Introduction
Head and neck cancer (HNC) is the sixth most common
type of cancer in humans; it can develop in any of the following
organs: Lips, oral cavity (mouth), nasal cavity (inside the nose),
paranasal sinuses, pharynx and larynx. An estimated 300,400 new
cases of oral cavity cancer, and 145,400 cases of oral cavity
cancer-associated mortality occurred worldwide in 2012 (1). In cases of HNC, ~90% develop head and
neck squamous cell carcinoma (HNSCC), which is the leading cause of
HNC worldwide (2). The development
of HNSCC has been associated with certain environmental and
lifestyle risk factors, including alcohol consumption, tobacco
smoking, UV light exposure, use of chemicals and viral infection
(3). Furthermore, when patients
develop this disease, they also experience maxillofacial deformity
and psychological changes in addition to the common symptoms of
cancer. As with other types of cancer, the development of HNSCC is
also associated with multiple genetic alterations, including the
overexpression of certain oncogenes and the reduced expression of
tumor suppressor genes (4,5).
KAI1, which is also known as CD82, and antigens R2,
C33, IA4 and 4F9, are members of the tetraspanin family of
proteins. KAI1 is located in chromosome 11p11.2, and it encodes 267
amino acids with the transmembrane proteins in eukaryotes (6,7). Its
expression was first identified during T-cell activation (8). Later, it was found to elicit the
suppression of prostate cancer metastasis using a somatic cell
hybridization technique (9).
Previous studies have also reported that the expression of KAI1 is
correlated with the progression of several types of human cancer.
It has been categorized as a metastatic suppressor in various types
of human cancer, including cancer of the prostate (10,11),
bladder (12,13), breast (14,15),
colon (16,17), pancreas (18) and lungs (19,20).
Previous studies have also reported that there is a significant
association between downregulated expression of KAI1 and primary
oral tumors, which are associated with lymph nodes (21–23).
However, there have been no reports on the outcome of the
overexpression of KAI1 in oral cancer. Therefore, the present study
aimed to investigate the role of KAI1 in oral cancer by
transfecting its full-length cDNA into malignant oral cancer
cells.
Materials and methods
Isolation and cloning of KAI1
cDNA
The complete DNA sequence of KAI1 was amplified from
the human brain cDNA library using the polymerase chain reaction
protocol (6). The primers used for
KAI1 amplification were as follows: Forward
5′-ATGGGCTCAGCCTGTATCAAAG-3′ and reverse
3′-GTACTTGGGGACCTTGCTGTA-5′. Following amplification, the KAI1 cDNA
was digested with EcoRI and SalI restriction enzymes (BLKW
Biotechnology, Beijing, China). It was then ligated with the
pIRES2-EGFP vector overnight at 16°C. Thus, the recombinant
plasmid, pIRES2-EGFP-KAI1 was constructed. This plasmid was then
purified using a QIAGEN Plasmid Purification kit (cat. no. 12125;
Qiagen, Inc., Valencia, CA, USA).
Cell culture, transfection and
selection
293T and OSCC-15 cells were purchased from American
Type Culture Collection (Manassas, VA, USA). These cells were
cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich,
Merck Millipore, Darmstadt, Germany), which was supplemented with
10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The cell culture was incubated at 37°C in a
humidified atmosphere containing 5% CO2. The cultured
cells were passaged every 2 days and fresh medium was added to the
culture.
The 293T cells were transfected with the
pIRES2-EGFP- KAI1 plasmid in a low serum culture medium (Opti-MEM;
Gibco; Thermo Fisher Scientific, Inc.) using Lipofectamine™ 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol, at room temperature.
Following transfection, 90% confluent cells were cultured in a
CO2 incubator at 37°C. After 24, 48 and 72 h of
transfection, the expression of KAI1 was evaluated in the 293T
cells using western blot analysis. This plasmid and the pIRES2-EGFP
vector were transfected into the OSCC-15 cells. At 24 h
post-transfection, the cells were washed with phosphate-buffered
saline (PBS) and placed in fresh medium containing 600 mg/ml G418
antibiotics (Invivogen; Thermo Fisher Scientific, Inc.). The cells
were grown for 7–10 days (24) at
37°C. Subsequently, stable clones expressing KAI1 were selected via
GFP detection. Fluorescence was examined under a fluorescence
microscope (BX51-32FB3F01; Olympus Corporation, Tokyo, Japan) For
performing additional functional assays, the cells were divided
into three groups: OSCC-15 cells as the blank control group;
OSCC-15 cells, which only expressed the pIRES2-EGFP vector as the
negative control (NC) group; and OSCC-15 cells stably expressing
the KAI1 gene as the experiment group. All transfection experiments
were repeated at least three times.
