Introduction
Fucoidan is a series of sulfated polysaccharides
derived from marine brown algae, predominantly composed of sulfate
and L-fucose (1,2). Numerous biological activities of
fucoidan have been previously described, including anti-oxidant and
antitumor properties, and roles in the regulation of metabolism and
inflammatory modulation (3). Such
properties make fucoidan a potential pharmaceutical and
nutraceutical candidate for health control and disease prevention.
The immunomodulatory effect is a primary property of fucoidan.
Immune cells, including macrophages, natural killer cells,
dendritic cells (DCs) and lymphocytes (4–6),
appear to be potential targets of fucoidan. For example, our
previous study suggested that fucoidan promoted the maturation of
human monocyte-derived DCs, altered the expression of cytokines and
co-stimulatory molecules, and drove their differentiation toward
the Th1-polarization phenotype (4). The modulation of immune cells by
fucoidan provides various properties for its clinical use.
Macrophages serve important roles in eliciting and
modulating the immune response (7). As the biggest population of innate
immunocytes in tissue, macrophages recognize dangerous signals, and
present them to T cells or B cells to initiate adaptive immunity.
Additionally, macrophages regulate the function of adaptive
immunity through cell-to-cell interaction or fluid-phase
modulation, via cytokines, chemokines, reactive radicals and nitric
oxide (8). Therefore, the
biological activities of macrophages, including migration,
phagocytosis and secretion of cytokines, are critical to the
outcome of an immune response. Notably, macrophage subsets differ
distinctly in these biological properties and their functions range
from inflammatory to anti-inflammatory (9). In addition to diversity, plasticity
is another characteristic of macrophages. This means that they can
be phenotypically and functionally polarized to distinct subsets
responding to microenvironmental signals or exogenous supplements
(10,11).
Fucoidan is recognized as a ligand of macrophage
scavenger receptors (12),
therefore macrophages may be a primary target in the
immunomodulatory effects of fucoidan. However, although previous
studies have reported the effects of fucoidan on various properties
of macrophages, including tumoricidal activity, phagocytosis and
cytokine production (13–15), controversy remains in these
observations. For example, although previous results suggested that
fucoidan promoted pro-inflammatory cytokine production in
monocytes/macrophages (12,16,17),
a previous study demonstrated attenuating effects of a
low-molecular-weight fucoidan on pro-inflammatory cytokine
production, including interleukin-1 (IL-1) and tumor necrosis
factor-α (TNF-α), in a dose-dependent manner from 1–100 µg/ml
(18). Additionally, fucoidan
exhibited bifunctional effects on another inflammatory mediator,
inducible nitric oxide synthase (iNOS) (19). Specifically, a low concentration of
fucoidan (10 µg/ml) increased basal iNOS levels in macrophages,
while higher fucoidan concentrations (100 or 300 µg/ml) suppressed
induction of iNOS expression by lipopolysaccharide (LPS). How
fucoidan modulates macrophage functions and how the inconsistency
occurs is not clear. Therefore, further investigation of the
effects of fucoidan on macrophages would improve the understanding
of its immunomodulatory property.
Colony-stimulating factor-1 (CSF-1) is a primary
regulator of the proliferation, survival and function of
macrophages (20). Exogenous CSF-1
induced monocytes/macrophages to express various cytokines,
including TNF-α and IL-1β (21,22).
In addition, CSF-1-treated monocytes/macrophages exhibited
migratory changes due to altered chemokine receptor expression
(23–25). However, to the best of our
knowledge, no previous studies have investigated the role of CSF-1
in the modulation of macrophages by fucoidan. The present study
investigated the effect of fucoidan on pro-inflammatory cytokine
production and on the migratory properties of THP-1-derived
macrophages, and aimed to verify the role of CSF-1 in the
modulatory function of fucoidan.
Materials and methods
Reagents and antibodies
Recombinant human CSF-1 (rhCSF-1) and interferon-γ
(IFN-γ) were purchased from R&D Systems, Inc. (Minneapolis, MN,
USA). LPS and phorbol 12-myristate 13-acetate (PMA) were obtained
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Monoclonal anti-human M-CSF neutralizing antibodies (NAb; cat. no.
AF216; 100 ng/ml) were obtained from R&D Systems, Inc.
Preparation of fucoidan
Fucoidan purified from F. vesiculosus was purchased
from Sigma-Aldrich (Merck Millipore) and dissolved in PBS. The
potential contamination of endotoxin in fucoidan was detected by
QCL-1000® Chromogenic LAL end-point assay (Lonza
Walkersville, Inc., Walkersville, MD, USA) according to the
manufacturer's manual. The detection limit of the kit was 0.1
EU/ml. The endotoxin level of 100 µg/ml fucoidan preparation was
<0.1 EU/ml.
Cell culture and generation of
THP-1-derived macrophages
THP-1 human acute monocytic leukemia cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in RPMI 1640 medium, (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
heat-incubated 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 µg/ml streptomycin
at 37°C in an incubator with 95% air and 5% CO2.
Generation of THP-1-derived macrophages was
performed as previously described (26). Briefly, 1×106 THP-1
cells were treated with 100 ng/ml PMA for 48 h. To generate
M1-polarized THP-1 macrophages, THP-1 cells were cultured with 100
ng/ml PMA for 6 h and then 100 ng/ml LPS and 20 ng/ml IFN-γ were
added for 42 h. In certain cases, fucoidan (100 µg/ml), rhCSF-1
(100 ng/ml) or CSF-1 NAb (2 µg/ml) was added along with LPS and
IFN-γ for 42 h.
Cell Counting kit-8 (CCK-8) assay for
cell viability
Cell viability was measured by CCK-8 (Wuhan Boster
Biological Technology, Ltd., Wuhan, China). Briefly, THP-1 cells
(1×104/well) were seeded into 96-well plates along with
PMA (100 ng/ml) for 6 h and then various concentrations of fucoidan
(50, 100 and 200 µg/ml) were supplemented into the wells. Cell
proliferation was measured after different periods of time, ranging
from 6 to 72 h, using the CCK-8 assay according to the
manufacturer's instructions.
