Introduction
Alzheimer's disease (AD) is characterized by a
widespread functional disturbance of the human brain. AD
pathogenesis is driven by the production and deposition of
β-amyloid peptides (Aβs) (1). Aβs
are peptides composed of 36–43 amino acids, which are the
predominant components of the amyloid plaques observed in the
brains of AD patients. Aβ is formed from the cleavage of amyloid
precursor protein (APPs) by β- and γ-secretases. The formation of
amyloid plaques is a major factor in AD, thus, determining the
underlying mechanism by which Aβ induces neuronal cell death is
crucial. A recent study demonstrated that dying cells in AD brains
and cultures of neurons exposed to Aβ exhibit the characteristics
of apoptosis (2). However, the
specific signaling pathways by which Aβ triggers cell apoptosis
have not been well defined.
Hyperlipidemia is a risk factor for atherosclerosis,
as well as for neurodegenerative diseases, including AD, as
evidenced by epidemiological, clinical, and animal studies.
Kivipelto et al (3) and
Solomon et al (4) selected
1449 residents for a 21-year follow-up study and demonstrated that
total cholesterol was an independent risk factor for AD in
middle-aged adults. Lipid-lowering treatment can reduce the risk of
neurodegenerative diseases (5–7).
Chan et al (8) observed
that sphingomyelin and cholesterol esters were notably increased in
AD patients and transgenic AD mouse brain tissues. Kosari et
al (9) demonstrated that
hippocampus-dependent memory was markedly impaired in rats fed with
a HFD for 12 weeks. Furthermore, El-Sayyad et al (10) observed a higher rate of neuronal
apoptosis in rats with hyperlipidemia compared with those fed a
normal diet.
Despite years of intensive research, the link
between hyperlipidemia and AD has yet to be established, although
it has previously been suggested that genes regulating lipid
metabolism may also be important in AD (11). Proprotein convertase
subtilisin/kexin type 9 (PCSK9) encodes a protein formerly termed
neuronal apoptosis-regulated convertase-1, a proprotein convertase
belonging to the subtilase subfamily (12). PCSK9 is highly expressed in the
liver, where it negatively regulates the low-density lipoprotein
(LDL) receptor (LDLR) in hepatocytes and, thus, is important in
controlling circulating levels of LDL-cholesterol (LDL-C) (13). PCSK9 affects neural development and
participates in neuronal apoptosis (12). Our previous study reported that
high PCSK9 expression levels, induced by oxidized LDL (oxLDL), were
positively associated with a high apoptotic ratio in human
umbilical vein endothelial cells (14).
The present study investigated whether PCSK9 was
involved in the effects of hyperlipidemia on hippocampal neuronal
apoptosis by establishing an apoE(−/−) hyperlipidemic mouse model.
Furthermore, the current study aimed to investigate a novel
association between lipid metabolism and AD.
Materials and methods
Animals and diets
A total of 18 male apoE(−/−) mice (age, 6 weeks;
average weight, 21±2.7 g), were purchased from Nanjing Qingzilan
Technology Co., Ltd. (Nanjing, China) and maintained in a
temperature-controlled environment (22–25°C, 45% humidity) with a
12 h light-dark cycle. Mice were randomly assigned to one of two
groups: HFD group, fed with food consisting of 2% (w/w)
cholesterol, 10% (w/w) lard, and 0.5% (w/w) cholic acid; and a
normal diet (ND) group. All mice were allowed ad libitum
access to their designated diet for 12 weeks. All animal procedures
were conducted in accordance with the International Guidelines for
the Ethical Use of Laboratory Animals and the National Institutes
of Health Guide for the Care and Use of Laboratory Animals
(15).
Plasma lipid analysis
At the end of the feeding period, animals were
anesthetized with 10% chloral hydrate (350 mg/kg body weight).
Blood was collected using cardiac puncture into tubes containing
0.1% EDTA following an overnight fast. Blood was then centrifuged
at 5,500 × g for 12 min at 4°C to separate the plasma. The
plasma was immediately used to determine the levels of LDL-C,
high-density lipoprotein cholesterol (HDL-C), total cholesterol
(TC), and triglyceride (TG) using an automatic biochemistry
analyzer (AU480 Chemistry system; Beckman Coulter, Inc., Brea, CA,
USA).
Tissue preparation for morphological
analyses
Following reaching surgical tolerance, the
anesthetized animals were sacrificed by cervical dislocation, and
were transcardially perfused with physiological saline for 60 sec,
followed by perfusion with a fixative (4% w/v formaldehyde) for 15
min at 22°C. Following perfusion, the brains were rapidly dissected
without the olfactory bulb and cerebellum. The brains were
post-fixed overnight (18–22 h) at 4°C in the formaldehyde solution
used for perfusion supplemented with 20% (w/v) sucrose. Tissues
were then immersed in 30% sucrose solution with 0.1 M sodium
cacodylate buffer at pH 7.3 for an additional day at 4°C. Frozen
hippocampal slices were cryosectioned into 8-µm thick sections.
Hematoxylin and eosin staining
Frozen hippocampal sections were stained with
hematoxylin and eosin (H&E) and examined under a light
microscope to observe cellular morphology changes in the mouse
hippocampus.
Oil red O staining
Frozen brain sections were stained with Oil Red O
solution [60% Oil Red O stock solution (5 mg/ml isopropanol)/40%
water] for 15 min. Following thoroughly washing twice with
distilled water, sections were counterstained with hematoxylin
solution for 30 sec. The staining of lipid droplets in the brain
was quantified using a phase contrast microscope and ImagePro Plus
6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Immunohistochemistry
Brain hippocampal tissues were obtained from the HFD
and ND groups in accordance with the protocols approved by the
Animal Investigative Review Committee of the University of South
China. The primary antibodies used were against PCSK9 (1:100; cat.
no. 55206-1-AP; Proteintech Group, Inc., Chicago, IL, USA),
β-secretase 1 (BACE1; 1:50; cat. no. ab108394; Abcam, Cambridge,
MA, USA), caspase-3 (1:50; cat. no. 19677-1-AP; Proteintech Group,
Inc.), B-cell lymphoma 2 (Bcl-2; 1:50; cat. no. BS1031; Bioworld
Technology, Inc., St. Louis Park, MN, USA), and Bcl-2-associated X
protein (Bax; 1:50; cat. no. 60267-1-Ig; Proteintech Group, Inc.).
