Introduction
Age-related macular degeneration (AMD) is the
leading cause of irreversible sight loss in the elderly, with risk
factors including aging, genetic characteristics, smoking, obesity
and hypertension (1,2). The two clinical classifications of
AMD are dry (nonneovascular) and wet (neovascular) AMD (3–5). Dry
AMD affects the majority of patients with AMD, and a small number
of dry AMD cases progress to become wet AMD by abnormal growth of
blood vessels from the choroid into the macula. A major
pathological hallmark of dry AMD is the presence of age-dependent
degenerative damage to the retinal pigment epithelium (RPE), a
monolayer of hexagonal epithelial cells located adjacent to, and
physically interacting with, retinal photoreceptors, forming the
outer blood-retinal barrier (BRB) (6). RPE is a common barrier for solutes
and fluids from the choroidal vasculature that must access the
inner retina (7,8). Strict control of fluids and solutes
across the BRB is achieved by well-developed tight junctions, which
mean that the liquid is not able to penetrate the barrier between
the two cells. Zonula occludens-1 and claudin-1 are the
most-studied tight junction proteins, with most attention focusing
on their relation to the BRB (9,10).
RPE cells are important in retinal physiology and
pathology (9). Previous studies
have revealed that abnormal distribution or expression of tight
junction proteins in RPE cells are involved in AMD pathogenesis
(10). Increased BRB permeability
allows toxic substances and microorganisms to cross the choroidal
vasculature, resulting in the activation of Toll-like receptor 4
(TLR4) (9). TLRs are a family of
signal transduction molecule, which are transmembrane proteins
usually expressed by sentinel cells and recognize structurally
conserved molecules from micro organisms. TLR4-mediated signaling
pathways have been revealed to activate nuclear transcription
factor-κB (NF-κB), suggesting that TLR-4 is critical to the
regulation of multiple proinflammatory genes, including cytokines,
chemokines, cyclooxygenase-2 (COX-2), interleukin (IL)-6, IL-8 and
inducible nitric oxide synthase (iNOS) (11). Therefore, the activation of NF-κB
has been proposed as a cause of ocular inflammatory disease. AMD is
a multifactorial disease that has several risk factors, including
aging, genetic characteristics and smoking. The involvement of
oxidative stress and inflammatory changes have been highlighted in
multiple studies (4). Therefore,
inhibiting inflammation and improving BRB function is the focus of
pathophysiology research regarding AMD.
5,7-dihydroxy-8-methoxyflavone, or wogonin, is a
naturally-derived ingredient isolated from the roots of
Scutellaria baicalensis Georgi, commonly known as Huang-Qin.
In traditional Chinese medicine, this substance has been used to
treat allergies, inflammatory diseases and tumors (11–13).
Previous studies have demonstrated that wogonin suppresses
LPS-induced expression of iNOS, tumor necrosis factor-α (TNF-α),
NO, and IL-1β in microglia via inhibition of NF-κB activation
(14,15). It has also been revealed that
wogonin (10−6-10−5 M) inhibits IL-6 and IL-8
gene expression and down-regulates the inflammation-associated
protein COX-2 through suppression of NF-κB binding in a murine skin
inflammation model (16,17). This evidence demonstrates the
benefit of wogonin treatment in inflammatory diseases, but little
is known about the function of wogonin in relation to AMD.
The human RPE cell line ARPE-19 has been
demonstrated to show structural and functional properties that are
characteristic to RPE cells in vivo, and so is ideal to use
for in vitro studies (18).
The present study investigated both the anti-inflammatory function
of wogonin in LPS-induced ARPE-19 cells, and the molecular
mechanisms through which it modulates inflammation.
Materials and methods
Cell culture and treatments
ARPE-19 cells (American Type Culture Collection,
Manassas, VA, USA) were seeded in Dulbecco's modified Eagle's
medium/F-12, a human amniotic membrane nutrient mixture (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.). Cultures were grown in
a humidified incubator at 37°C in an atmosphere of 5%
CO2. Culture medium was changed every 2 days. An
additional 2 µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was added to the medium for the LPS
groups for 24 h, as previously described (19). ARPE-19 cells (5×104
cells/well) were cultured in 96-well plates for 24 h at 37°C, then
pre-treated with different concentrations of wogonin (0–50 µM) for
24 h, followed by 24 h LPS stimulation. The concentrations of
wogonin used to treat ARPE-19 cells were based on the results of
previous studies (19–21).
Measurement of transepithelial
electrical resistance (TEER)
TEER was used to measure the paracellular
permeability of cell monolayers. ARPE-19 cells (5×104
cells/well) were cultured on microporous filter membranes (0.4 µm
pore size and 6.5 mm diameter; Corning Incorporated, Corning, NY,
USA) of apical chambers until the confluent monolayer achieved a
TEER >300 Ωcm2 (~15–18 days), indicating a tight
monolayer. A voltmeter (Millicell-ERS; Merck Millipore) was used to
measure TEER as previously described (22): TEER (Ωcm2)=[total
resistance-blank resistance (Ω)]x[area(cm2)].
