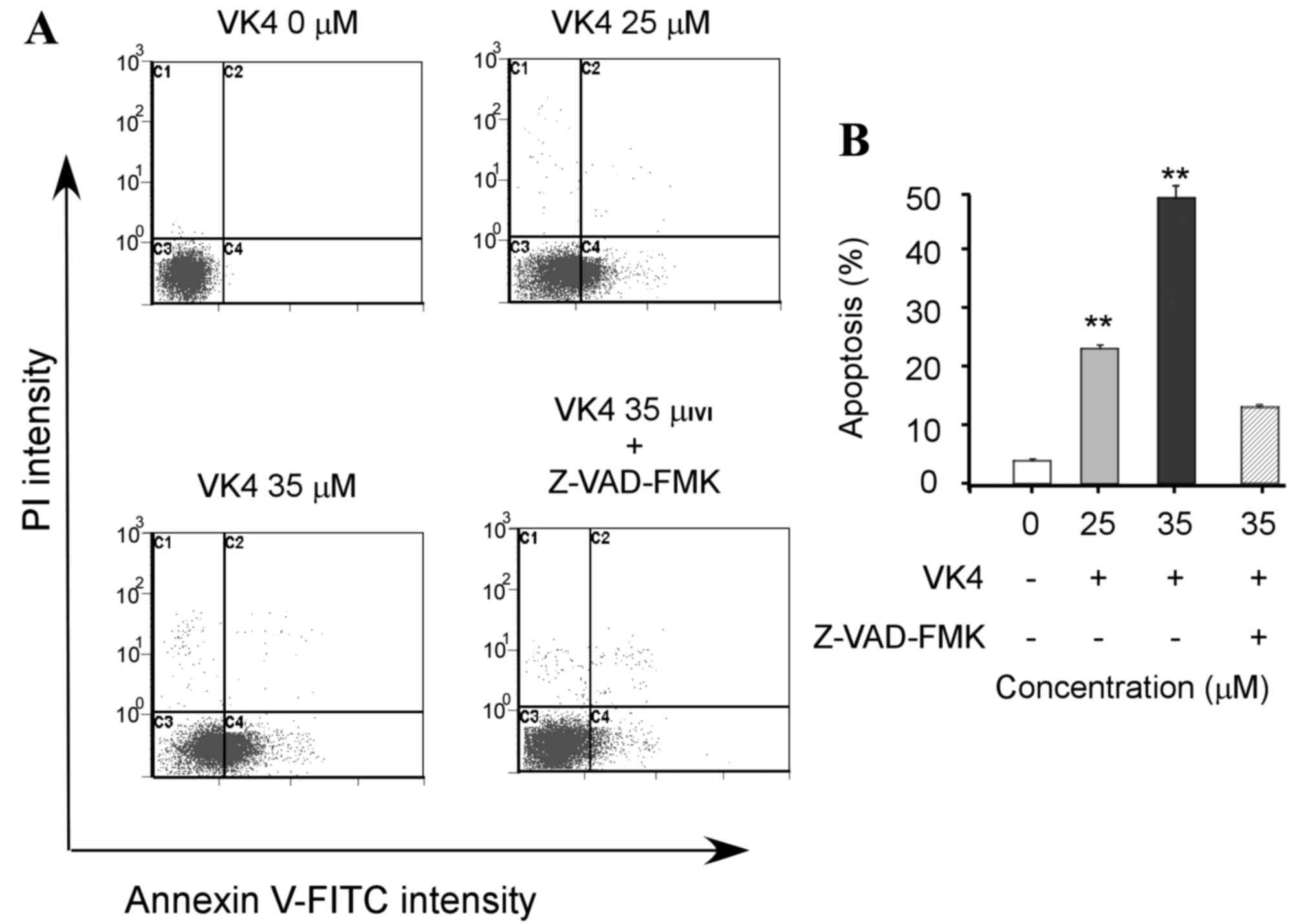
causes of cell growth inhibition (1). The effect of VK4 on U2OS cell
apoptosis was determined by annexin V-FITC/PI staining and flow
cytometry analysis. As shown in Fig.
2, VK4 induced apoptosis in U2OS cells in a dose-dependent
manner. In addition, pretreatment of cells with the pan-caspase
inhibitor Z-VAD-FMK, significantly attenuated the apoptotic effects
of VK4 (P<0.05). This indicates that VK4 induces apoptosis in
U2OS cells via caspase activation.
