Introduction
Osteoporotic fractures are a specific type of bone
fracture resulting from mild impact, which may have serious
consequences in osteoporotic patients. The estimated overall
lifetime risk of any osteoporotic fracture is 13–22% in males and
40–50% in females (1); therefore,
osteoporosis-associated fractures are a primary clinical and public
health concern. Despite significant improvements in treatment
strategies, a large number of patients suffer from delayed healing,
fracture nonunion or other serious complications. Clinical
investigations have increasingly focused on examining the ideal
biological microenvironment for fracture healing and the prevention
of nonunion. The extensive proliferation and differentiation
potential of mesenchymal stem cells (MSCs) render them suitable for
bone tissue engineering. However, the effectiveness of bone tissue
engineering using MSCs relies on the predesigned scaffold and
osteogenic factors used. Osteogenic factors, including bone
morphogenetic proteins (BMPs), have been revealed as crucial
scaffolding proteins in bone tissue engineering for osteogenic
differentiation resulting in functional bone tissue (2).
BMP9 has been suggested to be the most
osteo-inductive of various forms of recombinant BMPs, and may
improve osteogenic differentiation of BMSCs in vitro and
in vivo (3). Unlike BMP2
and BMP7, BMP9 may effectively induce orthotopic ossification that
is not inhibited by BMP3, a known inhibitor of BMP2- and
BMP7-mediated osteogenesis (4).
Previous studies have indicated that BMP9 may induce osteogenic
differentiation of primary osteoblasts, pre-osteoblasts and other
directed-differentiated stem cells (5,6),
suggesting a potential role for BMP9 in promoting the shift of
directed-differentiated stem cells towards osteoblastic
differentiation. Additionally, previous in vivo studies have
indicated that adenovirus (Ad)BMP9 or AdBMP9-transfected human MSCs
induce spinal fusion in rodents (7,8).
Furthermore, injection of AdBMP9-transduced osteoblast progenitors
or viral vectors into the quadriceps of athymic mice effectively
induced orthotopic ossification (4). Shui et al (9) demonstrated that subcutaneous
implantation of BMP9-engineered cells with a type 1 collagen
(COL-1) sponge or hydroxyapatite-tricalcium phosphate scaffold
resulted in robust and mature cancellous bone masses, compared with
minimal bone formation using demineralized bone matrix. However,
few studies have focused on the effect of BMP9 on fracture healing
in osteoporotic rats. The present study hypothesized that BMP9 may
mediate callus formation and fracture healing in osteoporotic rats.
The osteoconductive activities and bone regeneration potential of a
gelatin sponge containing AdBMP9 were evaluated in rats with
osteoporotic fractures.
Materials and methods
Isolation and culture of bone marrow
stromal cells (BMSCs)
All animal procedures were approved by the Animal
Research Ethics Committee of Chongqing Medical University
(Chongqing, China). Female Sprague Dawley rats (n=50; age, 3
months; weight, 276.6±18.9 g) were obtained from the Animals
Research Center of Chongqing Medical University (ARCCMU)
(Chongqing, China). The animals were maintained at a constant
temperature (22±2°C) and humidity (50%) under a 12-h light/dark
cycle with free access to water and food. Primary BMSCs were
harvested from the femora of rats as previously described (10). Animals were anesthetized with an
intraperitoneal injection of sodium pentobarbital (200 mg/kg body
weight, Sigma-Aldrich Merck Millipore, Darmstadt, Germany) and
sacrificed by cervical dislocation. Cells at passage four were
plated at 1×105/well in 24-well plates and cultured in
the Dulbecco's modified Eagle's medium (DMEM) or basal medium Eagle
supplemented with 10% fetal bovine serum (FBS) and 5%
penicillin-streptomycin (all purchased from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Recombinant adenoviruses expressing
BMP-9
The AdEasy Adenoviral Vector system, was provided by
Dr. Tongchuan He, was used to generate recombinant adenoviruses
expressing BMP9 as previously described (11). The coding region of human BMP9 was
amplified and cloned into an adenoviral shuttle vector to generate
recombinant adenoviruses in Human Embryonic Kidney 293 cells
[HEK293; American Type Culture Collection (ATCC), Manassas, VA,
USA]. Adenoviruses were purified by cesium chloride gradient
centrifugation at 176,000 × g for 20 h at 10°C. The virus
titer was assessed by measuring absorbance at a wavelength of 260
nm prior to use. The resulting adenoviruses were designated as
AdBMP9. Analogous adenoviruses expressing only monomeric green
fluorescent protein (GFP; AdGFP) served as a control.
