Introduction
Nonalcoholic fatty liver disease (NAFLD) is an
oxidative stress-associated liver lesion, which is caused by
abnormal lipid metabolism, eliminating excessive drinking and other
specific factors causing liver damage. At present, the incidence of
NAFLD is increasing and at increasingly younger ages (1). This increase appears to be pandemic,
as it is beginning to affect populations in developing countries
due to the increased western lifestyle (2). NAFLD has become an important factor
in chronic liver injury following viral hepatitis (3). The pathogenesis of NAFLD remains to
be fully elucidate, however, it is closely associated with hepatic
lipid deposition, high levels of reactive oxygen species (ROS) and
oxidative stress damage caused by ROS (4,5).
In the formation of NAFLD, the redox state is out of
balance causing the associated oxidoreductases in tissues and body
fluids to alter correspondingly (6), which includes xanthine oxidase (XOD)
and paraoxonase 1 (PON1). XOD is an important enzyme in the
metabolism of nucleic acids in the body, which are released into
the circulation ahead of the enzyme, alanine aminotransferase
(ALT), when liver injury occurs. XOD can catalyze xanthine, which
cannot be further decomposed or metabolized, and accumulates due to
liver cell damage, leading to an oxidation reaction and the
production of peroxides. XOD is important in the generation of free
radicals (7). Serum concentrations
of XOD directly reflect the quantity of free radicals generated in
liver tissue, and the increase in free radicals is important in the
pathogenesis and development of NAFLD (8).
PON1 is an antioxidant enzyme synthesized and
secreted by the liver, which can hydrolyze lipid peroxides. PON1
has a potent antioxidant function through specific binding with
high-density lipoprotein (HDL). It is significant in protection
against atherosclerosis, coronary heart disease and diabetes
(9–11). Studies of liver disease have shown
that PON1 can inhibit lipid peroxidation, target inflammation,
relieve the progress of liver fibrosis and reduce the degree of
liver damage by reducing hepatic oxidative stress (12,13).
The activity of PON1 in the serum is decreased significantly when
liver damage occurs (14,15).
At present, there are few reports on alterations of
XOD and PON in NAFLD. The present study hypothesized that the
activities of XOD and PON1 reflect the oxidation and antioxidant
capacities in the body, namely the severity of oxidative stress
injury, which indirectly reflects the severity of NAFLD. In the
present study, an NAFLD rat model was established by feeding rats
with a high fat diet (HFD), and an in vitro NAFLD model was
established by treating L-02 human hepatocyte cells with oleic acid
(OA), with α-lipoic acid (α-LA) used for preventative intervention
in the NAFLD models. The present study aimed to observe alterations
in the activities of XOD and PON1 during modeling, examine the
preventive and therapeutic effects of α-LA on the NAFLD model in
vivo and in vitro, and determine the relevance between
the activities of XOD and PON1 and the severity of NAFLD.
Materials and methods
Chemicals and reagents
The assay kits used for measuring the serum levels
of ALT, aspartate transaminase (AST), total cholesterol (TC),
triglycerides (TG), HDL cholesterol (HDL-C), low-density
lipoprotein cholesterol (LDL-C), free fatty acids (FFAs), and liver
concentrations of TC, TG and FFAs, were purchased from Wako Pure
Chemical Industries, Ltd. (Osaka, Japan). The assay kits for
measuring XOD, malondialdehyde (MDA), superoxide dismutase (SOD),
catalase (CAT), glutathione (GSH), glutathione peroxidase (GPX) and
total antioxidant capacity (TAOC) were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Phenylacetate
was purchased from Aladdin Reagent Co., Ltd (Shanghai, China). OA
and Oil Red O were purchased from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). α-LA was purchased from STADA (Dresden,
Germany). 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) reagent was purchased from Roche Diagnostics GmbH
(Mannheim, Germany). DMEM culture medium was purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Animals and treatment
A total of 32 male Sprague-Dawley rats, weighing
180–200 g, were supplied by Jiangsu University Laboratory Animal
Center (Jiangsu, China). The animals were maintained at an ambient
temperature of 23.0±2°C and the natural circadian rhythm of light.
The rat model of NAFLD was established by feeding the rats with a
HFD) according to our previous study (16). Briefly, following acclimation for 1
week, the animals were randomly divided into four groups (8 rats
per group): Control, model, α-LA high dose (α-LAH) and
α-LA low dose (α-LAL). The rats in the control group
were fed a standard diet and rats in the other three groups were
fed a HFD for 12 weeks. The rats in the α-LAH and
α-LAL groups were administered with α-LA solution
intragastrically at doses of 20 and 40 mg/kg/day, respectively. The
rats in the control and model groups were administered with the
same volume of normal saline by intragastric administration. The
rats were weighed twice per week. At the end of weeks 4, 6, 8, 10
and 12, the rats were anesthetized with etherization, and blood was
collected from the fundus oculi venous plexus. Serum was isolated
(1,000 × g, centrifuged for 10 min, at 25°C) and frozen at
−80°C. At the end of the experiment, the rats were sacrificed by
cervical dislocation and entire livers were collected and weighed.
The liver index (LI) was calculated as follows: LI=liver
weight(g)/body weight(BW, g)x100%. Certain sections of the liver
tissue in the same positions were immersed in 10% buffered formalin
solution for histological examination. The remaining tissues were
frozen at −80°C for the production of hepatic tissue
homogenate.
NAFLD cell model construction
The L-02 cells were provided by the Experimental
Teaching Center of Medical Basis for Pharmacy, China Pharmaceutical
University (Nanjing, China). The in vitro NAFLD model was
established by treating the L-02 cells with OA. The L-02 cells were
seeded at a density of 5×105 cells/well in six-well
plates and incubated for 24 h at 37°C, 5% CO2 in DMEM
culture medium. The cells were switched to serum-free media
containing 60 µg/ml OA and incubated for a further 24 h. The cells
were stained using Oil Red O and examined for lipid accumulation by
inverted microscope (Nikon Corporation, Tokyo, Japan).
The L-02 cells were seeded into six-well plates at a
concentration of 5×105 cells/well and were incubated for
an initial 24 h. The cells then were divided into the following
three groups: Control, model and α-LA, and incubated for another 24
h. The control cells were switched to DMEM containing 2% fetal
bovine serum (FBS) at 37°C, 5% CO2. The model cells were
switched to media containing 2% FBS and 60 µg/ml OA. The
α-LA-treated cells were switched to media containing 2% FBS, 60
µg/ml OA and 50, 100, 200 or 400 µmol/l α-LA, respectively. Cell
viability was determined using an MTT assay and examined by
measuring the absorbance at 490 nm. The cells and culture
supernatants of all groups were collected for FFA, XOD and PON1
determination.