Measurements were repeated at least three times for each well and
each experiment was repeated for at least five different wells.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Following 24 h of LPS exposure, RT-qPCR was
performed to detect the mRNA expression levels of tight junction
components ZO-1 and claudin-1 in ARPE-19 cells. mRNA expression
levels of biological markers of inflammation, COX-2, iNOS and
TNF-α, were also determined. Total RNA was isolated from ARPE-19
cells using TRIzol (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. First-strand cDNA was synthesized
using the PrimeScript RT kid (cat. no. DRR0375, Takara Bio Inc.,
Otsu, Japan). qPCR was performed using an ABI Prism 7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with thermocycling conditions as follows: An initial
denaturation step at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 60°C for 60 sec and
extension at 72°C for 30 sec. The expression of each PCR product
was analyzed using the 2−ΔΔCq method relative to β-actin
(23). The SYBR Green real-time
PCR master mixes (cat. no. PA-012 and cat. no. PA-011) were from
SuperArray Bioscience Corporation (Frederick, MD, USA). The primers
of the target genes were as follows: ZO-1, forward
5′-AGCCTGCAAAGCCAGCTCA-3′ and reverse 5′-AGTGGCCTGGATGGGTTCATAG-3′;
claudin-1, forward 5′-GCATGAAGTGTATGAAGTGCTTGGA-3′ and reverse
5′-CGATTCTATTGCCATACCATGCTG-3′; TLR4, forward
5′-GAGCCGTTGGTGATCTTTG-3′ and reverse 5′-TGCCGTTTCTTGTTCTTCC-3′;
β-actin, forward 5′-GGCGGACTATGACTTAGTTG-3′ and reverse
5′-AAACAACAATGTGCAATCAA-3′; iNOS, forward 5′-AGAGAGATCGGGTTCACA-3′
and reverse 5′-CACAGAACTGAGGGTACA-3′; COX-2, forward
5′-TTAAAATGAGATTGTCCGAA-3′ and reverse
5′-AGATCACCTCTGCCTGAGTA-3′.
Immunofluorescence
Immunostaining of ARPE-19 cells were performed as
described (24). ARPE-19 cells
were fixed with 4% PFA for 1 h, washed with PBS containing 0.1%
Triton X-100 (PBST), and blocked at 37°C for 30 min in PBST
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.). ARPE-19
cells were incubated overnight at 4°C with ZO-1 antibody (cat. no.
8193; dilution, 1:100, Cell Signaling Technology, Inc., Danvers,
MA, USA) or claudin-1 antibody (cat. no. 4933; dilution, 1:100,
Cell Signaling Technology, Inc.), then incubated with fluorescein
isothiocyanate-conjugated goat anti-mouse immunoglobulin G
secondary antibody (cat. no. sc-2357; dilution, 1:5,000, Santa Cruz
Biotechnology, Inc.) at 37°C for 2 h. This was followed by DNA
staining using 4′,6-diamidino-2-phenylindole. Fluorescent signals
were visualized with the Leica TCS SP2 Confocal Spectral Microscope
(Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Enzyme-linked immunosorbent assay
(ELISA)
ARPE-19 cells were plated in 24-well culture plates
at a density of 5×104 cells per well and treated as they
were to measure the TEER. ARPE-19 cells were collected and
centrifuged at 1,500 × g at 4°C for 5 min following 24 h LPS
treatment in the presence or absence of wogonin. ELISA was
performed to measure IL-1β (cat. no. 432601), IL-6 (cat. no.
430506) and IL-8 (cat. no. 431506) according to the manufacturer's
instructions (BioLegend ELISA MAX™ Deluxe kit; BioLegend, Inc., San
Diego, CA, USA). ELISA was performed in triplicate in three
independent experiments.
Western blot
Total protein was extracted from ARPE-19 cells that
had been lysed with a buffer (1 M Tris-HCl pH 7.5, 1% Triton X-100,
10% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 0.5 M
EDTA, 10 µg/ml leupeptin, 10 µg/ml aprotinin, and 1 mM
phenylmethanesulfonyl fluoride or phenylmethylsulfonyl fluoride
[PMSF]) at 4°C for 2 h. The soluble proteins were separated by 10%
SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride filter membranes (Merck Millipore).
Primary antibodies were incubated overnight at 4°C, including
inhibitor of NF-κB (IκB; cat. no. 9242; dilution, 1:500; Cell
Signaling Technology, Inc.), phospho-IκB (cat. no. 9246; dilution,
1:500; Cell Signaling Technology, Inc.), TLR4 (cat. no. sc-M300;
dilution, 1:500, Santa Cruz Biotechnology, Inc.) or β-actin
antibody (cat. no. sc-1616; dilution, 1:500, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Horseradish
peroxidase-labelled secondary antibody (cat. no. sc-3901; dilution,
1:10,000, Santa Cruz Biotechnology, Inc.) was added at 37°C for 1
h. Following this the membranes were washed extensively in
Tris-buffered saline+Tween-20 (25 mmol/l Tris, pH 7.5, 150 mmol/l
NaCl and 0.1% Tween-20) for 1 h. The films were visualized using
femto LUCENT® (Geno Technology Inc., Saint Louis, MO,
USA). All experiments were repeated independently four times in
triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA). All values were expressed as the mean ± standard deviation.