Incubation without the primary antibodies served as the negative
control. The frozen 8-µm thick sections were blocked with
endogenous peroxidase blocking agent (Fuzhou Maixin Biotech, Co.,
Ltd., Fuzhou, China) for 10 min at room temperature and
subsequently incubated with the different primary antibodies for 30
min at room temperature. Following washing in distilled water, the
sections were incubated with biotinylated secondary antibodies
(cat. nos. KIT-0105R and KIT-0105M; Fuzhou Maixin Biotech, Co.,
Ltd.) and streptavidin-peroxidase (Proteintech Group, Inc.) for 10
min at room temperature. Subsequently, 3,3′-diaminobenzidine
peroxidase substrate solution (Fuzhou Maixin Biotech, Co., Ltd.)
was added until the desired color (brown) was developed. Apoptotic
cells in the mouse hippocampus were detected using a Hoechst 33258
Staining kit (Beyotime Institute of Biotechnology, Haimen, China)
in accordance with the manufacturer's protocols.
Modified Bielschowsky staining for
substance P (SP)
Frozen slides were immersed in 2% silver nitrate
solution for 30 min at 37°C in the dark. Subsequently, the silver
nitrate solution was removed and the slides were washed three times
in distilled water for 3 min. The stain was deoxidized with 10%
reducing agent until the color of the slides turned pale yellow.
The slides were rinsed again three times in distilled water, and
excess water was removed. Ammoniacal silver solution was added to
each slide for 30 sec. The excess solution was removed, and the
slides were immersed in 10% reducing agent for 2 min. The slides
were rotated a number of times until the yellow dye was stabilized.
The slides were then rinsed in tap water, fixed in 5% sodium
thiosulfate for 3 min, air-dried, cleared in xylene, and finally
coverslipped for light microscopic examinations.
Statistical analysis
Experimental results were expressed as the mean ±
standard error of the mean. Statistical analyses were conducted
using unpaired Student's t-test assuming unequal variance unless
otherwise indicated. Statistical analyses were conducted using SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of plasma lipid profiles
between ND and HFD mice
The apoE(−/−) mouse has been established as a
suitable model to investigate diet-induced hyperlipidemia.
ApoE(−/−) mice (age, 6 weeks) were randomly assigned to receive
either ND or HFD for 12 weeks. The plasma lipid profiles, including
TC, TG, LDL-C, and HDL-C contents, were determined for the two
groups of mice. The data for plasma lipid levels are presented in
Table I. Plasma TG, TC, LDL-C, and
HDL-C concentrations were significantly increased in HFD-fed mice
compared with ND-fed mice. These results indicated that a
diet-induced hyperlipidemic model was successfully established.
 | Table I.Plasma concentrations of lipids in
apoE(−/−) mice given normal diet and high-fat diet. |
Table I.
Plasma concentrations of lipids in
apoE(−/−) mice given normal diet and high-fat diet.
| Serum lipid | Normal diet
(n=9) | High-fat diet
(n=9) |
|---|
| TG (mmol/l) | 0.70±0.12 |
1.33±0.09a |
| TC (mmol/l) | 8.75±0.52 |
27.89±4.56a |
| HDL-C (mmol/l) | 1.63±0.15 |
11.48±1.97a |
| LDL-C (mmol/l) | 1.47±0.08 |
8.57±2.56a |
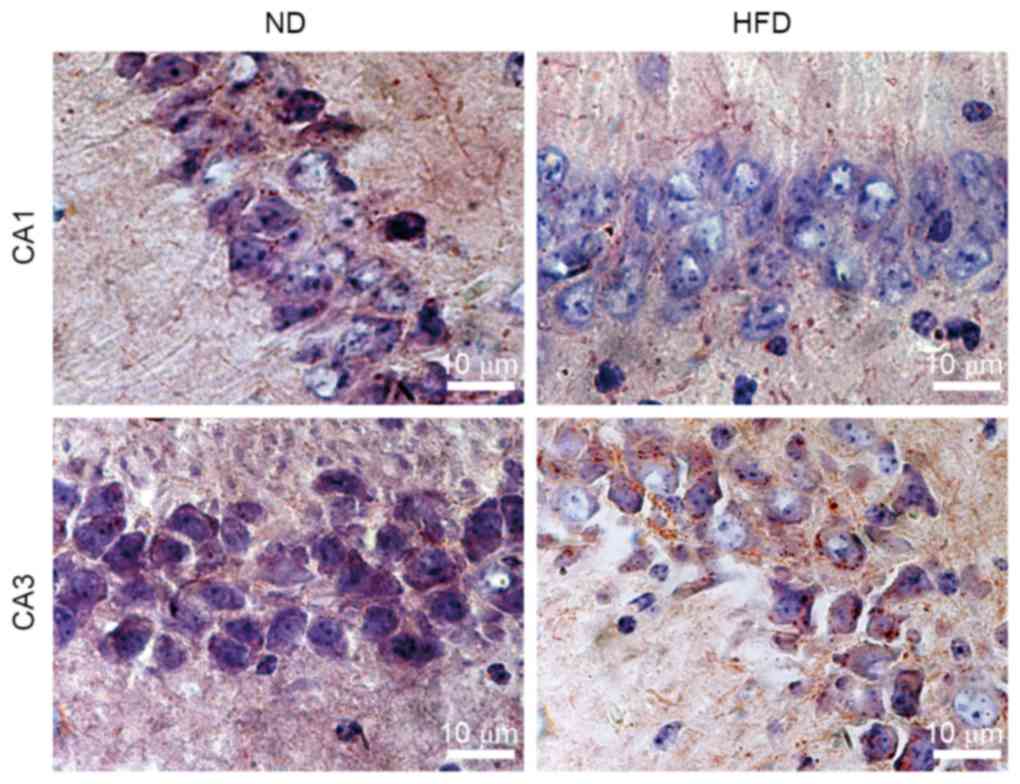
Effects of HFD on lipid accumulation
in the hippocampus of ApoE(−/−) mice
Lipids are essential to brain functions, including
membrane morphology, signal transduction, membrane fluidity, and
cell survival. Lipid abnormalities in the brain may contribute to
AD risk or severity. However, the mechanism by which hyperlipidemia
affects lipid accumulation in the hippocampus, which is a central
target of AD plaque pathology, remains to be elucidated. In the
present study, hippocampal sections were stained with Oil Red O to
identify the sites of lipid accumulation in apoE(−/−) mice fed with
ND and HFD. The majority of the Oil Red O-stained particles were
distributed as clusters in the hippocampus tissues. Lipid
accumulation increased in hippocampus CA3 neurons in HFD-fed
apoE(−/−) mice compared with ND-fed apoE(−/−) mice. However, the
difference in lipid contents between ND- and HFD-fed mice was not
notable in hippocampus CA1 of apoE(−/−) mice (Fig. 1). The results indicated that the
lipid contents of the hippocampus can be increased by HFD-induced
hyperlipidemia.