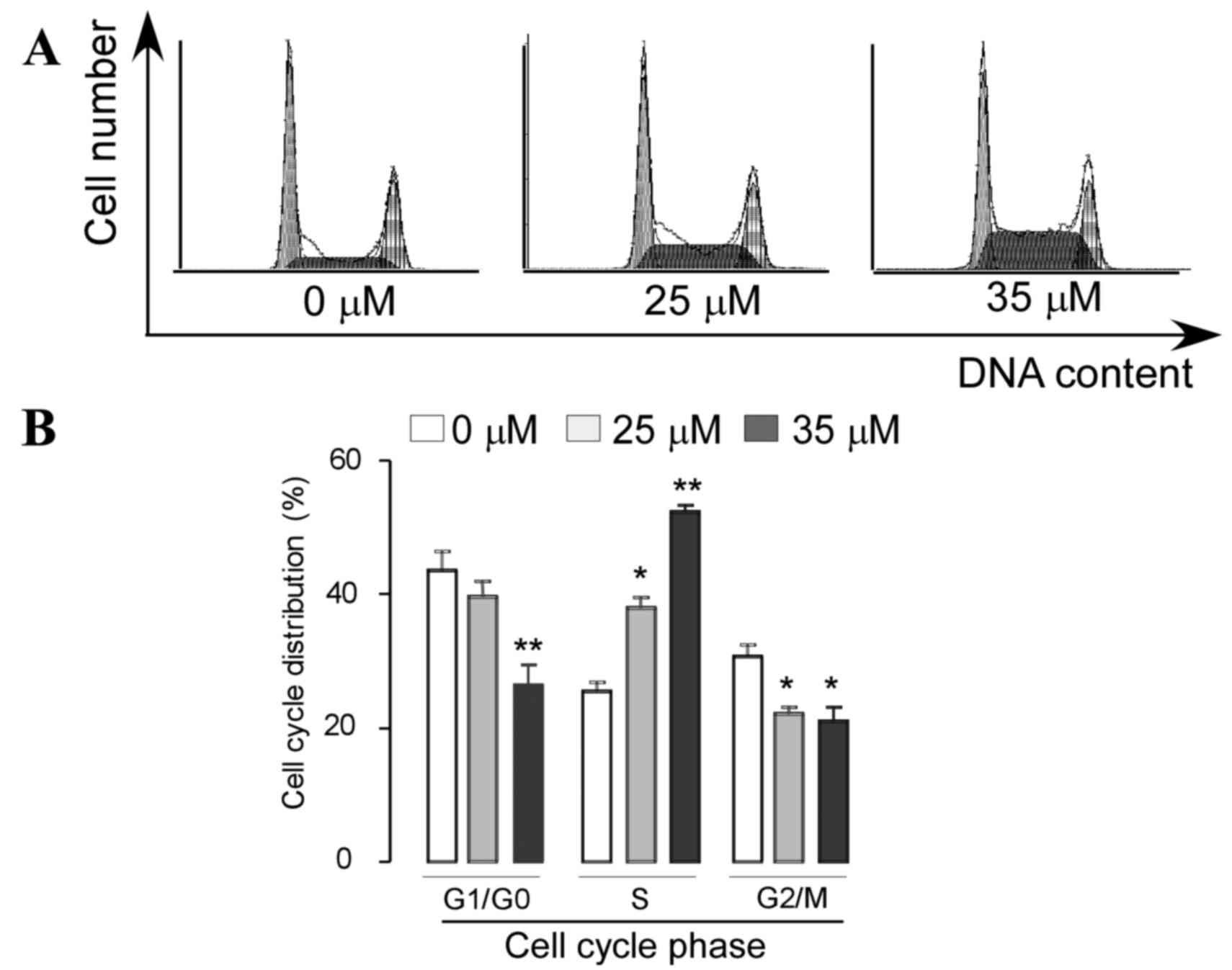
Induction of cell cycle arrest was
examined using PI staining and flow cytometry analysis
As shown in Fig. 3,
a dose-dependent increase in the percentage of cells arrested in S
phase was observed following treatment with VK4. Treatment with 25
and 35 µM VK4 significantly increased the percentage of cells in S
phase from 23.73±1.94 to 38.9±1.53 and 51.23±0.93%, respectively. A
corresponding decrease in the percentage of cells in G0/G1 phase
from 45.48±2.43 to 38.13±1.82 and 28.36±2.91%, and G2/M phase from
28.5±1.21 to 22.16±0.78 and 18.26±1.69% was observed following
treatment with 25 and 35 µM VK4, respectively (P<0.05).