Determination of biochemical
indicators and redox indices
The serum levels of ALT, AST, TC, TG, HDL-C, LDL-C
and FFAs were determined using a 7600 Automatic Biochemistry
Analyzer (Hitachi, Tokyo, Japan) in accordance with the
manufacturer's protocols. The serum enzymatic activities of XOD,
PON1, SOD, CAT and GPX, and the levels of TAOC, MDA and GSH were
determined using a colorimetric method with commercial kits. The
activity of the PON1 enzyme was evaluated using phenylacetate as a
substrate to determine arylesterase activity. Briefly, the
arylesterase activity was measured using 1 mmol phenylacetate
(Aladdin, Beijing, China) as the substrate in 20 mmol/l Tris-HCl
buffer (pH 8.0) with 1 mmol/l CaCl2; the activity was
expressed as kU/l.
Preparation of the liver homogenates was performed
on ice. The liver tissues were homogenized in ice-chilled 0.9% NaCl
to yield 10% (w/v) homogenate and centrifuged at 1,000 × g
for 10 min at 4°C, with the resulting supernatant used for the
detection of TC, TG and FFAs, and the XOD, PON1, SOD, CAT, GPX,
TAOC, MDA and GSH redox indices.
Histological examination
The liver tissues were fixed in 10% formaldehyde
solution, embedded in paraffin, sliced into 5 µm thick sections,
and stained with hematoxylin and eosin (H&E). Two pathologists
performed the assessment of fatty degeneration and focal necrosis
independently. Pathological evaluations by optical microscope
(Olympus Corporation, Tokyo, Japan) were based on lobular
inflammation, necrosis, which was scored as follows: 0, 0 foci; 1,
<2 foci; 2, 2–4 foci; and 3, >4 foci per field
(magnification, ×200), and steatosis, which was scored as follows:
0, <5%; 1, 6–33%; 2, 34–66%; and 3, >66%.
Oil Red O staining
The L-02 cell monolayers were gently rinsed twice
with phosphate-buffered saline, fixed with 60% isopropanol for 1
min at room temperature, stained with 0.5% Oil Red O-isopropanol
for 10 min and then washed with distilled water. The cells were
visualized using a bright-field optical microscope (CX-41; Olympus
Corporation, Tokyo, Japan). Three typical high-power fields were
selected at random and the integral optical density (IOD) of the
red lipid droplets was analyzed using ImagePro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. All values are
expressed as the mean ± standard deviation. Comparison of different
groups was performed using Student's t-test and one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Alterations in the BWs and LIs of
rats
With the extension of the duration of HFD
administration, the BWs and LIs of rats in the model group were
significantly higher, compared with those in the controls
(P<0.01/P<0.05). α-LA significantly decreased the BWs of the
rats, compared with those in model group (P<0.01; Table I).
 | Table I.Alterations in the BW and LI of
rats. |
Table I.
Alterations in the BW and LI of
rats.
|
| BW (g) |
|
|---|
|
|
|
|
|---|
| Group | Week 4 | Week 8 | Week 12 LI (%) | LI (%) |
|---|
| Control | 327.00±8.49 | 425.63±18.21 | 471.88±30.85 | 2.67±0.20 |
| Model |
385.63±26.34b |
461.75±39.73a |
514.13±46.10a |
4.04±0.16b |
|
α-LAL |
306.88±11.46c |
399.13±30.613c |
421.63±27.26c |
3.55±0.25c |
|
α-LAH |
297.38±18.62c |
364.13±14.10c |
375.63±15.61c |
3.17±0.21c |
Alterations in serum liver function
and lipid indices
The ingestion of HFD by the rats in the model group
resulted in significant increases in ALT, AST, TC, TG, LDL-C and
FFAs, and a decrease in the level of HDL-C, compared with the rats
in the control group (P<0.01/P<0.05). The serum levels of
ALT, AST, TC, TG and FFAs in the rats in the α-LA group were
significantly lower, compared with those in the model rats, and the
levels of HDL-C and LDL-C in the α-LA group were higher and lower,
respectively, compared with those in the model rats, although
without statistical significance (Fig.
1A and B).
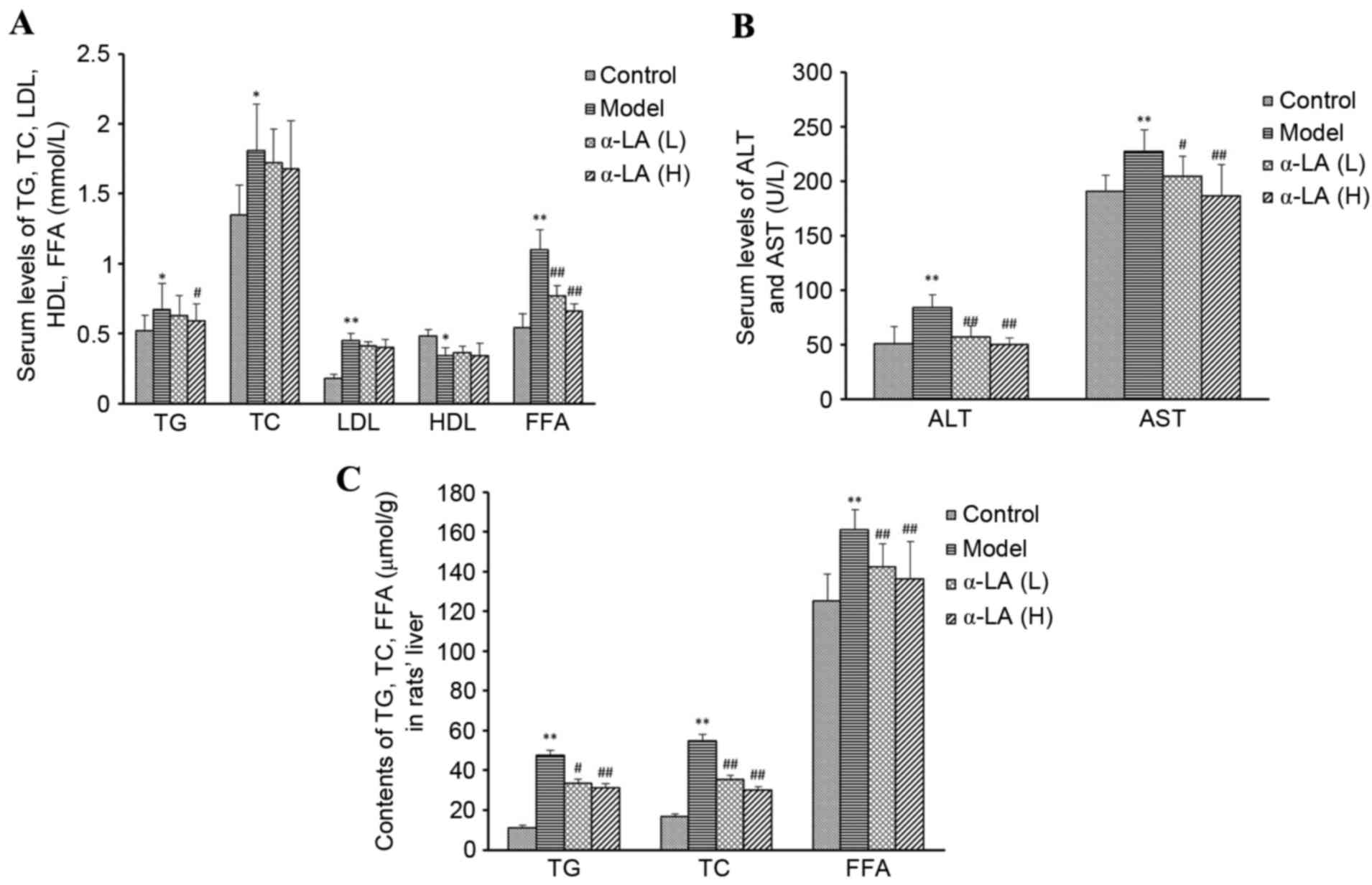 | Figure 1.Alterations in serum levels of ALT,
AST, TC, TG, HDL, LDL and FFAs, and intrahepatic TG, TC, and FFA
content. After 12 weeks of feeding a high fat diet, liver function
and serum lipids of Sprague-Dawley rats were detected. (A) Levels
of TC, TG, HDL, LDL and FFAs in serum. (B) Levels of ALT, AST in
serum. (C) Levels of TC, TG and FFAs in liver tissues. *P<0.05
and **P<0.01, vs. control group; #P<0.05 and
##P<0.01, vs. model group. ALT, alanine
aminotransferase; AST, aspartate transaminase; TC, total
cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL,
low-density lipoprotein; FFAs, free fatty acids. |
Alterations in hepatic lipids
The intrahepatic concentrations of TC, TG and FFAs
in the model rats were significantly higher, compared with those in
the control rats (P<0.01), and were significantly lower in the
α-LA rats, compared with the model rats (P<0.01). These results
showed that α-LA improved hepatic lipid metabolism (Fig. 1C).