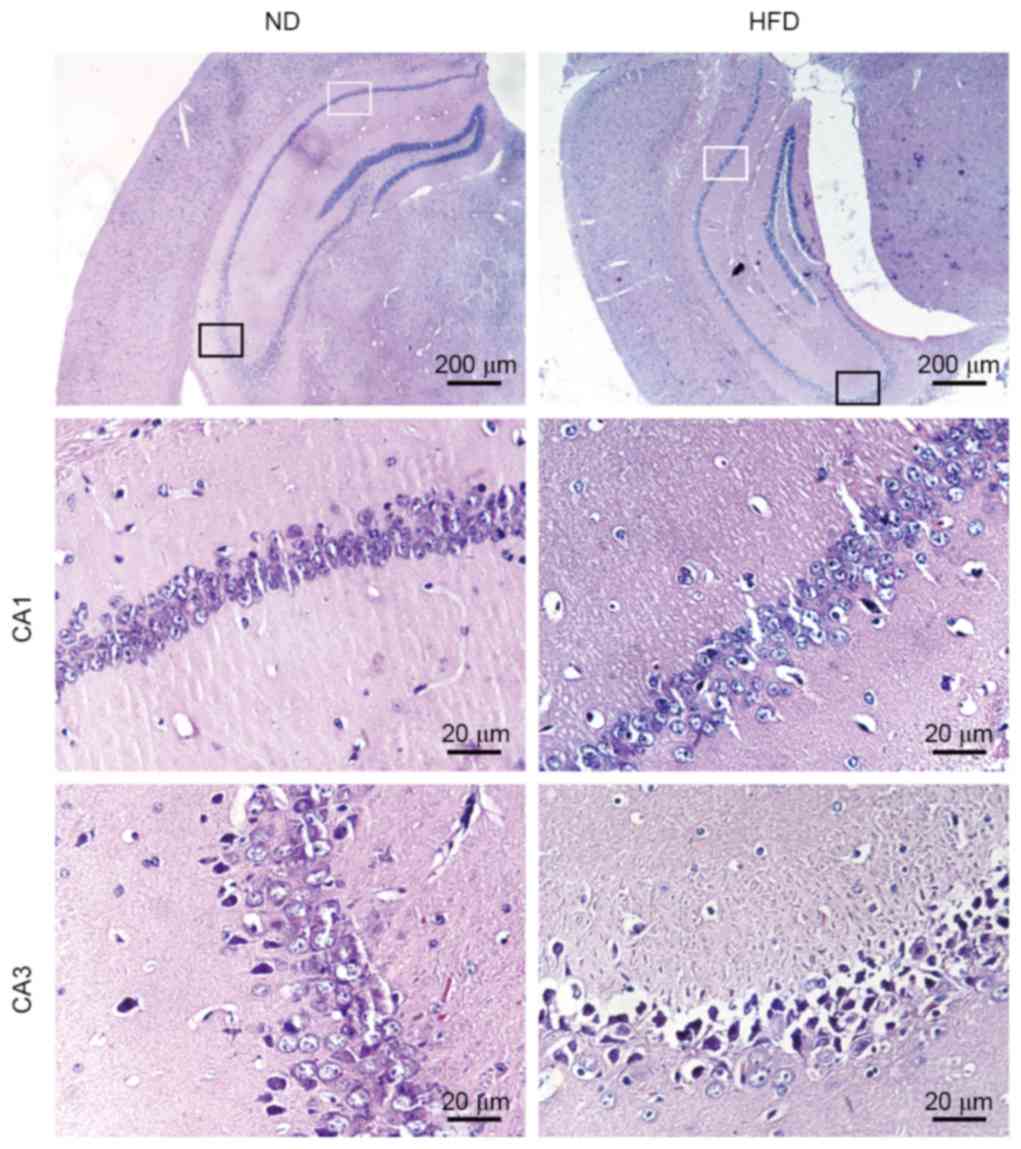
Effects of HFD on histology of the
hippocampus in apoE(−/−) mice
The histological changes in the hippocampus were
observed in the two groups of mice following H&E staining of
the brain sections. ND-fed apoE(−/−) mice exhibited normal
histological appearance and neuronal distribution in CA1 and CA3
hippocampal areas. Degenerative changes were observed in CA3
hippocampal areas of HFD-fed apoE(−/−) mice, which exhibited
pyknotic cells with reduced neuron count. Enlarged intercellular
spaces between CA1 and CA3 pyramidal cells were frequently observed
in HFD-fed apoE(−/−) mice. Furthermore, a number of cells in the
CA3 area of HFD-fed apoE(−/−) mice lost typical cell structure
compared with ND-fed mice (Fig.