Cell migration assay
THP-1-derived macrophages were cultured in a 6-well
plate, then collected and resuspended in serum-free RPMI 1640
medium at a density of 1×106/ml. Then, 100 µl serum-free
medium (1×105 cells) was added into the upper
compartment of Transwell inserts (24-well plate, 8 µm pores; BD
Biosciences, Franklin Lakes, NJ, USA). RPMI 1640 medium (600 µl)
containing 10% FBS was added into the lower chamber of the
Transwell plate. After incubating at 37°C in 5% CO2 for
12 h, the migrated cells on the lower surface of the filter were
fixed by 10% formalin for 15 min at room temperature and stained
with eosin. Five random fields of each well were imaged using a
light microscope (magnification, ×100), and cell numbers were
counted.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). Total RNA from THP-1-derived macrophages was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and the cDNA was synthesized
by reverse transcription. Total RNA (1.0 µg) was transcribed into
cDNA with oligo dT16 primers and Moloney murine leukemia virus
reverse transcriptase according to the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc.). GAPDH was used as an
internal control. The primers for TNF-α, IL-1β, IL-6, CSF-1 and
GAPDH are listed in Table I. The
reaction mixture, including 5 µl 2X SYBR® Green qPCR
Master mix (Thermo Fisher Scientific, Inc.), 1 µl forward primer, 1
µl reverse primer, 1 µl cDNA and 2 µl double distilled
H2O, was incubated at 94°C for 30 sec, 60°C for 30 sec
and 72°C for 45 sec for 30 cycles (LightCycler 2.0; Roche
Diagnostics GmbH, Mannheim, Germany). The mRNA level of each sample
was measured by the 2−∆∆Cq method (27).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Primer sequences |
|---|
| TNF-α | F: 5′-ATG AGC ACT GAA
AGC ATG ATC C-3′ |
|
| R: 5′-GAG GGC TGA TTA
GAG AGA GGT C-3′ |
| IL-1β | F: 5′-TGA TGG CTT ATT
ACA GTG GCA ATG-3′ |
|
| R: 5′-GTA GTG GTG GTC
GGA GAT TCG-3′ |
| IL-6 | F: 5′-CAC ACA GAC
AGC CAC TCA CC-3′ |
|
| R: 5′-GCT CTG GCT
TGT TCC TCA CT-3′ |
| CSF-1 | F: 5′-GGA GAC CTC
GTG CCA AAT TA-3′ |
|
| R: 5′-GGC CTT GTC
ATG CTC TTC AT-3′ |
| GAPDH | F: 5′-GGT GGT CTC
CTC TGA CTT CAA CAG-3′ |
|
| R: 5′-GTT GCT GTA
GCC AAA TTC GTT GT-3′ |
Enzyme-linked immunosorbent assay
(ELISA)
THP-1-derived macrophages were generated and
cultured in complete medium for another 24 h. Then, culture
supernatants were collected. TNF-α (cat. no. DTA00C), IL-1β (cat.
no. DLB50), IL-6 (cat. no. D6050) and CSF-1 (cat. no. DMC00)
concentrations in culture supernatants were determined by ELISA
kits (R&D Systems, Inc.), according to the manufacturer's
instructions.
Statistical analysis
Data were primarily presented as the mean + standard
deviation. The SPSS software package (version 13.0; SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analysis. The
distribution of the samples was determined via Kolmogorov-Smirnov
test. The results of experiments were analyzed by unpaired t-test
or one-way analysis of variance wherever appropriate. Tukey
post-hoc comparison was performed when statistical
significance was detected between datasets. P<0.05 was
considered to indicate a statistically significant difference.
Results
Fucoidan modifies the morphology and
migration activity of THP-1-derived macrophages
THP-1 human acute monocytic leukemia cell line is a
widely-used model for monocyte/macrophage differentiation (28). PMA, an agonist of protein kinase C,
induces the THP-1 cells to acquire a macrophage-like
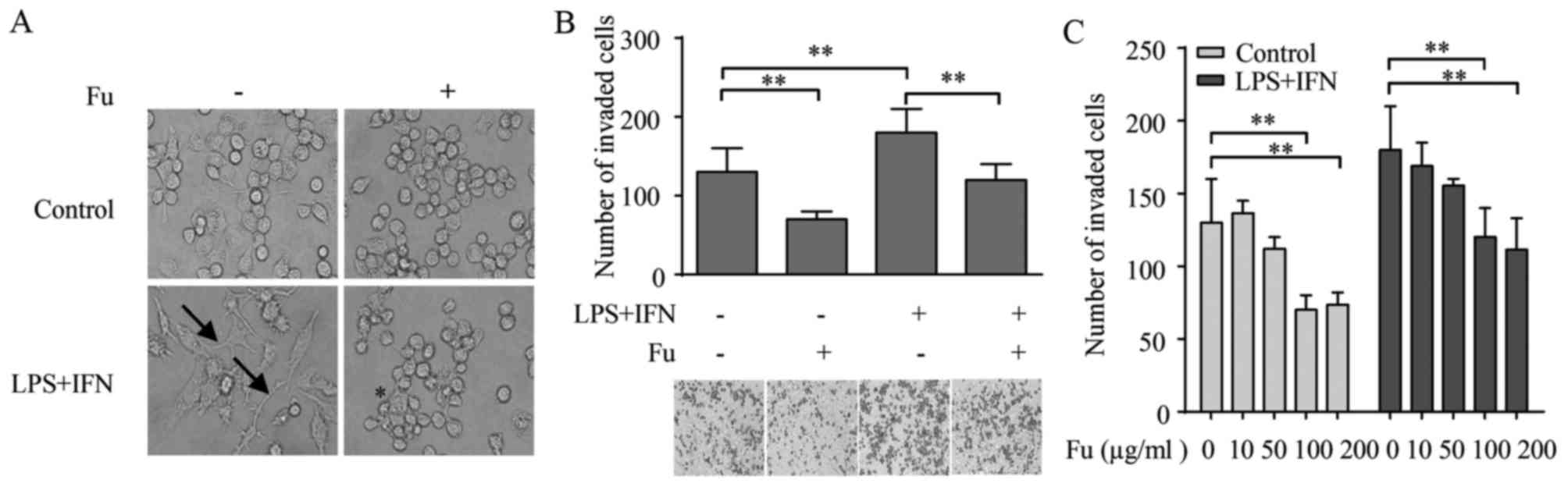
characteristic, which can be distinguished by morphology (29). As demonstrated in Fig. 1A, the PMA-treated THP-1 cells
became attached and adopted an amoeboid morphology, exhibiting
similar morphology to primary human macrophages. Therefore, this
study referred to them as THP-1-derived macrophages. When LPS (100
ng/ml) and IFN-γ (20 ng/ml) were added for 42 h alongside PMA
stimulation, the morphology of THP-1-derived macrophages was
markedly changed from a round/spindle shape to a dendritic-like
shape with large filopodia, resembling the classically activated M1
macrophages (Fig. 1A).