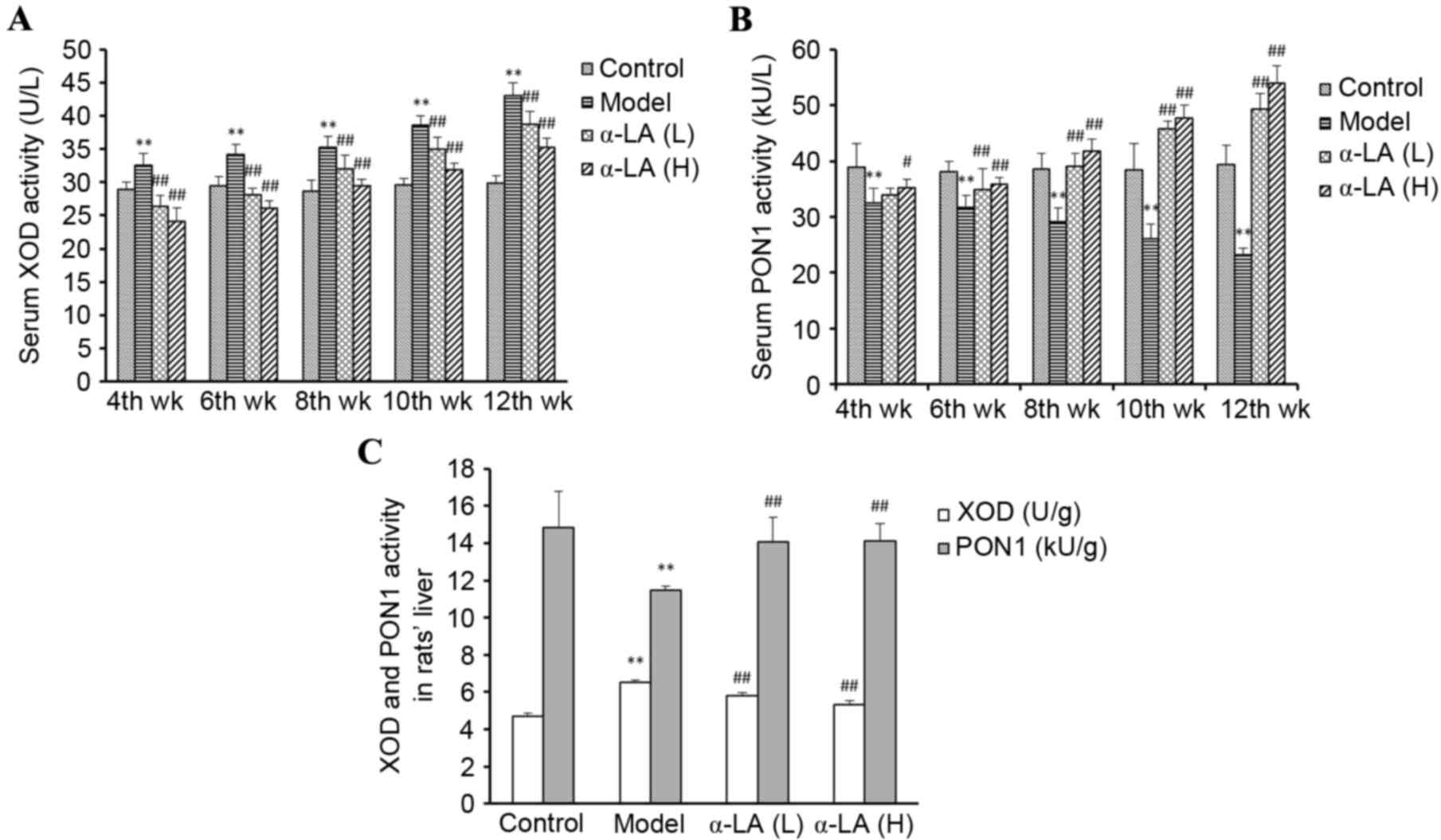
Alterations in the activities of XOD
and PON1
Compared with the rats in the control group, the
serum activity of XOD was significantly higher and that of PON1 was
significantly lower in the rats in the model group (P<0.01).
With the increased duration of HFD feeding, these alterations
became gradually more marked. In the model group, there were
significant differences between the week 4, 6, 8, 10 and 12
XOD/PON1 activities (P<0.01). The serum activities of XOD and
PON1 in the α-LA group rats were significantly lower and higher,
respectively, compared with those in the model group rats
(P<0.01; Fig. 2A and B). The
hepatic tissue activities of XOD and PON1 were also measured. The
HFD significantly increased the hepatic activity of XOD and
decreased the hepatic activity of PON1, compared with the control
group (P<0.01), and α-LA returned the activities of XOD and PON1
to normal (Fig. 2C).
Alterations in redox indices
The oxidative stress statuses of the rats were
evaluated by analyzing the serum and tissue levels of SOD, CAT,
GPX, TAOC, MDA and GSH. Compared with the control group rats, the
serum and tissue levels of SOD, CAT, GPX, TAOC and GSH were
significantly lower, and the level of MDA was significantly higher
in the model group rats (P<0.01). Compared with the model group
rats, the serum and tissue levels of SOD, CAT, GPX, TAOC and GSH
were significantly higher, and the level of MDA was significantly
lower in the α-LA group rats (P<0.01; Fig. 3A-F).