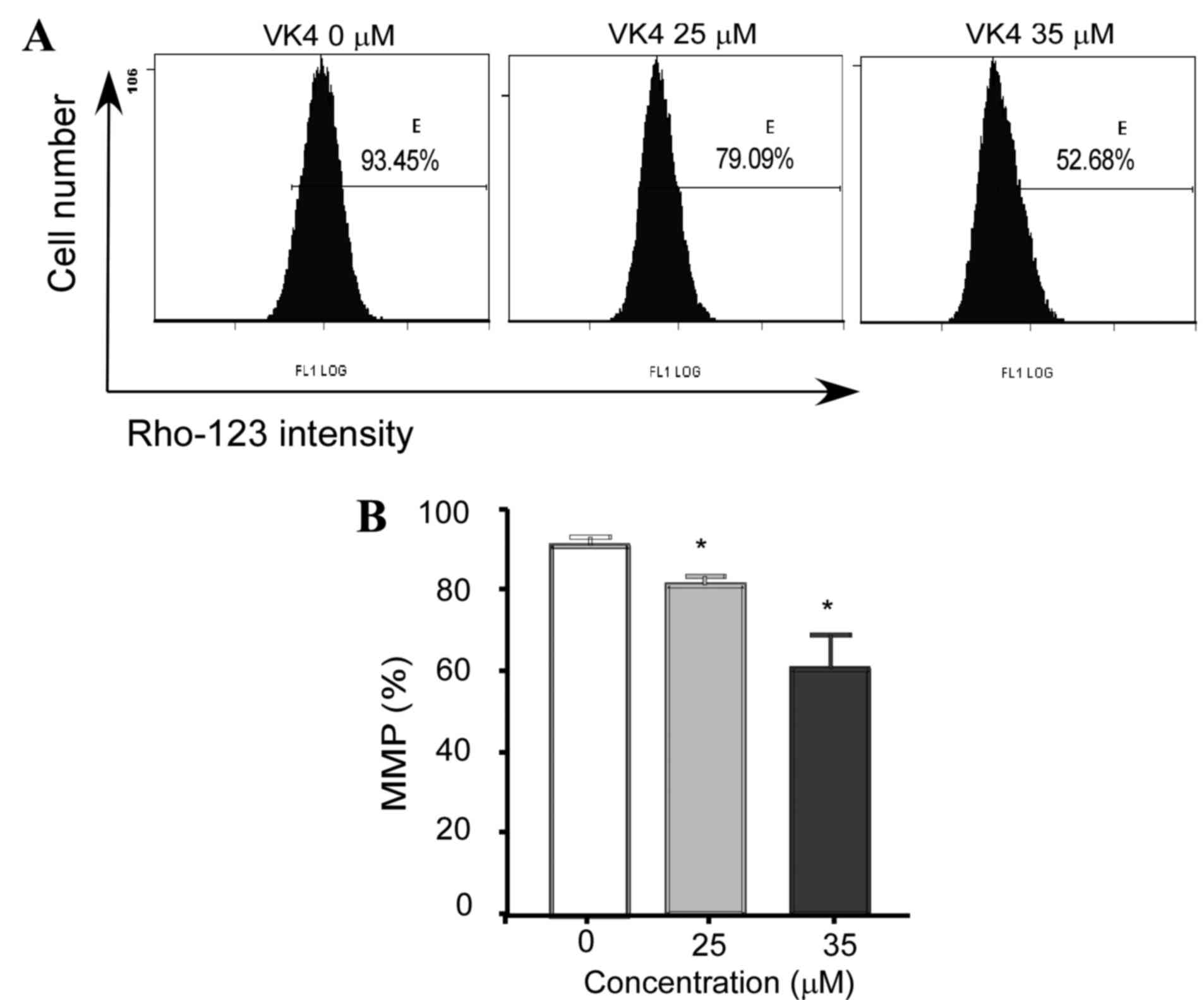
VK4 induces ROS generation and
disrupts the MMP in U2OS osteosarcoma cells
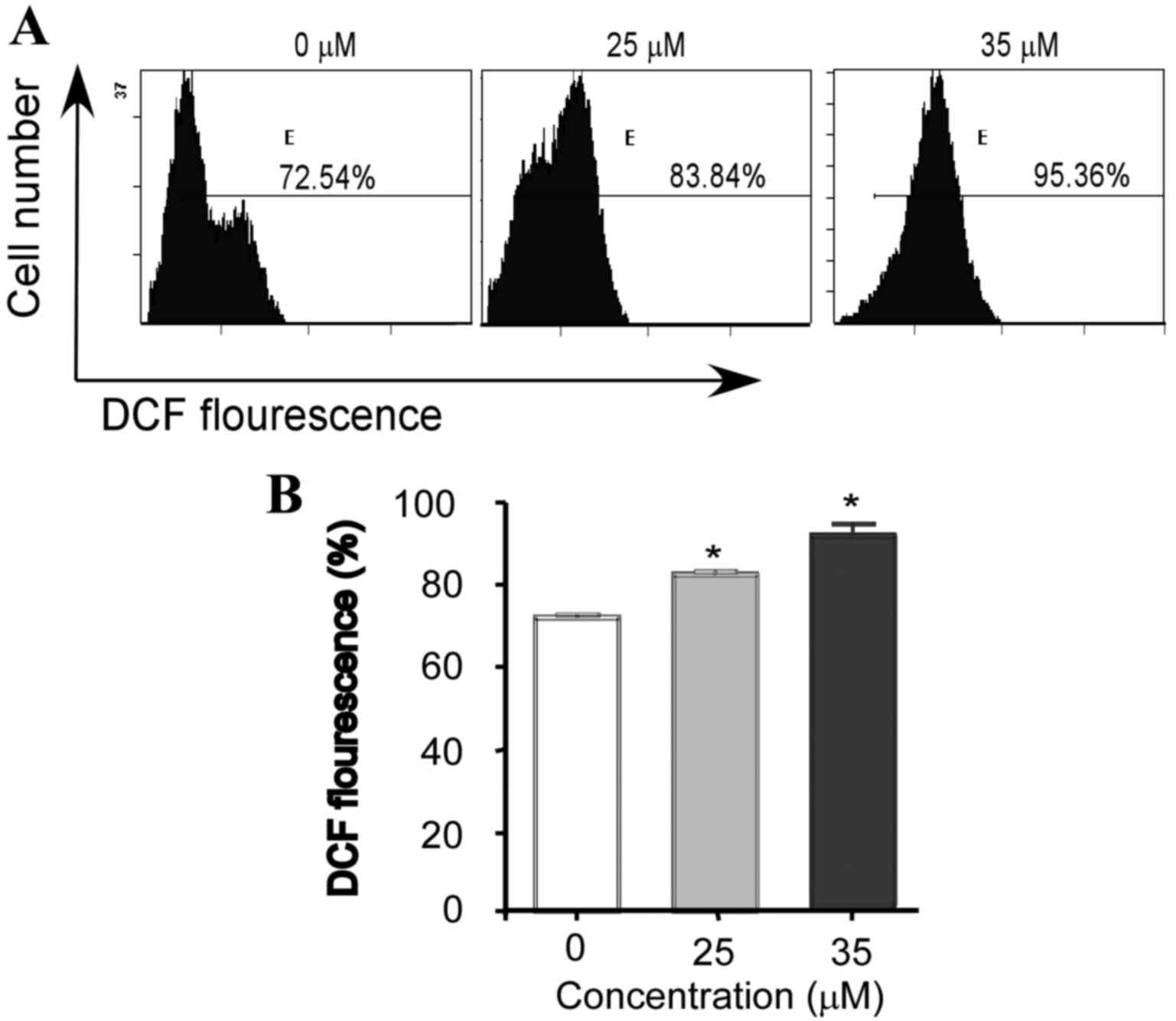
Intracellular ROS generation in U2OS cells was
evaluated by flow cytometry analysis using DCFH-DA. As shown in
Fig. 4, the level of ROS in U2OS
cells treated with 25 and 35 µM VK4 was significantly increased
when compared with untreated controls (82.6±1.2 and 95.1±1.8 vs.
71.2±0.9% respectively; P<0.05).
Depolarization of the MMP is a characteristic
feature of apoptosis (1).
Excessive intracellular ROS production has been demonstrated to
induce apoptosis by disrupting the MMP (15). In order to investigate the role of
ROS in VK4-mediated apoptosis, the MMP in U2OS cells was examined
using Rho-123 and flow cytometry analysis. As shown in Fig. 5, a significant reduction in MMP was
observed in cells following treatment with 25 and 35 µM VK4 when