When fucoidan alone or combined with LPS and IFN-γ
was added during the differentiation of THP-1-derived macrophages,
the morphology was modified. Specifically, fucoidan increased the
percentage of rounded-shaped cells in the group stimulated by PMA
only. The morphology of the LPS and IFN-γ stimulated group adopt
striking changes compared with the PMA only treated group, with
loss of filopodia and acquisition of amoeboid-like morphology
(Fig. 1A).
The morphology of macrophages is associated with
their migratory activity (30). As
fucoidan modulated the morphology of THP-1-derived macrophages, the
effect of fucoidan on their migratory properties was analyzed using
a Transwell assay. The result indicated that LPS and IFN-γ
increased the number of migrated THP-1-derived macrophages compared
with untreated cells (P=0.0039). Notably, compared with groups that
did not receive fucoidan, when treated by fucoidan, there were
significant decreases in the number of migrated macrophages in the
LPS/IFN-γ-stimulated (P=0.0161) and unstimulated (P=0.0342) groups
(Fig. 1B).
It was also examined whether the effects of fucoidan
on macrophage migration was dependent on its concentration. For
this purpose, THP-1-derived macrophages were treated with different
concentrations of fucoidan from 10–200 µg/ml during the
differentiation process, and their migratory properties were
measured. Fig. 1C demonstrates
that the attenuation of fucoidan on migration was dose-dependent,
as its effects became significant compared with 0 µg/ml fucoidan at
a concentration of 100 µg/ml in the unstimulated control (P=0.0135)
and LPS/IFN-γ treated groups (P=0.0171), and no significant
differences in migrated cell numbers were detected between 100 and
200 µg/ml.
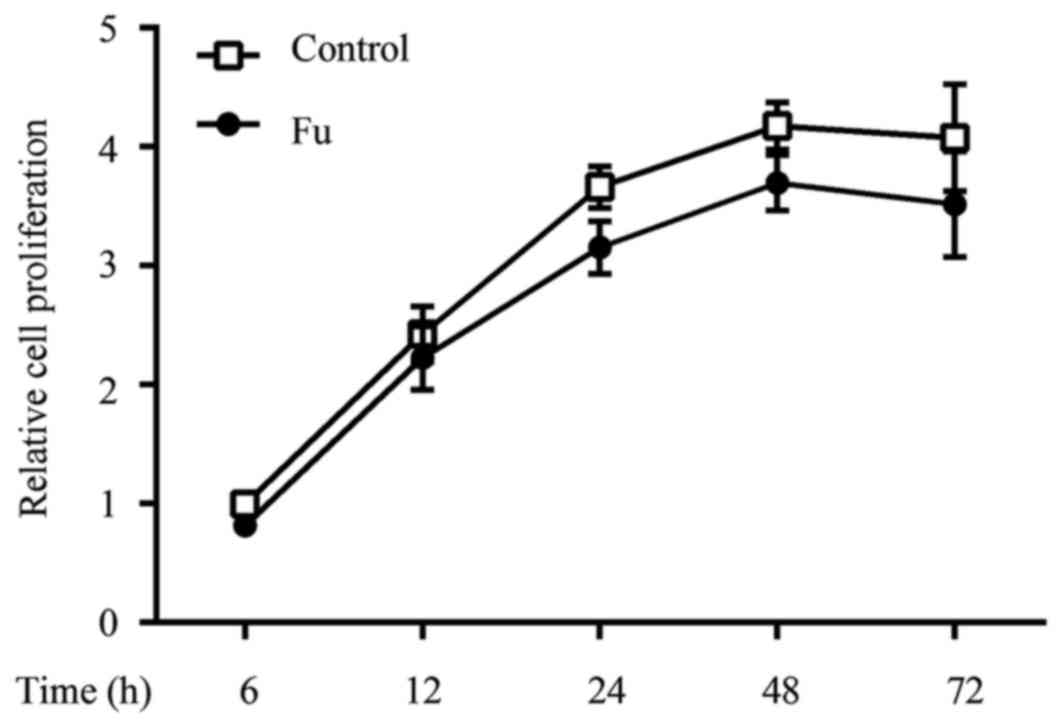
To determine whether fucoidan exhibited cytotoxic
effects, THP-1 cells were treated with various concentrations of
fucoidan (50, 100 and 200 µg/ml) for different periods of time in
the presence of PMA (100 ng/ml), and then cell viability was
determined by using the CCK-8 assay. Fucoidan did not exhibit
significant cytotoxic effects on THP-1-derived macrophages up to 72
h of incubation, at a concentration of 200 µg/ml (data not shown).
The cell proliferation curve of fucoidan at a concentration of 100
µg/ml is shown in Fig. 2.