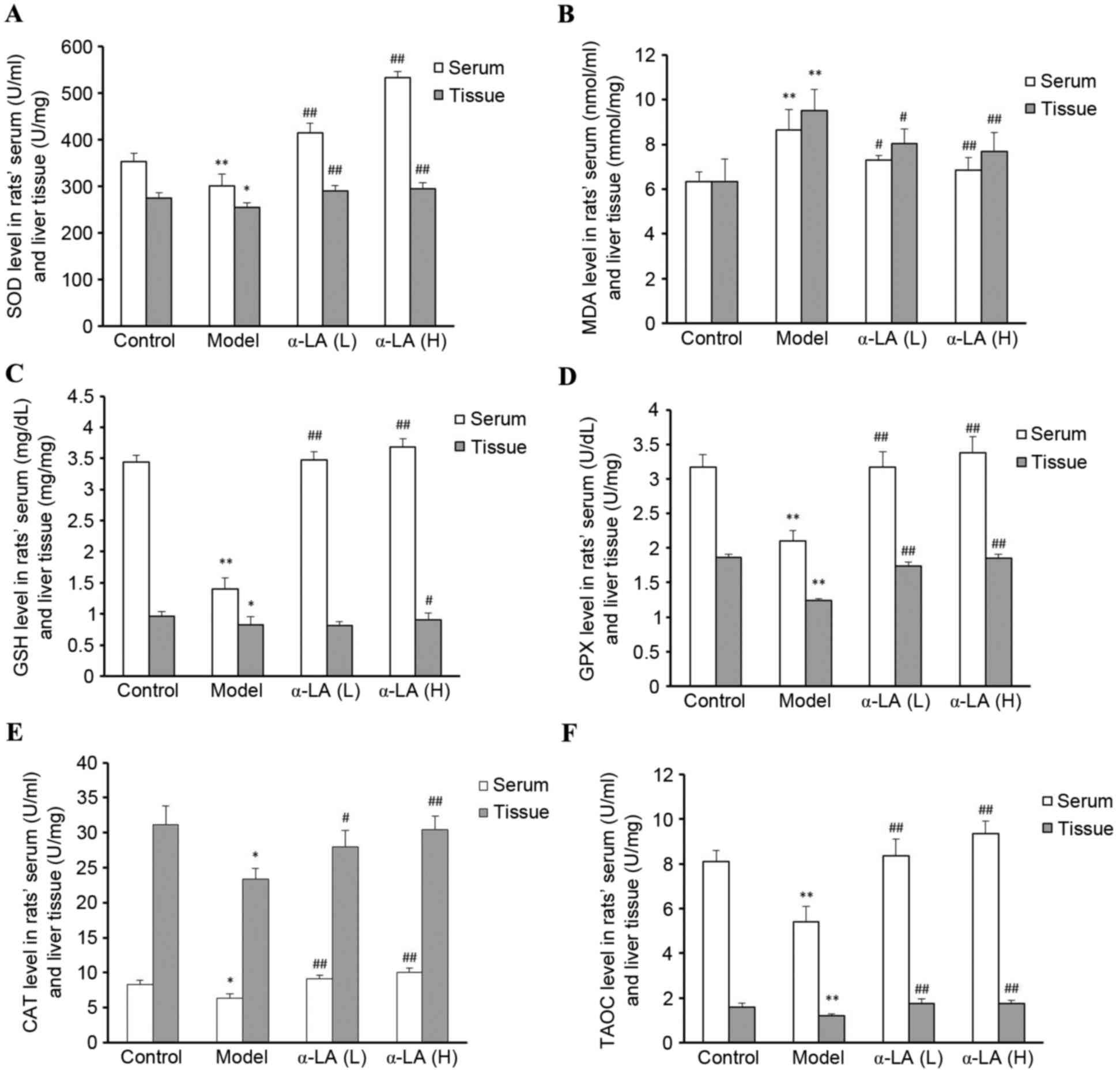 | Figure 3.Alterations in the levels of (A) SOD,
(B) MDA, (C) GSH, (D) GPX, (E) CAT and (F) TAOC in serum and
tissue. After 12 weeks of feeding a high fat diet, serum and liver
tissues of each group were collected and the levels of SOD, CAT,
GPX, TAOC, MDA and GSH were detected. *P<0.05 and **P<0.01,
vs. control group; #P<0.05 and
##P<0.01, vs. model group. SOD, superoxide dismutase;
CAT, catalase; GSH, glutathione; GPX, glutathione peroxidase; TAOC,
total antioxidant capacity; MDA, malondialdehyde. |
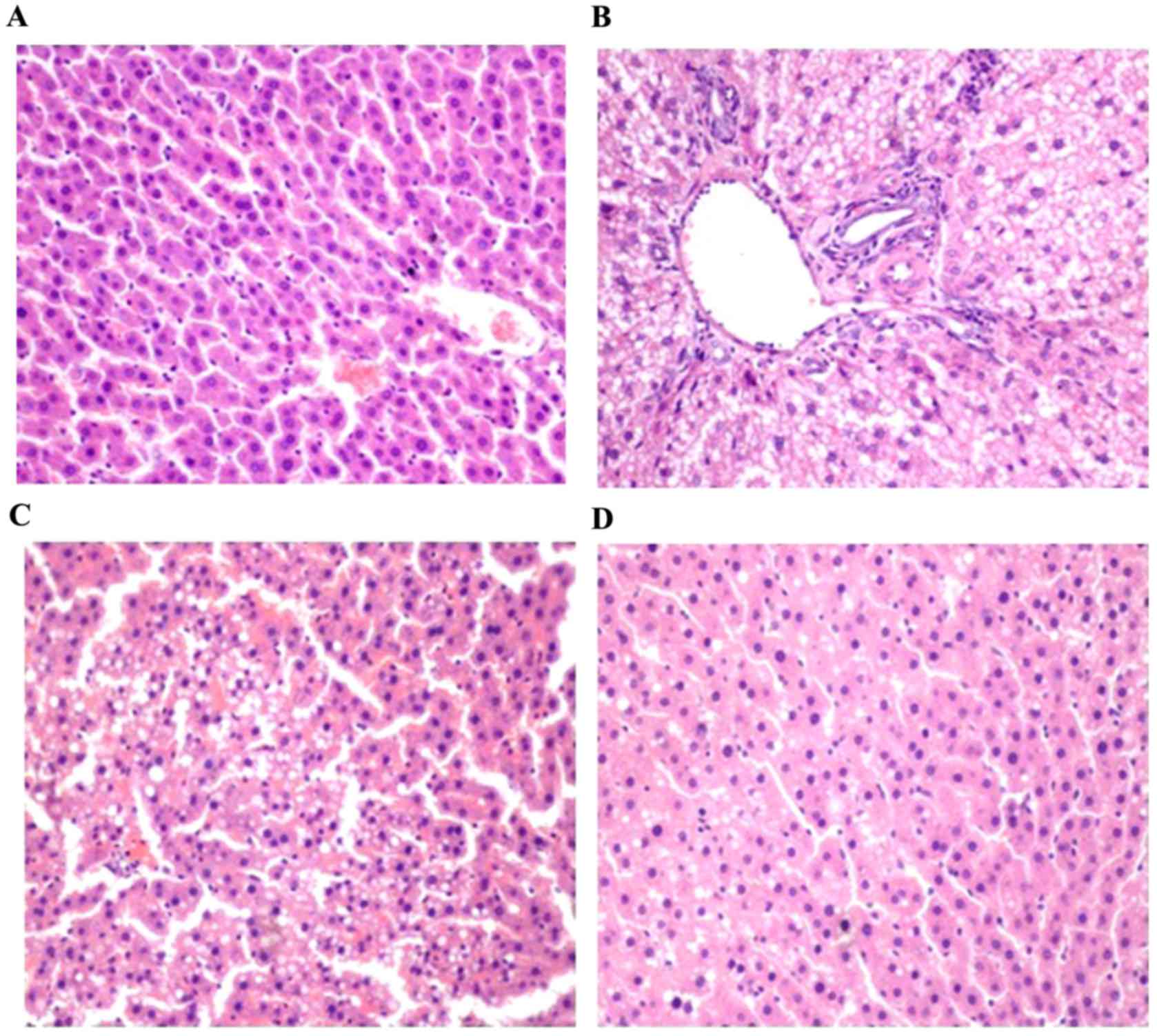
Histological observations
Macroscopic examination of the livers of the rats in
the control group showed a normal red, smooth and shiny appearance.