Preparation of conditioned medium
(CM)
The CM was prepared as previously described
(12). HCT116 human colon
carcinoma cells (ATCC) susceptible to adenovirus infection were
used to secrete overexpressed proteins into the medium (13,14).
Cells were infected with an optimal titer of AdGFP or AdBMP9 for 24
h. The culture medium was subsequently replaced with serum-free
DMEM; the CM of AdGFP or AdBMP9 was collected 48 h after infection
and used immediately.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
BMSCs were seeded into a 24-well plate and cultured
for 24 h. Cells were subsequently treated with AdGFP or AdBMP9 for
24 h in the presence of 8 µg/ml polybrene (Sigma-Aldrich; Merck
Millipore) and observed via microscopy (Nikon Corporation, Tokyo,
Japan). Following treatment with DMEM, AdGFP or AdBMP9 for 2 or 7
days, total RNA was isolated using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) for expression
analysis of BMP9 forward 5′-CGCAGCCTTAACCTCAGC-3′, reverse
5′-GTTGGAGGCAGGCGTAGA-3′, runt-related transcription factor 2
(RUNX2) forward 5′-GCCAATCCCTAAGTGTGGCT-3′, reverse
5′-AACAGAGAGCGAGGGGGTAT-3′ and COL-1 forward
5′-CAGTCGCTTCACCTACAGCA-3′, reverse 5′-GGTGGAGGGAGTTTACACGA-3′. The
cDNA templates were synthesized by RT reaction with hexamer and
Superscript II RT (Invitrogen; Thermo Fisher Scientific, Inc.). The
first strand cDNA products were further diluted 5- to 10-fold and
used as PCR templates. Semi-quantitative PCR was carried out as
described previously (15).
Protein extraction and western blot
analysis
Protein extraction and western blotting was
performed as previously described (16). To extract total proteins, cells
treated with DMEM, AdGFP or AdBMP9 for 2 or 7 days were washed with
cold PBS (4°C) and lysed in 300 µl RIPA lysis buffer
(Sigma-Aldrich; Merck Millipore). Total proteins (30 µg) were
separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride
membranes, blocked in 10% skimmed milk, and probed at 4°C for 24 h
with the following primary antibodies: Mouse anti-BMP9 (cat. no.
sc-514211; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit anti-RUNX2 (cat. no. sc-10758; 1:1,000; Santa Cruz
Biotechnology, Inc.), mouse anti-COL-1 (cat. no. ab6308; 1:1,000;
Abcam, Hong Kong, China) and anti-GAPDH (cat. no. sc-32233;
1:1,000; Santa Cruz Biotechnology, Inc.), which served as the
control. The membranes were subsequently incubated with the
appropriate horseradish peroxidase-conjugated secondary antibodies,
including goat anti-rabbit IgG (cat. no. sc-2004; 1:3,000; Santa
Cruz Biotechnology, Inc.) or goat anti-mouse IgG (cat. no. sc-2005;
1:3,000; Santa Cruz Biotechnology, Inc.), at 25°C for 1 h. Images
of target bands were developed using the Thermo Scientific™
SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific, Inc.). The Quantity One version 4.62 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was applied to assess the
gray value and the rate of grey value between target band and
control band was regarded as the relative expression level of
target protein.
Alkaline phosphatase (ALP) activity
assay
ALP activity was assessed using a modified Great
EscAPe™ SEAP Chemiluminescence assay (Clontech Laboratories, Inc.,
Mountainview, CA, USA) and/or histochemical staining as described
previously (17). BMSCs were
treated with DMEM, CM-GFP or CM-BMP9 for 7, 9 and 11 days. Each
Chemiluminescence assay was performed in triplicate and ALP
activity was normalized against total cellular protein levels.