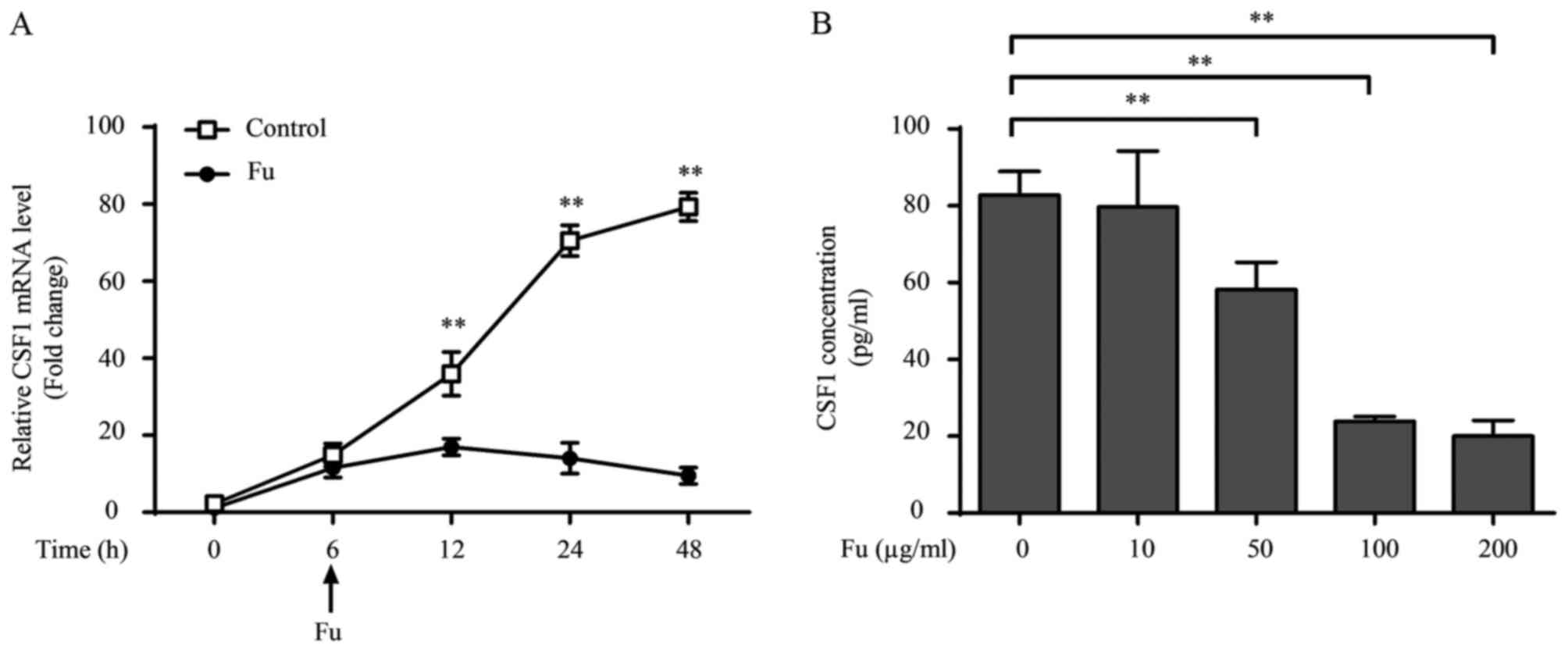
Fucoidan affects cytokine production
of THP-1-derived macrophages
It was also investigated whether fucoidan modified
the cytokine production in THP-1-derived macrophages. Consistent
with previous studies (28), LPS
and IFN-γ treatment increased pro-inflammatory cytokine production,
including TNF-α, IL-1β and IL-6, compared with unstimulated cells
(Fig. 3A). Notably, when fucoidan
was added, cytokine transcription and secretion were decreased
compared with untreated cells. Additionally, fucoidan also reversed
the augmented effect of LPS and IFN-γ on the cytokine production of
THP-1-derived macrophages (Fig.
3A).
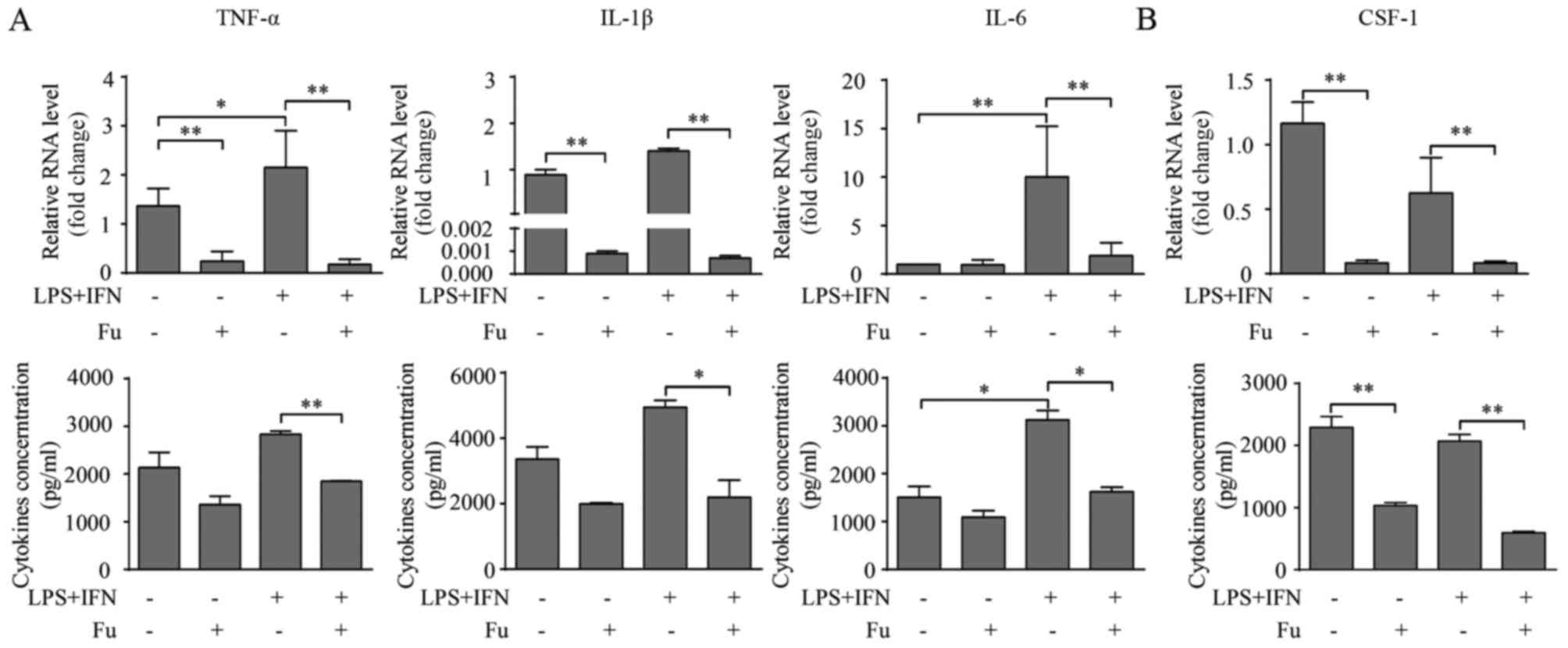 | Figure 3.Fu modifies the secretory activity of
THP-1-derived macrophages. (A) The transcription and secretion of
pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 with or without
Fu treatment, was measured by RT-qPCR (upper) and ELISA (lower).