Western blot analysis
Western blot analysis was performed to measure the
protein expression of KAI1. The protein was extracted from the
cultured cells by incubation in radioimmunoprecipitation assay
buffer (Norgen Biotek Corp., Thorold, ON, Canada) for 30 min at
37°C; the lysis buffer contained a protease inhibitor (2 µg/ml),
aprotinin (2 µg/ml), leupeptin, 1M phenylmethylsulfonyl fluoride,
1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl
sulfate (SDS) in 1X PBS. Following lysis, purification of the
supernatant was achieved by performing centrifugation at 1,4000 × g
for 5 min at 4°C; following which a fixed quantity of protein (10
µl) from each sample was suspended in a loading buffer (1% SDS and
1% dithiothreitol). This suspension was heated to 100°C for 5 min
for denaturing of the proteins. The 50 µg (5 µg/µl) protein samples
were then electrophoresed via 10% SDS polyacrylamide gel
electrophoresis, following which the samples were transferred onto
a nitrocellulose membrane (cat. no. 88018; Pierce; Thermo Fisher
Scientific, Inc.). Following blocking of the membrane with 5%
non-fat dry milk in Tris-Buffered Saline and Tween, containing 10
mmol/l Tris-HCl (pH 8.0) 100 mmol/l NaCl and 0.05% Tween, the
membrane was incubated overnight at 4°C with anti-KAI1 polyclonal
antibody (1:200; ab140238; Abcam, Cambridge, UK). Following
incubation, the membrane was washed twice with PBS for 5 min and
incubated with immunoglobulin G-horseradish peroxidase secondary
antibodies (1:2,000; ab6789; Abcam) for 2 h at 4°C. The expression
of glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:800;
sc-365062; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; or
anti-GAPDH; 1;2,000; ab9485; Abcam) was measured in the control
group, and each experiment was performed in triplicate. Gel-Pro
Analyzer 5.0 (Media Cybernetics, Rockville, MD, USA) was used to
analyze the protein expression.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The MTT colorimetric assay was used to determine the
relative proliferation rate of the tumor cells. The cells (1×104)
were seeded in 96-well plates and were cultured overnight at 37°C
in a humidified atmosphere containing 5% CO2. The
following day, 1X MTT reagent was added to each well; the
concentration of this reagent was 5 mg/ml in a volume of 20 µl. The
cells were incubated for another 4 h, and the absorbance of the
cell cultures at 570 nm were recorded. The experiments were
repeated three times. The relative proliferation rate of the cells
was calculated using the following equation: Proliferation rate (%)
= (experimental absorbance value / control absorbance value)
×100%.
Invasion assay
The cell invasion assay was performed in a 96-well
Transwell plate with polycarbonate filters (8-µm pore size; EMD
Millipore, Billerica, MA, USA). These filters were coated with 50
mg/l of Matrigel (EMD Millipore), which was previously diluted at a
ratio of 1:8 (3.9 µg/µl, 60–80 µl) in a serum-free medium. The
coated filters were air-dried for 24 h. The transfected OSCC-15
cells (5×104) were trypsinized and washed in PBS. The cells were
then suspended in 0.1% serum-containing medium and were loaded into
the upper chamber of the filter. The conditioned medium, as a
chemoattractant, was added into the lower chamber of the filter.
The cells were incubated at 37°C in 5% CO2 for 18 h,
following which the cells, which had invaded the lower surface of
the filter were fixed with methanol. Subsequently, the cells were
washed in PBS and stained with hexamethyl-pararosaniline. The
experiment was repeated three times using different cell lines. For
quantification, the cells were counted under a light microscope
(Olympus Corporation) in five predetermined fields (magnification,
×200).
The cell invasion inhibition rate was calculated
using the following equation: Cell invasion inhibition rate =
(number of migrated cells in control group-number of migrated cells
in experimental group / number of migrated cells in control group)
×100.
Apoptotic assay
At 72 h post-transfection, the transfected and
non-transfected OSCC-15 cells (105–106 for each sample) were
trypsinized, collected and washed twice with cold 1X PBS. Each
sample was then re-suspended in 100 µl of 5% Annexin V-FITC (ADL,
San Diego, CA, USA) reagent. This suspension was incubated at 37°C
for 15 min in the dark. Subsequently, 10 µl propidium iodide (PI)
was added to the suspension, and the cells were incubated for a
further 30 min. The apoptotic cells were stained with Annexin
V-FITC and analyzed using flow cytometry. The experiments were
performed in triplicate.
Tumor xenograft growth
BALB/c nude male mice (5-week-old) were obtained
from the Animal Laboratory of the Fourth Military Medical
University (Shaanxi, China). The animals were housed in a
specific-pathogen-free laboratory (22°C, natural 12-h light/dark
cycle, free access to food/purified water) and were divided into
three groups, each containing 21 mice. Non-transfected cells
(OSCC-15), cells transfected with the NC plasmid (OSCC-15 + NC),
and cells transfected with CD82 (OSCC-15 + CD82) were
subcutaneously inoculated into the nude mice. Briefly, on day 1,
100 µl cell suspension (1×106 cells) was subcutaneously inoculated
into the right rear flank of the nude mice. At 7 days
post-injection, tumor appearance was detected in these mice.
Consequently, the size of the tumors was measured every 3 days
using a precision caliper; the measurements were recorded for 25
days. The tumor volume was calculated using the following formula:
Volume (V)=a (length)xb2(width)x0.52. At 30 days post-cellular
injection, the mice were anesthetized with 4% hydrous ammonia
aldehyde (intraperitoneal injection, 0.01 ml/g) sacrificed by
cervical dislocation. Subsequently, the tumors were excised and the
weights of the tumors were recorded. The tumor inhibitory rate was
calculated using the following formula:
(Vb-Va/Va)x100%. Vb
represents the volume of the experiment group; Va
represents the volume of the control group. The experimental
procedure involving these animals was conducted in accordance with
the Guide for the Care and Use of Laboratory Animals by the
National Institutes of Health (NIH Pub. No. 85–23, revised 1996)
(NIH pub. no. 85–23, revised 1996) (25). The experimental study design was
approved by the Animal Care and Use Committee of The Fourth
Military Medical University.