2). These results suggested that hyperlipidemia may result in
structural damage and neuronal loss in the hippocampus of HFD-fed
apoE(−/−) mice.
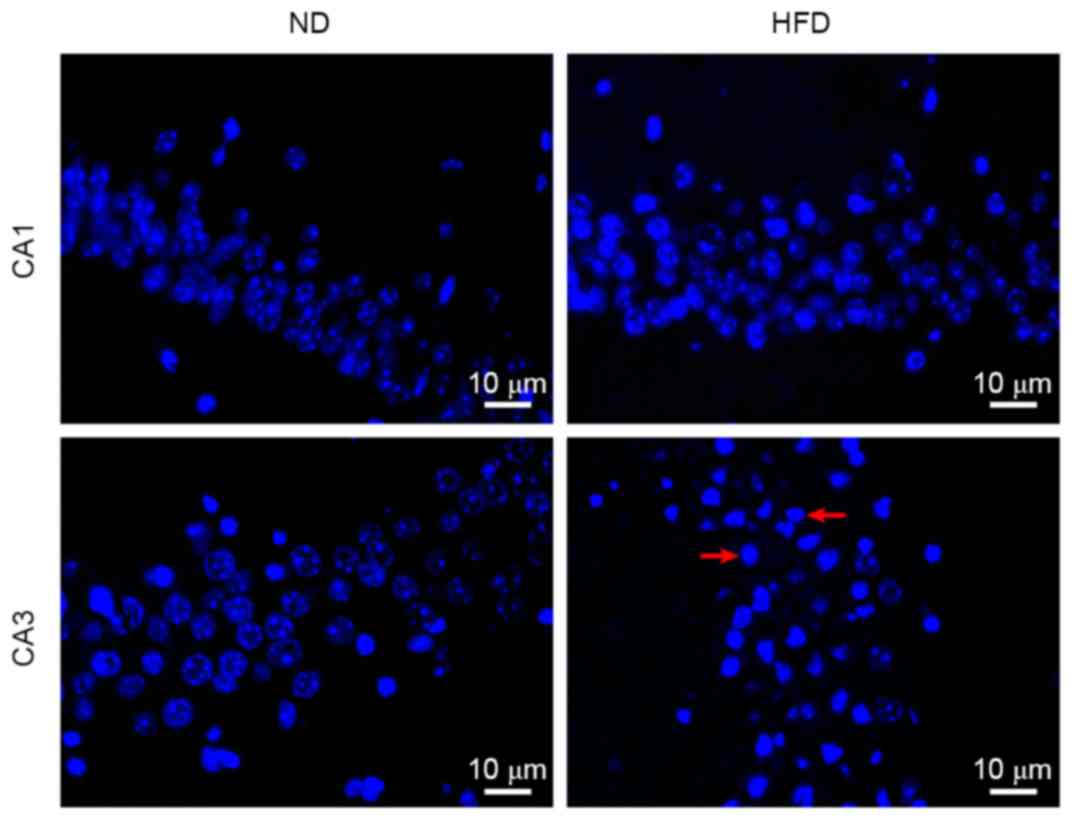
Effects of HFD on neuronal apoptosis
in the hippocampus of apoE(−/−) mice
Marked neuronal loss in the hippocampus is often
observed in AD brains. To observe whether neuronal apoptosis may be
induced by hyperlipidemia, a Hoechst 33258 staining assay was
performed to detect cell apoptosis in the hippocampus. Neuronal
apoptosis was observed in a number of cells in the hippocampus CA3
of ND-fed apoE(−/−) mice. By contrast, a large number of
hippocampus CA3 neuronal cells underwent apoptosis in HFD-fed
apoE(−/−) mice. Although individual apoptotic cells were observed
in hippocampus CA1 of HFD-fed apoE(−/−) mice, no notable difference
was observed between the percentage of apoptotic cells in
hippocampus CA1 between ND- and HFD-fed mice (Fig. 3). These results demonstrated that
apoptosis was the predominant cause of neuronal death in the
hippocampus, and hyperlipidemia increases neuronal apoptosis in
hippocampus CA3 of apoE(−/−) mice.
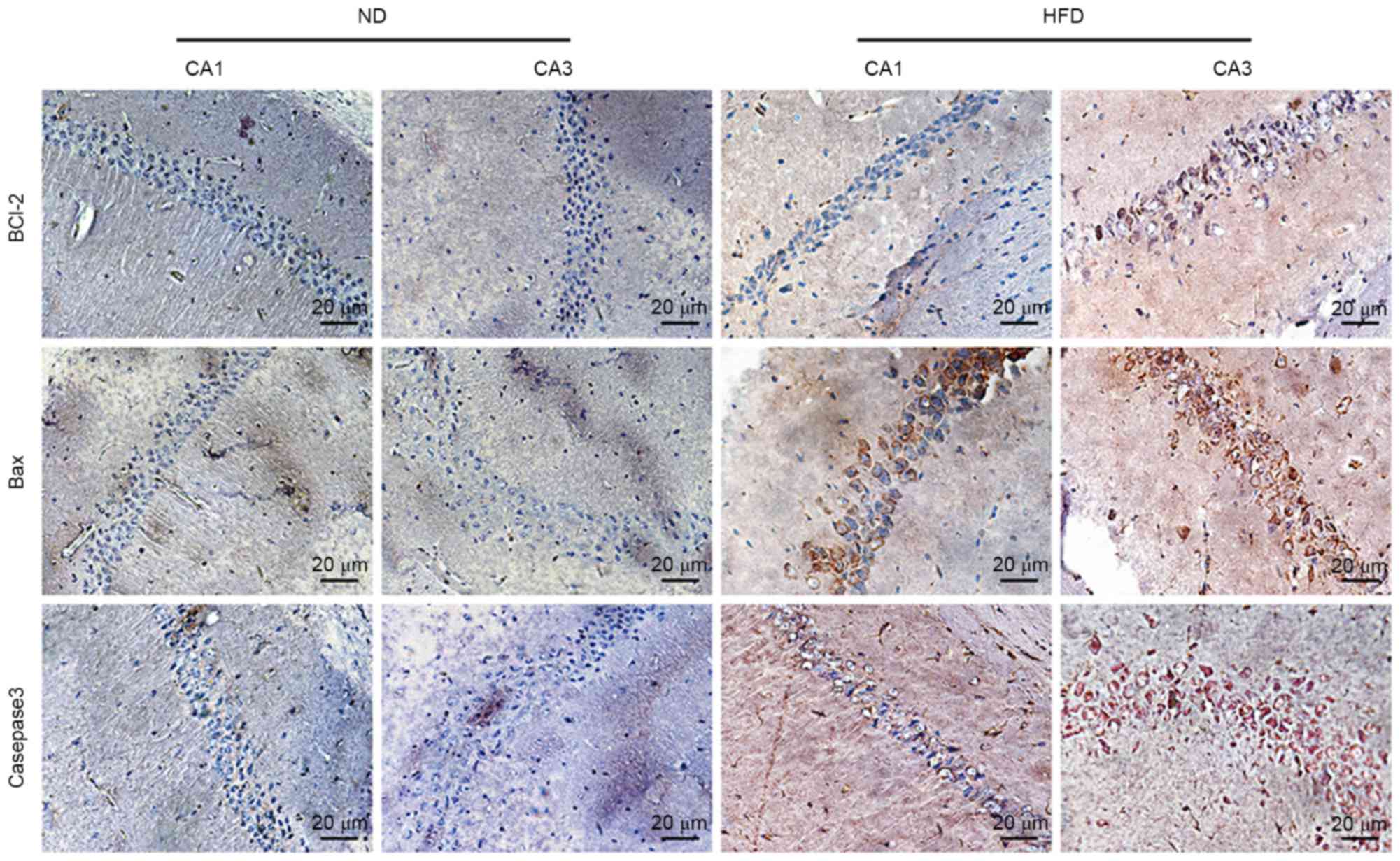
Effects of HFD on Bcl-2, Bax, and
caspase-3 expression levels in the hippocampus of apoE(−/−)
mice
To investigate the underlying molecular mechanisms
of hyperlipidemia-induced apoptosis of hippocampal neurons,
