Introduction
Melanoma differentiation-associated gene-7 (MDA-7),
also termed interleukin-24 (IL-24), as one of the IL-10 gene family
members, exhibits profound anticancer toxicity, with no adverse
effect on normal cells (1). The
ectopic overexpression of MDA-7/IL-24, either by a plasmid or a
recombinant adenovirus (Ad.MDA-7), induces apoptosis in a wide
range of tumor cells, however normal cells are not affected
(1–3).
Previous studies have demonstrated that the human
MDA-7/IL-24 protein performs physiological functions through
interacting with two heterodimeric cytokine receptor complexes,
IL-20 receptor 1 (IL-20R1)/IL-20R2 and IL-22R1/IL-20R2 (4,5).
Unlike the mammalian-produced protein, adenoviral and
bacterial-synthesized MDA-7/IL-24, with glutathione S-transferase
(GST) tag (GST-IL-24), suppresses tumor cell growth and promotes
apoptosis in an IL-20 receptor-independent manner (5). It has been suggested previously that
cancer cells may internalize GST-MDA-7/IL-24 independent of the
receptor attachment (6,7). By contrast with the GST-MDA-7/IL-24
and Ad.MDA-7 protein products, the purified mammalian-made
MDA-7/IL-24 protein did not exert any toxic effect on cells lacking
IL-20 receptors (8,9). Thus, IL-24 exhibits its
death-inducing function partly via a receptor-dependent pathway as
a classical cytokine (in the case of secreted soluble protein), but
also via an intracellular non-receptor-mediated manner (in the case
of GST-MDA-7/IL-24 and Ad.MDA-7) (5).
Theoretically, specific targeting of IL-24 protein
to tumor tissues/cells may significantly enhance its antitumor
effect in vivo. The arginine-glycine-aspartic acid (RGD)
peptide is a conserved motif on certain ligands with affinity to
integrins (10). The αvβ3 integrin
is significantly overexpressed in angiogenic endothelial cells and
certain tumor cells, such as melanoma, breast cancer, prostate
cancer and hepatocellular carcinoma. Since the αvβ3 integrin is a
marker for neovascularization, it has been developed as an
encouraging target for cancer therapy (11,12).
The phage display technique has demonstrated that the ACDCRGDCFCG
(RGD-4C) peptide sequences selectively bind to the αvβ3 integrin
(13,14). Because of the specific interaction
between RGD sequence and integrins, targeting delivery of the RGD
fused cytokine has been exhibited as an encouraging antineoplastic
approach (15–17).
In this regard, it is hypothesized that in
comparison with the native form, the RGD coupled IL-24 sequence may
harbor an enhanced antitumor activity as it can bind receptors on
neighbor cells following secretion. This modification retains the
natural intracellular activity of IL-24, but also improves its
bystander effect via protein attachment to neighboring cell
receptors (18). Accordingly, the
current study constructed a novel plasmid vector expressing RGD
fused to IL-24 with the aim of improving tumor targeting, then its
efficacy was evaluated in vitro. HepG2 hepatocellular
carcinoma line and LX-2 human liver stellate cell were selected as
tumor and normal cells, respectively, to compare apoptosis
induction properties of the modified and the native IL-24.
Materials and methods
Cell lines and culture
LX-2 human liver stellate cell line was provided by
Dr Scott L. Friedman (Mount Sinai School of Medicine, NY, USA). The
cell line is characterized as an immortal, non-malignant cell line
that retains key features of the hepatic stellate lineage. It was
included in the current study as a negative control/normal cell.
The HepG2 human liver cancer cell line and Ad-293 cell line were
purchased from the National Cell Bank, Pastor Institute of Tehran
(Tehran, Iran). All cells were maintained in Dulbecco's modified
Eagle's medium containing 10% fetal bovine serum, 10 mM glutamine,
100 U/ml penicillin, and 100 µg/ml streptomycin (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) under 5% CO2
atmosphere and 37°C condition.
Construction of
pcDNA3.1/RGD-IL-24
The original MDA-7 plasmid (pGEX-5X1/GST-IL-24) was
a gift from Dr Stephanie Kreis (Laboratoire de Biologie et
Physiologie Integree University of Luxembourg, Esch-sur-Alzette,
Luxembourg) (19). The plasmids,
expressing IL-24 and SP.RGD.IL-24, were constructed by stepwise
cloning. Using the P1 and P2 primers, which harbored the
BamHI and XhoI recognition sites (Table I), the normal MDA-7/IL-24 was
amplified by Taq DNA polyemerase (Thermo Fisher Scientific, Inc.)
using the following cycling conditions: Denaturation at 95°C for 5
min; followed by 35 cycles at 95, 58, and 72°C for 15 sec, 40 sec,
and 1 min, respectively. The amplified segment was directly cloned
into the pcDNA3.1 (Addgene, Inc., Cambridge, MA, USA) expression
vector. The resulting vector was designated as pc/IL-24.
 | Table I.Primers used in the study. |
Table I.