Matrix mineralization assay
Matrix mineralization was detected by Alizarin Red S
(Sigma-Aldrich; Merck Millipore) staining as described previously
(15). BMSCs were treated with
DMEM, CM-GFP or CM-BMP9 for 28 days. The staining of calcium
mineral deposits was recorded under bright-field microscopy. To
quantify the matrix mineralization, Alizarin Red S was extracted
with 1 ml/well 100 mmol/l cetylpyridinium chloride (Sigma-Aldrich;
Merck Millipore) and absorbance was measured at a wavelength of 570
nm.
Osteoporotic fracture models
Previous studies have indicated that body weight
(BW) and/or body mass index have an effect on bone mineral density
and osteoporotic fractures (18–22).
To reduce potential experimental errors caused by body weight
variation in the present study, rats were randomly divided into
sham (n=10) and ovariectomized (OVX; n=40) groups, and anesthetized
with an intraperitoneal injection of sodium pentobarbital (200
mg/kg body weight) prior to surgery. Rats in the OVX group were
bilaterally ovariectomized and rats in the sham group underwent a
sham operation. After 3 months without any treatment for
osteoporosis induction, 10 randomly selected animals in the OVX
group and all 10 animals in the sham group were euthanized as
previously described (23). The BW
and uterus weight (UW) of rats were recorded, and femoral bone
mineral density (BMD) was measured using dual energy X-ray
absorptiometry (DXA). Following this, the femora were fixed in 4%
paraformaldehyde at 4°C for 48 h and stored in 70% ethanol. DXA was
performed using the Dual-Energy X-ray Absorption (XR-46; Norland
Corp., Fort Atkinson, WI, USA) with Small Animal software version
2.5.0 (Norland Corporation, Fort Atkinson, WI, USA).
The remaining 30 OVX rats were randomized into two
groups: AdGFP and AdBMP9 (n=15 per group). Rats were anaesthetized
as previously described. To create an open fracture, the left femur
was prepared for surgery under standard sterile conditions. The
patella was deflected laterally and a hole was drilled through the
intercondylar notch of the femur using a mini electric drill (drill
bit, 1.0-mm diameter). The periosteum of the femur was
circumferentially incised and elevated, and a transverse osteotomy
was made at the distal tuberositas deltoidea of the left femur with
a mini electric saw. A 1-mm diameter Kirschner wire (length, 3 cm;
bend, 90°; handle, 3 mm) was subsequently buried beneath the muscle
and inserted through the hole across the fracture ends. Gelatin
sponges (5×10-mm strips, Xiang En Jiangxi Medical Technology
Development Co., Nanchang, China) soaked with 300 µl AdGFP or
AdBMP9 (2.2×1012 viral particles/ml) were wrapped around
the fracture site circumferentially. The muscle and skin incisions
were sutured with 4–0 nylon suture (Johnson & Johnson Medical
Ltd., Wokingham, UK). Following surgery, plain X-rays were
performed to confirm a proper fracture pattern. All rats were
subsequently sacrificed after 4 weeks.
Radiographic analysis
After 4 weeks, the rats were anesthetized as
previously described and radiographed in lateral planes for X-ray
analysis using the RADspeed General Radiographic system (50 kv; 200
mv; 32 msev; Shimadzu Corporation, Kyoto, Japan) for high-precision
focus detection.
Micro-computed tomography (CT)
analysis
For analysis of fracture healing, animals (n=5 per
group) were scanned using an eXplore Locus SP Pre-Clinical Specimen
Micro-CT (GE Healthcare Life Sciences, Chalfont, UK) with an
isotropic voxel resolution of 14 µm (24). Rats were fastened onto a foam board
and scanned perpendicular to the long bone axis with a tube voltage
of 80 kV and current of 80 mA. A constrained 3D Gaussian filter was
used to partially improve noise volumes. The fracture line of each
specimen was measured as the central position for the region of
interest, with the upper and lower 5 mm as the two margins.