compared with untreated controls (79.3±1.75% and 53.40±2.9% vs.
93.03±1.35%, respectively; P<0.05).
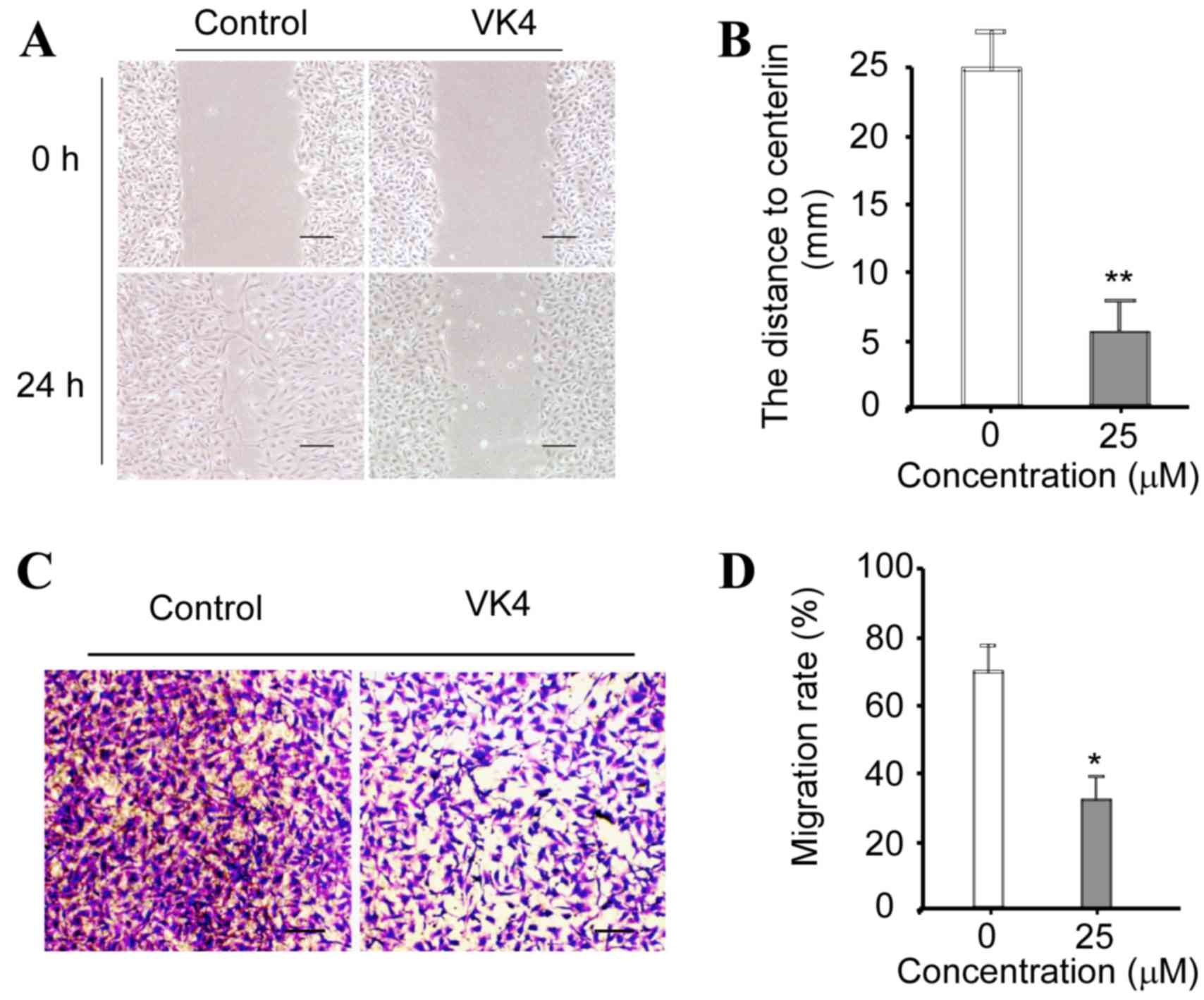
VK4 suppresses the migration of U2OS
cells in vitro
The effect of VK4 on the migration of U2OS cells
in vitro was determined using wound healing and Transwell
migration assays, respectively. As shown in Fig. 6A and B, the distance to the center
of the ‘wound’ was significantly reduced in cells incubated with 25
µM VK4 for 24 h when compared with untreated controls. As shown in
Fig. 6C and D, the migration rate
of cells was significantly reduced following treatment with 25 µM
VK4 when compared with untreated controls (P<0.05). Taken
together, these data indicate that VK4 inhibits the migration of
U2OS cells in vitro.
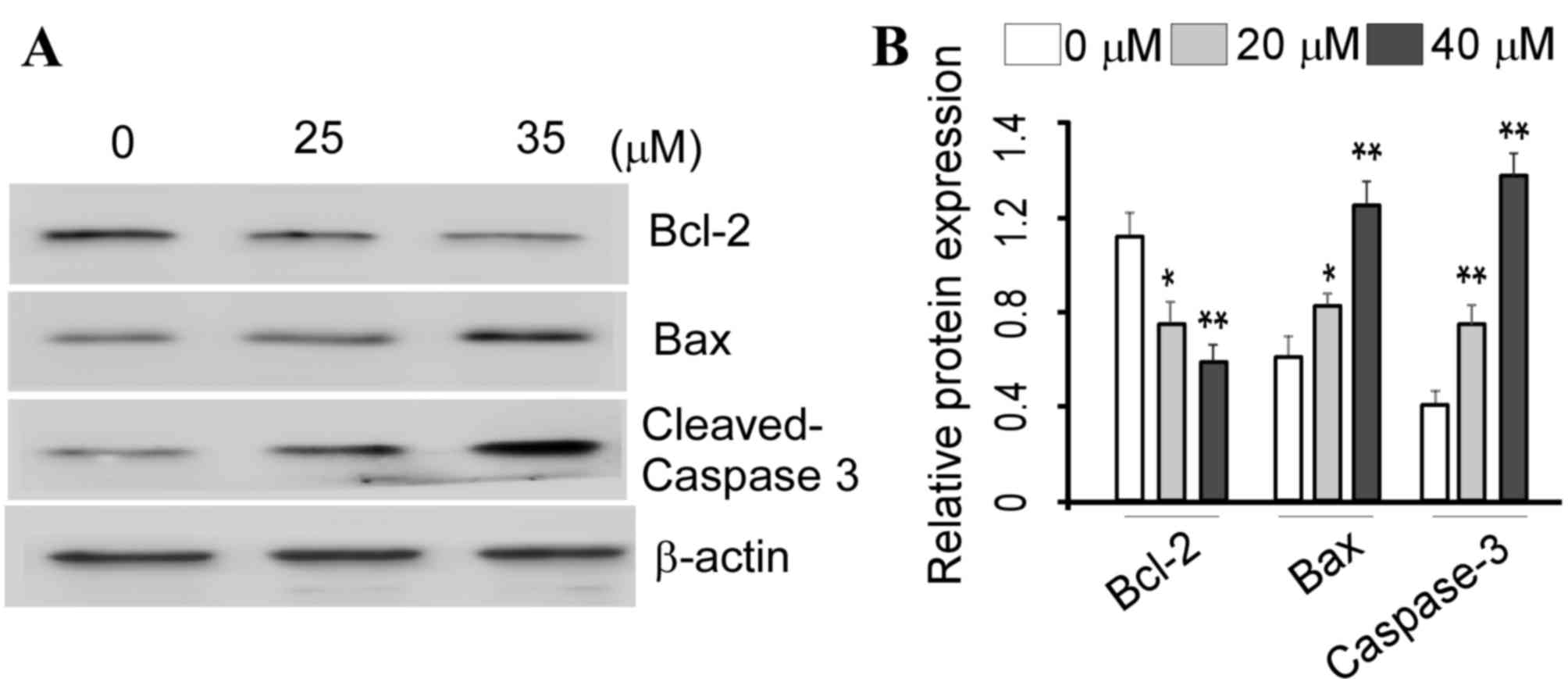
VK4 induces apoptosis in U2OS cells
through the mitochondrial pathway
ROS generation and mitochondrial membrane
dissipation are characteristic features of mitochondrial apoptosis
(11). Therefore, in order to
investigate the molecular mechanisms underlying the pro-apoptotic
effects of VK4 on U2OS cells, the protein expression levels of
Bcl-2, Bax and caspase-3 were examined. As shown in Fig. 7, VK4 significantly increased the
protein expression of pro-apoptotic protein Bax, and significantly
decreased the expression of anti-apoptotic protein Bcl-2 in a
dose-dependent manner when compared with untreated controls
(P<0.05). Cleavage of caspase-3 is a major hallmark of apoptosis
(1). Therefore, the protein
expression of cleaved caspase-3 in VK4-treated U2OS cells was
examined. As shown in Fig. 7, VK4
treatment significantly increased the expression of cleaved
caspase-3 in a dose-dependent manner, when compared with untreated
controls (P<0.05). Taken together, these results indicate that
VK4 may induce the intrinsic apoptosis pathway in U2OS cells.