By contrast, the livers from the model group were yellowish brown,
enlarged and greasy. The livers from the α-LA group showed a more
normal appearance, and the size and texture of the livers in the
α-LA group were intermediary between those of the control group and
model group. Examination of the liver tissues using light
microscopy in the tissues of the control group stained with H&E
showed normal polyhedral hepatocytes with central nuclei and an
eosinophilic cytoplasm (Fig. 4A).
Compared with the rats in the control group with a normal liver
histology, the rats in the model group developed various degrees of
diffuse hepatic steatosis, characterized by the accumulation of
fat, hepatocyte ballooning, loss of cytoplasmic eosin, eccentric
nuclei, intralobular inflammation and necrosis (Fig. 4B). α-LA treatment markedly
attenuated the histopathological characteristics of NAFLD observed
in the model group. There was mild microvesicular steatosis in the
tissues, and the steatosis was almost absent in the remainder of
the samples, with intact architecture and no inflammatory foci.
Compared with the α-LAL group (Fig. 4C), the improvement was more marked
in the α-LAH group (Fig.
4D). The histological data showed that the model group
exhibited grade 2–3 focal necrosis and grade 1–2 hepatic steatosis,
whereas the normal control group exhibited grade 0–1 focal necrosis
and grade 0 hepatic steatosis (Table
II).
 | Table II.Summary of histological scoring for
liver tissue sections of different treatment groups. |
Table II.
Summary of histological scoring for
liver tissue sections of different treatment groups.
| Group | Focal necrosis | Hepatic
steatosis |
|---|
| Control | 0.125±0.35 | 0 |
| Model |
2.75±0.46a |
1.63±0.52a |
|
α-LAL |
2.00±0.53b |
1.00±0.53b |
|
α-LAH |
1.63±0.52c |
0.63±0.52c |
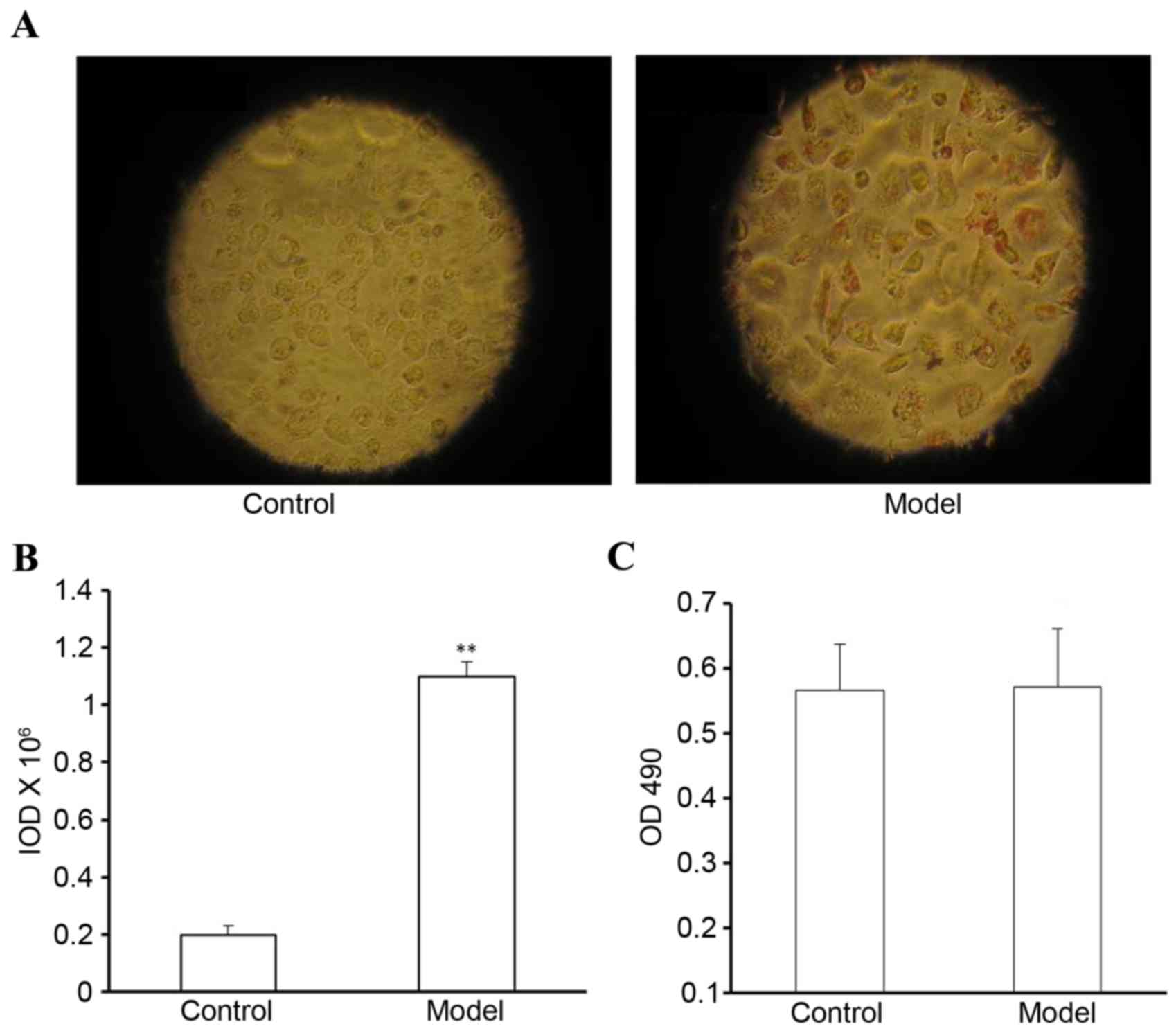
OA induces lipid accumulation in L-02
cells
The L-02 cells were incubated in DMEM containing 60
µg/ml OA mixture for 24 h and were then stained with Oil Red O. A
substantial quantity of red lipid droplets was observed in the L-02
cells of the model group (Fig.