Data was analyzed with one-way ANOVA tests followed by Tukey's
range tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
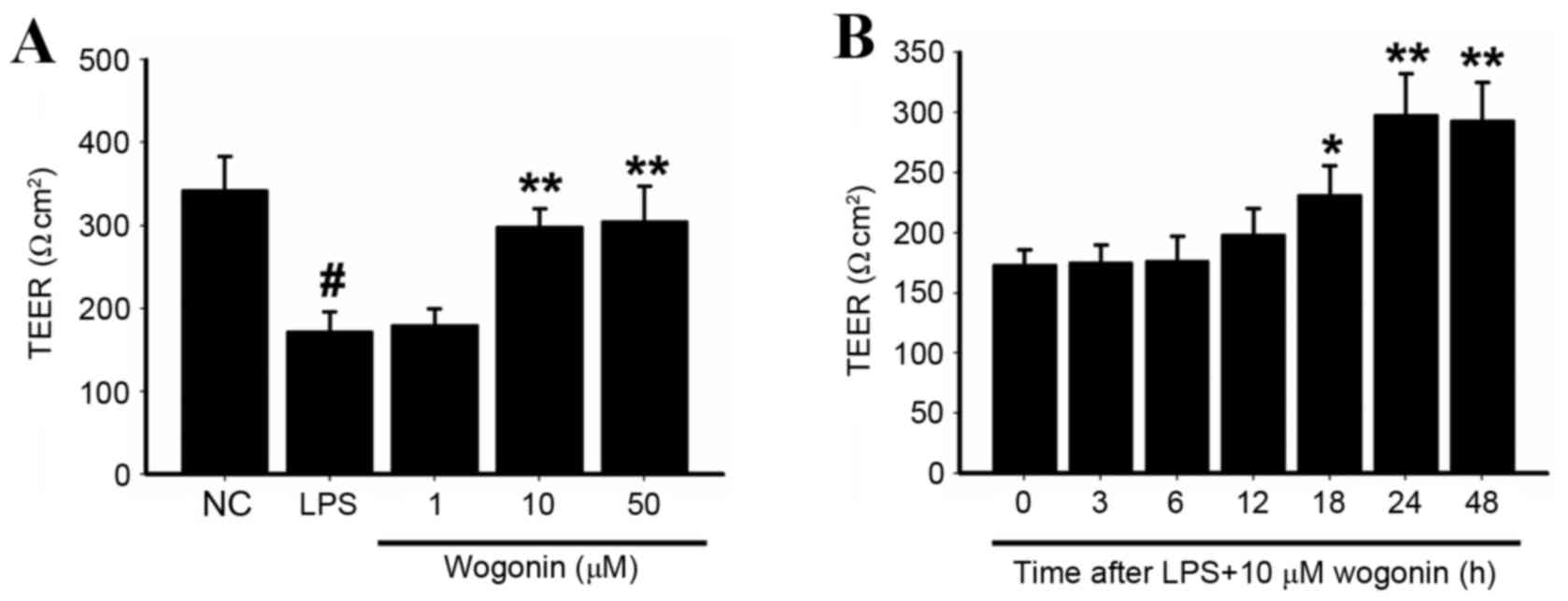
The effect of wogonin on TEER changes
in LPS-stimulated ARPE-19 cells
TEER was significantly reduced in cells stimulated
with LPS compared with the unstimulated negative control (NC) cells
(P<0.05; Fig. 1A). The effect
of different concentrations of wogonin on LPS-stimulated ARPE-19
cells was then investigated. Significant increases in TEER were
observed in cells treated with 10 and 50 µM wogonin compared with
the LPS-only group (P<0.05 and P<0.05 respectively; Fig. 1A). Treatment with 10 µM wogonin
following LPS-stimulation resulted in increasing TEER as time
increased, peaking at 24 h (P<0.05 at 18, 24 and 48 h; Fig. 1B).
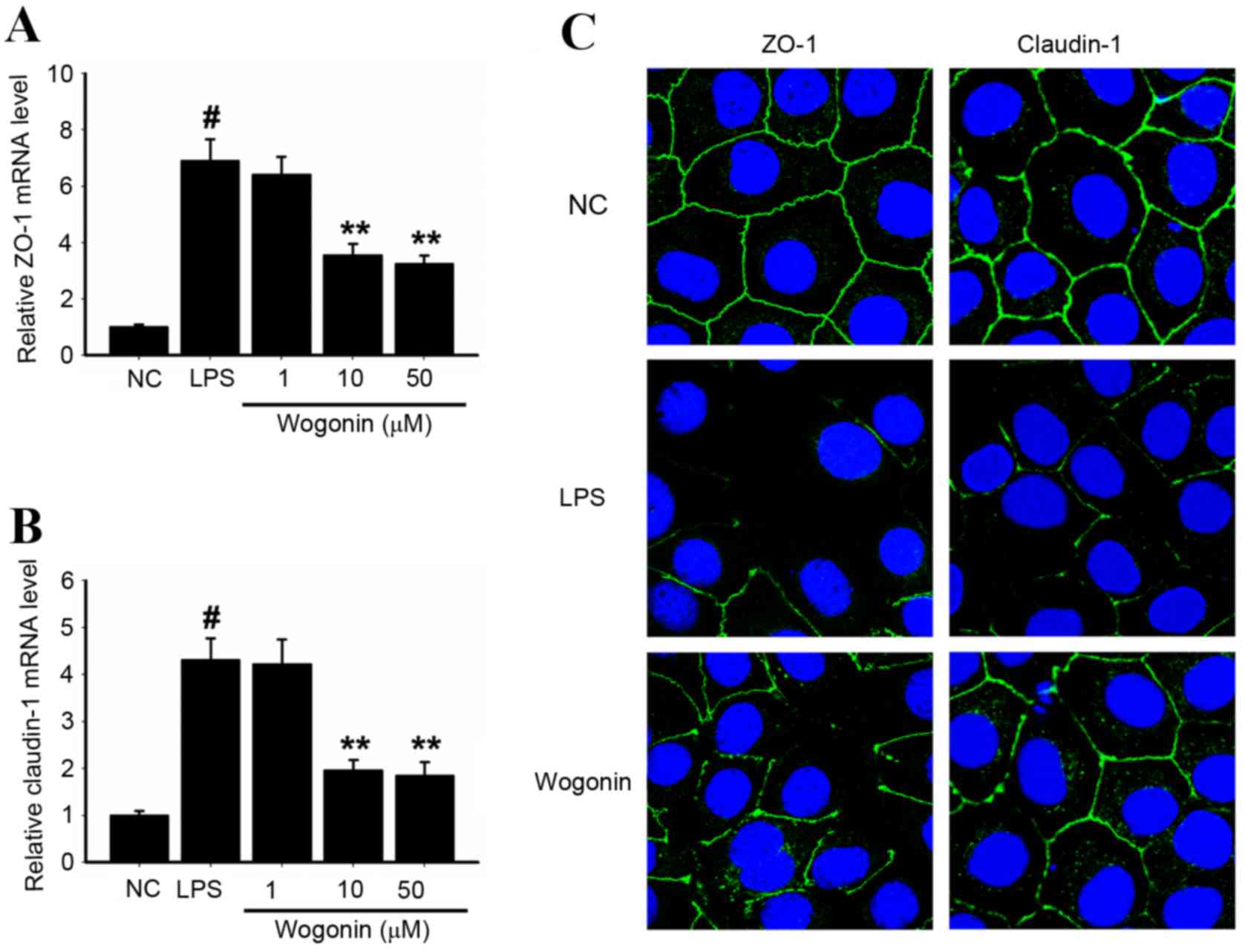
The effect of wogonin on the expression of ZO-1 and
claudin-1 in LPS-stimulated ARPE-19 cells. ZO-1 and claudin-1 mRNA
expression levels were significantly increased in ARPE-19 cells