Discussion
VK4 is a synthetic hydrophilic menadione compound,
which is used clinically as a treatment for hemostasis (14). Jiang et al (14) demonstrated that VK4 induced
cytotoxic effects in human prostate carcinoma PC-3 cells. However,
the molecular mechanisms underlying VK4-induced cytotoxicity in
PC-3 cells remain largely unknown. Therefore, the aim of the
present study was to determine whether VK4 inhibits the growth of
U2OS osteosarcoma cells in vitro. Consistent with Jian et
al (14), VK4 was observed to
inhibit the growth of U2OS cells in a dose-dependent manner.
Apoptosis and cell cycle arrest are the two major
causes of cell growth inhibition in cancer cells (16,17).
A number of studies indicate that the majority of chemotherapeutic
drugs inhibit tumor cell growth via induction of apoptosis
(18–20). In the present study, VK4 arrested
the cell cycle of U2OS osteosarcoma cells at S phase and induced
apoptosis in cells in a dose-dependent manner. Apoptosis, a form of
programmed cell death, is a highly controlled and evolutionarily
conserved process characterized by cell shrinkage, membrane
blebbing, loss of plasma membrane integrity, DNA fragmentation and
cleavage of caspase-3 (21,22).
Consistent with these features of apoptosis, VK4-treated U2OS cells
exhibited cell shrinkage and cleavage of caspase-3. Apoptosis can
be divided into the following three major categories: i)
Mitochondrial caspase-dependent apoptosis or intrinsic apoptosis;
ii) extrinsic apoptosis; and iii) caspase-independent apoptosis
(23,24). In order to gain further insight
into the molecular mechanisms underlying VK4-induced apoptosis,
annexin V/PI staining of VK4-treated U2OS cells exposed to a
pan-caspase inhibitor Z-VAD-FMK was performed. Treatment of cells
with Z-VAD-FMK significantly inhibited the apoptotic effects of
VK4, which suggests that VK4 may induce caspase-dependent apoptosis
in U2OS cells.
It is well established that the mitochondria serve
key roles in the regulation of cell death and proliferation through
the production of ROS (14,25).
Mitochondrial apoptosis is associated with ROS production and MMP
dissipation (26). In the present
study, VK4 increased ROS generation and decreased the MMP in U2OS
cells in a dose-dependent manner.
ROS may lead to extensive oxidative damage,
including mitochondrial damage, lipid peroxidation and DNA damage.
In addition, ROS are known to function as second messengers to
activate diverse redox-sensitive signaling cascades, such as the
mitochondrial intrinsic apoptotic cascade, through interaction with
Bcl-2 family proteins (1). The
Bcl-2 protein family includes a wide variety of antiapoptotic
proteins, such as Bcl-2, and pro-apoptotic proteins, such as Bax,
which are key factors involved in mediating the permeabilization of
the mitochondrial outer membrane and regulating apoptosis (27,28).
Under normal conditions, Bax is present in the cytosol, and is
negatively regulated by the antiapoptotic protein Bcl-2. Thus,
Bcl-2/Bax is considered to be a molecular regulator that controls
cell fate. In the presence of a pro-apoptotic stimulus, the
Bcl-2/Bax ratio decreases and the cell undergoes apoptosis. ROS
have been demonstrated to inhibit the anti-apoptotic protein Bcl-2
and activate the pro-apoptotic protein Bax (29–31).
In the present study, VK4 treatment increased the level of ROS and
dissipated the MMP in U2OS cells. Therefore, the protein expression
levels of Bcl-2 and Bax were examined. VK4 treatment significantly
increased the expression of Bax and significantly decreased the
expression of Bcl-2. These results suggest that VK4 induces the
intrinsic apoptotic cell death signaling pathway in U2OS
osteosarcoma cells. Consistent with these observations, Jiang et
al (14) demonstrated a
similar effect of VK4 on Bcl-2 family protein expression
modulation.