Statistical analysis
SPSS software version 19.0 (IBM SPSS, Armonk, NY,
USA) was used to perform the statistical analysis. The expression
of was assessed using the χ-test. The values are presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of pIRES2-EGFP-KAI1 plasmid
transfection efficiency
In the 293T and OSCC-15 cells transfected with the
pIRES2-EGFP-KAI1 plasmid, the overexpression of KAI1/CD82 was
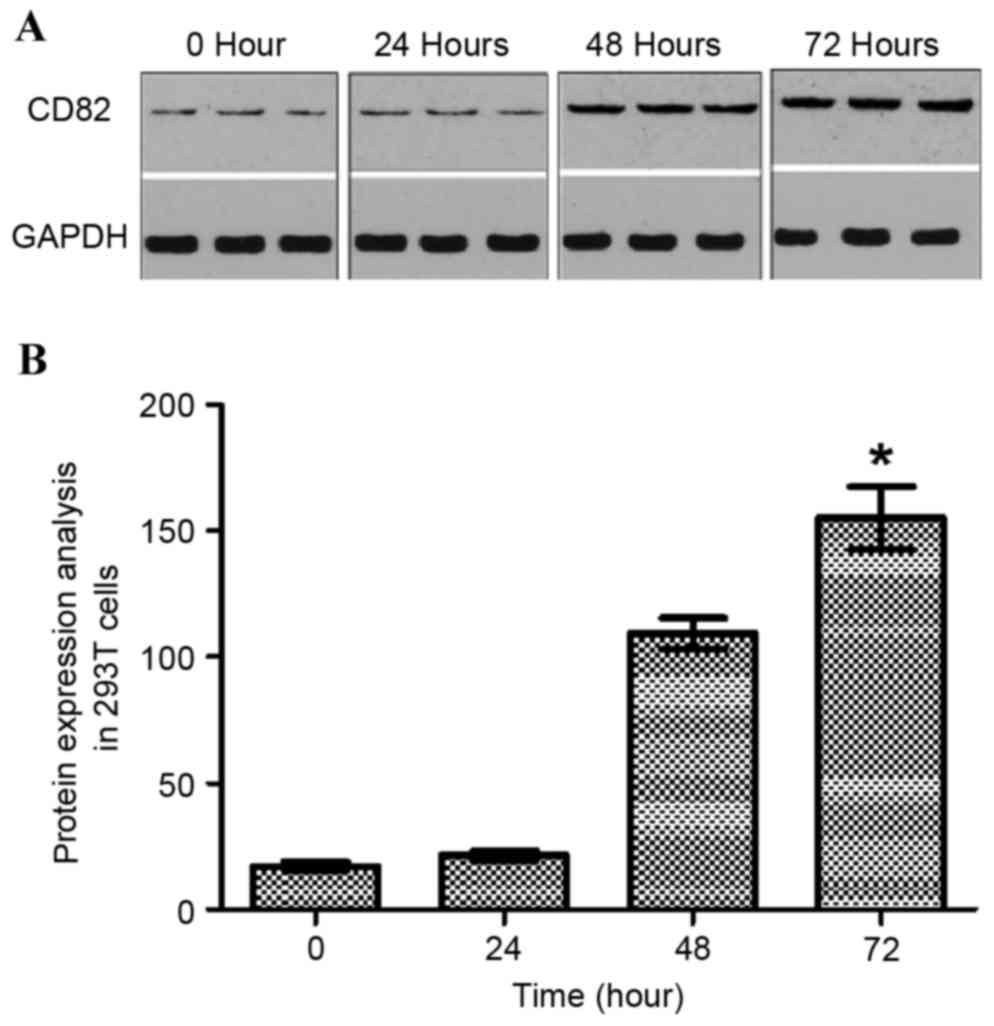
analyzed using western blot analysis (Fig. 1A). The data indicated that the
expression of KAI1 increased with increasing duration following
transfection of the 293T cells with the pIRES2-EGFP-KAI1 plasmid.
At the time point of 72 h, the maximum expression of KAI1 was
found. As shown in Fig. 1B, the
expression of KAI1/CD82 was normalized with GAPDH. The expression
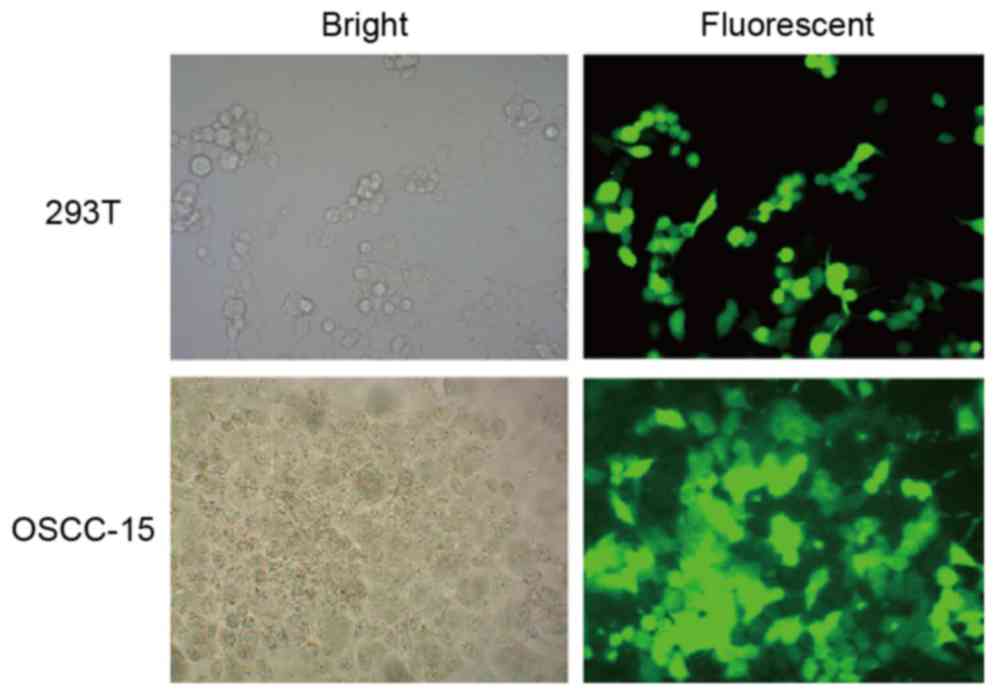
of KAI1 was also verified on the basis of GFP expression, which was
determined using fluorescent microscopy following the selection of
stable cells from the transfected 293T cells (Fig. 2, above) and OSCC-15 cells (Fig. 2, below). These representative
images in the fluorescent fields indicated that the GFP signal was
relatively high in ~90% of the cells.