Primers used in the study.
| Primer name | Sequence (5′-3′) |
|---|
| P1 | CCCCCGGATCCGCCATGAATTTTCAACAGAGa |
| P2 | GGGGCTCGAGTCAGAGCTTGTAGAATTTCTb |
| P3 |
GGGGCCCAGGGCCAAGAATTC |
| S1 | TTTGGATCCATGTGGTGGAGACTGTGGTGGCTGCTTCTGTTGCTGCTTCTGTTGTGGCCTATGGTGTGGGCTc |
| S2 |
AGTGGAATTCTTGGCCCTGGGCCCCCGCCGCAGAAGCAGTCGCCCCGACAGTCGCAGGCAGCCCACACCATAGGCCACAd |
| Bax F1 |
GCCCTTTTGCTTCAGGGTTTCA |
| Bax R1 |
CAGCTTCTTGGTGGACGCAT |
| Gadd153 F |
CACCTCCTGGAAATGAAGAGGAAG |
| Gadd153 R |
GAGGTGCTTGTGACCTCTGC |
| PGK1 F1 |
TAAAGCCGAGCCAGCCAAAA |
| PGK1 R1 |
CTCCTACCATGGAGCTGTGG |
The modified IL-24 coding sequence with proceeding
RGD4C sequence was constructed by replacing the intrinsic IL-24
signal peptide sequence with a fusion of the artificial signal
peptide RGD4C sequence. The secrecon is a
bioinformatically-designed signal sequence with a high secretion
potency, which is composed of 21 amino acids (20). The cleavage site of the secrecon in
the fusion sequence was predicted just before the RGD sequence
using SignalP v.4.0 software (http://www.cbs.dtu.dk/services/SignalP/) (21).
The fusion segment of the artificial signal peptide
RGD4C was made by extending the forward (S1) and the reverse (S2)
primers. This sequence was termed SP-RGD4C. The S1 and S2 primers
had an overlapping complementary 20-base sequence in their 3′ ends
(double underline), which allows the primers to extend each other.
Additionally, the IL-24 coding region was amplified without its
intrinsic signal peptide sequence by the P2 and P3 primers. As the
forward primer (P3) was designed immediately following the signal
sequence, the amplified segment did not contain the signal
sequence. The S2 primer had 25 overlapped bases in the 5′end
(underlined) with the IL-24 sequence immediately following the
signal sequence. Therefore, the IL-24 without intrinsic signal
sequence was fused to the SP-RGD4C using the synthesis by overlap
extension polymerase chain reaction (PCR) method. The total
sequence was termed SP.RGD.IL-24. Due to the features of the S1 and
P2 primers with the BamHI and the XhoI recognition
sites, respectively, SP.RGD.IL-24 final amplicons were adjustable
for the directed cloning into the pcDNA3.1 expression vector. The
vector, containing the SP.RGD.IL-24 sequence, was termed
pc/SP.RGD.IL-24. The gene insertion and the integrity of the
constructs were assessed through PCR, restriction analysis and
sequencing.
DNA transfection
Cells were plated 1 day before transfection. A total
of 4×105 cells were seeded per well in 6-well plates and
transfected with 1 µg plasmid vectors using Lipofectamine-LTX™
(Thermo Fisher Scientific, Inc.) transfection reagent according to
the manufacturer's instructions. The cell lines were transfected
separately with empty pCDNA3.1 (pc), pc/IL-24 or pc/SP.RGD.IL-24.
To optimize plasmid transfection method and estimate transfection
rate, a control GFP-expressing plasmid (pAdenovator-CMV5-IRES-EGFP
vector; Qbiogene; MP Biomedicals, LLC, Santa Ana, CA, USA) was also
included. The percentage of GFP expression was observed using
fluorescent microscopy and used to estimate the transfection
rate.
Enzyme-linked immunosorbent assay
(ELISA)
In order to evaluate the gene expression potency of
the new constructs, after cultivating the transfected and
non-transfected Ad-293 for 72 h, the supernatants were collected
and centrifuged at 3,000 × g for 5 min at room temperature.
The concentrations of IL-24 or SP.RGD.IL-24 in Ad-293 supernatants
were quantified using an IL-24 human ELISA kit (Abcam, Cambridge,
MA, USA; cat. no. ab171345), following the manufacturer's
instructions. All the experiments were performed in triplicate and
the mean value was included in the analysis.
Cell attachment screening
As the final product of the novel SP.RGD.IL-24
sequence was proposed to anchor to integrin receptors, a simple
attachment assay was performed to determine the potency. For this
purpose, an ELISA-based method was performed. In this regard, at
first Ad-293 cells were transfected with each of the pc.1/IL-24 or
pc/SP.RGD.IL-24 constructs. After 72 h, the supernatants of these
cells were collected and the concentrations of the gene products
were estimated using an IL-24 ELISA kit. The gene products were
then diluted to the same concentrations. HepG2 cells were plated in
a 6-well plate (4×105 cells per well), 2 days prior to
the attachment assay. The HepG2 cell is among the types of liver
cell to express integrins, thus, it can support the attachment of
RGD-modified protein.
Subsequently, the supernatant of cells was removed
and the plated cells were washed twice with PBS. Then, different
supernatants from transfected Ad-293 cells, containing different
gene products, but with the same concentration, were added to each
well of HepG2 cells. After 2 h, the supernatants were removed
slowly and the concentrations of the protein products were
estimated by the IL-24 ELISA kit again. The reduced amount of the
protein concentrations were estimated as the cell attachment
capability of the gene products. The assays were performed in
triplicate.
cDNA synthesis and quantitative PCR
(qPCR)
Total cellular RNA was isolated from cells 36 h
after transfection by using the Total RNA Isolation System kit
(Promega Corporation, Madison, WI, USA) in accordance with the
manufacturer's instructions. The relative RNA integrity was, then,
checked by visualizing the ribosomal RNA bands via gel
electrophoresis and using a NanoDrop (Thermo Fisher Scientific,
Inc., Pittsburgh, PA, USA). Total RNA (~1 µg) was subjected to
reverse transcription using the cDNA Synthesis Premix (GeneAll
Biotechnology Co. Ltd, Seoul, South Korea), according to the
manufacturer's instructions.
qPCR was performed on the ABI 7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using qPCR SYBR GreenMaster mix (Jena Bioscience GmbH, Jena,
Germany). Thermal cycling was performed using the following
conditions: 95°C for 5 min as the first denaturation step, followed
by 40 cycles at 95, 60, and 72°C for 15 sec, 40 sec, and 1 min,
respectively. Each individual run was followed by a melting curve
analysis for 65–95°C to ensure the homogeneity of the PCR products.
