Introduction
Hepatocellular carcinoma (HCC) is the most common
form of liver cancer and the third most lethal cancer worldwide
(1). Over 500,000 new cases of HCC
are diagnosed each year worldwide, with ~50% of cases diagnosed in
China alone (2). Multiple gene
mutations, insertion of viral DNA and epigenetic alterations are
involved in the genetic mechanisms underlying hepatocarcinogenesis
(3). Surgery is currently the
primary approach for the treatment of HCC; however, a consistent
effective chemotherapeutic option for this disease is required
(1).
Sorafenib is a small molecule inhibitor that targets
multiple kinases, including Raf kinases, the vascular endothelial
factor receptor and the platelet-derived growth factor receptor,
and is approved for the treatment of advanced HCC in Chinese
clinics (4). The efficacy of
sorafenib for the improvement of median survival rates was first
presented in 2008 (5). Following
this, sorafenib was identified as useful for the treatment of HCC
in several additional studies, either alone or in combinational
treatment (6,7). However, unsatisfactory response rates
and adverse side effects, such as diarrhea and jaundice, have been
reported (8). The search for an
efficient adjuvant agent to sorafenib has recently invited
significant attention in the field.
Digitoxin is a cardiac glycoside that has been
widely studied and used for its cardiotonic effects (9). Since the 1990s, the anticancer effect
of digitoxin has been demonstrated in multiple cancer types
including prostate, breast and pancreatic cancers (10,11).
However, the precise anticancer mechanisms of digitoxin remain
elusive. It has been proposed that, at nanomolar concentrations,
digitoxin activates the Na+/K+ adenosine
triphosphatase signalosome to regulate cellular events, including
apoptosis, cell movement, cell proliferation and tight junction
regulation (10). Signaling
pathways, including the p53, mitogen-activated protein
kinase/extracellular signal-regulated kinase (ERK) and
phosphatidyl-inositol-3-kinase pathways, have been demonstrated to
be affected by digitoxin (10). As
the target downstream signaling pathways of digitoxin overlap with
those targeted by sorafenib, and the activity of digitoxin on HCC
has not yet been reported, the combination of these drugs may
provide a novel treatment strategy for HCC. Therefore, the aim of
the present study was to investigate the effects of the
combinational treatment of digitoxin and sorafenib on HCC cells,
and to examine the relevant underlying molecular mechanisms.
Materials and methods
Cell culture
Human HCC cell lines BEL-7402 and HepG2 were
purchased from the Shanghai Institute of Biochemistry and Cell
Biology (Chinese Academy of Sciences, Shanghai, China). These cell
lines were maintained in Dulbecco's Modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Equitech-Bio, Kerrville, Texas,
USA), 100 U/ml penicillin G and 100 Ug/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.). Digitoxin was purchased from
Sigma-Aldrich; Merck-Millipore (Darmstadt, Germany). Sorafenib was
purchased from Bayer AG (Leverkusen, Germany). Cells were treated
with different concentrations of digitoxin (0, 10, 20, 40, 80 and
160 nmol/l) and with various concentrations of sorafenib (0, 1, 2,
4, 8 and 16 µmol/l) prior to the MTT assay. For wound healing
assays, western blot analyses and the acridine orange/ethidium
bromide (AO/EB) assay, the cells of control group cultured in RPMI
1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.), whereas
cells in the experimental groups were treated with 10 nmol/l
digitoxin and 8 µmol/l sorafenib.
MTT assay
A total of 5,000 cells were seeded in a 96-well
plate in 200 µl medium and incubated for 24, 48 and 72 h at 37°C in
5% CO2 incubator. Cell viability was determined using an
MTT assay kit (cat. no. 0793; Amresco, LLC, Solon, OH, USA)
according to the manufacturer's instructions. The absorbance was
read at 490 nm on a microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). All assays were performed in triplicate. For
statistical analysis of cell viability, one-way analysis of
variance with the Dunnett's post-hoc test was used.
Scratch wound healing assay
HepG2 and BEL-7402 cells were seeded in a six-well
plates at a density of 2×103 cells/well and incubated overnight
until they reached confluency. The monolayer of cells were
scratched with a sterile pipette tip to create a wound. Cells were
washed twice with serum-free media to remove floating cells, and
cultured in complete media containing serum. Three groups of cells
were treated with digitoxin and/or sorafenib, while the control
group was left untreated. The cells migrating from the leading edge
were imaged at 0 and 48 h. A total of three fields of view for each
well were analyzed and experiments were performed in triplicate.
The migration index was calculated using the following formula:
Migration index = (g0 h - g48 h) / g0
h x100%, where g0 h and g48 h represent
the wound width at 0 and 48 h, respectively.