stimulated with LPS compared with NC (P<0.05 and P<0.05,
respectively; Fig. 2A and B,
respectively). Treatment with 10 and 50 µM wogonin for 24 h
resulted in significantly reduced ZO-1 and claudin-1 expression in
LPS-stimulated ARPE-19 cells compared with LPS stimulated/wogonin
untreated cells (P<0.05; Fig. 2A
and B). The changes observed in immunofluorescence staining
demonstrated similar trends to those observed in RT-qPCR analysis:
The groups treated with wogonin following LPS stimulation exhibited
higher ZO-1 and claudin-1 expression than the group treated with
LPS alone (Fig. 2C). These results
indicated the protective effects of wogonin against inflammation in
the LPS-induced ARPE-19 cells.
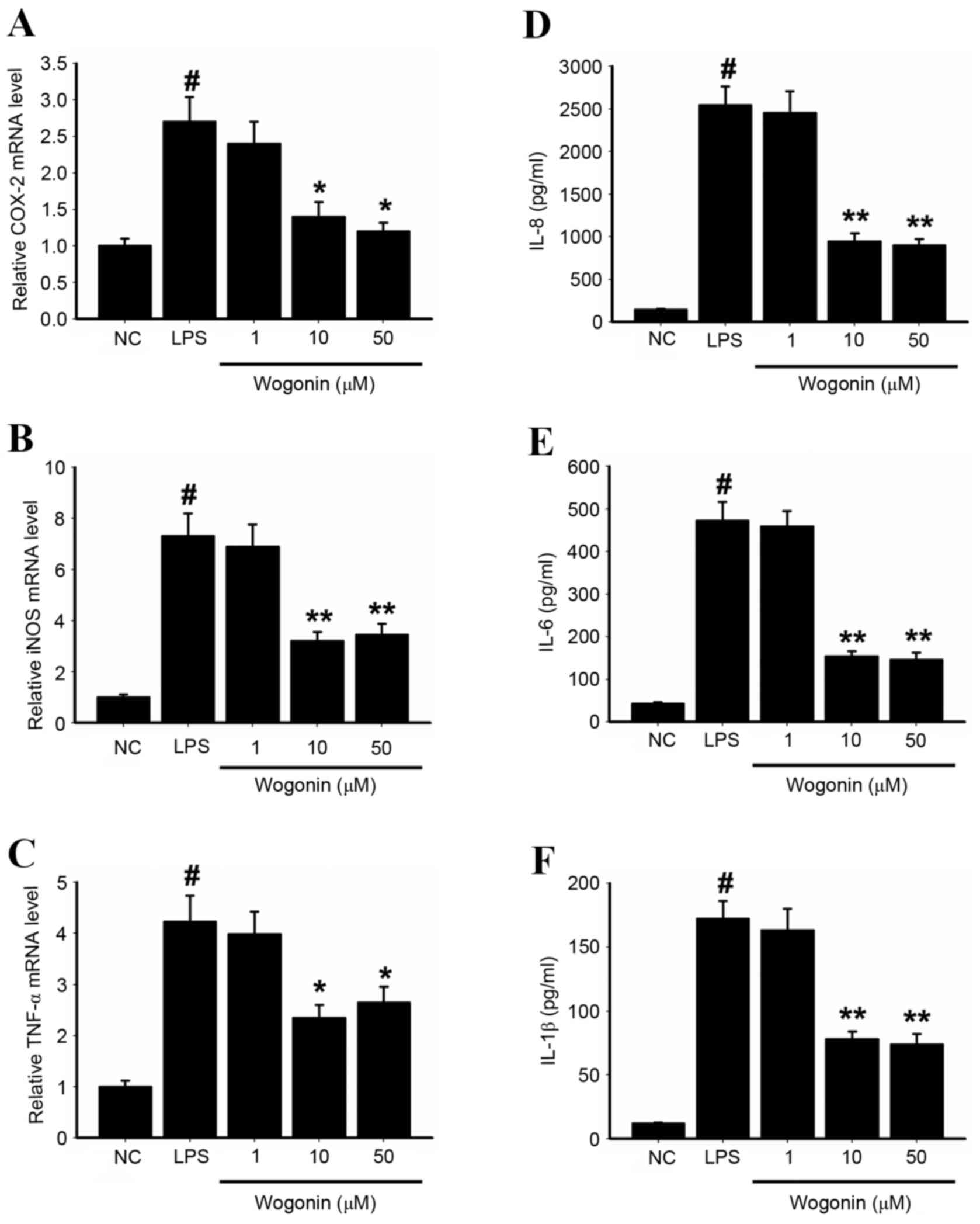
Key inflammatory mediators in
LPS-stimulated ARPE-19 cells are inhibited by wogonin
Expression levels of COX-2, iNOS and TNF-α mRNA were
significantly increased in the LPS-stimulated cells compared with