apoptosis-associated protein expression in the hippocampus of
apoE(−/−) mice was observed via immunohistochemistry. The
expression of caspase-3, which is the major executioner caspase
during the demolition phase of apoptosis, was markedly higher in
CA3 of HFD-fed mice than in ND-fed mice, and pro-apoptotic protein
Bax also increased in CA1 and CA3 of HFD-fed apoE(−/−) mice.
Notably, anti-apoptotic protein Bcl-2 slightly increased in CA3 of
HFD-fed apoE(−/−) mice (Fig. 4).
These results indicated that hyperlipidemia-induced hippocampal
neuronal apoptosis of apoE(−/−) mice may be controlled via the
Bcl-2/Bax-caspase-3 signaling pathway.
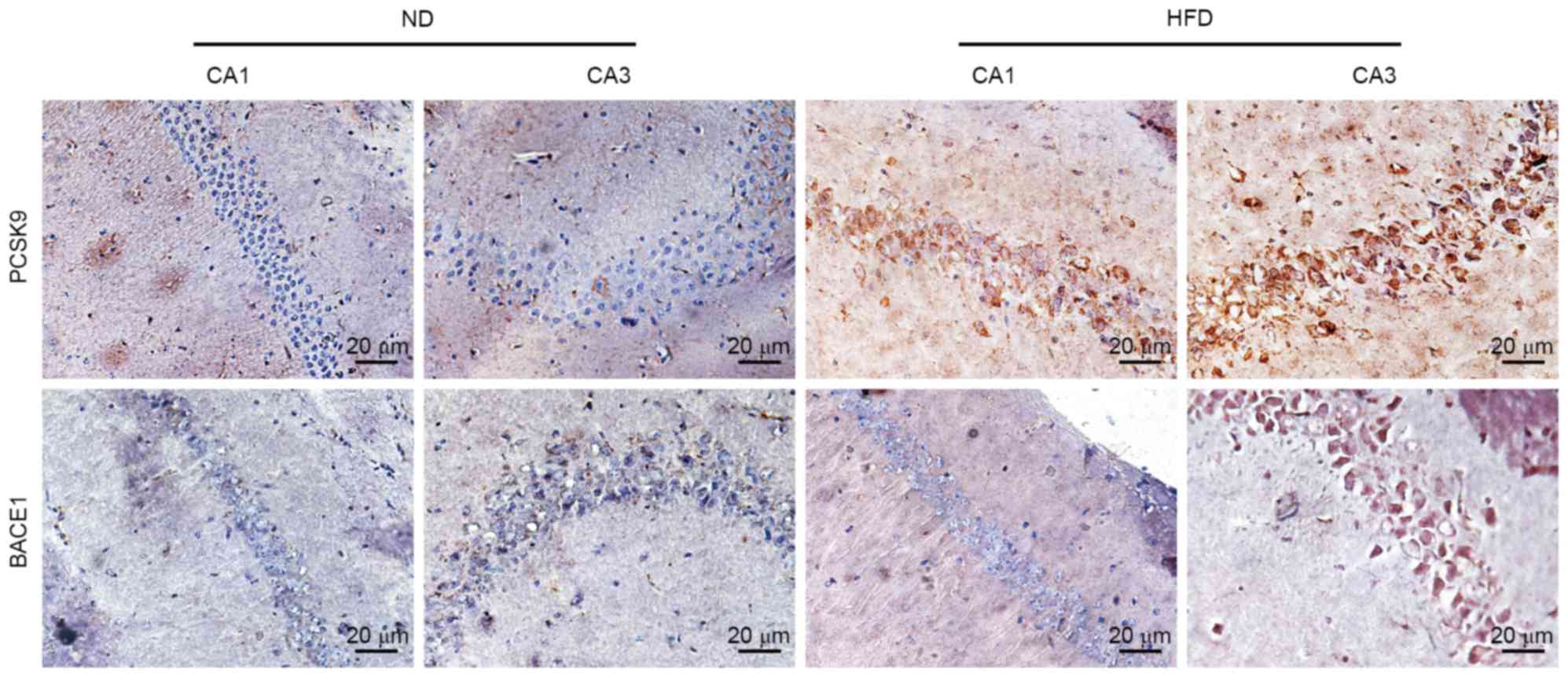
Effects of HFD on PCSK9 and BACE1
expression in the hippocampus of ApoE(−/−) mice
PCSK9, an apoptosis-associated protein, may degrade
BACE1, the predominant enzyme cleaving APP to generate Aβ. Aβ is
another key apoptosis inducer in neurodegenerative diseases. To
determine whether PCSK9 and BACE1 are involved in hippocampal
neuronal apoptosis, changes in PCSK9 and BACE1 expression levels in
the hippocampus of apoE(−/−) mice were observed by
immunohistochemistry. PCSK9 expression in CA1 and CA3 of HFD-fed
apoE(−/−) mice notably increased compared with ND-fed apoE(−/−)
mice. Furthermore, BACE1 expression in CA3 of HFD-fed apoE(−/−)
mice notably increased (Fig. 5).