Western blot analysis
BEL-7402 and HepG2 cells were exposed to sorafenib
(8 µM) and/or digitoxin (10 nM) for 24 or 48 h prior to western
blot analysis. A total of ~1×106 cells were homogenized in 100–200
µl radioimmunoprecipitation assay lysis buffer (Beyotime Institute
of Biotechnology, Haimen, China) containing protease inhibitors
(Roche Diagnostics, Basel, Switzerland) at 4°C for 30 min prior to
western blot analysis, as described previously (12). The polyvinylidene difluoride (PVDF)
membranes were incubated for 2 h at 37°C or overnight at 4°C with
the following primary antibodies (dilution, 1:500): Anti-p44/42
(ERK; cat no. 9102; Cell Signaling Technology, Inc., Danvers, MA,
USA), anti-phosphorylated (p)-p44/42 ERK (cat. no. sc-16982; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-vascular
endothelial growth factor (VEGF; cat no. sc-4570; Santa Cruz
Biotechnology, Inc.), anti-hypoxia inducible factor (HIF)-1α (cat.
no. MA-516; Thermo Fisher Scientific, Inc.), anti-HIF-2α (cat. no.
ab8365; Abcam, Cambridge, UK) and anti-β-actin (cat. no. 4970; Cell
Signaling Technology, Inc.). The horseradish peroxidase-conjugated
goat anti-rabbit IgG (cat. no. 12–348) and goat anti-mouse IgG
(cat. no. 12–349) secondary antibodies were purchased from
Sigma-Aldrich; Merck Millipore. The PVDF membranes were incubated
with the secondary antibodies (dilution, 1:5,000) for 1 h at 37°C.
Protein bands were detected using an enhanced chemiluminescence kit
(Thermo Fisher Scientific Inc.) and were detected by Chemic Genius
Bioimaging System (Syngene, Frederick, MD). GeneSnap (version,
7.12) and GeneTools (version, 4.3.5) software programs (Syngene)
were used for quantification.
AO/EB assay
Cells were seeded in six-well plates to a final
concentration of 2×105 cells/well. Following 24 h, cells were
exposed to digitoxin (10 nmol/l) and/or sorafenib (8 µmol/l) for 48
h at 37°C in 5% CO2, before they were stained with AO/EB
dye mixture containing AO (200 µg/ml; Sigma-Aldrich; Merck
Millipore) and EB (200 µg/ml; Sino-American Biotechnology Company,
Luoyang, China). A total of six fields of view for each group were
observed and counted under a fluorescence microscope (Nikon
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis and visualization of the data
was achieved using the GraphPad Prism software program (version,
6.04; GraphPad Software Inc., La Jolla, CA, USA) and the Bliss
additive model (13). The Bliss
additive model was used to classify the effects of combining
digitoxin and sorafenib as additive, synergistic or antagonistic.
Combined inhibition was calculated using the following equation:
Ebliss = EA + EB - EA × EB, where EA and EB represent the
fractional inhibitions obtained by drug A (digitoxin) alone and
drug B (sorafenib) alone at specific concentrations. Data were
analyzed using the Student's t-test and one-way analysis of
variance with the Dunnett's post-hoc test. Samples were analyzed in
triplicate, and experiments were repeated three times. P<0.05
was considered to indicate a statistically significant
difference.
Results
Sorafenib and digitoxin synergized to
suppress the viability, but not migration of HCC cells
The effect of a range of concentrations of sorafenib
(0, 1, 2, 4, 8 and 16 µM) and digitoxin (0, 10, 20, 40, 80 and 160
nM) on the viability of HCC cells was first examined using an MTT
assay. For the BEL-7402 and HepG2 cells, the half maximal
inhibitory concentration values for sorafenib were ~8 µM and for
digitoxin were ~80 nM (Fig. 1A and
B). Considering the high cardiotoxicity of digitoxin in
clinical practice (14) and the
recent model demonstrating that digitoxin is sufficient to activate
intracellular downstream signaling cascades at 10 nM (10), the effect of sorafenib in
combination with a low concentration of digitoxin (10 nM) on HCC
cells was examined in the present study. Sorafenib (8 µM) and
digitoxin (10 nM) significantly inhibited the viability of BEL-7402
and HepG2 cells (P<0.001 and P<0.01, respectively; Fig. 1C and D) when compared with the
established additive combinatorial inhibition that was based on
single agent data and the Bliss additive model. This suggests a
synergy between the two reagents at these concentrations.