Effect of the overexpression of
KAI1/CD82 on cell growth
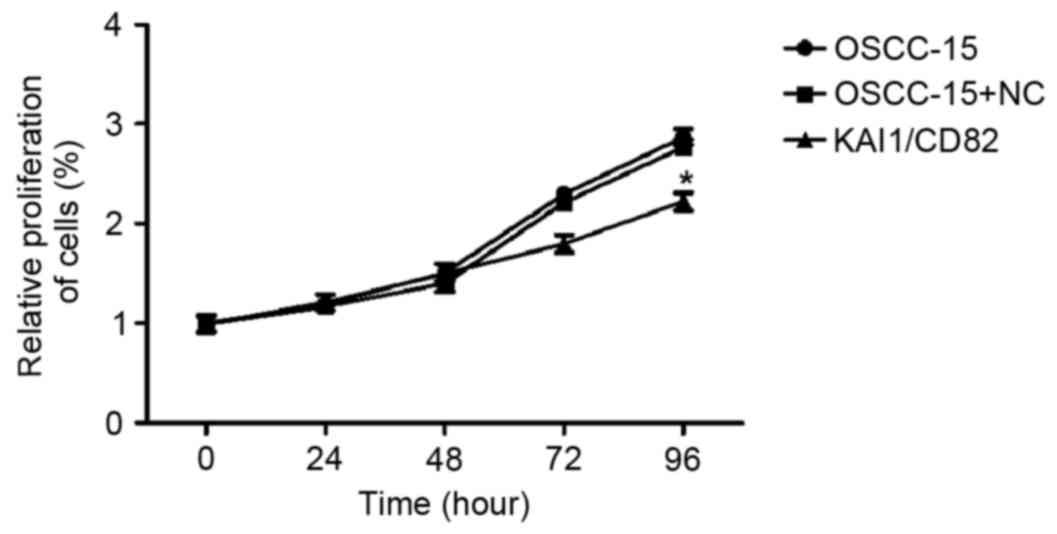
The present study performed an MTT assay to
determine the relative proliferation rate of the three groups of
cells (OSCC-15 wild-type; OSCC-15 transfected with the control
vector and OSCC-15 transfected with KAI1-expressing vector). The
results indicated that the overexpression of KAI1/CD82 in OSCC-15
cells markedly inhibited cell proliferation. The inhibitory effect
was most marked 96 h following plating of the cells (Fig. 3).
Effect of the overexpression of
KAI1/CD82 on cell invasion
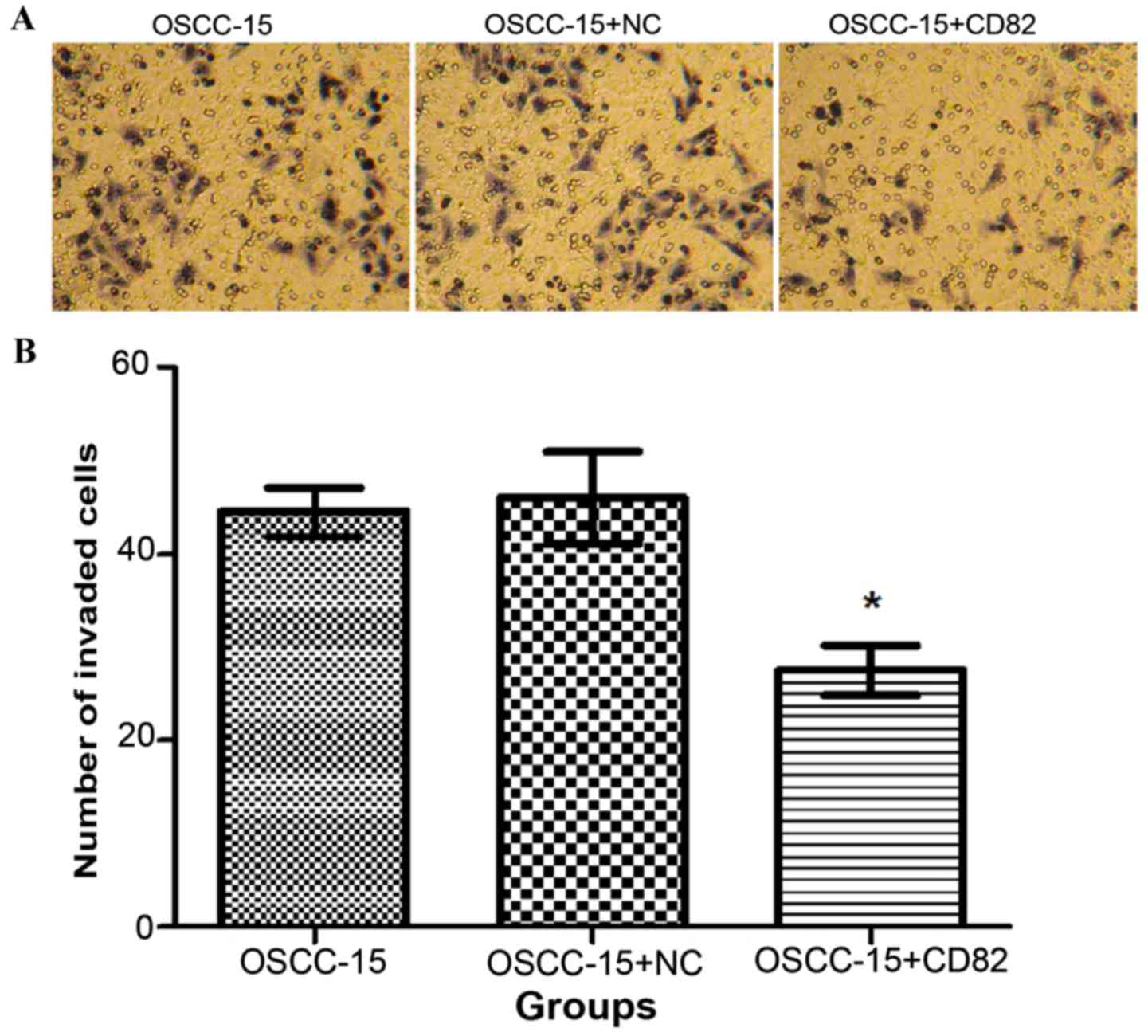
Following 24 h of culture of cells in the three
groups, the number of cells in each group, which migrated through
the Transwell polycarbonate membrane, were counted. As shown in