These results suggested that PCSK9 and BACE1 may be associated with
hyperlipidemia-induced hippocampal neuronal apoptosis.
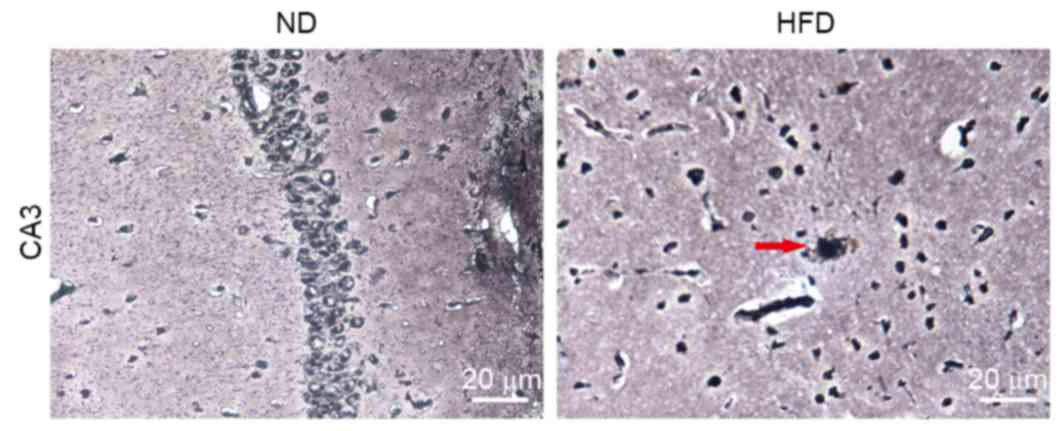
Effects of HFD on amyloid plaques in
the hippocampus of apoE(−/−) mice
To further elucidate the role of BACE1 in
hyperlipidemia-induced hippocampal neuronal apoptosis, the changes
in levels of SP in the brains in ND- and HFD-fed apoE(−/−) mice
were compared using modified Bielschowsky staining, which allowed
visualization of diffuse plaques in the brains. Small amyloid
plaques were observed in the hippocampus of HFD-fed apoE(−/−) mice
but not in ND-fed apoE(−/−) mice (Fig.
6). This finding was consistent with the expression pattern of
BACE1.
Discussion
Neurodegenerative diseases, which affect tens of
millions of people annually worldwide, are characterized by the
physical decay and eventual loss of neurons. AD is one of the most
common neurodegenerative diseases, which predominantly affects the
bilateral frontotemporal lobe and hippocampus. Histopathology
indicates deposition of senile plaques, neurofibrillary tangles,
lost neurons, and glial hyperplasia. Neuronal apoptosis is one of
the major pathological characteristics of AD. The majority of cases
of AD have a genetic element, although sporadic cases of this
disease have been reported. To date, no specific treatment is able
to reverse the progression of AD. Limiting this harmful disease is
one of the most critical challenges in current medicine.
Hyperlipidemia is a risk factor of AD and other
neurodegenerative diseases, as evidenced by epidemiological
(3,4), clinical, and experimental studies
(8–10). In the present study, 18
six-week-old apoE(−/−) mice were randomly divided into two groups:
ND and HFD. After 12 weeks of feeding, hippocampal tissues were
collected for frozen sectioning. Compared with the ND group,
pyramidal cells in the hippocampus of apoE(−/−) mice fed with HFD
were disordered. In addition, intercellular spaces increased, cells
swelled, and nuclei became smaller and hyperchromatic, exhibiting
karyopyknosis, particularly in CA3 areas. By contrast, CA1
indicated no notable changes. These results suggested that
hyperlipidemia results in structural damage in the hippocampus of
HFD-fed apoE(−/−) mice. Oil red O staining demonstrated that
HFD-fed apoE(−/−) mice exhibited slightly increased lipid
accumulation levels compared with ND-fed apoE(−/−) mice in
hippocampus CA3. This staining also demonstrated that plasma lipids
can pass through the blood-brain barrier (BBB) into the brain
tissues when hyperlipidemia occurs, resulting in increased lipid
deposits in the pyramidal cells of the hippocampus. Stranahan et
al (16) also observed that
free cholesterol levels in the hippocampus were markedly increased
following induction of hyperlipidemia in Sprague-Dawley (SD) rats
by feeding with high-fat and high-sugar diets for 3 months.
Generally, blood lipids cannot completely pass through BBB,
however, previous studies have indicated that the permeability of
BBB in apoE(−/−) mice is 3.7 times of ordinary mice (17,18).