Cortical bone, callus bone and non-bony tissue were segmented by
thresholding algorithm, following the manufacturer's protocol. Bone
analysis was conducted using MicroView software version 2.1.2 (GE
Healthcare Life Sciences). The output density data (Hounsfield
units) was converted to mineral content (mg/cc) using the density
data from the phantoms. For femoral analysis, bone volume (BV),
BV/total volume (TV), bone mineral content (BMC) and BMD were
calculated.
Biomechanical assessment
The fractured femora of each rat was harvested with
saline-moistened gauze and stored at −80°C. Prior to biomechanical
assessment, the intramedullary wires were removed. The femora (n=5
per group) were subsequently subjected to a three-point bending
assessment using the Instron 4302 Universal Testing system
(Instron, Norwood, MA, USA). The fracture samples were prepared to
remove the soft tissue. To maintain consistency between samples,
all mechanical tests were conducted by one individual. The femora
were placed in the material testing machine on two supports
separated by a distance of 1.5 mm, and the testing area was defined
as the central part of the callus. A compression load was applied
at a rate of 2 mm/min until breakage. The data of ultimate load at
failure (N) and stiffness (N/mm) were monitored by a connected
computer.
Histological analysis
The specimens evaluated by micro-CT analysis were
decalcified with 10% EDTA disodium salt for 4 weeks. The tissues
were subsequently embedded in paraffin and 5-µm thick consecutive
sections were cut using a hand-operated microtome (Leica
Microsystems GmbH, Wetzlar, Germany). The sections were stained
with hematoxylin and eosin (H&E) or Masson's trichrome, and the
images were quantified as described in our previous study (15).
Callus protein extraction and western
blot analysis
A total of 18 female Sprague Dawley rats (age,
6-months; weight, 321.3±7.7 g) were obtained from ARCCMU and housed
in the aforementioned conditions. Rats were randomly divided into
AdGFP (n=9) and AdBMP9 (n=9) groups and the open fracture was
created. All the fractured animals were treated with AdGFP or
AdBMP9. At each time-point (days 7, 14 and 28), three animals per
group were sacrificed, and the callus at the fracture site was
collected and stored in liquid nitrogen. The frozen tissue samples
harvested from the callus were homogenized and proteins were
extracted for western blot analysis of BMP9, RUNX2 and COL-1
expression levels as described above.
Statistical analysis
Statistical analysis was performed using SPSS
software version 20.0 (IBM SPSS, Armonk, NY, USA). All results are
expressed as the mean ± standard error. Differences between two
groups were assessed using the unpaired Student's t-test.
Comparisons of multiple groups were performed using one-way
analysis of variance, followed by Turkey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
AdBMP9 promotes osteoblastic
differentiation of BMSCs in vitro
BMSCs infected with AdBMP9 or AdGFP for 24 h were
observed via microscopy (Fig. 1A).
BMP9 mRNA and protein expression levels were significantly enhanced
in the AdBMP9 group compared with the AdGFP and control groups
(P<0.001; Fig. 1B). To
investigate the BMP9-induced osteogenic differentiation of BMSCs,
ALP activity was measured following treatment of BMSCs with CM-BMP9
or CM-GFP for 7, 9 and 11 days (Fig.
1C). The activity of ALP was significantly enhanced in the
CM-BMP9-treated group, peaking at day 9 (P<0.001). In addition,
cells were stained with Alizarin Red S at day 28 (Fig. 1D), and the matrix mineralization
was markedly induced by BMP9 as demonstrated by significantly
greater Alizarin Red S staining in the AdBMP9, compared with the
AdGFP and control, groups (P<0.001). Furthermore, whether BMP9
modulates the expression levels of the two osteogenesis-regulating
proteins, RUNX2 and COL-1, was investigated. It was revealed that
stable overexpression of BMP9 in BMSCs effectively upregulated
RUNX2 (day 2) and COL-1 (day 7) mRNA and protein expression levels
compared with the AdGFP and control groups (P<0.001; Fig. 1B).