Fig. 4A and B, the total number of
KAI1/CD82-transfected cells, which migrated was significantly
lower, compared with the numbers in the blank and negative control
groups (P<0.05). This data indicated that the overexpression of
KAI1/CD82 in OSCC-15 cells was associated with reduced invasive
capability.
Effect of the overexpression of
KAI1/CD82 on cell apoptosis
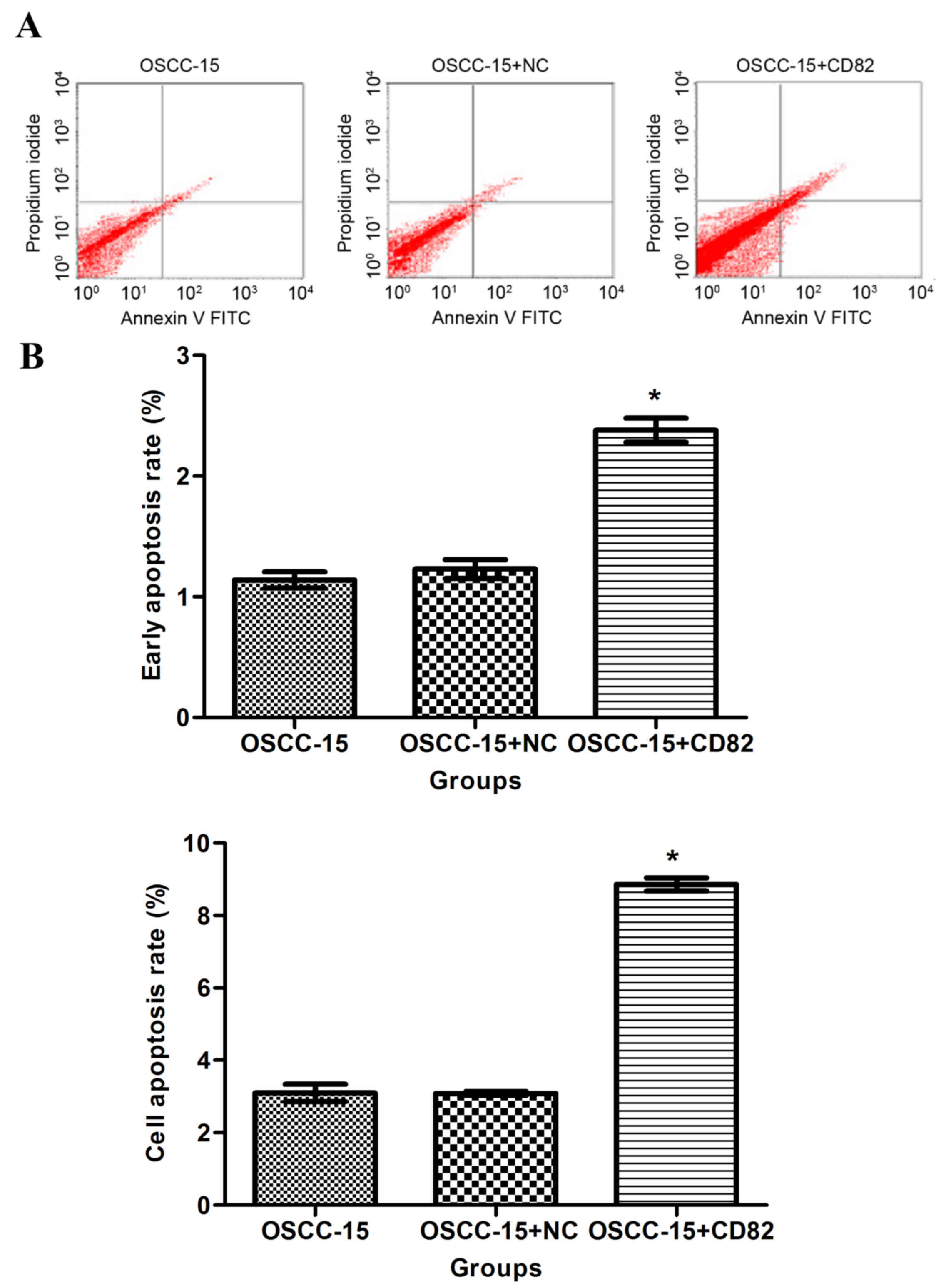
The present study determined the effect of KAI1/CD82
on apoptosis by labeling the cells with Annexin-V staining. As
shown in Fig. 5A and B, the
percentage of Annexin-V-positive staining was significantly higher
in the KAI1/CD82-overexpressing cells, compared with the OSCC-15
cells in the blank control or vector control groups. This data
indicated that the overexpression of KAI1/CD82 accelerated the
apoptosis of OSCC-15 cells.