NC (P<0.05, P<0.05 and P<0.05; Fig. 3A-C). COX-2, iNOS and TNF-α mRNA
expression levels in LPS-stimulated cells were slightly decreased
with 1 µM wogonin treatment compared with the group stimulated with
LPS alone, and significantly decreased by 10 µM and 50 µM wogonin
treatments (P<0.05; Fig. 3A-C).
The ELISA results indicated that protein expression levels of
IL-1β, IL-6 and IL-8 were also significantly increased in
LPS-stimulated ARPE-19 cells compared with NC, and significantly
reduced in LPS-stimulated cells treated with 10 and 50 µM wogonin
compared with LPS-stimulated/wogonin-untreated cells (P<0.05;
Fig. 3D-F). This suggests that
wogonin suppresses inflammatory activity in LPS-stimulated ARPE-19
cells.
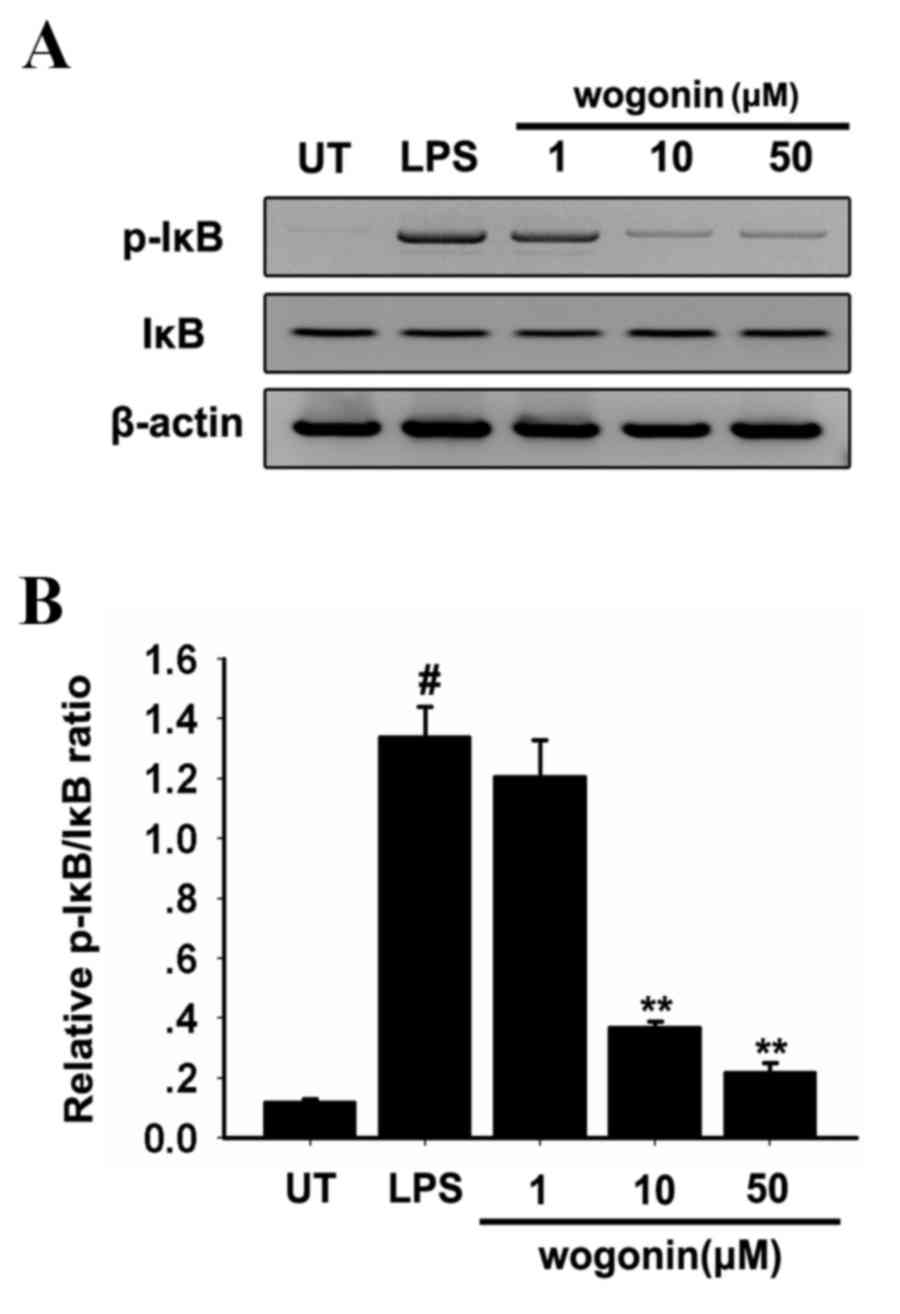
Effect of wogonin on NF-κB activation
in LPS-stimulated ARPE-19 cells
NF-κB is central to the regulation of several genes
involved in the inflammatory response (24). Activation of NF-κB by LPS is
induced by a cascade of events leading to the activation of IκB.