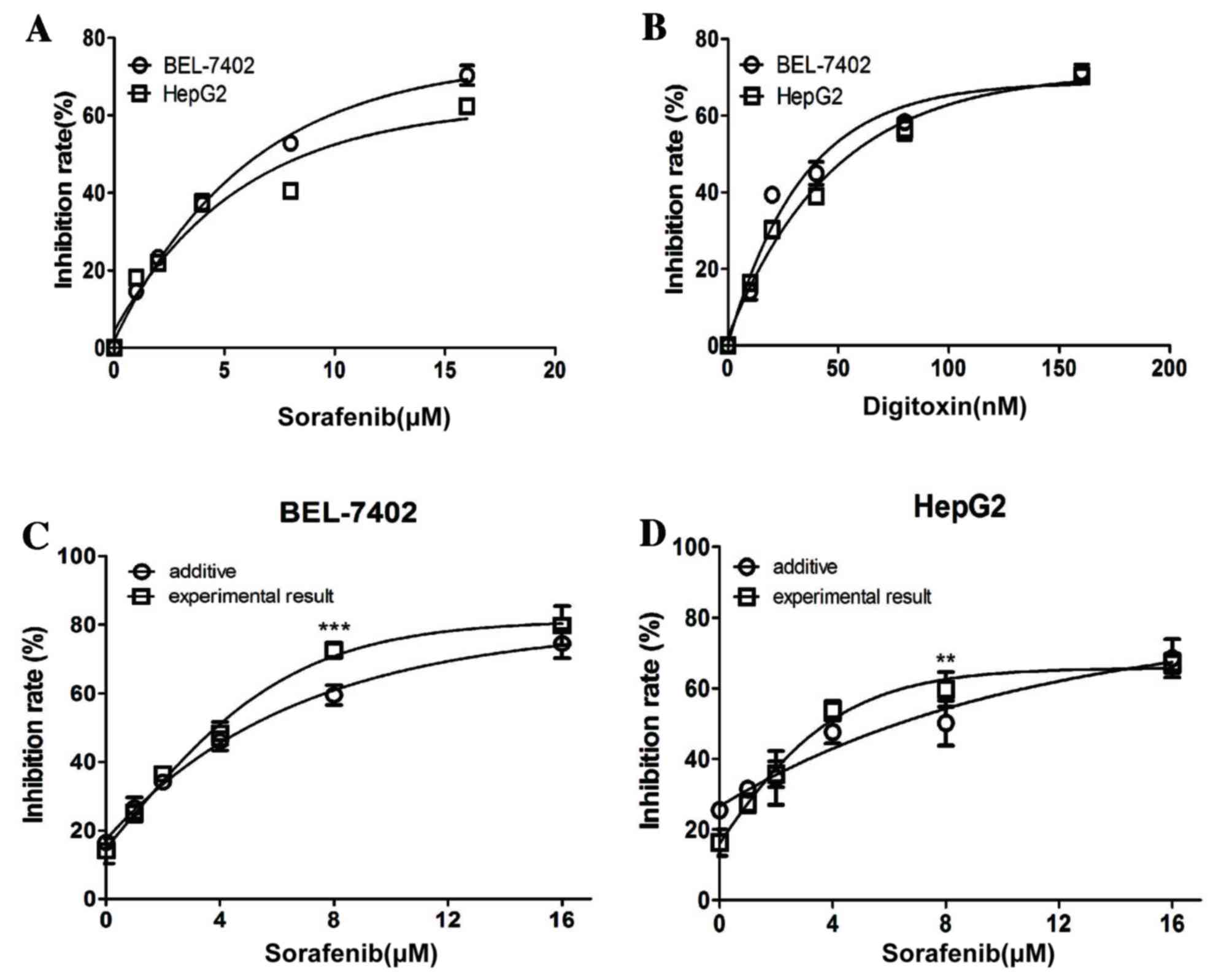 | Figure 1.Sorafenib and digitoxin
synergistically-inhibits the proliferation of hepatocellular
carcinoma cell lines. BEL-7402 and HepG-2 cells were exposed to a
series of concentrations of (A) sorafenib (0, 1, 2, 4, 8 and 16 µM)
or (B) digitoxin (0, 10, 20, 40, 80 and 160 nM) for 48 h prior to
MTT assay analysis. (C) BEL-7402 and (D) HepG-2 cells were exposed
to a series of concentrations of sorafenib (0, 1, 2, 4, 8 and 16
µM) in combination with digitoxin (10 nM) for 48 h prior to MTT
assay analysis. The additive inhibition for each combination was
calculated using the Bliss additive model. Each experiment was