Effect of the overexpression of
KAI1/CD82 on xenograft tumor growth
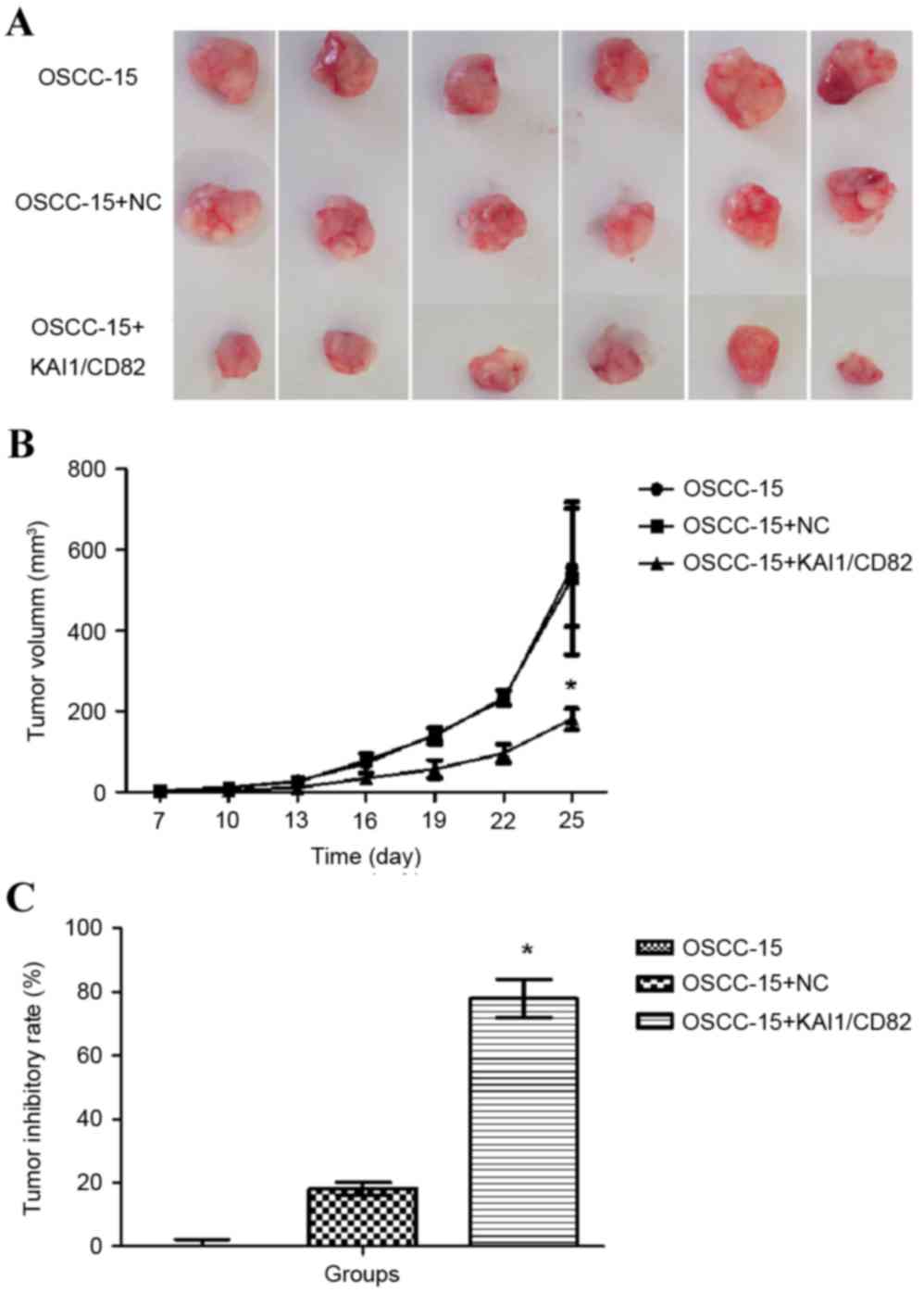
To further determine the effect of the
overexpression of KAI1/CD82 on the tumor growth of OSCC-15 cells,
the present study used a xenograft tumor model. The
KAI1/CD82-overexpressing OSCC-15 cells and negative controls were
subcutaneously injected into nude mice, and the tumor volumes were
recorded over a period of 1 month. As shown in Fig. 6A, the overexpression of KAI1/CD82
significantly reduced the tumor volumes and weights of the OSCC-15
cells, compared with those of the control vector or blank control
groups. Based on the tumor volumes, the tumor inhibitory rate was
calculated, which revealed that the overexpression of KAI1/CD82 was
associated with the highest tumor inhibitory rate among the three
groups (Fig. 6B and C). These
results confirmed that overexpression of KAI1/CD82 had a
suppressive effect on the tumorigenicity of oral cancer.