5A). However, no lipid droplets were detected in the L-02 cells
of the control group (Fig. 5A). As
shown in Fig. 5B, the IOD value in
the model cells was significantly higher, compared with that in the
control cells (P<0.01). The cytotoxicity of OA towards the L-02
cells was analyzed using an MTT assay. The results revealed no
significant difference between the control group and the model
group in terms of cell viability (P>0.01; Fig. 5C).
a-LA reduces the accumulation of FFAs
in NAFLD model cells
To determine the effect of α-LA on FFA accumulation
in the OA-treated L-02 cells, the L-02 cells were treated in
different groups, and the extracellular and intracellular FFAs were
quantified. Compared with the control cells, the levels of
extracellular and intracellular FFAs were significantly higher
following treatment with 60 µg/ml OA (P<0.01). Compared with the
cells in the model group, α-LA significantly reduced the FFA
concentrations (P<0.01; Fig.
6A).
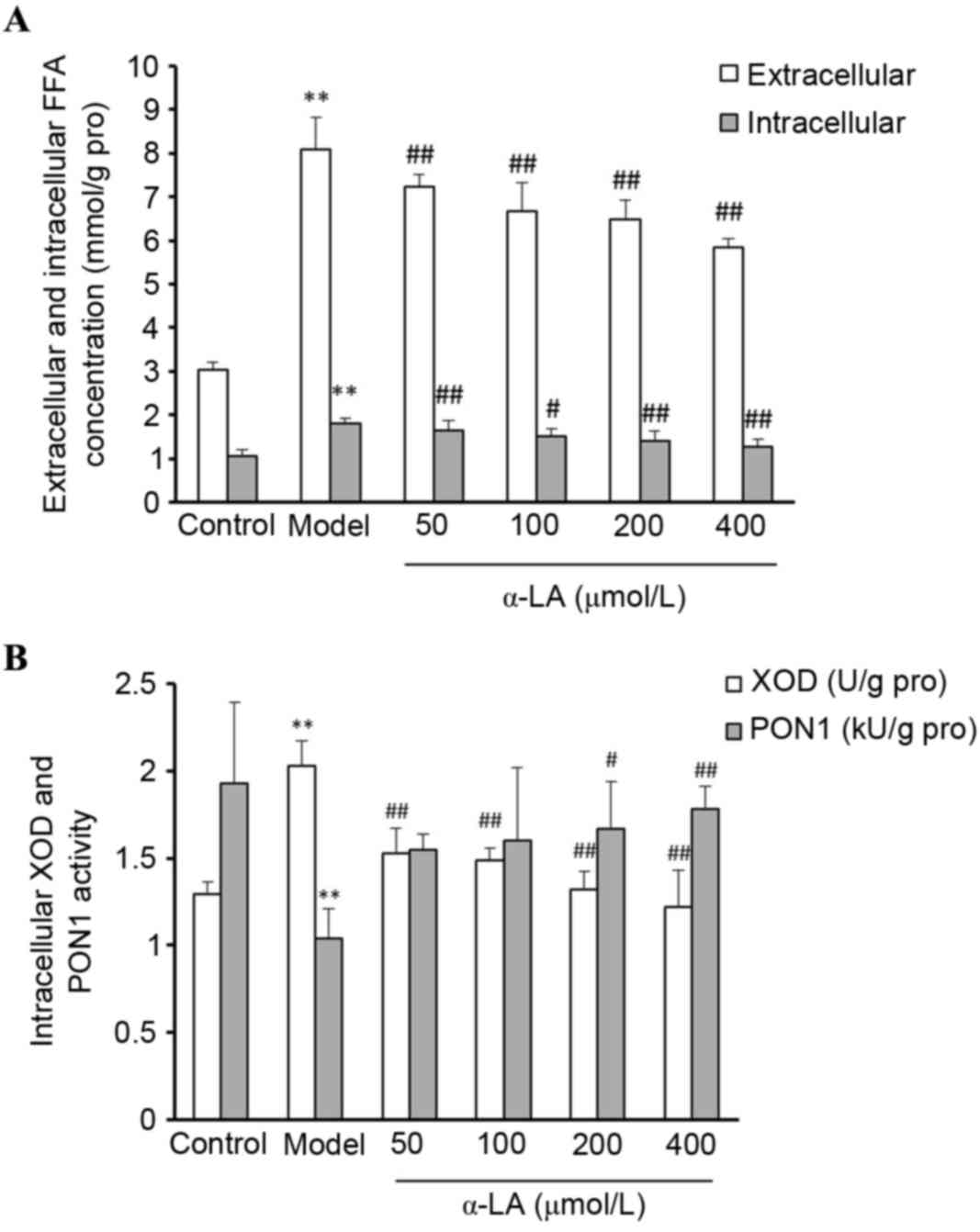 | Figure 6.Alterations in the activities of
FFAs, XOD and PON1 in L-02 cells. Control cells were incubated in
media containing 2% FBS. Model cells were incubated in media
containing 2% FBS and 60 µg/ml OA. α-LA treated cells were switched
to media containing 2% FBS, 60 µg/ml OA and 50, 100, 200 or 400
µmol/l α-LA, respectively. (A) Extracellular and intracellular FFA
activity. (B) Intracellular XOD and PON1 activity. **P<0.01. vs.
control group; #P<0.05 and ##P<0.01,
vs. model group. OA, oleic acid; α-LA, α-lipoic acid; FFAs, free
fatty acids; XOD, xanthine oxidase; PON1, paraoxonase 1. |
Alterations in the activities of XOD
and PON1 in L-02 cells
As shown in Fig.
6B, compared with the control cells, the intracellular XOD
activity was significantly higher and the activity of PON1 was
significantly lower in the model cells (P<0.01). Compared with
control cells, the intracellular XOD activity was significantly
lower in the α-LA treated cells (P<0.01) and the activity of
PON1 was significantly higher, until the α-LA concentration reached
200 µmol/l (P<0.01).
Discussion
NAFLD is a clinical pathological syndrome without a
history of excessive alcohol consumption, characterized by fatty
denaturation and lipid accumulation in hepatocytes.
Histopathologically, it exhibits the following characteristics:
Simple fatty liver, steatohepatitis, fatty liver fibrosis and
hepatocirrhosis, which may exist alone or in combination, and the
clinical manifestations of NAFLD are diverse (17). The pathogenesis of NAFLD remains to
be fully elucidated, and the current hypothesis of ‘two hits’ has
been well accepted (4,18). In the two hits hypothesis, insulin
resistance is the basis of pathogenesis, and ROS are the core link
of pathogenesis. The substantial quantity of ROS generated is
beyond the removal capacity of the anti-oxidative system.