This increase in permeability results in the passage of
nonessential small molecules into the brain, leading to brain
function disorders. This may be attributed to HFD-induced AD of
apoE(−/−) mice (17). The
increased raw material for lipid synthesis in the blood passes
through the BBB freely, possibly increasing the lipid contents in
the brain. Furthermore, lysophosphatidic acid, the predominant
bioactive lipid of oxLDL, may damage BBB, leading to the occurrence
of AD (19).
Although hyperlipidemia has been identified as a
risk factor of AD, the mechanism of hyperlipidemia-induced AD has
not been completely elucidated. It has been demonstrated that
following feeding C57BL/6 and LDLR-/− mice with a HFD for 8 weeks,
the expression levels of a number of cytokines, including tumor
necrosis factor-α, interleukin-1, interleukin-6, nitric oxide
synthase-2, and cyclooxygenase-2, increased along with BACE1
expression, suggesting that hypercholesterolemia results in nerve
inflammation and influences the APP metabolic pathway, resulting in
neurodegenerative diseases (20).
Stranahan et al (16)
observed that the levels of lipid peroxidation products
4-hydroxynonenal-lysine and 4-hydroxynonenal-histidine were locally
increased in the hippocampus subsequent to feeding SD rats high-fat
and high-sugar diets for 3 months to induce hyperlipidemia,
indicating cell membrane-associated oxidative stress. On the basis
of the above results, the present study suggests that
hyperlipidemia can increase lipid deposition and promote cell
apoptosis, leading to neuronal degeneration.
Hoechst 33258 staining results indicated that
pyramidal cells in hippocampus CA3 of HFD-fed apoE(−/−) mice
exhibited greater apoptosis than ND-fed apoE(−/−) mice, indicating
that hyperlipidemia increased neuronal apoptosis in the
hippocampus. Furthermore, the expression of PCSK9, BACE1,
caspase-3, and Bax significantly increased, whereas Bcl-2
expression increased only marginally, indicating that
hyperlipidemia resulted in neuronal apoptosis in the hippocampus of
apoE(−/−) mice possibly via the Bcl-2/Bax-caspase-3 signaling
pathway.
PCSK9 is a newly identified gene associated with
blood cholesterol metabolism. Its predominant biological function
is the degradation of LDLR in hepatic cells. PCSK9 function has
been comprehensively investigated, revealing its regulatory
functions, such as regulation of neuronal apoptosis in addition to
lipid metabolism (21). A previous
study demonstrated that when cerebellar granule neurons were
damaged, PCSK9 expression increased, activating the caspase-3 and
caspase-9 signaling pathways, possibly leading to apoptosis
(21). The current study observed
that PCSK9 expression in hippocampal CA3 of HFD-fed apoE(−/−) mice
was significantly increased compared with that in the ND-fed group.
Combined with previous experimental findings, the results of the
present study suggest that PCSK9 is important in neuronal apoptosis
of HFD-fed apoE(−/−) mice.
Numerous studies have confirmed that the large
number of Aβ deposits in the brain is an important risk factor for
the occurrence of AD (22,23). As previously mentioned, BACE1 is
the predominant enzyme producing Aβ from APP. Aβ-induced neuronal
apoptosis leads to AD. BACE1 protein expression levels and enzyme
activities increased in the majority of sporadic AD patients. The
present study also compared the changes of substance P levels in
the brains of ND- and HFD-fed apoE(−/−) mice using modified
Bielschowsky staining, and a slightly increased SP deposition was
observed in the hippocampus of HFD-fed apoE(−/−) mice. This is
consistent with previous studies that have observed that HFD can
lead to an increase in SP deposition (24–26).
Although numerous studies have verified the association between
lipids and Aβ, the underlying mechanism remains to be
elucidated.
PCSK9 expression was demonstrated to be negatively
associated with BACE1 level in certain studies (27,28),
however, other reports have indicated no association between PCSK9
and BACE1 (29). Thus, the
correlation between PCSK9 and BACE1 requires further elucidation.
The present study investigated the association between PCSK9 and
BACE1 and observed that PCSK9 and BACE1 expression increased in
HFD-fed apoE(−/−) mice. Furthermore, BACE1 mRNA and protein levels
increased subsequent to feeding C57BL/6 and LDLR-/−mice a HFD for 8
weeks (20). Thus, the association
between PCSK9 and BACE1 requires further investigation at a
cellular level.
In conclusion, the present study demonstrated that
hyperlipidemia can induce apoptosis of hippocampal neurons in
apoE(−/−) mice, and that PCSK9 may be involved in this process.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81370376
and 81200217), the Visiting Scholar Foundation of Key Laboratory of
Biorheological Science and Technology (Chongqing University),
Ministry of Education (grant nos. CQKLBST- 2014–002 and
CQKLBST-2015-004), the Construct Program of the Key Discipline in
Hunan Province (grant no. 2011001), and the ‘225’ High-level Health
Professionals Grant in Hunan Province (grant no. 201313).
References
|
1
|
Mawuenyega KG, Sigurdson W, Ovod V,
Munsell L, Kasten T, Morris JC, Yarasheski KE and Bateman RJ:
Decreased clearance of CNS beta-amyloid in Alzheimer's disease.
Science. 330:17742010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akhter R, Sanphui P, Das H, Saha P and
Biswas SC: The regulation of p53 up-regulated modulator of
apoptosis by JNK/c-Jun pathway in β-amyloid-induced neuron death. J
Neurochem. 134:1091–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kivipelto M, Helkala EL, Laakso MP,
Hänninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A,
Tuomilehto J, Nissinen A and Soininen H: Apolipoprotein E epsilon4
allele, elevated midlife total cholesterol level, and high midlife
systolic blood pressure are independent risk factors for late-life
Alzheimer disease. Ann Intern Med. 137:149–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solomon A, Kåreholt I, Ngandu T, Winblad
B, Nissinen A, Tuomilehto J, Soininen H and Kivipelto M: Serum
cholesterol changes after midlife and late life cognition:
Twenty-one-year follow-up study. Neurology. 68:751–756. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shepardson NE, Shankar GM and Selkoe DJ:
Cholesterol level and statin use in Alzheimer disease: I. Review of
epidemiological and preclinical studies. Arch Neurol. 68:1239–1244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pregelj P: Involvement of cholesterol in
the pathogenesis of Alzheimer's disease: Role of statins. Psychiatr
Danub. 20:162–167. 2008.PubMed/NCBI
|
|
7
|
Longenberger J and Shah ZA: Simvastatin
and other HMG-CoA reductase inhibitors on brain cholesterol levels
in Alzheimer's disease. Curr Alzheimer Res. 8:434–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan RB, Oliveira TG, Cortes EP, Honig LS,
Duff KE, Small SA, Wenk MR, Shui G and Di Paolo G: Comparative
lipidomic analysis of mouse and human brain with Alzheimer disease.