The phosphoglycerate kinase 1 gene was used as a reference gene to
normalize the expression levels. Based on the similar efficiency of
the qPCRs, the relative quantification of genes was measured using
the 2−ΔΔCq method and represented as fold change in
expression (22). The assays were
performed in triplicate independently. The primer sequences,
employed in the qPCR assays, are listed in Table I.
Apoptosis analysis
An apoptosis assay was performed using a propidium
iodide (PI)/annexin V-APC staining kit (eBioscience, Inc., San
Diego, CA, USA), according to the manufacturer's protocols. In
brief, the transfected cells were trypsinized and harvested 48 h
after transfection, washed with PBS, and resuspended in 1% binding
buffer (200 µl). The cells of different wells were, then, aliquoted
equally and stained sequentially with annexin V-conjugated APC and
PI for 15 min in the dark. Subsequently, 300 µl binding buffer was
added to the mixture and the acquisition was immediately performed
on a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). The analyses were completed by using Cell Quest Pro 5.1
software package (BD Biosciences). The sum of early and late
apoptosis cells percentages was considered as the total percentage
of apoptotic cells for further analysis. The assays were performed
in three independent experiments and subsequently, the means of
each group were statistically compared.
Statistical analysis
In all of the experiments, the statistical
differences between the means were evaluated by one-way analysis of
variance followed by Tukey post test evaluation. P<0.05 was
considered to indicate a statistically significant difference.
Values are presented as the mean ± standard deviation.
Results
Construction and expression of
pcDNA3.1/IL-24 and pcDNA3.1/SP.RGD.IL-24
Following cloning of two different sequences into
the pcDNA3.1 plasmid, all PCR reactions, restriction analysis and
sequencing result confirmed the vector integrity, sequence
accuracy, and the correct direction of inserts. The sequencing
results confirmed that SP.RGD.IL-24 had an artificial signal
sequence, followed by the RGD4C sequence, and the sequence of IL-24
beyond its intrinsic signal sequence.
The IL-24 gene, with an intrinsic signal peptide,
produces a secretory protein, which is a common feature of all
cytokines. Additionally, the novel SP.RGD.IL-24 sequence, with an
artificial signal peptide, was expected to similarly produce a
secretory protein. As the most reliable test for the protein
secretion assay, the concentration of IL-24 in the supernatants of
the transfected cells was quantified by ELISA. The results
demonstrated that the concentration of IL-24 in the supernatant of
the A-293 cells transfected with pc/SP.RGD.IL-24, was fairly
similar to pc/IL-24, albeit the former construct exhibited less
production (P>0.05). As expected, the concentration of IL-24 in
the supernatant of the transfected cells, with an empty pcDNA3.1,
was negligible (data not shown).
Tethered RGD motif targets IL-24 to
cancer cells
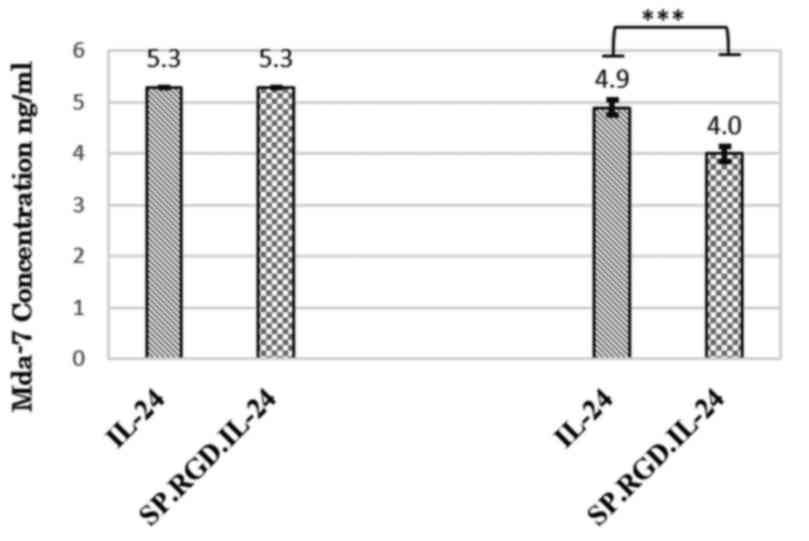
To assess whether the RGD motif of SP.RGD.IL-24 is
functional and if is accessible to its cognate integrin, αvβ3, an
ELISA based method was employed. Reduction of gene products in the
presence of HepG2 cells indirectly indicated that adhesion of
SP.RGD.IL-24 to integrin αvβ3 is increased compared with IL-24
(Fig. 1).