(B) The transcription (upper) and secretion (lower) of CSF-1 was
measured. The RT-qPCR data were normalized to the control and
presented as the fold change. Each bar represents the mean +
standard deviation (n=3, *P<0.05, **P<0.01). Fu, fucoidan;
TNF-α, tumor necrosis factor-α; IL, interleukin; LPS,
lipopolysaccharide; IFN, interferon; CSF, colony-stimulating
factor; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction. |
CSF-1 is an important regulator associated with the
biological behavior of macrophages, promoting their proliferation,
migration and cytokine production (20). Therefore the current study also
investigated whether fucoidan affected the production of CSF-1 in
THP-1-derived macrophages. The results indicated that THP-1-derived
macrophages produce CSF-1 (~2 ng/1×106 cells over 24 h).
LPS and IFN-γ treatment marginally, but not significantly,
decreased CSF-1 transcription, and this attenuation was not
observed at the secretory level (Fig.
3B). Notably, fucoidan significantly attenuated CSF-1
transcription and CSF-1 secretion in LPS/IFN-γ-treated and
untreated groups (P<0.01; Fig.
3B).
The effect of exposure length and the concentration
of fucoidan on cytokine production were also examined. Inhibitory
effects of fucoidan on CSF-1 transcription occurred 6 h after 100
µg/ml of fucoidan was added into the culture medium (12 h,
P=0.0057; Fig. 4A). Additionally,
fucoidan decreased CSF-1 secretion in a dose-dependent manner, with
the CSF-1 levels significantly decreased by 50 µg/ml fucoidan
compared with 0 µg/ml (P=0.0061), and a more significant decrease
was observed at a concentration of 100 µg/ml (P=0.0032; Fig. 4B). Dose-dependent effects of
fucoidan on the secretion of TNF-α and IL-1β were also observed
(data not shown).
CSF-1 is involved in the production of
pro-inflammatory cytokines modified by fucoidan
Previous investigation revealed that CSF-1 promoted
pro-inflammatory cytokine production in monocytes/macrophages
(21). Because fucoidan
significantly decreased CSF-1 secretion in THP-1-derived
macrophages, it was also determined whether the reduced CSF-1 level
was responsible for the attenuation of pro-inflammatory cytokine
expression. Initially, CSF-1 NAb (2 µg/ml) was added with LPS and
IFN-γ during the differentiation of THP-1-derived macrophages. PCR
and ELISA results demonstrated that blocking endogenous CSF-1
expression significantly decreased pro-inflammatory cytokine
production in macrophages compared with the control groups
(P<0.05; Fig. 5A).
Additionally, rhCSF-1 (100 ng/ml) was added with/without fucoidan
to THP-1-derived macrophages in the presence of LPS and IFN-γ.