Discussion
KAI1, also known as CD82, is a member of the TM4SF
protein family and has is considered to be a metastatic-suppressor
gene (26). In general, TM4SF
family proteins are known to be involved in the regulation of cell
morphology, cell proliferation, fusion, motility, cell signaling
and the immune system (27–29).
In particular, KAI1/CD82 has been reported to suppress metastasis
in a Dunning rat model of prostatic adenocarcinoma (6). Yang et al (24) reported that the overexpression of
KAI1 in breast cancer cells resulted in the suppression of in
vitro invasion and in vivo metastasis. KAI1/CD82
interacts with other tetraspanin proteins, integrins and chemokines
to regulate the migration, adhesion and signaling of cells
(30). Jee et al (19,31)
reported that the upregulation of KAI1/CD82 protein is responsible
for a significant reduction in the invasion and metastatic
potential of non-small cell lung cancer cells. In addition, the
overexpression of KAI1/CD82 is associated with the attenuation of
integrin signaling. Odintsova et al (32) reported that KAI1/CD82 contributes
to epithelial growth factor (EGF)-induced signaling, and described
its association with EGF receptor (EGFR) and tetraspanin, which is
critical for EGFR desensitization. In the present study, the effect
of the overexpression of KAI1 in oral cancer was examined by
transfecting KAI1/CD82 cDNA into the OSCC-15 malignant oral cancer
cell line. It was found that the overexpression of KAI1/CD82 had an
inhibitory effect on in vitro cell growth and invasion.
Similarly, a previous study demonstrated that the expression of
KAI1/CD82 significantly suppressed the in vitro invasion of
breast cells (29). Schoenfeld
et al (33) reported that
KAI1/CD82 is also involved in the induction of apoptosis as it
produces reactive oxygen intermediates and regulates the
pro-apoptotic cdc42-signaling pathway. This is in agreement with
the results of the present study study. The data obtained in the
present study also indicated that the overexpression of KAI1/CD82
accelerated the apoptosis of OSCC-15 cells.
The xenograft tumor model used in the present study
revealed that the overexpression of KAI1/CD82 decreased tumor
volumes. Therefore, the overexpression of KAI1/CD82 appeared to
have a suppressive effect on the tumorigenicity of oral cancer
cells. Although KAI1/CD82 has been extensively investigated for its
involvement in the progression of different types of human cancer,
how it suppresses the in vivo tumorigenicity of oral cancer
cells remains to be fully elucidated. To the best of our knowledge,
the present study is the first to clearly demonstrate how the
overexpression of KAI1/CD82 is able to significantly suppress the
tumorigenicity of oral cancer cells.
In conclusion, KAI1/CD82 was found to be pivotal in
the regulation of oral cancer cells. In relation to the clinical
and therapeutic implications of oral cancer, this is an important
finding.
Acknowledgements
This study was supported partially by the National
Natural Science Foundation of China (grant no. 81072230), the
Project of Scientific and Technological Research Development of
Shaanxi Province (grant no. 2014K12-16) and the Scientific Research
Starting Foundation for Doctors of Xi'an Medical University (grant
no. 2016DOC20).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choudhury JH and Ghosh SK: Promoter
hypermethylation profiling identifies subtypes of head and neck
cancer with distinct viral, environmental, genetic and survival
characteristics. PLoS One. 10:e01298082015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen C, Mendez E, Houck J, Fan W,
Lohavanichbutr P, Doody D, Yueh B, Futran ND, Upton M, Farwell DG,
et al: Gene expression profiling identifies genes predictive of
oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev.
17:2152–2162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raudenska M, Sztalmachova M, Gumulec J,
Fojtu M, Polanska H, Balvan J, Feith M, Binkova H, Horakova Z,
Kostrica R, et al: Prognostic significance of the tumour-adjacent
tissue in head and neck cancers. Tumour Biol. 36:9929–9939. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong JT, Lamb PW, Rinker-Schaeffer CW,
Vukanovic J, Ichikawa T, Isaacs JT and Barrett JC: KAI1, a
metastasis suppressor gene for prostate cancer on human chromosome
11p11.2. Science. 268:884–886. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogt AB, Spindeldreher S and Kropshofer H:
Clustering of MHC-peptide complexes prior to their engagement in
the immunological synapse: Lipid raft and tetraspan microdomains.
Immunol Rev. 189:136–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaugitsch HW, Hofer E, Huber NE, Schnabl E
and Baumruker T: A new superfamily of lymphoid and melanoma cell
proteins with extensive homology to Schistosoma mansoni antigen
Sm23. Eur J Immunol. 21:377–383. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichikawa T, Ichikawa Y and Isaacs JT:
Genetic factors and suppression of metastatic ability of prostatic
cancer. Cancer Res. 51:3788–3792. 1991.PubMed/NCBI
|
|
10
|
Liu W, Iiizumi-Gairani M, Okuda H,
Kobayashi A, Watabe M, Pai SK, Pandey PR, Xing F, Fukuda K, Modur
V, et al: KAI1 gene is engaged in NDRG1 gene-mediated metastasis
suppression through the ATF3-NFkappaB complex in human prostate
cancer. J Biol Chem. 286:18949–18959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JJ, Jin YB, Lee YJ, Lee JS, Lee YS,
Ko YG and Lee M: KAI1 suppresses HIF-1α and VEGF expression by
blocking CDCP1-enhanced Src activation in prostate cancer. BMC
Cancer. 12:812012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu Y, Yang JL, Markovic B, Jackson P,
Yardley G, Barrett J and Russell PJ: Loss of KAI1 messenger RNA
expression in both high-grade and invasive human bladder cancers.