Effect of the modified SP.RGD.IL-24 on
the expression level of pro-apoptotic DNA damage inducible
transcript 3 (Gadd153) and BCL2 associated X apoptosis regulator
(Bax) genes
Three prepared plasmids were transfected into LX-2
cells (as the normal control) and HepG2 liver cancer cells. Ad-293,
LX-2 and HepG2 cells exhibited different rates of susceptibility to
transfection. The GFP signal counting under microscope (data not
shown) indicated that transfection rate for Ad-293, LX-2 and HepG2
cells were estimated to be ~80, 70 and 50% respectively, during
experiments. Subsequently, qPCR was employed to compare the
expression level of two pro-apoptotic genes (Gadd153 and Bax),
following transfection of plasmids expressing IL-24 and the
modified SP.RGD.IL-24 in LX-2 and HepG2 cells. Notably, the
attained data exhibited a significant difference of the response to
plasmids in normal and tumor cells. The expression data analysis
demonstrated that the expression of the modified SP.RGD.IL-24,
similarly to IL-24, significantly upregulated the Gadd153 (2.5
fold) and Bax (1.9 fold) expression levels in RNA extracted from
plasmid-transfected LX-2 and HepG2 cells, compared with the empty
plasmid when assayed on HepG2 (P<0.05). However, the gene
expression induced by the SP.RGD.IL-24 construct was significantly
greater than that induced by IL-24. As a result, Gadd153 and Bax
exhibited 74 and 73% overexpression, respectively (P<0.05). In
contrast to the HepG2 cells, the expression of IL-24 or
SP.RGD.IL-24 in LX-2 cells did not have a significant effect on
Gadd153 and Bax mRNA expression levels compared with the empty
plasmid (P>0.05; Fig. 2).
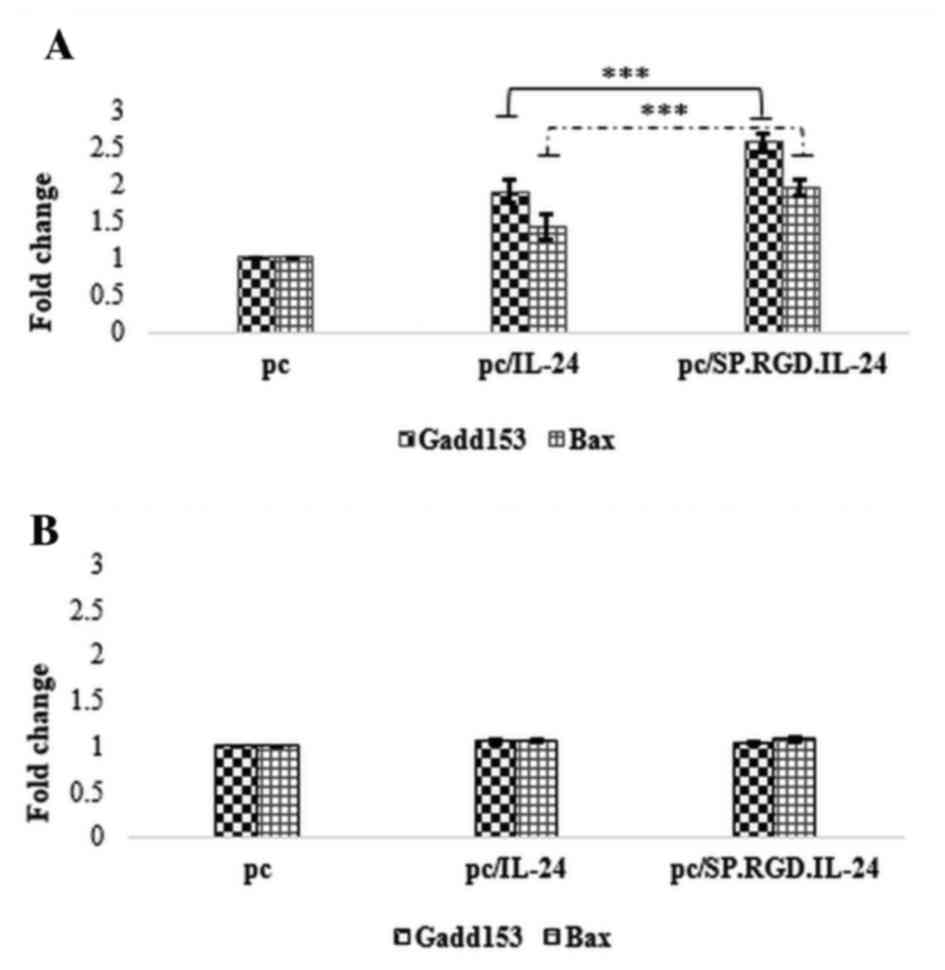 | Figure 2.Effect of IL-24 and the modified
SP.RGD.IL-24 on the expression level of Gadd153 and Bax
pro-apoptotic genes in HepG2 and LX-2 cells. Gadd153 and Bax mRNA
expression levels were evaluated by reverse
transcription-quantitative polymerase chain reaction relative to
the phosphoglycerate kinase 1 as a reference gene, in HepG2 and
LX-2 cells, 36 h after the transfection with pcDNA3.1, pc/IL-24 or
pc/SP.RGD.IL-24 plasmids. (A) Gadd153 and Bax mRNA expression
levels in HepG2 cells, transfected with either pc/IL-24 or
pc/SP.RGD.IL-24 plasmids were significantly higher (P<0.05) than
the cells transfected with pcDNA3.1. Gadd153 and Bax mRNA
expression levels in HepG2 cells, transfected with pc/SP.RGD.IL-24
plasmids were higher (74 and 73%, respectively) than the cells,
transfected with pc/IL-24. (B) Gadd153 and Bax mRNA expression
levels in LX-2 cells, transfected with pcDNA3.1, pc/IL-24 or
pc/SP.RGD.IL-24 plasmids did not exhibit significant differences
(P>0.05). The columns represent the means of three different
experiments, and the bars indicate the standard deviation.