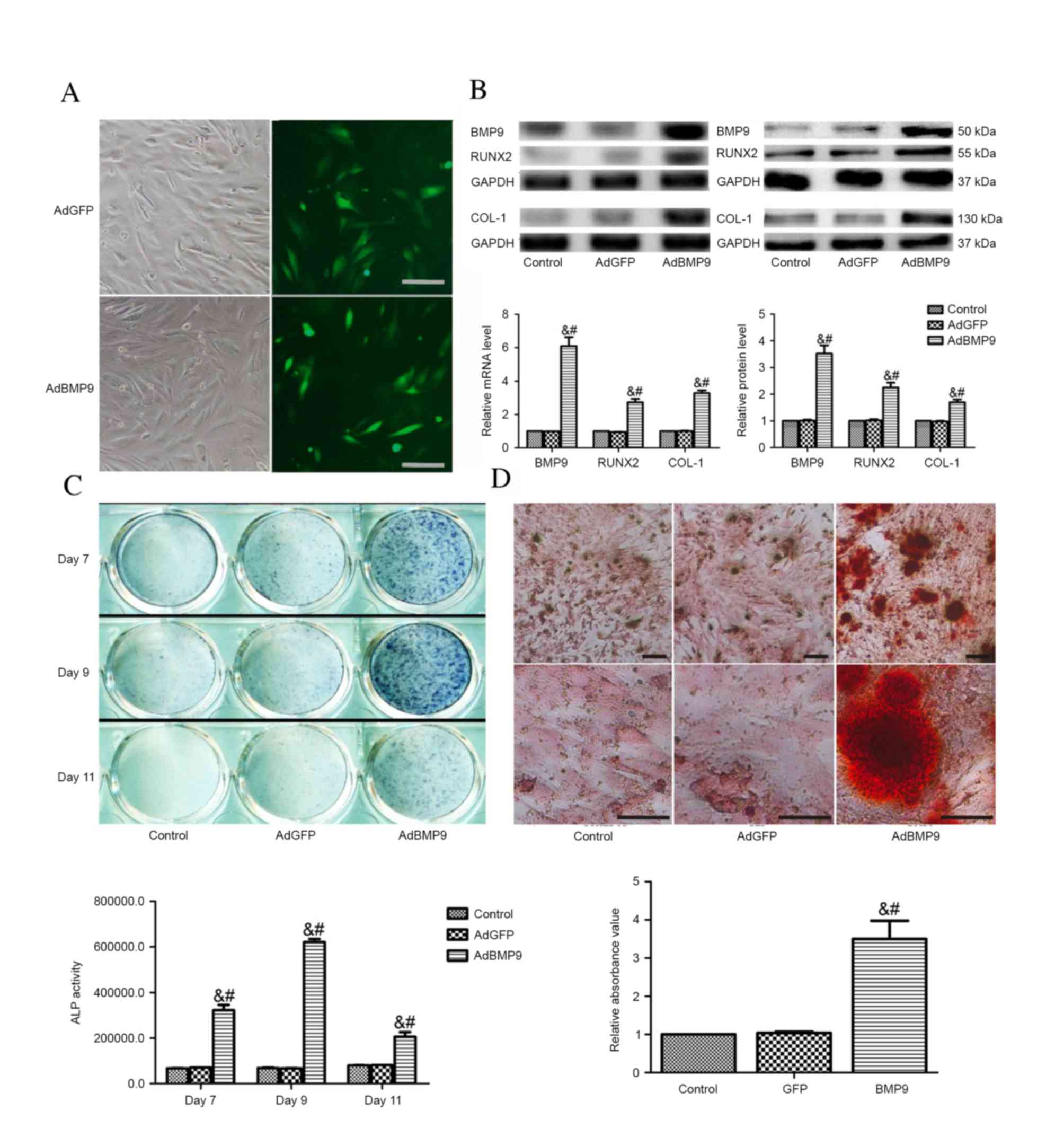 | Figure. 1AdBMP9 induces osteoblastic
differentiation in vitro. (A) Representative images of the
morphology of BMSCs transfected with AdBMP9 and AdGFP, and GFP
signal, as observed via fluorescence microscopy 3 days following
transfection (magnification, ×100; scale bar=100 µm). (B) mRNA and
protein expression level analysis of BMP9, RUNX2 (day 2) and COL-1
(day 7) in BMSCs transfected with AdGFP or AdBMP9, or without
transfection as a control. (C) ALP activity assay following BMSC
treatment with CM-BMP9 or CM-GFP for 7, 9 and 11 days. (D) Matrix
mineralization assay following CM-BMP9 or CM-GFP treatment for 28
days, visualized using Alizarin Red S staining. Magnification, ×40
(top row) or ×200 (bottom row); scale bar=200 (top row) or 20 µm
(bottom row). Each assay was performed in triplicate. Data are
presented as the mean ± standard error. &P<0.001
vs. control group, #P<0.001 vs. AdGFP group. Ad,
adenoviral; BMSCs, bone marrow stem cells; GFP, green fluorescent
protein; BMP9, bone morphogenic protein 9; CM, conditioned medium;
ALP, alkaline phosphatase; RUNX2, runt-related transcription factor