Consequently, oxidative stress occurs together with substantial
peroxide formation, and the peroxide leads to hepatocyte damage,
which induce inflammatory reactions during which inflammatory cells
infiltrate the liver parenchyma (19). Therefore, NAFLD occurs as a result
of an imbalance between oxidative stress and resistance against
oxidative stress, and results in imbalance of the redox system in
the body. Oxidative stress persists in NAFLD and several studies
have demonstrated that severe lipid peroxidation exist in patients
with NAFLD (20,21). It is important for clarification of
the pathogenesis, diagnosis and sequelae of NAFLD to investigate
alterations in oxidase and antioxidase, and to examine the redox
state of the body. The findings of the experiments performed in the
present study using an animal model of NAFLD confirmed this. It was
found that the oxidative product, MDA, was generated in high
levels, however, anti-oxidative capacity decreased substantially
due to reductions in oxidants, including SOD, GSH and CAT. The
alterations in these indices indicated that the NAFLD rat model had
been successfully established. The state of oxidative stress in the
body can be evaluated by detecting the metabolites in the
associated tissues and body fluids during oxidative stress, which
can assist in judging the severity and prognosis of NAFLD.
The tissue with the highest expression levels of XOD
is the liver (22,23). Usually, 90% of XOD is present in
hepatocytes in the form of its precursor, xanthine dehydrogenase
(XD), and is generally inactive. In cases of tissues in a
pathological state, including oxidative stress, XD converts to XOD,
the activity of which increases significantly. XOD then catalyzes
the oxidation of xanthine; generating numerous free radicals, which
mediate the peroxidation of lipids, and trigger the occurrence and
progression of NAFLD (24). As the
increase in the activity of XOD leads to the production of
increased free radicals, the activity of XOD in the serum reflects
the production of free radicals in tissues directly, and represents
the degree of liver damage due to oxidative stress. The present
study showed that an increase in peroxidative products and XOD
activity in the NAFLD rats resulted from excess XOD, which was
released into the blood circulation of NAFLD rats, causing the
production of free radicals, which were involved in the onset and
progression of NAFLD.
PON1 is an anti-oxidative enzyme, which the liver
synthesizes and secretes, and has high levels of activity in the
liver and the blood (25). PON1
can bind to HDL to inhibit oxidative modification (26), preventing against the accumulation
of oxidative products on LDL (27)
to reduce the lipid peroxidation-induced damage in endothelial
cells (28), the production of
peroxides and oxidative phosphatide, and the incidence of
atherosclerosis (29). It had been
found that activity of PON1 is negatively correlated with LDL-C and
TG, and that the PON1 gene plasmid can increase the activity of
PON1 in the serum, and inhibit lipid accumulation in the blood and
the liver (30). If the PON1 gene
plasmid is used to treat NAFLD, the activity of PON1 in the serum
increases, whereas the activities of TC, LDL-C and TG are decreased
to suppress lipid accumulation in the blood and the liver,
mitigating the liver damage induced by a HFD. The results of the
present study showed that the activity of PON1 decreased markedly
in the serum of the NAFLD model rats, whereas the levels of TC,
LDL-C and TG increased. It has also been found that LDL and HDL in
the plasma are more readily oxidized in PON1-knockout mice
(31). In NAFLD, reduced PON1 and
increased MDA activity can be considered a biochemical marker for
lipid peroxidation (14). PON1 may
be closely associated with lipid peroxidation and oxidative
stress.
NAFLD is a complex pathological procedure of chronic
liver damage involving several factors, and oxidative stress is
important in the occurrence and progression of NAFLD. The present
study hypothesized that alterations in the activities of XOD and
PON1 are associated with the severity of NAFLD. The results of the
present study showed that the activities of XOD in the serum,
tissues and cells of the model groups were higher, compared wit
those in the control group, whereas the activities of PON1 were
lower, compared with those in the control group. Notable fat
denaturation and inflammatory necrosis were present in the liver
tissues of the model group. Alterations in the activities of XOD
and PON1 were time-dependent, and with increasing duration in the
formation of the animal model, the activities of XOD and PON1
increased and decreased, respectively.
In the body, α-LA is a sole water-soluble and
fat-soluble antioxidant, and has the capacity to remove free
radicals and resist oxidation (32–34),
which has lead to it being applied widely in biomedicine and
associated areas. Following the administration of α-LA in the
present study, the state of oxidative stress, indicated by the
levels of SOD, CAT, GPX, TAOC, MDA and GSH, in the serum and liver
tissues of the NAFLD rats were markedly relieved, the activities of
XOD and MDA decreased significantly, and the activities of SOD and
PON1 increased significantly, which indicated that α-LA had a
potent reversal effect on HFD-induced oxidative stress. The
administration of α-LA also improves lipid metabolism (35,36).
In the present study, α-LA reduced the levels of TC, TG and FFAs in
the serum and L-02 cells in the model groups. The results from the
pathological sections of the liver also showed decreased lipid
deposition in the hepatocytes, and relief of pathological damage of
hepatocytes, suggesting that α-LA had certain preventive effects.
The available evidence suggested that α-LA may delay the
development of NAFLD, to a certain extent, by resisting oxidative
stress and reducing the damage of lipid peroxidation.
In conclusion, in the occurrence and progression of
NAFLD, oxidative stress usually persists. XOD and PON1 were found
to represent a state of oxidative stress and anti-oxidative
capacity in the body, and indirectly reflect the severity of NAFLD,
to a certain extent. Despite debate regarding whether
anti-oxidative therapy is effective in NAFLD (37,38),
the results of the present study showed that the antioxidant, α-LA,
led to an improvement in the symptoms of NAFLD. However, why the
activities of XOD and PON1 alter NAFLD, and whether this alteration
is associated with the expression levels of XOD and PON1 remain to
be elucidated. Therefore, further investigations on the association
between XOD and PON1 and the occurrence and progression of NAFLD
are required, which may be useful for early diagnosis, treatment
and prognosis of the disease.
References
|
1
|
Singer C, Stancu P, Coşoveanu S and Botu
A: Non-alcoholic fatty liver disease in children. Curr Health Sci
J. 40:170–176. 2014.PubMed/NCBI
|
|
2
|
Masarone M, Federico A, Abenavoli L,
Loguercio C and Persico M: Non alcoholic Fatty liver: Epidemiology
and natural history. Rev Recent Clin Trials. 9:126–133.
2014.PubMed/NCBI
|
|
3
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mehta K, Van Thiel DH, Shah N and Mobarhan
S: Nonalcoholic fatty liver disease: Pathogenesis and the role of
antioxidants. Nutr Rev. 60:289–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J, Ren W, Li A, Ding Y, Guo W, Su D,
Hu C, Xu K, Chen H, Xu X, et al: Fat mass and obesity-associated
gene enhances oxidative stress and lipogenesis in nonalcoholic
fatty liver disease. Dig Dis Sci. 58:1004–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Surapaneni KM and Jainu M: Comparative
effect of pioglitazone, quercetin and hydroxy citric acid on the
status of lipid peroxidation and antioxidants in experimental
non-alcoholic steatohepatitis. J Physiol Pharmacol. 65:67–74.
2014.PubMed/NCBI
|
|
7
|
Doehner W and Landmesser U: Xanthine
oxidase and uric acid in cardiovascular disease: Clinical impact
and therapeutic options. Semin Nephrol. 31:433–440. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sumida Y, Niki E, Naito Y and Yoshikawa T:
Involvement of free radicals and oxidative stress in NAFLD/NASH.
Free Radic Res. 47:869–880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mackness M and Mackness B: Targeting
paraoxonase-1 in atherosclerosis. Expert Opin Ther Targets.