***P<0.05, comparison indicated by brackets. pc, pcDNA3.1;
IL-24, interleukin-24; Gadd153, DNA damage inducible transcript 3;
Bax, BCL2 associated X apoptosis regulator. |
Apoptosis induction by the modified
SP.RGD.IL-24
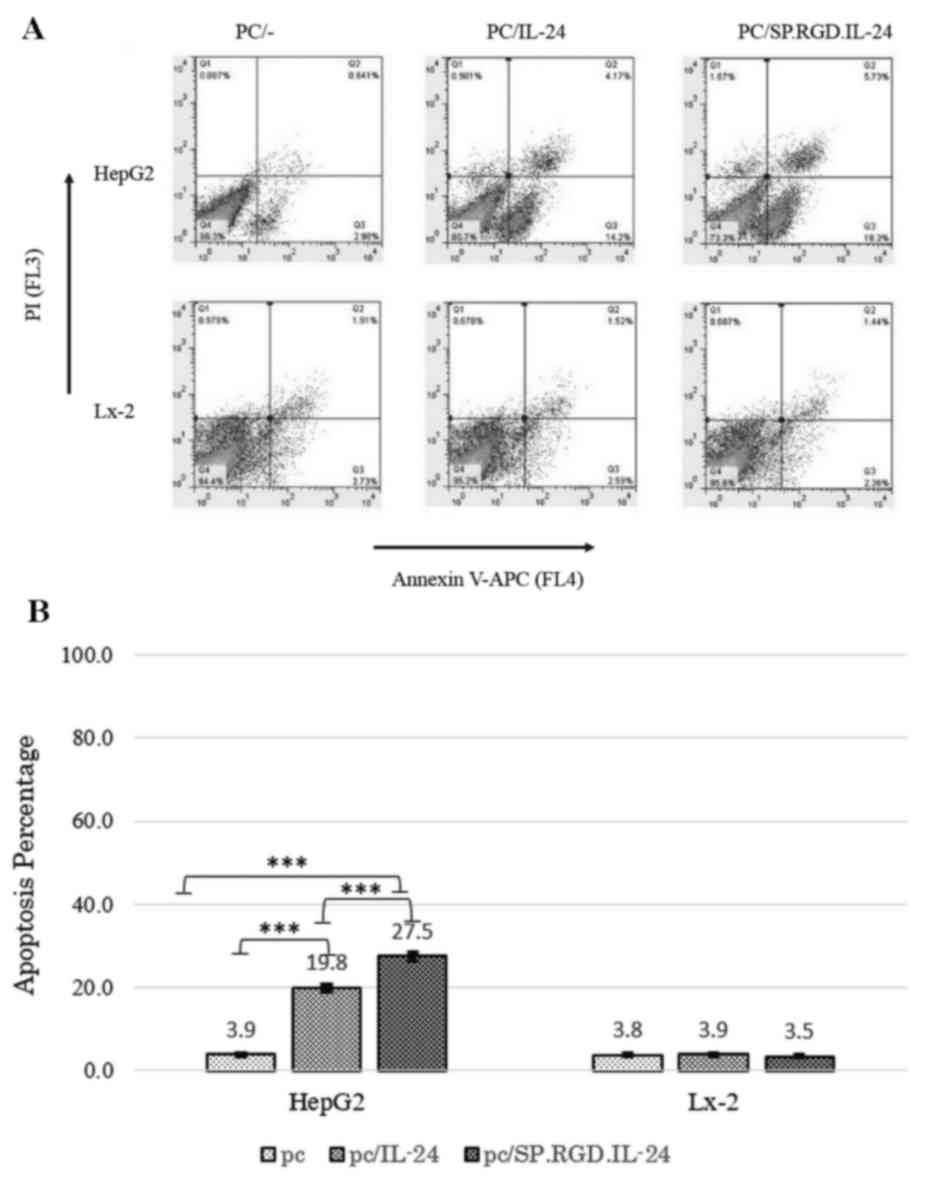
The annexin/PI method was used to investigate the
apoptosis induction of different constructs by flow cytometry. As a
result, in the analysis, the Q2 and Q3 quadrants were determined as
the early and late apoptosis-induced cells, respectively (Fig. 3A). The percentage of total
apoptosis (early and late stages) for the acquired population
following HepG2 transfection were as follows: 19.8±4.6% for the
native IL-24 (P=0.00051), 27.5±4.8% for the modified SP.RGD.IL-24,
and 3.9±1.4% for the empty plasmid (Fig. 3). Modified SP.RGD.IL-24 had
significantly higher apoptosis induction potency when compared with
unmodified IL-24 (P<0.05). Additionally, the amount of necrosis
events was negligible according to the data from the Q1 section
(Fig. 3A). In transfected LX-2
cells, no significant changes in the apoptotic population was
detected compared with the control group, and the quantity of cells
in Q2 and Q3 was similar (<4%) in all groups (Fig. 3).
In brief, the results demonstrated that the modified
SP.RGD.IL-24 was more efficient in the induction of apoptosis in
HepG2 cells compared with the IL-24. However, SP.RGD.IL-24, similar
to IL-24, had no detectable effect on apoptosis of non-cancerous
LX-2 cells.