performed in triplicate, and data are presented as the mean ±
standard error. **P<0.01 and ***P<0.001 vs. additive. |
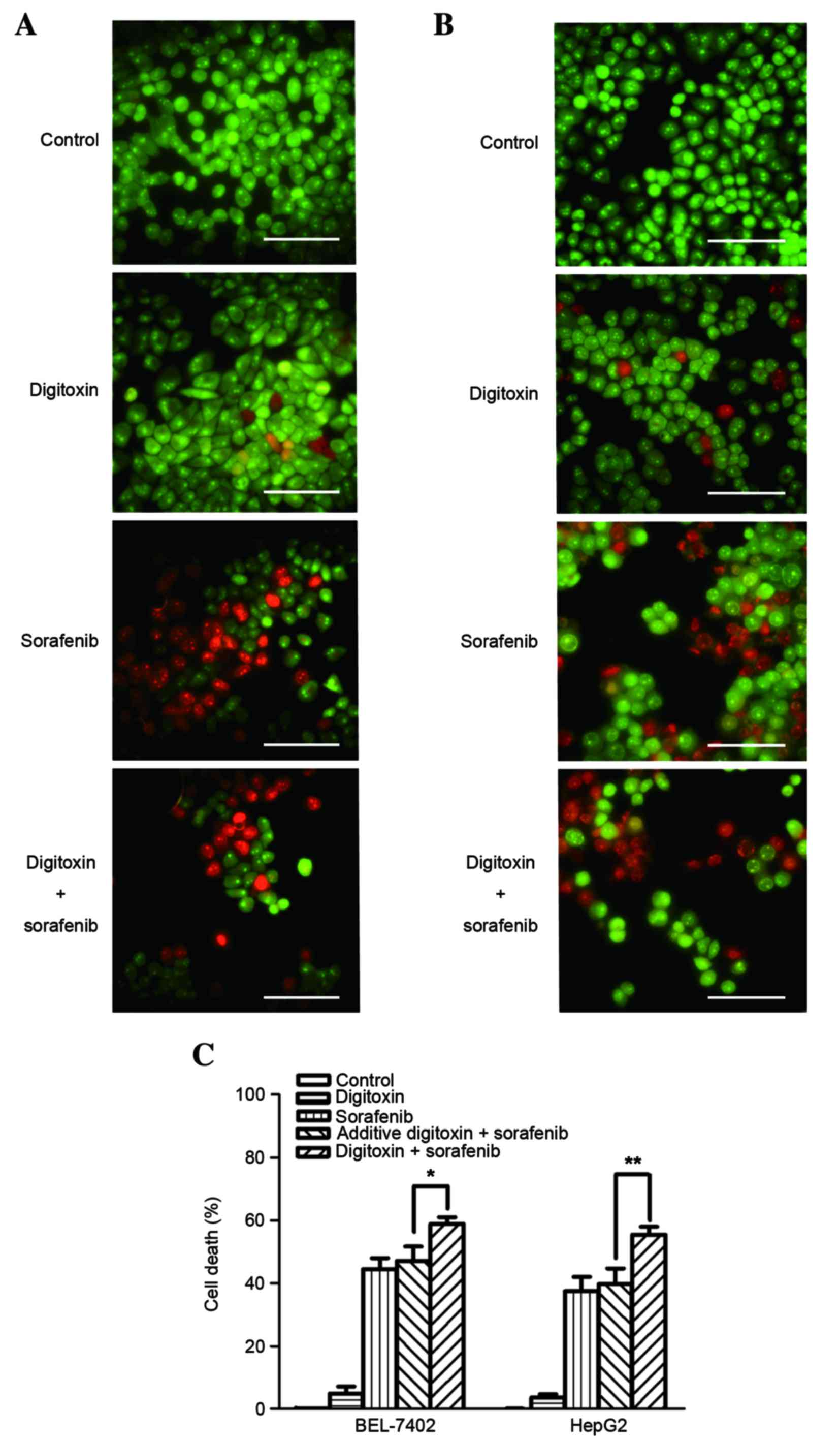
AO/EB staining was then used to examine cell death
(Fig. 2A and B). Consistent with
the MTT assay results, the death rate of BEL-7402 and HepG2 cells
exposed to 8 µM sorafenib plus 10 nM digitoxin was significantly
higher when compared with the additive cell death rate (P=0.041 and
P=0.0057, respectively; Fig. 2C).
In addition to cell death, cell migration of VSMC and NSCLC has
been demonstrated to be affected by digitoxin treatment, while the
effect of digitoxin on migration of hepatocellular carcinoma cell