J Biol Chem. 287:2678–2688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kosari S, Badoer E, Nguyen JC, Killcross
AS and Jenkins TA: Effect of western and high fat diets on memory
and cholinergic measures in the rat. Behav Brain Res. 235:98–103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Sayyad HI, El Sherbiny MA, Sobh MA, El
Naga AM Abou, Ibrahim MA and Mousa SA: Protective effects of Morus
alba leaves extract on ocular functions of pups from diabetic and
hypercholesterolemic mother rats. Int J Biol Sci. 7:715–728. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JH, Hsieh CJ, Huang YL, Chen YC, Chen
TF, Sun Y, Wen LL, Yip PK and Chu YM: Genetic polymorphisms of
lipid metabolism gene SAR1 homolog B and the risk of Alzheimer's
disease and vascular dementia. J Formos Med Assoc. 115:38–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seidah NG, Benjannet S, Wickham L,
Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A and Chretien
M: The secretory proprotein convertase neural apoptosis-regulated
convertase 1 (NARC-1): Liver regeneration and neuronal
differentiation. Proc Natl Acad Sci USA. 100:928–933. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benjannet S, Rhainds D, Essalmani R, Mayne
J, Wickham L, Jin W, Asselin MC, Hamelin J, Varret M, Allard D, et
al: NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and
effects on the low density lipoprotein (LDL) receptor and LDL
cholesterol. J Biol Chem. 279:48865–48875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS
and Liu LS: PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL
via Bcl/Bax caspase9-caspase3 pathway. Mol Cell Biochem.
359:347–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington (DC): 2011,
PubMed/NCBI
|
|
16
|
Stranahan AM, Cutler RG, Button C,
Telljohann R and Mattson MP: Diet-induced elevations in serum
cholesterol are associated with alterations in hippocampal lipid
metabolism and increased oxidative stress. J Neurochem.
118:611–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moghadam A Hafezi, Thomas KL and Wagner
DD: ApoE deficiency leads to a progressive age-dependent
blood-brain barrier leakage. Am J Physiol Cell Physiol.
292:C1256–C1262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Methia N, André P, Hafezi-Moghadam A,
Economopoulos M, Thomas KL and Wagner DD: ApoE deficiency
compromises the blood brain barrier especially after injury. Mol
Med. 7:810–815. 2001.PubMed/NCBI
|
|
19
|
Frisardi V, Panza F, Seripa D, Farooqui T
and Farooqui AA: Glycerophospholipids and
glycerophospholipid-derived lipid mediators: A complex meshwork in
Alzheimer's disease pathology. Prog Lipid Res. 50:313–330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thirumangalakudi L, Prakasam A, Zhang R,
Bimonte-Nelson H, Sambamurti K, Kindy MS and Bhat NR: High
cholesterol-induced neuroinflammation and amyloid precursor protein
processing correlate with loss of working memory in mice. J
Neurochem. 106:475–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bingham B, Shen R, Kotnis S, Lo CF,
Ozenberger BA, Ghosh N, Kennedy JD, Jacobsen JS, Grenier JM,
DiStefano PS, et al: Proapoptotic effects of NARC 1 (=PCSK9), the
gene encoding a novel serine proteinase. Cytometry A. 69:1123–1131.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klein WL, Krafft GA and Finch CE:
Targeting small Abeta oligomers: The solution to an Alzheimer's
disease conundrum? Trends Neurosci. 24:219–224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fernández-Vizarra P, Fernández AP,
Castro-Blanco S, Serrano J, Bentura ML, Martínez-Murillo R,
Martínez A and Rodrigo J: Intra- and extracellular Abeta and PHF in
clinically evaluated cases of Alzheimer's disease. Histol
Histopathol. 19:823–844. 2004.PubMed/NCBI
|
|
24
|
Shie FS, Jin LW, Cook DG, Leverenz JB and
LeBoeuf RC: Diet-induced hypercholesterolemia enhances brain A beta
accumulation in transgenic mice. Neuroreport. 13:455–459. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Refolo LM, Malester B, LaFrancois J,
Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K and Pappolla
MA: Hypercholesterolemia accelerates the Alzheimer's amyloid
pathology in a transgenic mouse model. Neurobiol Dis. 7:321–331.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Umeda T, Tomiyama T, Kitajima E, Idomoto
T, Nomura S, Lambert MP, Klein WL and Mori H: Hypercholesterolemia
accelerates intraneuronal accumulation of Aβ oligomers resulting in
memory impairment in Alzheimer's disease model mice. Life Sci.
91:1169–1176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jonas MC, Costantini C and Puglielli L:
PCSK9 is required for the disposal of non-acetylated intermediates
of the nascent membrane protein BACE1. EMBO Rep. 9:916–922. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ko MH and Puglielli L: Two endoplasmic
reticulum (ER)/ER Golgi intermediate compartment-based lysine
acetyltransferases post-translationally regulate BACE1 levels. J
Biol Chem. 284:2482–2492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu M, Wu G, Baysarowich J, Kavana M,
Addona GH, Bierilo KK, Mudgett JS, Pavlovic G, Sitlani A, Renger
JJ, et al: PCSK9 is not involved in the degradation of LDL
receptors and BACE1 in the adult mouse brain. J Lipid Res.
51:2611–2618. 2010. View Article : Google Scholar : PubMed/NCBI
|