Discussion
It remains unclear how MDA-7/IL-24 exerts its
cancer-specific cell killing activity. MDA-7/IL-24 predominantly
exhibits antitumor activity via ER stress and other intracellular
apoptosis pathways. An additional scenario proposes that IL-24
exerts its activity, as a classical cytokine, in an extracellular
manner, known as the bystander effect. It was demonstrated that
soluble MDA-7/IL-24 acts through a cell signaling pathway by
interacting with the IL-20/IL-22 receptor complexes (23). Thus, the primary objective of the
current study was to improve the apoptotic properties of IL-24
through combining intracellular activity with its
receptor-dependent function, bystander effect. To achieve this an
RGD coupled IL-24 sequence was cloned in such a way to perform the
apoptosis activity like the native form and to also increase the
bystander effect due to an RGD peptide sequence in the
N-terminal.
The toxic bystander effect of secreted cytokines may
be improved by targeting of cancer cells using peptides. Pei et
al inserted a glycine code between glutamic acid and the
arginine code of IL-24 to produce a modified RGD-IL-24 with an
internal mutated RGD sequence. This modification enhanced the
attachment of the product to cancer cells and improved its
apoptosis-inducing function (24).
The most effective and established RGD-associated
motif is the RGD4C peptide. The sequence of this motif was added to
that of the IFN-α gene. Then, the modified IFN-α.RGD was introduced
into tumor vessels by a DNA plasmid. The modified IFN-α.RGD gene
therapy exhibited a more effective suppression of tumor development
than the wild-type IFN-α gene therapy (17).
In our previous study, it was demonstrated that
adding the RGD4C sequence to the carboxyl end of IL-24 adversely
decreases its antitumor function. The modeling analysis revealed
that this kind of modification strongly disrupts the IL-24 binding
to the relevant receptor in silico (25). However, RGD4C motif in the
amino-terminal of MDA-7/IL-24 protein, which was produced in
bacteria, did not have any effect on its apoptosis-inducing
activity (26). On the basis of
these findings, a vector expressing modified RGD-IL-24 cDNA with a
proceeding RGD4C-coding sequence was constructed.
Even though with limited studies regarding the
application of exogenous/soluble IL-24 protein (24,27)
a question may arise that why exogenous protein was not used
instead of the plasmid vector in the current study? It was
demonstrated that direct application of soluble IL-24 protein for
tumor killing exhibited less cytotoxic effect, when compared with
protein endogenously expressed from a plasmid. This difference is
partly related to the more effective interaction of endogenous
IL-24 protein with endoplasmic chaperone protein BiP/GRP78, which
induces endoplasmic stress (28).
Here, the modified SP.RGD.IL-24 was designed so that
the intrinsic signal sequence of IL-24 is exchanged with a fusion
of the artificial signal sequence (secrecon) and the RGD4C
sequence. Since the signal sequence of IL-24 is long (49 amino
acids), a shorter signal sequence with a higher secretion potency
was used. As the secrecon, a bioinformatically-designed signal
sequence (20), was employed for
the first time fused with IL-24, it was not clear if it would
retain the secretion property of SP-RGD-IL-24. The ELISA result
using the supernatant of the transfected Ad-293 cells demonstrated
suitable secretion of the modified RGD-IL-24, indicating the
correct action of the secrecon. The expression level of the
modified and the native IL-24 were comparable as determined by
ELISA. However, the data also indicated that these types of
modifications on IL-24 cause a relative decrease in protein
secretion, albeit not at a significant level.
An ELISA-based assay was also applied to observe if
the modified SP.RGD.IL-24 product, compared to the native IL-24,
has increased adhesion properties for cancerous HepG2 cells. This
method demonstrated that the targeting of SP.RGD.IL-24 to HepG2
cells was increased compared with native IL-24 product.
It was demonstrated that the ectopic expression of
IL-24 leads to an enhanced expression of pro-apoptotic genes,
including Gadd153 and Bax (29,30).
The upregulation of Bax gene expression is a well-established
marker of apoptosis with roles in the intrinsic and extrinsic
apoptosis pathways, and was thus, selected for expression analysis
in the current study (31).
Gadd153 is involved in ER stress-associated apoptosis and its
expression is known to be induced following endogenous IL-24
expression (6). The ability of the
modified SP.RGD.IL-24 and the unmodified IL-24 to induce expression
of these pro-apoptotic genes was compared following the
transfection of HepG2 cell lines with the IL-24 constructs. qPCR
analysis demonstrated that the modified SP.RGD.IL-24 upregulated
the expression of pro-apoptotic genes more efficiently than the
unmodified IL-24. This finding demonstrated that the RGD
modification did have a detrimental impact on the function of the
modified IL-24 protein. However, neither the modified SP.RGD.IL-24,
nor the native IL-24, had an observable effect on the expression of
these pro-apoptotic genes in the LX-2 human liver stellate cell
line. To the best of our knowledge, the effect of IL-24 on normal
liver stellate cell apoptosis has not been previously investigated.
This mode of IL-24 cytokine action on human stellate cell may
strongly support its safety prolife for employment in human
patients.
Annexin/PI staining and flow cytometry analysis, in
accordance with the expression results, revealed that the modified
SP.RGD.IL-24, compared with the unmodified IL-24 gene, had a
greater capacity to induce apoptosis in the HepG2 cancer cell line.