is still unknown (15,16). Sorafenib has been reported to
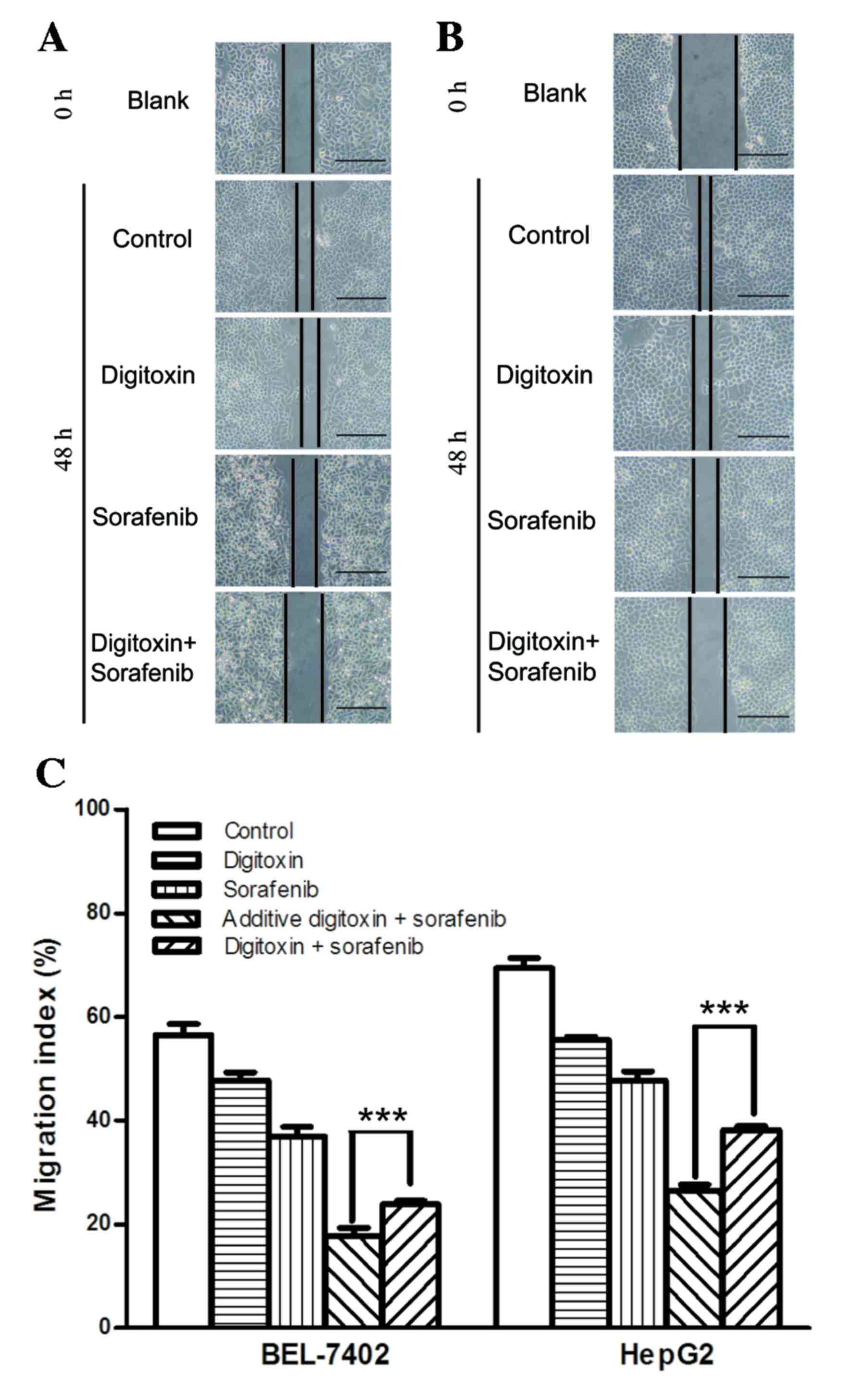
inhibit migration of hepatocellular carcinoma cells (17). Therefore, the present study
investigated the effect of sorafenib and digitoxin on cell
migration using a scratch wound healing assay (Fig. 3A and B). Although the cell
migration inhibition, generated by 8 µM sorafenib was enhanced in
BEL-7402 and HepG2 cells, when cells were exposed to sorafenib plus
10 nM digitoxin, a significant increase in cell migration was
observed when compared with the additive inhibition. This suggests
that sorafenib and digitoxin antagonized each other in cell
migration suppression (Fig.
3C).
Sorafenib plus digitoxin treatment