The rate of tyrosine phosphorylation of p65 and degradation of IκB
could measure the effects of wogonin on LPS-induced NF-κB
activation. LPS stimulation was observed to significantly increase
the serine phosphorylation of IκB (Fig. 4). Treatment of LPS-stimulated
ARPE-19 cells with 10 and 50 µM wogonin significantly decreased IκB
activation in response to LPS compared with the cells stimulated
with LPS alone (P<0.05; Fig.
4). Wogonin may, therefore, significantly inhibit LPS-induced
NF-κB transcriptional activity in ARPE-19 cells.
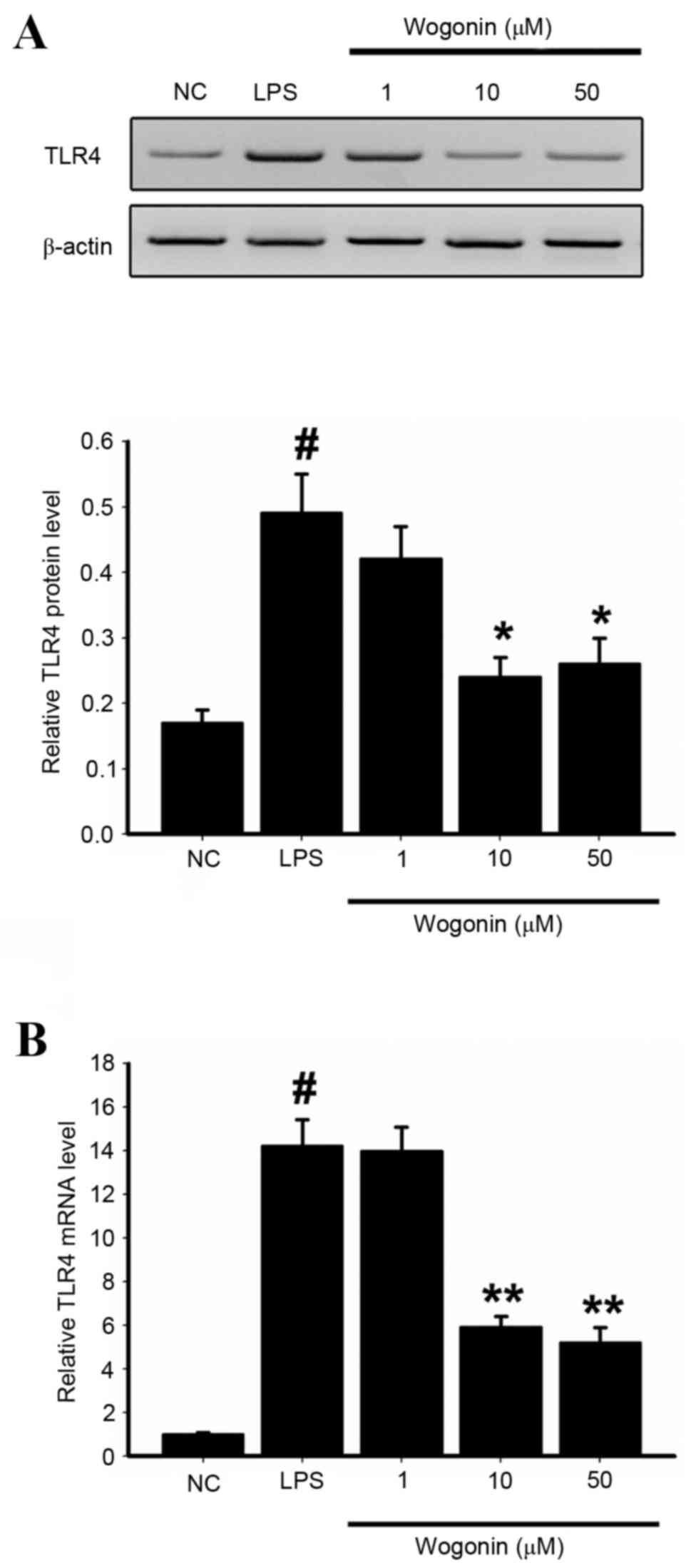
Effect of wogonin on TLR4 expression
in LPS-induced ARPE-19 cells
As activation of TLR4 stimulates the activation of
NF-κB, the effects of wogonin on TLR4 expression were examined.