In the vast majority of previous cases, adenovector was employed as
the expression/delivery vector of IL-24 in vitro and in
vivo. The result of the adenovirus expression vector indicated
suitable production of IL-24 and apoptosis induction (33.5%)
(32–35). However, in the present study a
plasmid construct was employed to express the modified and native
IL-24. The previously obtained results supported the critical role
of adenovector expression vector in increasing the induction of
apoptosis by IL-24 (32–35). By contrast to adenovectors, plasmid
expression of IL-24 is considered less effective and, thus,
apoptosis induction decreases. Increased levels of apoptosis are
expected when these plasmid constructs are replaced by an
adenovector-expression system. However, the modified SP.RGD-IL-24
or native IL-24 did not induce apoptosis in the LX-2 human liver
fibroblast cell line.
In conclusion, the findings of the current study
indicated the following: i) The new RGD modified construct retained
sufficient expression and secretion propensity; ii) SP.RGD-IL-24
attachment to the cognate receptor and integrin was increased
compared with native IL-24; and iii) SP.RGD-IL-24 triggered
apoptosis in a HCC-associated cell more effectively than native
IL-24, and did not affect apoptosis in normal stellate cells.
Collectively, the obtained findings supported that the newly
generated construct may be utilized in an adenoviral vector as a
method of gene therapy.
Acknowledgements
The authors would like to highly appreciate all the
help of the members of the Clinical Microbiology Research Center at
Shiraz University of Medical Sciences, especially Dr Mehdi Kalani
for her assistance in flow cytometry analysis. The authors also
wish to thank other colleagues in the Clinical Microbiology
Research Center at Shiraz University of Medical Sciences, including
Mr. Javad Moayedi, Ms. Maryam Mousavi, Mr. Saeed Amirzadeh, Ms.
Maryam Nejabat and Mr. Amir Arastefar.
References
|
1
|
Ekmekcioglu S, Ellerhorst J, Mhashilkar
AM, Sahin AA, Read CM, Prieto VG, Chada S and Grimm EA:
Down-regulated melanoma differentiation associated gene (mda-7)
expression in human melanomas. Int J Cancer. 94:54–59. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ellerhorst JA, Prieto VG, Ekmekcioglu S,
Broemeling L, Yekell S, Chada S and Grimm EA: Loss of MDA-7
expression with progression of melanoma. J Clin Oncol.
20:1069–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Sun A, Xu R, Tao X, Dong Y, Lv X
and Wei D: Cell-penetrating and endoplasmic reticulum-locating
TAT-IL-24-KDEL fusion protein induces tumor apoptosis. J Cell
Physiol. 231:84–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parrish-Novak J, Xu W, Brender T, Yao L,
Jones C, West J, Brandt C, Jelinek L, Madden K, McKernan PA, et al:
Interleukins 19, 20, and 24 signal through two distinct receptor
complexes. Differences in receptor-ligand interactions mediate
unique biological functions. J Biol Chem. 277:47517–47523. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Persaud L, De Jesus D, Brannigan O,
Richiez-Paredes M, Huaman J, Alvarado G, Riker L, Mendez G, Dejoie
J and Sauane M: Mechanism of Action and Applications of Interleukin
24 in Immunotherapy. Int J Mol Sci. 17(pii): E8692016.PubMed/NCBI
|
|
6
|
Lebedeva IV, Emdad L, Su ZZ, Gupta P,
Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, et al:
mda-7/IL-24, novel anticancer cytokine: Focus on bystander
antitumor, radiosensitization and antiangiogenic properties and
overview of the phase I clinical experience (Review). Int J Oncol.
31:985–1007. 2007.PubMed/NCBI
|
|
7
|
Emdad L, Lebedeva IV, Su ZZ, Gupta P,
Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D and Fisher
PB: Historical perspective and recent insights into our
understanding of the molecular and biochemical basis of the
antitumor properties of mda-7/IL-24. Cancer Biol Ther. 8:391–400.
2009.PubMed/NCBI
|
|
8
|
Nishikawa T, Ramesh R, Munshi A, Chada S
and Meyn RE: Adenovirus-mediated mda-7 (IL24) gene therapy
suppresses angiogenesis and sensitizes NSCLC xenograft tumors to
radiation. Mol Ther. 9:818–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pataer A, Bocangel D, Chada S, Roth JA,
Hunt KK and Swisher SG: Enhancement of adenoviral MDA-7-mediated
cell killing in human lung cancer cells by geldanamycin and its
17-allyl- amino-17-demethoxy analogue. Cancer Gene Ther. 14:12–18.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruoslahti E: The RGD story: A personal
account. Matrix Biol. 22:459–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X, Lu D, Scully M and kakkar V: The
role of integrins in cancr and integrin therapeutic agent for
cancer therapy. Perspect Medicin Chem. 2:57–73. 2008.PubMed/NCBI
|
|
12
|
Brooks PC, Clark RA and Cheresh DA:
Requirement of vascular integrin alpha v beta 3 for angiogenesis.
Science. 264:569–571. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Assa-Munt N, Jia X, Laakkonen P and
Ruoslahti E: Solution structures and integrin binding activities of
an RGD peptide with two isomers. Biochemistry. 40:2373–2378. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arap W, Pasqualini R and Ruoslahti E:
Cancer treatment by targeted drug delivery to tumor vasculature in
a mouse model. Science. 279:377–380. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dickerson EB, Akhtar N, Steinberg H, Wang
ZY, Lindstrom MJ, Padilla ML, Auerbach R and Helfand SC:
Enhancement of the antiangiogenic activity of interleukin-12 by
peptide targeted delivery of the cytokine to alphavbeta3 integrin.