inhibited the ERK and hypoxia responsive pathways
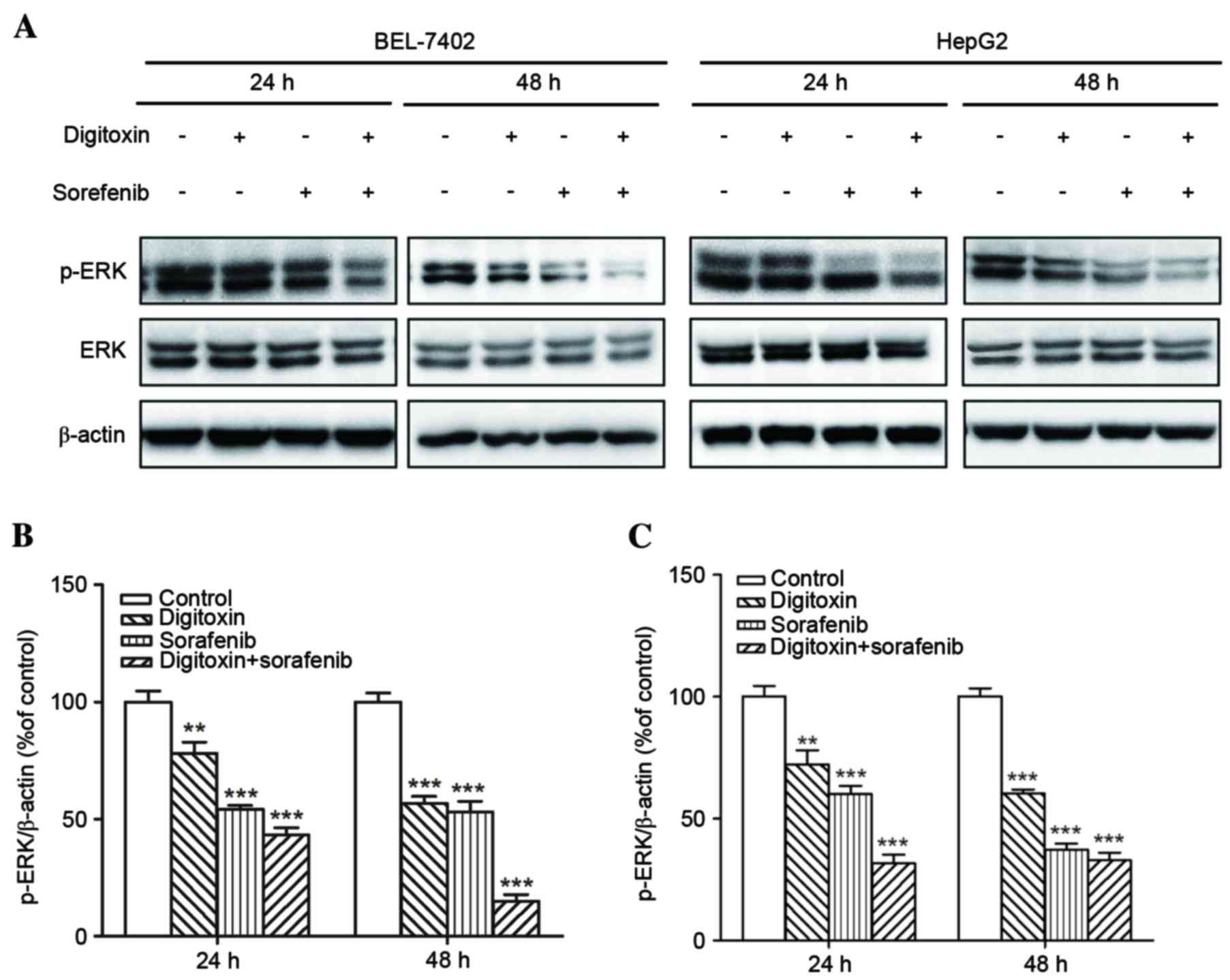
ERK is an important downstream effector of Raf
kinases, and is affected by digitoxin (18). Therefore, the present study
examined the expression of p-ERK in HCC cells exposed to 8 µM
sorafenib and/or 10 nM digitoxin. p-ERK expression inhibition was
more efficient in BEL-7402 and HepG2 cells treated with sorafenib
plus digitoxin, when compared with either agent alone (Fig. 4). Previous studies have
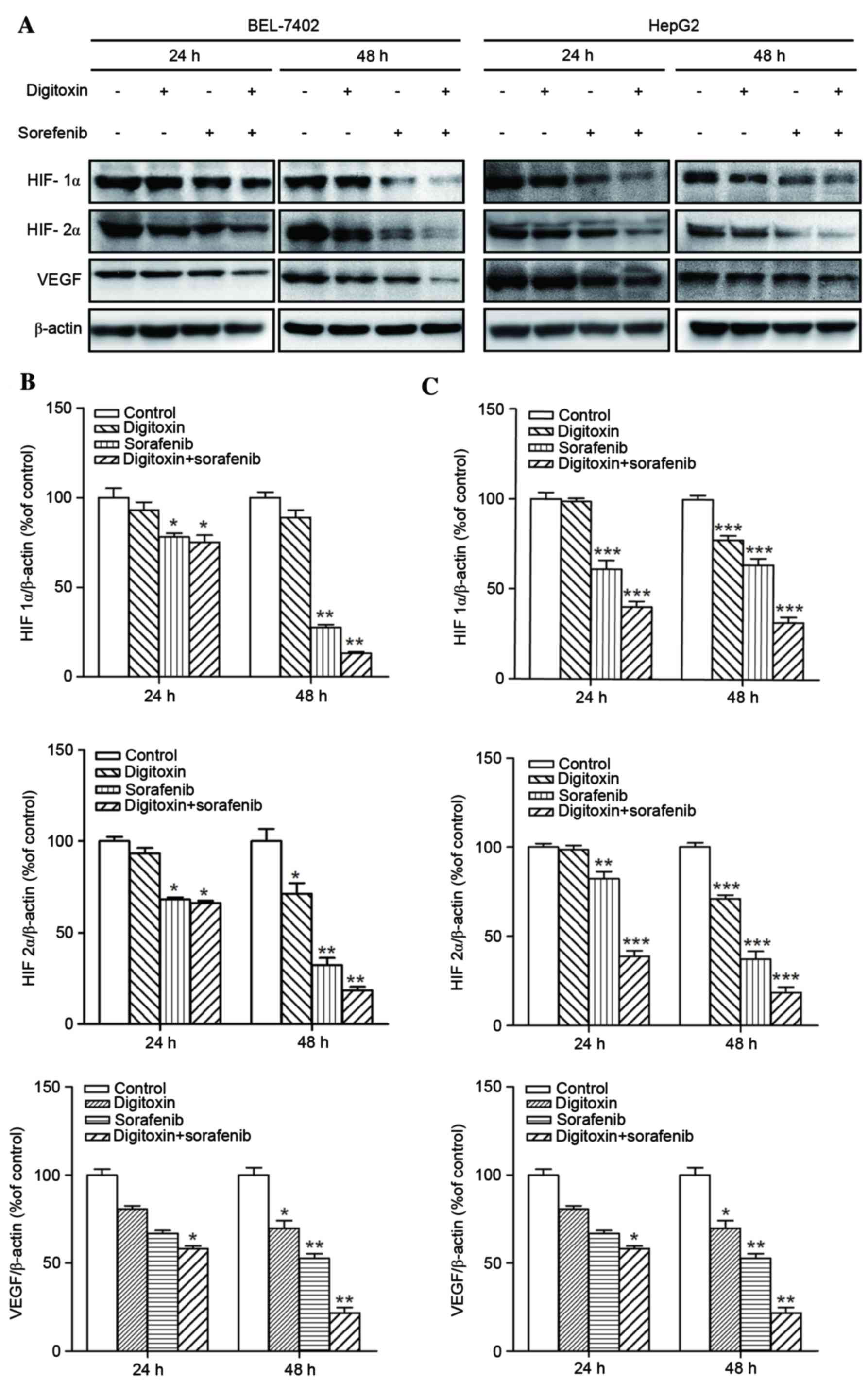
demonstrated that HIF-1α, HIF-2α and VEGF are therapeutic targets
of either digitoxin or sorafenib in several cancer types (19,20).