Clin Cancer Res. 3:1045–1049. 1997.PubMed/NCBI
|
|
13
|
You J, Madigan MC, Rowe A, Sajinovic M,
Russell PJ and Jackson P: An inverse relationship between KAI1
expression, invasive ability, and MMP-2 expression and activity in
bladder cancer cell lines. Urol Oncol. 30:502–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Christgen M, Bruchhardt H, Ballmaier M,
Krech T, Länger F, Kreipe H and Lehmann U: KAI1/CD82 is a novel
target of estrogen receptor-mediated gene repression and
downregulated in primary human breast cancer. Int J Cancer.
123:2239–2246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christgen M, Christgen H, Heil C, Krech T,
Langer F, Kreipe H and Lehmann U: Expression of KAI1/CD82 in
distant metastases from estrogen receptor-negative breast cancer.
Cancer Sci. 100:1767–1771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rowe A and Jackson P: Expression of
KITENIN aKAI1/CD82 binding protein and metastasis enhancer, in
bladder cancer cell lines: Relationship to KAI1/CD82 levels and
invasive behaviour. Oncol Rep. 16:1267–1272. 2006.PubMed/NCBI
|
|
17
|
Hashida H, Takabayashi A, Tokuhara T, Taki
T, Kondo K, Kohno N, Yamaoka Y and Miyake M: Integrin alpha3
expression as a prognostic factor in colon cancer: Association with
MRP-1/CD9 and KAI1/CD82. Int J Cancer. 97:518–525. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo XZ, Friess H, Shao XD, Liu MP, Xia YT,
Xu JH and Buchler MW: KAI1 gene is differently expressed in
papillary and pancreatic cancer: Influence on metastasis. World J
Gastroenterol. 6:866–871. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jee BK, Park KM, Surendran S, Lee WK, Han
CW, Kim YS and Lim Y: KAI1/CD82 suppresses tumor invasion by MMP9
inactivation via TIMP1 up-regulation in the H1299 human lung
carcinoma cell line. Biochem Biophys Res Commun. 342:655–661. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takeda T, Hattori N, Tokuhara T, Nishimura
Y, Yokoyama M and Miyake M: Adenoviral transduction of MRP-1/CD9
and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung
cancer model. Cancer Res. 67:1744–1749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uzawa K, Ono K, Suzuki H, Tanaka C,
Yakushiji T, Yamamoto N, Yokoe H and Tanzawa H: High prevalence of
decreased expression of KAI1 metastasis suppressor in human oral
carcinogenesis. Clin Cancer Res. 8:828–835. 2002.PubMed/NCBI
|
|
22
|
Farhadieh RD, Smee R, Ow K, Yang JL,
Russell PJ, Crouch R, Jackson P and Jacobson IV: Down-regulation of
KAI1/CD82 protein expression in oral cancer correlates with reduced
disease free survival and overall patient survival. Cancer Lett.
213:91–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyazaki T, Kato H, Kimura H, Inose T,
Faried A, Sohda M, Nakajima M, Fukai Y, Masuda N, Manda R, et al:
Evaluation of tumor malignancy in esophageal squamous cell
carcinoma using different characteristic factors. Anticancer Res.
25:4005–4011. 2005.PubMed/NCBI
|
|
24
|
Yang X, Wei LL, Tang C, Slack R, Mueller S
and Lippman ME: Overexpression of KAI1 suppresses in vitro
invasiveness and in vivo metastasis in breast cancer cells. Cancer
Res. 61:5284–5288. 2001.PubMed/NCBI
|
|
25
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special report: The 1996 guide for the care and use
of laboratory animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu WM and Zhang XA: KAI1/CD82, a tumor
metastasis suppressor. Cancer Lett. 240:183–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skubitz KM, Campbell KD, Iida J and
Skubitz AP: CD63 associates with tyrosine kinase activity and
CD11/CD18, and transmits an activation signal in neutrophils. J
Immunol. 157:3617–3626. 1996.PubMed/NCBI
|
|
28
|
Lagaudrière-Gesbert C, Le Naour F,
Lebel-Binay S, Billard M, Lemichez E, Boquet P, Boucheix C,
Conjeaud H and Rubinstein E: Functional analysis of four
tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in
costimulation, cell adhesion, and migration: Only CD9 upregulates
HB-EGF activity. Cell Immunol. 182:105–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toyo-oka K, Yashiro-Ohtani Y, Park CS, Tai
XG, Miyake K, Hamaoka T and Fujiwara H: Association of a
tetraspanin CD9 with CD5 on the T cell surface: Role of particular
transmembrane domains in the association. Int Immunol.
11:2043–2052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malik FA, Sanders AJ and Jiang WG:
KAI-1/CD82, the molecule and clinical implication in cancer and
cancer metastasis. Histol Histopathol. 24:519–530. 2009.PubMed/NCBI
|
|
31
|
Jee BK, Lee JY, Lim Y, Lee KH and Jo YH:
Effect of KAI1/CD82 on the beta1 integrin maturation in highly
migratory carcinoma cells. Biochem Biophys Res Commun. 359:703–708.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Odintsova E, Sugiura T and Berditchevski
F: Attenuation of EGF receptor signaling by a metastasis
suppressor, the tetraspanin CD82/KAI-1. Curr Biol. 10:1009–1012.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schoenfeld N, Bauer MK and Grimm S: The
metastasis suppressor gene C33/CD82/KAI1 induces apoptosis through
reactive oxygen intermediates. FASEB J. 18:158–160. 2004.PubMed/NCBI
|