Mol Cancer Res. 2:663–673. 2004.PubMed/NCBI
|
|
16
|
Curnis F, Gasparri A, Sacchi A, Longhi R
and Corti A: Coupling tumor necrosis factor-alpha with alphaV
integrin ligands improves its antineoplastic activity. Cancer Res.
64:565–571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Craig R, Cutrera J, Zhu S, Xia X, Lee YH
and Li S: Administering plasmid DNA encoding tumor vessel-anchored
IFN-alpha for localizing gene product within or into tumors. Mol
Ther. 16:901–906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sauane M, Su ZZ, Gupta P, Lebedeva IV,
Dent P, Sarkar D and Fisher PB: Autocrine regulation of mda-7/IL-24
mediates cancer-specific apoptosis. Proc Natl Acad Sci USA.
105:9763–9768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khodadad M, Hosseini SY, Shenavar F,
Erfani N, Bina S, Ahmadian S, Fattahi MR and Hajhosseini R:
Construction of expressing vectors including melanoma
differentiation-associated gene-7 (mda-7) fused with the RGD
sequences for better tumor targeting. Iran J Basic Med Sci.
18:780–787. 2015.PubMed/NCBI
|
|
20
|
Barash S, Wang W and Shi Y: Human
secretory signal peptide description by hidden Markov model and
generation of a strong artificial signal peptide for secreted
protein expression. Biochem Biophys Res Commun. 294:835–842. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petersen TN, Brunak S, von Heijne G and
Nielsen H: SignalP 4.0: Discriminating signal peptides from
transmembrane regions. Nat Methods. 8:785–786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dent P, Yacoub A, Hamed HA, Park MA, Dash
R, Bhutia SK, Sarkar D, Gupta P, Emdad L, Lebedeva IV, et al:
MDA-7/IL-24 as a cancer therapeutic: From bench to bedside.
Anticancer Drugs. 21:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pei DS, Yang ZX, Zhang BF, Yin XX, Li LT,
Li HZ and Zheng JN: Enhanced apoptosis-inducing function of
MDA-7/IL-24 RGD mutant via the increased adhesion to tumor cells. J
Interferon Cytokine Res. 32:66–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bina S, Shenavar F, Khodadad M, Haghshenas
MR, Mortazavi M, Fattahi MR, Erfani N and Hosseini SY: Impact of
RGD peptide tethering to IL24/mda-7 (Melanoma Differentiation
Associated Gene-7) on apoptosis induction in hepatocellular
carcinoma cells. Asian Pac J Cancer Prev. 16:6073–6080. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao B, Li W, Yang J, Guo G, Mao XH and
Zou QM: RGD-IL-24, a novel tumor-targeted fusion cytokine:
Expression, purification and functional evaluation. Mol Biotechnol.
41:138–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Q, Jin B, Zhang Y, Shi Y, Zhang C, Luo
D, Wang P, Duan C, Song H, Li X, et al: Secreted recombinant human
IL-24 protein inhibits the proliferation of esophageal squamous
cell carcinoma Eca-109 cells in vitro and in vivo. Oncol Rep.
35:2681–2690. 2016.PubMed/NCBI
|
|
28
|
Sieger KA, Mhashilkar AM, Stewart A,
Sutton RB, Strube RW, Chen SY, Pataer A, Swisher SG, Grimm EA,
Ramesh R and Chada S: The tumor suppressor activity of MDA-7/IL-24
is mediated by intracellular protein expression in NSCLC cells. Mol
Ther. 9:355–367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su ZZ, Madireddi MT, Lin JJ, Young CS,
Kitada S, Reed JC, Goldstein NI and Fisher PB: The cancer growth
suppressor gene mda-7 selectively induces apoptosis in human breast
cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad
Sci USA. 95:14400–14405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarkar D, Su ZZ, Lebedeva IV, Sauane M,
Gopalkrishnan RV, Valerie K, Dent P and Fisher PB: mda-7 (IL-24)
Mediates selective apoptosis in human melanoma cells by inducing
the coordinated overexpression of the GADD family of genes by means
of p38 MAPK. Proc Natl Acad Sci USA. 99:10054–10059. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CJ, Xue XB, Yi JL, Chen K, Zheng JW,
Wang J, Zeng JP and Xu RH: Melanoma differentiation-associated
gene-7, MDA-7/IL-24, selectively induces growth suppression,
apoptosis in human hepatocellular carcinoma cell line HepG2 by
replication-incompetent adenovirus vector. World J Gastroenterol.
12:1774–1779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishikawa T, Ramesh R, Munshi A, Chada S
and Meyn RE: Adenovirus-mediated mda-7 (IL24) gene therapy
suppresses angiogenesis and sensitizes NSCLC xenograft tumors to
radiation. Mol Ther. 9:818–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He
L, Wang Y, Zhang J, Zhang Z, Huiwang J, et al: Potent antitumor
activity of oncolytic adenovirus expressing mda-7/IL-24 for
colorectal cancer. Hum Gene Ther. 16:845–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan X, Wu L, Cao J, Guo W, Wang Z, Han B
and Hu W: Recombinant adenovirus vector-mediated human MDA-7 gene
transfection suppresses hepatocellular carcinoma growth in a mouse
xenograft model. J Biomed Res. 26:53–58. 2012. View Article : Google Scholar : PubMed/NCBI
|