Osteosarcoma is characterized by resistance to
chemotherapy and the development of metastases. Although the use of
multi-agent chemotherapy in combination with surgery has improved
the five-year patient survival rate to 60–70% (1,32),
the five-year survival rate of patients with osteosarcoma that have
developed metastases is only 20%. In addition, current therapeutic
strategies are not effective for the treatment of these patients
(33). Therefore, the
identification of agents that not only inhibit the growth of tumor
cells, but also suppress the metastatic activity of cancer cells,
is highly desirable. In the present study, the effect of VK4 on
U2OS cell migration was investigated using wound healing and
Transwell migration assays. The results demonstrated that VK4
effectively inhibited the migration of U2OS cells in vitro.
However, a detailed mechanistic study is required to determine the
precise mechanisms underlying the anti-metastatic activity of VK4
in these cells.
In conclusion, the results of the present study
demonstrated that VK4 inhibits the growth and induces apoptosis of
U2OS osteosarcoma cells. To the best of our knowledge, this is the
first study that has provided evidence demonstrating the inhibitory
effects of VK4 on U2OS cell growth. Apoptosis was associated with
ROS generation, MMP dissipation, modulation of Bcl-2 family protein
expression and activation of caspase-3. In addition, VK4 suppressed
the migration of U2OS cells in vitro. These results suggest
that VK4 may present a potential compound in the development of
future therapies for the treatment of osteosarcoma. Further
investigation is required to confirm the contribution of VK4 as an
anti-tumor therapy using in vivo studies.
Acknowledgements
The present study was supported by Binzhou Medical
University Affiliated Hospital scientific research funds and
Binzhou Medical University (grant no. BY2015KYQD27).
References
|
1
|
Di W, Khan M, Rasul A, Sun M, Sui Y, Zhong
L, Yang L, Zhu Q, Feng L and Ma T: Isoalantolactone inhibits
constitutive NF-κB activation and induces reactive oxygen
species-mediated apoptosis in osteosarcoma U2OS cells through
mitochondrial dysfunction. Oncol Rep. 32:1585–1593. 2014.PubMed/NCBI
|
|
2
|
Sun L, Li Y, Li H, Zhang J, Li B and Ye Z:
Analysis of chemotherapy dosage and dosage intensity and survival
outcomes of high-grade osteosarcoma patients younger than 40 years.
Clin Ther. 36:567–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Zhu X, Li H, Li B, Sun L, Xie T,
Zhu T, Zhou H and Ye Z: Piperine inhibits proliferation of human
osteosarcoma cells via G2/M phase arrest and metastasis by
suppressing MMP-2/−9 expression. Int Immunopharmacol. 24:50–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JS, DuBois SG, Boscardin WJ, Wustrack
RL and Goldsby RE: Secondary malignant neoplasms among children,
adolescents, and young adults with osteosarcoma. Cancer.
120:3987–3993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao C, Wei JJ, Wang ZY, Ding HM, Li D, Yan
SC, Yang YJ and Gu ZP: Perifosine induces cell apoptosis in human
osteosarcoma cells: New implication for osteosarcoma therapy? Cell
Biochem Biophys. 65:217–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li YS, Deng ZH, Zeng C and Lei GH: Role of
osteopontin in osteosarcoma. Med Oncol. 32:4492015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei G, Wang M and Carr BI: Sorafenib
combined vitamin K induces apoptosis in human pancreatic cancer
cell lines through RAF/MEK/ERK and c-Jun NH2-terminal kinase
pathways. J Cell Physiol. 224:112–119. 2010.PubMed/NCBI
|
|
8
|
Mamede AC, Tavares SD, Abrantes AM,
Trindade J, Maia JM and Botelho MF: The role of vitamins in cancer:
A review. Nutr Cancer. 63:479–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baran I, Ganea C, Scordino A, Musumeci F,
Barresi V, Tudisco S, Privitera S, Grasso R, Condorelli DF, Ursu I,
et al: Effects of menadione, hydrogen peroxide, and quercetin on
apoptosis and delayed luminescence of human leukemia Jurkat
T-cells. Cell Biochem Biophys. 58:169–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Qi Z, Qian J, Bi F, Lv J, Xu L,
Zhang L, Chen H and Jia R: Induction of apoptosis in hepatocellular
carcinoma Smmc-7721 cells by vitamin K(2) is associated with p53
and independent of the intrinsic apoptotic pathway. Mol Cell
Biochem. 342:125–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oshiro Y, Takada Y, Enomoto T, Fukao K,
Ishikawa S and Iijima T: A resected case of metachronous liver
metastasis from lung cancer producing alpha-fetoprotein (AFP) and
protein induced by vitamin K absence or antagonist II (PIVKA-II).
Hepatogastroenterology. 1:1144–1147. 2004.
|
|
12
|
Akiyoshi T, Matzno S, Sakai M, Okamura N
and Matsuyama K: The potential of vitamin K3 as an anticancer agent
against breast cancer that acts via the mitochondria-related
apoptotic pathway. Cancer Chemother Pharmacol. 65:143–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan F, Yu Y, Guan R, Xu Z, Liang H and
Hong L: Vitamin K2 induces mitochondria-related apoptosis in human
bladder cancer cells via ROS and JNK/p38 MAPK signal pathways. PLoS
One. 11:e01618862016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang Y, Yang J, Yang C, Meng F, Zhou Y,
Yu B, Khan M and Yang H: Vitamin K4 induces tumor cytotoxicity in
human prostate carcinoma PC-3 cells via the mitochondria-related
apoptotic pathway. Pharmazie. 68:442–448. 2013.PubMed/NCBI
|
|
15
|
Vermeer C: Vitamin K: The effect on health
beyond coagulation-an overview. Food Nutr Res. 56:2012.