Western blotting revealed that basal TLR4 expression was low in the
NC group (Fig. 5A). The protein
expression levels of TLR4 were significantly increased in
LPS-stimulated ARPE-19 cells compared with NC cells (P<0.05;
Fig. 5A). However, treatment of
LPS-stimulated cells with 10 and 50 µM wogonin resulted in
significantly lower TLR4 protein expression levels than
LPS-stimulated cells (P<0.05 and P<0.05, respectively;
Fig. 5A). RT-qPCR analyses of mRNA
expression levels were consistent with the protein results
(Fig. 5B). This suggests that
wogonin has a significant inhibitory effect on LPS-stimulated TLR4
expression in ARPE-19 cells. Therefore, wogonin may inhibit the
activation of inflammation-associated cytokines through the
TLR4/NF-κB pathway, which may be the molecular mechanism underlying
the protective effect of wogonin on RPE cells.
Discussion
The present study presents three novel findings.
First, wogonin inhibits inflammation in LPS-stimulated ARPE-19
cells, which results in the protection of the tight junction
proteins ZO-1 and claudin-1. By protecting endothelial tight
junctions, wogonin treatment helps maintain an intact BRB.
Secondly, wogonin attenuated the LPS-induced inflammatory response
via the inhibition of IL-1β, IL-6, IL-8, COX-2, iNOS and TNF-α gene
expression. This is consistent with the findings of previous
studies, that demonstrated the ability of wogonin to inhibit
IL-1β-induced IL-6 and IL-8 expression via the suppression of NF-κB
binding activities (19). Thirdly,
wogonin inhibited the activation of the TLR4/NF-kB pathway, which
is also associated with the inflammatory response. Previous studies
have demonstrated that wogonin acts as a potent inhibitor of
several other kinases involved in signal transduction (23). This is consistent with the findings
of the present study, with wogonin revealed to attenuate AMD.
Lipopolysaccharide (LPS), also known as endotoxin,
is the major cell wall constituent of gram-negative bacteria, and
functions as a microglia activator via induction of TLR4 (25). In vitro assays have
demonstrated that LPS induces inflammation in RPE, followed by
subsequent destruction of the outer BRB (25,26).
Therefore, cultured ARPE-19 cells were exposed to LPS to induce
inflammation. Inflammation depends largely on gene expression and
shares key regulators, including COX-2, iNOS and TNF-α (24). TLR4-mediated NF-κB signaling is
thought to be central to the regulation of numerous inflammatory
responses, and considered to be one of the indicators for ocular
inflammatory disease (24).
Activation of the NF-κB transcription pathway is considered to be
essential for expression of pro-inflammatory genes encoding enzymes
such as COX-2, iNOS and TNF-α (27). It has previously been demonstrated
that suppression of TLR4/NF-kB signaling by anti-inflammatory
agents reduces RPE cell damage (23). The present study also observed that
TLR4/NF-kB signaling pathway activation in AMD was attenuated by
wogonin treatment. The obtained data were in agreement with
previous studies demonstrating that wogonin suppresses neutrophil
infiltration and reduces injury-induced IL-1β, IL-6, IL-8 and COX-2
expression, thereby ameliorating RPE damage (24).
The most abundant activated form of NF-κB is a
heterodimer of p50 and p65, çontaining transcriptional activation
domains necessary for gene induction (28). In resting cells, the NF-κB
heterodimer is held in the cytosol through interaction with IκB
inhibitory proteins. Following exposure to pro-inflammatory stimuli
IκB becomes phosphorylated, ubiquitinated, and then degraded
(28,29). The present study provides further
support for targeting this mechanism of NF-kB activation to provide
protection from inflammation. Wogonin reduced the stimulation
effect of LPS on the phosphorylation of IκB and the expression of
iNOS, COX-2 and TNF-α together. This suggests that NF-κB was
activated via phosphorylation of IκB.
In conclusion, these findings indicate that the
upstream factors (TLR4/NF-kB) and downstream factors (COX-2, iNOS,
TNF-α activity and IL-1β, IL-6, IL-8 expression) of the TLR4/NF-kB
pathway were associated with the neuroprotective effects of RPE
cells and may be involved in the pathogenesis of AMD. Further
studies are required to explore the potential efficacy of wogonin
in other ocular diseases against inflammatory responses.
References
|
1
|
Lu L, Hackett SF, Mincey A, Lai H and
Campochiaro PA: Effects of different types of oxidative stress in
RPE cells. J Cell Physiol. 206:119–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding X, Patel M and Chan CC: Molecular
pathology of age-related macular degeneration. Prog Retin Eye Res.
28:1–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson DH, Mullins RF, Hageman GS and
Johnson LV: A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol. 134:411–431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salminen A, Kauppinen A, Hyttinen JM,
Toropainen E and Kaarniranta K: Endoplasmic reticulum stress in
age-related macular degeneration: Trigger for neovascularization.
Mol Med. 16:535–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowak JZ: Age-related macular degeneration
(AMD): Pathogenesis and therapy. Pharmacol Rep. 58:353–363.