As a result, the expression of these proteins in cells exposed to
sorafenib and/or digitoxin was examined in the present study.
Similar to p-ERK expression, greater suppression of HIF-1α, HIF-2α
and VEGF expression was observed following treatment of cells with
sorafenib plus digitoxin, when compared to application with either
agent alone (Fig. 5).
Discussion
The development of novel anticancer agents is a long
and expensive process, which is often associated with a high
failure rate (21,22). Drugs that have already been
approved for the treatment of other diseases in the clinic, may be
tested for their anticancer properties more rapidly and with less
expense. This process of drug ‘repurposing’ has the potential of
accelerating anticancer drug development. For instance, the
anticancer properties of the rheumatoid arthritis drug auranofin
(23) or the oral hypoglycemic
agent metformin (24) have
recently been discovered, and clinical trials investigating the
application of these drugs in cancer treatment are currently
ongoing. As it has been studied for a number of years, the clinical
profile of digitoxin is already well established (25), which may facilitate the rapid
initiation of clinical trials investigating its effectiveness in
combination with sorafenib. Therefore, digitoxin represents an
attractive adjuvant agent. The anticancer effects of digitoxin have
been understood for decades (26);
however, its narrow therapeutic window caused by high
cardiotoxicity, hinders its application in cancer as a single
agent. The present study demonstrated that, even at a very low
concentration (10 nM), digitoxin is sufficiently potent to
synergize with sorafenib to initiate cell death, which suggests
that it may be a promising candidate for combinational treatment
with sorafenib for HCC. However, sorafenib was not observed to
synergize with digitoxin in the cell migration assay indicating
that the synergy primarily relied on the signaling pathways
associated with cell death Future studies will aim to explore these
effects in vivo using animal models to further validate
these results.
In response to the sorafenib and/or digitoxin
treatment, the expression alterations of four signaling proteins,
p-ERK, HIF-1α, HIF-2α and VEGF, were examined. In single agent
treatment, sorafenib suppressed HIF-1α and HIF-2α expression more
than digitoxin in the cell lines examined. Considering the
difference in cell death induction between 10 nM digitoxin and 8 µM
sorafenib, it is possible that the hypoxia pathway may be more
critical for cell viability among the four proteins tested in HCC.
Conversely, the expression changes of ERK and VEGF coincides better
with cell migration alterations, which suggests they may be more
involved in regulating cell migration in HCC.
The results of the present study demonstrated that
sorafenib in combination with digitoxin suppresses HCC cell
viability, which may be mediated through inhibition of p-ERK,
HIF-1α, HIF-2α and VEGF expression. This is consistent with the
findings of a previous study demonstrating that digitoxin exhibits
greater toxicity in lung cancer cells when compared with primary or
non-tumorigenic epithelial cells (27). However whether this selectivity
remains in patients with HCC, is unknown. Toxicity in normal liver
epithelial cells will need to be taken into account in future in
vivo or in vitro experiments, when evaluating the
effectiveness of combined sorafenib and digitoxin treatment.
Acknowledgements
The present study was fully supported by the
National Natural Science Foundation of China for Distinguished
Young Scholars (grant nos. 31301172 and 31201008), the Natural
Science Foundation of Fujian Province (grant no. 2014J01122), the
Scientific Foundation of Fuzhou University (grant no.
2014-S-139-7), the Open Scientific Foundation of Fujian Key
Laboratory (grant no. 2014ZDSY2002) and was partially supported
from the University of Macau Start-Up Research Grant (grant no.
SRG2014-00006-FHS) & Multi-Year Research Grant (grant no.
MYRG2015-00065-FHS). Ms. Libin Guo, Ms. Bin Li and Mr. Henrique
Neves are in receipt of PhD studentships from the Faculty Health
Sciences University of Macau.