|
|
16
|
Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To
Y, Lee WH, Li M, Chu KH and Toh M: Cucurbitacin B induces apoptosis
and S phase cell cycle arrest in BEL-7402 human hepatocellular
carcinoma cells and is effective via oral administration. Cancer
Lett. 294:118–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan M, Zheng B, Yi F, Rasul A, Gu Z, Li
T, Gao H, Qazi JI, Yang H and Ma T: Pseudolaric acid B induces
caspase-dependent and caspase-independent apoptosis in u87
glioblastoma cells. Evid Based Complement Alternat Med.
2012:9575682012.PubMed/NCBI
|
|
18
|
Rasul A, Di J, Millimouno FM, Malhi M,
Tsuji I, Ali M, Li J and Li X: Reactive oxygen species mediate
isoalantolactone-induced apoptosis in human prostate cancer cells.
Molecules. 18:9382–9396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Min Z, Wang L, Jin J, Wang X, Zhu B, Chen
H and Cheng Y: Pyrroloquinoline quinone induces cancer cell
apoptosis via mitochondrial-dependent pathway and down-regulating
cellular Bcl-2 protein expression. J Cancer. 5:609–624. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao X, Liu X and Su L: Parthenolide
induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung
cancer cells. J Exp Clin Cancer Res. 33:32014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A,
Wu Q, Zhang J and Hong Y: Caspases: A molecular switch node in the
crosstalk between autophagy and apoptosis. Int J Biol Sci.
10:1072–1083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu HJ, Pu JL, Krafft PR, Zhang JM and Chen
S: The molecular mechanisms between autophagy and apoptosis:
Potential role in central nervous system disorders. Cell Mol
Neurobiol. 35:85–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou X, Zhang J, Jia Q, Ren Y, Wang Y, Shi
L, Liu N, Wang G, Pu P, You Y and Kang C: Reduction of miR-21
induces glioma cell apoptosis via activating caspase 9 and 3. Oncol
Rep. 24:195–201. 2010.PubMed/NCBI
|
|
24
|
Huang C, Chen X, Guo B, Huang W, Shen T,
Sun X, Xiao P and Zhou Q: Induction of apoptosis by Icariside II
through extrinsic and intrinsic signaling pathways in human breast
cancer MCF7 cells. Biosci Biotechnol Biochem. 76:1322–1328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arciuch VG Antico, Elguero ME, Poderoso JJ
and Carreras MC: Mitochondrial regulation of cell cycle and
proliferation. Antioxid Redox Signal. 16:1150–1180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang R, Lee IK, Piao MJ, Kim KC, Kim AD,
Kim HS, Chae S, Kim HS and Hyun JW: Butin
(7,3′,4′-trihydroxydihydroflavone) reduces oxidative stress-induced
cell death via inhibition of the mitochondria-dependent apoptotic
pathway. Int J Mol Sci. 12:3871–3887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaparou M, Choumerianou D, Perdikogianni
C, Martimianaki G, Kalmanti M and Stiakaki E: Enhanced levels of
the apoptotic BAX/BCL-2 ratio in children with acute lymphoblastic
leukemia and high-risk features. Genet Mol Biol. 36:7–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Renault TT, Floros KV, Elkholi R, Corrigan
KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla
JJ, Buettner C, et al: Mitochondrial shape governs BAX-induced
membrane permeabilization and apoptosis. Mol Cell. 57:69–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan M, Yu B, Rasul A, Al Shawi A, Yi F,
Yang H and Ma T: Jaceosidin Induces Apoptosis in U87 Glioblastoma
Cells through G2/M Phase Arrest. Evid Based Complement Alternat
Med. 2012:7030342012.PubMed/NCBI
|
|
30
|
Khan M, Yi F, Rasul A, Li T, Wang N, Gao
H, Gao R and Ma T: Alantolactone induces apoptosis in glioblastoma
cells via GSH depletion, ROS generation, and mitochondrial
dysfunction. IUBMB Life. 64:783–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan M, Li T, Khan MK Ahmad, Rasul A,
Nawaz F, Sun M, Zheng Y and Ma T: Alantolactone induces apoptosis
in HepG2 cells through GSH depletion, inhibition of STAT3
activation, and mitochondrial dysfunction. Biomed Res Int.
2013:7198582013.PubMed/NCBI
|
|
32
|
Buddingh EP, Schilham MW, Ruslan SE,
Berghuis D, Szuhai K, Suurmond J, Taminiau AH, Gelderblom H, Egeler
RM, Serra M, et al: Chemotherapy-resistant osteosarcoma is highly
susceptible to IL-15-activated allogeneic and autologous NK cells.
Cancer Immunol Immunother. 60:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakamoto A and Iwamoto Y: Current status
and perspectives regarding the treatment of osteo-sarcoma:
Chemotherapy. Rev Recent Clin Trials. 3:228–231. 2008. View Article : Google Scholar : PubMed/NCBI
|