RT-qPCR results revealed that rhCSF-1 significantly increased the
transcription of TNF-α, IL-1β and IL-6 with or without fucoidan
treatment compared with no rhCSF-1 treatment (P<0.01; Fig. 5B). The effects of rhCSF-1 on
cytokine secretion was somewhat different from the transcription
level, as rhCSF-1 did not significantly alter the cytokine
secretion of THP-1-derived macrophages without fucoidan treatment,
however, did reverse the attenuating effect of fucoidan on the
secretion of these cytokines (P<0.05; Fig. 5C).
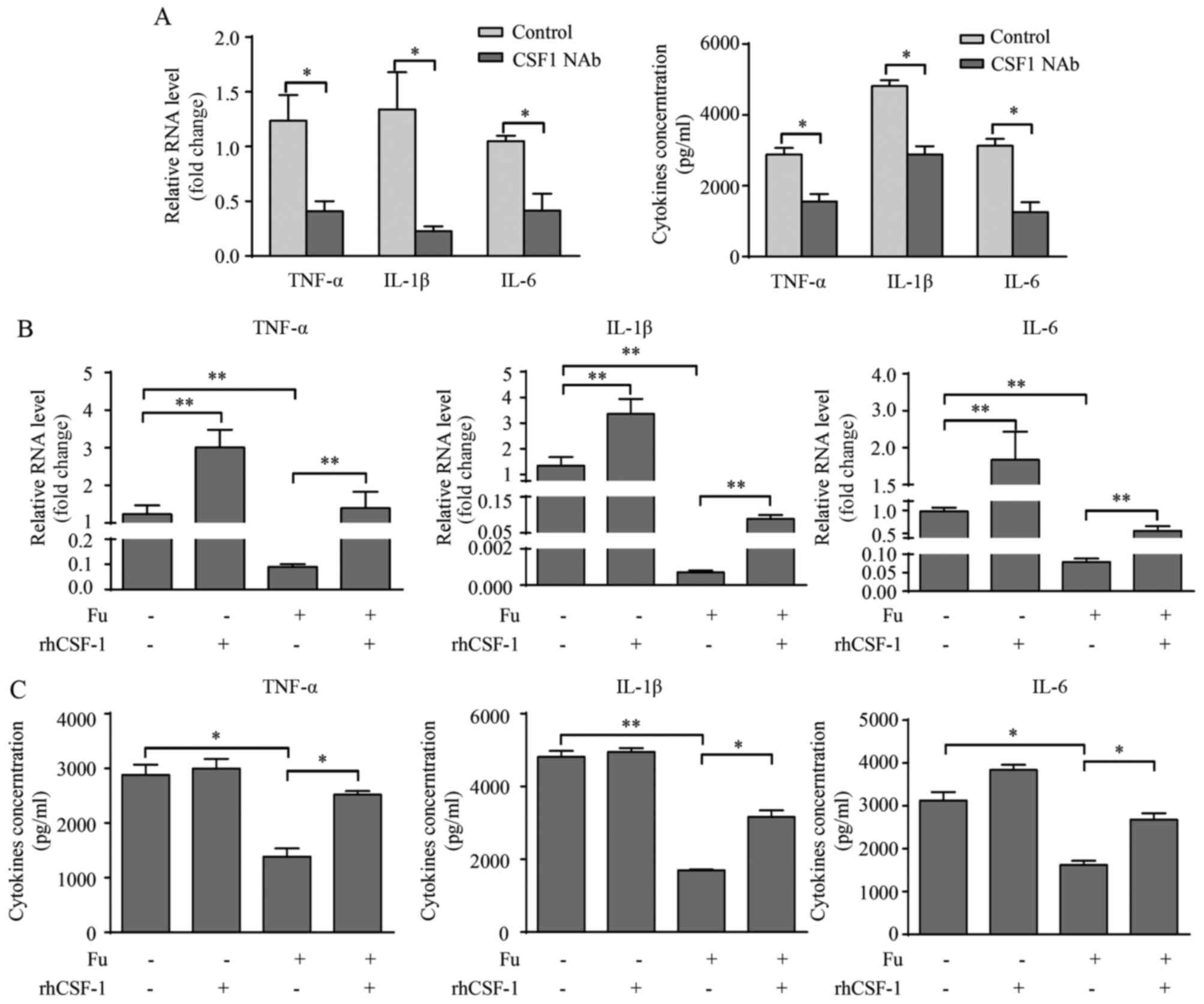 | Figure 5.CSF-1 is involved in Fu-modulated
cytokine production of THP-1-derived macrophages. (A) Transcription
and secretion of TNF-α, IL-1β and IL-6 with or without CSF-1 NAb
were measured by RT-qPCR (left) and ELISA (right). rhCSF-1 and/or
Fu were added to the THP-1-derived macrophages. (B) Transcription
and (C) secretion of TNF-α, IL-1β and IL-6 were measured. The
RT-qPCR data were normalized to the control and shown as the fold
change. Each bar represents the mean + standard deviation (n=3,
*P<0.05, **P<0.01). CSF, colony-stimulating factor; Fu,
fucoidan; TNF-α, tumor necrosis factor-α; IL, interleukin; NAb,
neutralizing antibody; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; rhCSF, recombinant human
colony-stimulating factor. |
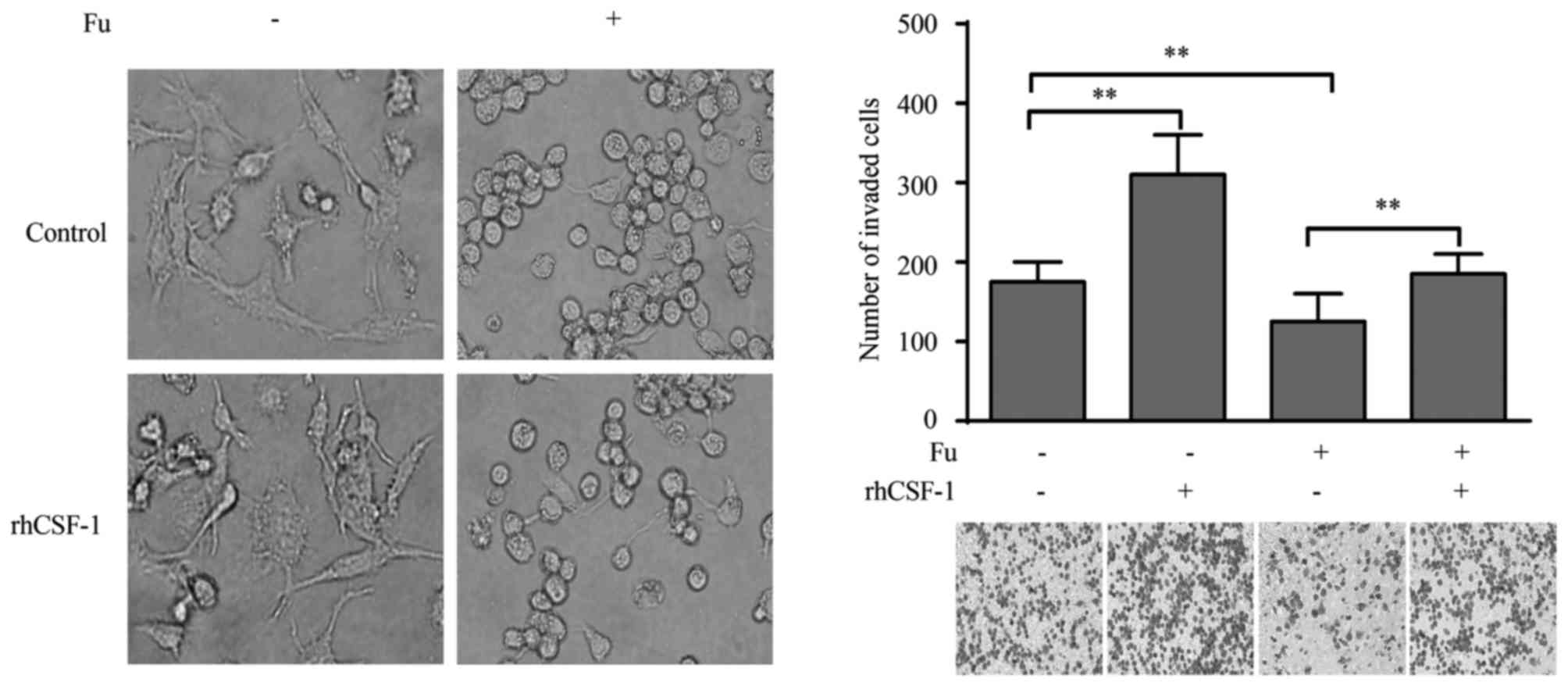
CSF-1 is associated with the migratory
activity of THP-1-derived macrophages modified by fucoidan
The current study also explored whether the altered
expression of CSF-1 was responsible for the migration of
THP-1-derived macrophages. Exogenous CSF-1 treatment did not cause
morphological change in macrophages, in either fucoidan treated or
untreated groups (Fig. 6A).
However, when stimulated with rhCSF-1, the migratory activity of
THP-1-derived macrophages was significantly increased compared with
the control (P<0.01; Fig. 6B).
More importantly, the suppression of migration by fucoidan was also
reversed by rhCSF-1 (P<0.01; Fig.
6B).
Discussion
The present study analyzed the effects of fucoidan
on the migration and cytokine production of THP-1-derived
macrophages, and aimed to examine the mechanisms involved in this
modulation. The current study demonstrated that fucoidan-treated
THP-1-derived macrophages exhibited morphological changes, reduced
migration and pro-inflammatory cytokine production. In addition,
fucoidan also decreased CSF-1 production and exogenous CSF-1
reversed the attenuating effect of fucoidan. Therefore, the results
indicate that fucoidan reduced the pro-inflammatory capacity of
THP-1-derived macrophages. This effect may be modulated by CSF-1
expression.