17:829–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang M, Lang X, Cui S, Zou L, Cao J, Wang
S and Wu X: Quantitative assessment of the influence of paraoxonase
1 activity and coronary heart disease risk. DNA Cell Biol.
31:975–982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manning PJ, Jong SA, Ryalls AR and
Sutherland WH: Paraoxonase 1 activity in chylomicrons and VLDL: The
effect of type 2 diabetes and meals rich in saturated fat and oleic
acid. Lipids. 47:259–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferre N, Marsillach J, Camps J, Mackness
B, Mackness M, Riu F, Coll B, Tous M and Joven J: Paraoxonase-1 is
associated with oxidative stress, fibrosis and FAS expression in
chronic liver diseases. J Hepatol. 45:51–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marsillach J, Camps J, Ferré N, Beltran R,
Rull A, Mackness B, Mackness M and Joven J: Paraoxonase-1 is
related to inflammation, fibrosis and PPAR delta in experimental
liver disease. BMC Gastroenterol. 9:32009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samy W and Hassanian MA: Paraoxonase-1
activity, malondialdehyde and glutathione peroxidase in
non-alcoholic fatty liver disease and the effect of atorvastatin.
Arab J Gastroenterol. 12:80–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karsen H, Binici I, Sunnetcioglu M, Baran
AI, Ceylan MR, Selek S and Celik H: Association of paraoxonase
activity and atherosclerosis in patients with chronic hepatitis B.
Afr Health Sci. 12:114–118. 2012.PubMed/NCBI
|
|
16
|
Mingyue Z, Bing W, Qilong D, Ruining Y and
Hong Q: Study on the relationship between xanthine oxidase
paraoxonase-1 and occurrence and development of nonalcoholic fatty
liver disease. Chinese Hepatology. 19:323–328. 2014.
|
|
17
|
Tuyama AC and Chang CY: Non-alcoholic
fatty liver disease. J Diabetes. 4:266–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hijona E, Hijona L, Arenas JI and Bujanda
L: Inflammatory mediators of hepatic steatosis. Mediators Inflamm.
2010:8374192010.PubMed/NCBI
|
|
20
|
Stiuso P, Scognamiglio I, Murolo M,
Ferranti P, De Simone C, Rizzo MR, Tuccillo C, Caraglia M,
Loguercio C and Federico A: Serum oxidative stress markers and
lipidomic profile to detect NASH patients responsive to an
antioxidant treatment: A pilot study. Oxid Med Cell Longev.
2014:1692162014.PubMed/NCBI
|
|
21
|
Koruk M, Taysi S, Savas MC, Yilmaz O,
Akcay F and Karakok M: Oxidative stress and enzymatic antioxidant
status in patients with nonalcoholic steatohepatitis. Ann Clin Lab
Sci. 34:57–62. 2004.PubMed/NCBI
|
|
22
|
Parks DA and Granger DN: Xanthine oxidase:
Biochemistry, distribution and physiology. Acta Physiol Scand
Suppl. 548:87–99. 1986.PubMed/NCBI
|
|
23
|
Sarnesto A, Linder N and Raivio KO: Organ
distribution and molecular forms of human xanthine
dehydrogenase/xanthine oxidase protein. Lab Invest. 74:48–56.
1996.PubMed/NCBI
|
|
24
|
Morita M, Ishida N, Uchiyama K, Yamaguchi
K, Itoh Y, Shichiri M, Yoshida Y, Hagihara Y, Naito Y, Yoshikawa T
and Niki E: Fatty liver induced by free radicals and lipid
peroxidation. Free Radic Res. 46:758–765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodrigo L, Hernández AF, López-Caballero
JJ, Gil F and Pla A: Immunohistochemical evidence for the
expression and induction of paraoxonase in rat liver, kidney, lung
and brain tissue. Implications for its physiological role. Chem
Biol Interact. 137:123–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karlsson H, Kontush A and James RW:
Functionality of HDL: Antioxidation and detoxifying effects. Handb
Exp Pharmacol. 224:207–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mackness MI, Arrol S and Durrington PN:
Paraoxonase prevents accumulation of lipoperoxides in low-density
lipoprotein. FEBS Lett. 286:152–154. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marsillach J, Camps J, Beltran-Debón R,
Rull A, Aragones G, Maestre-Martínez C, Sabench F, Hernández M,
Castillo DD, Joven J, et al: Immunohistochemical analysis of
paraoxonases-1 and 3 in human atheromatous plaques. Eur J Clin
Invest. 41:308–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gür M, Çaylı M, Uçar H, Elbasan Z, Şahin
DY, Gözükara MY, Selek Ş, Koyunsever NY, Şeker T, Türkoğlu C, et
al: Paraoxonase (PON1) activity in patients with subclinical
thoracic aortic atherosclerosis. Int J Cardiovasc Imaging.
30:889–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu AL and Wu SP: Single intravenous
injection of plasmid DNA encoding human paraoxonase-1 inhibits
hyperlipidemia in rats. Biochem Biophys Res Commun. 397:257–262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosenblat M, Vaya J, Shih D and Aviram M:
Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol
efflux via the ABCA1 transporter in association with increased HDL
binding to the cells: A possible role for lysophosphatidylcholine.
Atherosclerosis. 179:69–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gorąca A, Huk-Kolega H, Piechota A,
Kleniewska P, Ciejka E and Skibska B: Lipoic acid-biological
activity and therapeutic potential. Pharmacol Rep. 63:849–858.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rahimifard M, Navaei-Nigjeh M, Baeeri M,
Maqbool F and Abdollahi M: Multiple protective mechanisms of
alpha-lipoic acid in oxidation, apoptosis and inflammation against
hydrogen peroxide induced toxicity in human lymphocytes. Mol Cell
Biochem. 403:179–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park S, Karunakaran U, Jeoung NH, Jeon JH
and Lee IK: Physiological effect and therapeutic application of
alpha lipoic acid. Curr Med Chem. 21:3636–3645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carrier B, Wen S, Zigouras S, Browne RW,
Li Z, Patel MS, Williamson DL and Rideout TC: Alpha-lipoic acid
reduces LDL-particle number and PCSK9 concentrations in high-fat
fed obese Zucker rats. PLoS One. 9:e908632014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huerta AE, Navas-Carretero S,
Prieto-Hontoria PL, Martínez JA and Moreno-Aliaga MJ: Effects of
α-lipoic acid and eicosapentaenoic acid in overweight and obese
women during weight loss. Obesity (Silver Spring). 23:313–321.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leuschner UF, Lindenthal B, Herrmann G,
Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J and Berg T: NASH
Study Group: High-dose ursodeoxycholic acid therapy for
nonalcoholic steatohepatitis: A double-blind, randomized,
placebo-controlled trial. Hepatology. 52:472–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perra A, Pibiri M, Sulas P, Simbula G,
Ledda-Columbano GM and Columbano A: Alpha-lipoic acid promotes the
growth of rat hepatic pre-neoplastic lesions in the
choline-deficient model. Carcinogenesis. 29:161–168.
2008.PubMed/NCBI
|