2006.PubMed/NCBI
|
|
6
|
Chen C, Cano M, Wang JJ, Li J, Huang C, Yu
Q, Herbert TP, Handa JT and Zhang SX: Role of unfolded protein
response dysregulation in oxidative injury of retinal pigment
epithelial cells. Antioxid Redox Signal. 20:2091–2106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kucuksayan E, Konuk EK, Demir N, Mutus B
and Aslan M: Neutral sphingomyelinase inhibition decreases ER
stress-mediated apoptosis and inducible nitric oxide synthase in
retinal pigment epithelial cells. Free Radic Biol Med. 72:113–123.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshikawa T, Ogata N, Izuta H, Shimazawa
M, Hara H and Takahashi K: Increased expression of tight junctions
in ARPE-19 cells under endoplasmic reticulum stress. Curr Eye Res.
36:1153–1163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du M, Wu M, Fu D, Chen J, Wilson K and
Lyons TJ: Effects of modified LDL and HDL on retinal pigment
epithelial cells: A role in diabetic retinopathy? Diabetologia.
56:2318–2328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yeh CH, Yang ML, Lee CY, Li YC, Chen CJ
and Kuan YH: Wogonin attenuates endotoxin-induced prostaglandin E2
and nitric oxide production via Src-ERK1/2-NFκB pathway in BV-2
microglial cells. Environ Toxicol. 29:1162–1170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gibbons HM and Dragunow M: Microglia
induce neural cell death via a proximity-dependent mechanism
involving nitric oxide. Brain Res. 1084:1–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu H, Gao F, Shu G, Xia G, Shao Z, Lu H
and Cheng K: Wogonin inhibits the proliferation of myelodysplastic
syndrome cells through the induction of cell cycle arrest and
apoptosis. Mol Med Rep. 12:7285–7292. 2015.PubMed/NCBI
|
|
14
|
Piao HZ, Choi IY, Park JS, Kim HS, Cheong
JH, Son KH, Jeon SJ, Ko KH and Kim WK: Wogonin inhibits microglial
cell migration via suppression of nuclear factor-kappa B activity.
Int Immunopharmacol. 8:1658–1662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CM, Chen YH, Ong JR, Ma HP, Shyu KG
and Bai KJ: Functional role of wogonin in anti-angiogenesis. Am J
Chin Med. 40:415–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chi YS, Lim H, Park H and Kim HP: Effects
of wogonin, a plant flavone from Scutellaria radix, on skin
inflammation: In vivo regulation of inflammation-associated gene
expression. Biochem Pharmacol. 66:1271–1278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CM, Chang H, Chen YH, Li SY, Wu IH and
Chiu JH: Protective role of wogonin against
lipopolysaccharide-induced angiogenesis via VEGFR-2, not VEGFR-1.
Int Immunopharmacol. 6:1690–1698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burke JM: Epithelial phenotype and the RPE
Is the answer blowing in the Wnt? Prog Retin Eye Res. 27:579–595.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Xiong J, Zhan Y, Liu W and Wang X:
Wogonin inhibits LPS-induced inflammatory responses in rat dorsal
root ganglion neurons via inhibiting TLR4-MyD88-TAK1-mediated NF-κB
and MAPK signaling pathway. Cell Mol Neurobiol. 35:523–531. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen F, Wu R, Zhu Z, Yin W, Xiong M, Sun
J, Ni M, Cai G and Zhang X: Wogonin protects rat dorsal root
ganglion neurons against tunicamycin-induced ER stress through the
PERK-eIF2α-ATF4 signaling pathway. J Mol Neurosci. 55:995–1005.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu S, Zhao X, Zhao Q, Zheng Q, Fang Z,
Yang X, Wang H, Liu P and Xu H: Wogonin prevents rat dorsal root
ganglion neurons death via inhibiting tunicamycin-induced ER stress
in vitro. Cell Mol Neurobiol. 35:389–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang W, Xia T and Yu X: Wogonin suppresses
inflammatory response and maintains intestinal barrier function via
TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res.
64:423–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen W, Gao Y, Lu B, Zhang Q, Hu Y and
Chen Y: Negatively regulating TLR4/NF-κB signaling via PPARα in
endotoxin-induced uveitis. Biochim Biophys Acta. 1842:1109–1120.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paeng SH, Park WS, Jung WK, Lee DS, Kim
GY, Choi YH, Seo SK, Jang WH, Choi JS, Lee YM, et al: YCG063
inhibits Pseudomonas aeruginosa LPS-induced inflammation in human
retinal pigment epithelial cells through the TLR2-mediated
AKT/NF-κB pathway and ROS-independent pathways. Int J Mol Med.
36:808–816. 2015.PubMed/NCBI
|
|
26
|
Mateos MV, Kamerbeek CB, Giusto NM and
Salvador GA: The phospholipase D pathway mediates the inflammatory
response of the retinal pigment epithelium. Int J Biochem Cell
Biol. 55:119–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Breccia M and Alimena G: NF-κB as a
potential therapeutic target in myelodysplastic syndromes and acute
myeloid leukemia. Expert Opin Ther Targets. 14:1157–1176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He X, Wei Z, Zhou E, Chen L, Kou J, Wang J
and Yang Z: Baicalein attenuates inflammatory responses by
suppressing TLR4 mediated NF-κB and MAPK signaling pathways in
LPS-induced mastitis in mice. Int Immunopharmacol. 28:470–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wardill HR, Van Sebille YZ, Mander KA,
Gibson RJ, Logan RM, Bowen JM and Sonis ST: Toll-like receptor 4
signaling: A common biological mechanism of regimen-related
toxicities: An emerging hypothesis for neuropathy and
gastrointestinal toxicity. Cancer Treat Rev. 41:122–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|