THP-1-derived macrophages exhibit specialized
morphological and functional characteristics in response to
exogenous stimulations. Initially, morphological changes in
macrophages were observed when fucoidan was added; filopodia
formation was decreased and macrophages adopted a more
amoeboid-like morphology. Additionally, fucoidan reduced the
migratory properties of THP-1-derived macrophages, with/without LPS
and IFN-γ stimulation, however, the mechanisms of modulation
remained unresolved. Cell migratory activity is dependent on
various factors, for example, chemokine secretion, integrin
expression, expression of matrix metalloproteinases (MMPs) and
cytoskeleton organization (31).
Our previous study demonstrated that fucoidan increased
TNF-α-induced proMMP-9 in human monocyte U937 cells, suggesting
that fucoidan regulates MMPs expression (32). In addition, cytoskeleton
arrangement regulates the type of cell movement, and also the
migratory properties (33).
Because fucoidan significantly altered the morphology of
THP-1-derived macrophages, there was a possibility that fucoidan
regulated macrophage migration by reorganizing the cytoskeleton.
Another previous study reported that fucoidan-treated MDA-MB-231
breast cancer cells also adopt morphological changes similar to
LPS/IFN-γ-treated macrophages, loss of cell spreading occurred with
cytoskeleton reorganization and depletion of vinculin (34). Whether similar mechanisms occur in
THP-1-derived macrophages requires further investigation.
In addition to the migratory properties, fucoidan
also greatly attenuated pro-inflammatory cytokine production,
including TNF-α, IL-1β and IL-6. This attenuation by fucoidan was
significant, and the stimulating effects of LPS and IFN-γ were
almost fully reversed. The effects of fucoidan on cytokine
production by macrophages remains controversial, as certain studies
have reported that fucoidan induces pro-inflammatory cytokine
production, including TNF-α and IL-1, in macrophages (12), while others demonstrated an
attenuating effect (18). How this
paradox exists remains unclear but is acceptable, as the biological
activities of fucoidan are different depending on seaweed species
or the structural and compositional characteristics (35). The findings of the current study
enriched the evidence for the immunomodulatory effects of fucoidan,
particularly in macrophages. Furthermore, as the bioactive
functions of fucoidan vary, which is demonstrated by the present
and previous studies, a full understanding of the functions and
structural molecular features of fucoidan is required prior to its
clinical application.
CSF-1 is known as a primary regulator of
cytotoxicity, chemotaxis and cytokine production in
monocytes/macrophages, therefore, it is closely associated with
their inflammatory properties (21,22).
Macrophages are important producers and targets of CSF-1. The
present study demonstrated that THP-1-derived macrophages produced
high levels of CSF-1, and LPS and IFN-γ marginally, but not
significantly, decreased CSF-1 production. Importantly, fucoidan
greatly attenuated CSF-1 transcription and secretion in LPS and
IFN-γ treated/untreated groups. To the best of our knowledge, this
is the first time that fucoidan has been reported to regulate CSF-1
production in macrophages. In order to determine whether decreased
CSF-1 was involved in the migration and cytokine production of
macrophages, two experiments were we performed. CSF-1 NAb was added
during the differentiation process to block basic CSF-1 production
of macrophages. Pro-inflammatory cytokine production, which was
stimulated by LPS and IFN-γ, was significantly decreased following
blocking of CSF-1. Recombinant human CSF-1 (100 ng/ml) was then
added to THP-1-derived macrophages with/without fucoidan treatment.
rhCSF-1 treatment almost completely reversed the attenuation of
pro-inflammatory cytokine production by fucoidan, particularly
TNF-α and IL-6. Furthermore, THP-1-derived macrophages regained
their migratory activity following rhCSF-1 treatment. described
above experiments have limitations, as it is difficult to determine
whether rhCSF-1 has similar structural traits, decomposition time
and biological activities to endogenous CSF-1 secreted by
macrophages. Nevertheless, the present study demonstrated that
fucoidan attenuated CSF-1 production in THP-1-derived macrophages,
and CSF-1 concentration was positively associated with
pro-inflammatory cytokine production and migration. Therefore,
fucoidan-modulated CSF-1 production may be a potential mechanism
that mediates biological changes observed in this study.
In conclusion, the current study demonstrated that
fucoidan attenuated the migration and pro-inflammatory cytokine
production of THP-1-derived macrophages. Fucoidan-modulated CSF-1
production may be responsible for this attenuation. These results
provide novel insights into the biological properties of fucoidan
as an immunomodulatory agent, particularly its regulatory effects
on macrophages.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant nos. 31300752, 31470885,
31270971, 81072406 and 81300510).
Glossary
Abbreviations
Abbreviations:
|
CSF-1
|
colony-stimulating factor-1
|
|
PMA
|
phorbol-12-myristate-13-acetate
|
|
Fu
|
fucoidan
|
|
NAb
|
neutralizing antibody
|
References
|
1
|
Chizhov AO, Dell A, Morris HR, Haslam SM,
McDowell RA, Shashkov AS, Nifant'ev NE, Khatuntseva EA and Usov AI:
A study of fucoidan from the brown seaweed Chorda filum. Carbohydr
Res. 320:108–119. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bilan MI, Grachev AA, Ustuzhanina NE,
Shashkov AS, Nifantiev NE and Usov AI: Structure of a fucoidan from
the brown seaweed Fucus evanescens C. Ag. Carbohydr Res.
337:719–730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sang VT and Kim SJ: Fucoidans as a natural
bioactive ingredient for functional foods. J Function Foods.
5:16–27. 2013. View Article : Google Scholar
|
|
4
|
Yang M, Ma C, Sun J, Shao Q, Gao W, Zhang
Y, Li Z, Xie Q, Dong Z and Qu X: Fucoidan stimulation induces a
functional maturation of human monocyte-derived dendritic cells.
Int Immunopharmacol. 8:1754–1760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maruyama H, Tamauchi H, Hashimoto M and
Nakano T: Antitumor activity and immune response of Mekabu fucoidan
extracted from Sporophyll of Undaria pinnatifida. In Vivo.