References
|
1
|
El-Serag HB: Hepatocellular Carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feitelson MA, Sun B, Tufan NL Satiroglu,
Liu J, Pan J and Lian Z: Genetic mechanisms of
hepatocarcinogenesis. Oncogene. 21:2593–2604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, De Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR,
Bhagat N and Geschwind JF: Phase II trial of sorafenib combined
with concurrent transarterial chemoembolization with drug-eluting
beads for hepatocellular carcinoma. J Clin Oncol. 29:3960–3967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keating GM and Santoro A: Sorafenib: A
review of its use in advanced hepatocellular carcinoma. Drugs.
69:223–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belz G, Breithaupt-Grögler K and Osowski
U: Treatment of congestive heart failure-current status of use of
digitoxin. Eur J Clin Invest. 31 Suppl 2:10–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elbaz HA, Stueckle TA, Tse W, Rojanasakul
Y and Dinu CZ: Digitoxin and its analogs as novel cancer
therapeutics. Exp Hematol Oncol. 1:42012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
López-Lázaro M: Digitoxin as an anticancer
agent with selectivity for cancer cells: Possible mechanisms
involved. Expert Opin Ther Targets. 11:1043–1053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie JW, Chen PC, Zheng CH, Li P, Wang JB,
Lin JX, Lu J, Chen QY, Cao LL, Lin M, et al: Evaluation of the
prognostic value and functional roles of CD44v6 in gastric cancer.
J Cancer Res Clin Oncol. 141:1809–1817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buck E, Eyzaguirre A, Brown E, Petti F,
McCormack S, Haley JD, Iwata KK, Gibson NW and Griffin G: Rapamycin
synergizes with the epidermal growth factor receptor inhibitor
erlotinib in non-small-cell lung, pancreatic, colon, and breast
tumors. Mol Cancer Ther. 5:2676–2684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith TW: Digitalis toxicity: Epidemiology
and clinical use of serum concentration measurements. Am J Med.
58:470–476. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan G, Wang Q, Hu S, Wang D, Qiao Y, Ma G,
Tang C and Gu Y: Digoxin inhibits PDGF-BB-induced VSMC
proliferation and migration through an increase in ILK signaling
and attenuates neointima formation following carotid injury. Int J
Mol Med. 36:1001–1011. 2015.PubMed/NCBI
|
|
16
|
Lin SY, Chang HH, Lai YH, Lin CH, Chen MH,
Chang GC, Tsai MF and Chen JJ: Digoxin suppresses tumor malignancy
through inhibiting multiple Src-related signaling pathways in
non-small cell lung cancer. PLoS One. 10:e01233052015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ha TY, Hwang S, Moon KM, Won YJ, Song GW,
Kim N, Tak E, Ryoo BY and Hong HN: Sorafenib inhibits migration and
invasion of hepatocellular carcinoma cells through suppression of
matrix metalloproteinase expression. Anticancer Res. 35:1967–1976.
2015.PubMed/NCBI
|
|
18
|
Einbond LS, Shimizu M, Ma H, Wu HA,
Goldsberry S, Sicular S, Panjikaran M, Genovese G and Cruz E:
Actein inhibits the Na+-K+-ATPase and enhances the growth
inhibitory effect of digitoxin on human breast cancer cells.
Biochem Biophys Res Commun. 375:608–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Qian DZ, Tan YS, Lee K, Gao P,
Ren YR, Rey S, Hammers H, Chang D, Pili R, et al: Digoxin and other
cardiac glycosides inhibit HIF-1alpha synthesis and block tumor
growth. Proc Natl Acad Sci USA. 105:19579–19586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu M, Zheng YL, Xie XY, Liang JY, Pan FS,
Zheng SG and Lü MD: Sorafenib blocks the HIF-1α/VEGFA pathway,
inhibits tumor invasion and induces apoptosis in hepatoma cells.
DNA Cell Biol. 33:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pessetto ZY, Weir SJ, Sethi G, Broward MA
and Godwin AK: Drug repurposing for gastrointestinal stromal tumor.
Mol Cancer Ther. 12:1299–1309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woodcock J and Woosley R: The FDA critical
path initiative and its influence on new drug development. Annu Rev
Med. 59:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roder C and Thomson MJ: Auranofin:
Repurposing an old drug for a golden new age. Drugs RD. 15:13–20.
2015. View Article : Google Scholar
|
|
24
|
Kourelis TV and Siegel RD: Metformin and
cancer: New applications for an old drug. Med Oncol. 29:1314–1327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richards LG, Castle MC and Lage GL:
Glucuronidation of digitoxin and its derivatives by rat and rabbit
liver homogenates. Drug Metab Dispos. 5:469–473. 1977.PubMed/NCBI
|
|
26
|
Haux J: Digitoxin is a potential
anticancer agent for several types of cancer. Med Hypotheses.
53:543–548. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elbaz HA, Stueckle TA, Wang HY, O'Doherty
GA, Lowry DT, Sargent LM, Wang L, Dinu CZ and Rojanasakul Y:
Digitoxin and a synthetic monosaccharide analog inhibit cell
viability in lung cancer cells. Toxicol Appl Pharmacol. 258:51–60.
2012. View Article : Google Scholar : PubMed/NCBI
|