17:245–249. 2003.PubMed/NCBI
|
|
6
|
Oomizu S, Yanase Y, Suzuki H, Kameyoshi Y
and Hide M: Fucoidan prevents C epsilon germline transcription and
NFkappaB p52 translocation for IgE production in B cells. Biochem
Biophys Res Commun. 350:501–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Epelman S, Lavine KJ and Randolph GJ:
Origin and functions of tissue macrophages. Immunity. 41:21–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A, Biswas SK, Galdiero MR, Sica
A and Locati M: Macrophage plasticity and polarization in tissue
repair and remodelling. J Pathol. 229:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu HY, Chiu SL, Wen MH, Chen KY and Hua
KF: Ligands of macrophage scavenger receptor induce cytokine
expression via differential modulation of protein kinase signaling
pathways. J Biol Chem. 276:28719–28730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi EM, Kim AJ, Kim YO and Hwang JK:
Immunomodulating activity of arabinogalactan and fucoidan in vitro.
J Med Food. 8:446–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kasai A, Arafuka S, Koshiba N, Takahashi D
and Toshima K: Systematic synthesis of low-molecular weight
fucoidan derivatives and their effect on cancer cells. Org Biomol
Chem. 13:10556–10568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Senthilkumar K and Kim SK: Anticancer
effects of fucoidan. Adv Food Nutr Res. 72:195–213. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mytar B, Woloszyn M, Macura-Biegun A,
Hajto B, Ruggiero I, Piekarska B and Zembala M: Involvement of
pattern recognition receptors in the induction of cytokines and
reactive oxygen intermediates production by human
monocytes/macrophages stimulated with tumour cells. Anticancer Res.
24:2287–2293. 2004.PubMed/NCBI
|
|
17
|
Mytar B, Gawlicka M, Szatanek R, Woloszyn
M, Ruggiero I, Piekarska B and Zembala M: Induction of
intracellular cytokine production in human monocytes/macrophages
stimulated with ligands of pattern recognition receptors. Inflamm
Res. 53:100–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KJ, Yoon KY and Lee BY: Low molecular
weight fucoidan from the sporophyll of Undaria pinnatifida
suppresses inflammation by promoting the inhibition of
mitogen-activated protein kinases and oxidative stress in RAW264.7
cells. Fitoterapia. 83:1628–1635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JW, Yoon SY, Oh SJ, Kim SK and Kang
KW: Bifunctional effects of fucoidan on the expression of inducible
nitric oxide synthase. Biochem Biophys Res Commun. 346:345–350.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akagawa KS: Functional heterogeneity of
colony-stimulating factor-induced human monocyte-derived
macrophages. Int J Hematol. 76:27–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji XH, Yao T, Qin JC, Wang SK, Wang HJ and
Yao K: Interaction between M-CSF and IL-10 on productions of IL-12
and IL-18 and expressions of CD14, CD23, and CD64 by human
monocytes. Acta Pharmacol Sin. 25:1361–1365. 2004.PubMed/NCBI
|
|
22
|
Sweet MJ, Campbell CC, Sester DP, Xu D,
McDonald RC, Stacey KJ, Hume DA and Liew FY: Colony-stimulating
factor-1 suppresses responses to CpG DNA and expression of
toll-like receptor 9 but enhances responses to lipopolysaccharide
in murine macrophages. J Immunol. 168:392–399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ancuta P, Rao R, Moses A, Mehle A, Shaw
SK, Luscinskas FW and Gabuzda D: Fractalkine preferentially
mediates arrest and migration of CD16+ monocytes. J Exp Med.
197:1701–1707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sierra-Filardi E, Nieto C, Domínguez-Soto
A, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven
J, Ardavín C, Rodríguez-Fernández JL, et al: CCL2 shapes macrophage
polarization by GM-CSF and M-CSF: Identification of
CCL2/CCR2-dependent gene expression profile. J Immunol.
192:3858–3867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reimer MK, Brange C and Rosendahl A: CCR8
signaling influences Toll-like receptor 4 responses in human
macrophages in inflammatory diseases. Clin Vaccine Immunol.
18:2050–2059. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao P, Gao D, Wang Q, Song B, Shao Q, Sun
J, Ji C, Li X, Li P and Qu X: Response gene to complement 32
(RGC-32) expression on M2-polarized and tumor-associated
macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4.
Cell Mol Immunol. 12:692–699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daigneault M, Preston JA, Marriott HM,
Whyte MK and Dockrell DH: The identification of markers of
macrophage differentiation in PMA-stimulated THP-1 cells and
monocyte-derived macrophages. PloS One. 5:e86682010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuchiya S, Kobayashi Y, Goto Y, Okumura
H, Nakae S, Konno T and Tada K: Induction of maturation in cultured
human monocytic leukemia cells by a phorbol diester. Cancer Res.
42:1530–1536. 1982.PubMed/NCBI
|
|
30
|
Vogel DY, Heijnen PD, Breur M, De Vries
HE, Tool AT, Amor S and Dijkstra CD: Macrophages migrate in an
activation-dependent manner to chemokines involved in
neuroinflammation. J Neuroinflammation. 11:232014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Louis SF and Zahradka P: Vascular smooth
muscle cell motility: From migration to invasion. Exp Clin Cardiol.
15:e75–e85. 2010.PubMed/NCBI
|
|
32
|
Sun J, Feng A, Zhang Y, Sun S, Hu W, Yang
M, Wei F and Qu X: Fucoidan increases TNF-alpha-induced MMP-9
secretion in monocytic cell line U937. Inflamm Res. 59:271–276.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rougerie P, Miskolci V and Cox D:
Generation of membrane structures during phagocytosis and
chemotaxis of macrophages: Role and regulation of the actin
cytoskeleton. Immunol Rev. 256:222–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu JM, Bignon J, Haroun-Bouhedja F,
Bittoun P, Vassy J, Fermandjian S, Wdzieczak-Bakala J and
Boisson-Vidal C: Inhibitory effect of fucoidan on the adhesion of
adenocarcinoma cells to fibronectin. Anticancer Res. 25:2129–2133.
2005.PubMed/NCBI
|
|
35
|
Ale MT, Mikkelsen JD and Meyer AS:
Important determinants for fucoidan bioactivity: A critical review
of structure-function relations and extraction methods for
fucose-containing sulfated polysaccharides from brown seaweeds.
Marine drugs. 9:2106–2130. 2011. View Article : Google Scholar : PubMed/NCBI
|