2; COL-1, type 1 collagen. |
Animal health and DXA analysis
At the end of the experiment, three animals were
excluded. In the process of creating the open fracture, one rat in
the AdBMP9 group died from asphyxia. During fracture healing, one
rat in the AdBMP9 group was excluded due to osteomyelitis and one
in the AdGFP group due to distal migration of the nail. This left
14 rats in the AdGFP group and 13 rats in the AdBMP9 group.
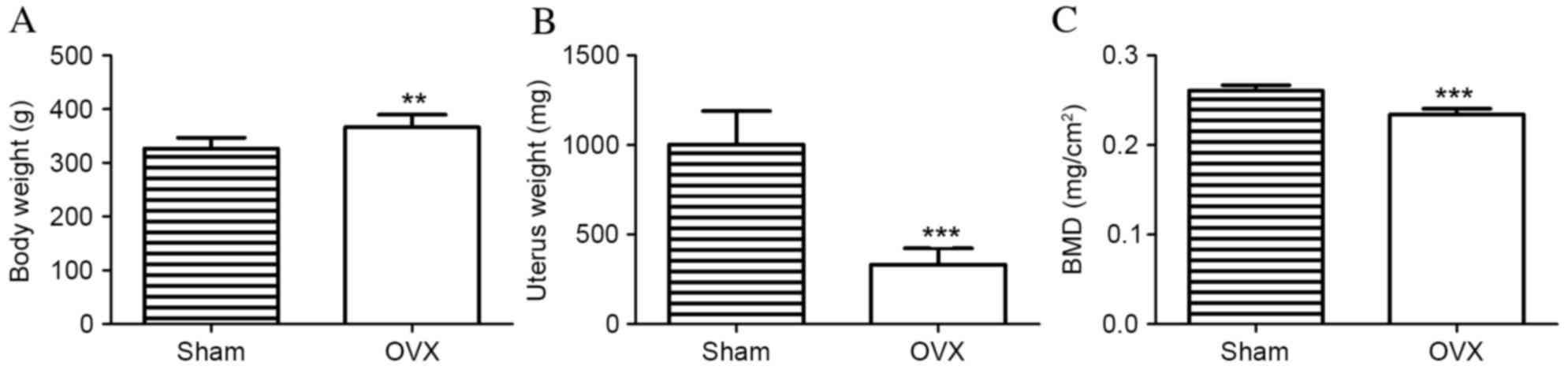
Ovariectomy resulted in significant alterations in
BW, UW and femoral BMD after 3 months. BW and UW differed
significantly between the sham and OVX groups. In the OVX group, BW
was significantly increased compared with the sham group
(366.5±7.32 vs. 326.8±6.35 g; P=0.001; Fig. 2A). However, the UW of rats in the
sham group was significantly increased compared with the OVX group
(1,002±59.02 vs. 331.7±28.89 mg; P<0.001; Fig. 2B). The femoral BMD value in the OVX
group was significantly reduced compared with the sham group
(0.2341±0.0020 vs. 0.2608±0.0019 mg/cm2; n=10;
P<0.001; Fig. 2C). These
results demonstrated the successful establishment of osteoporosis
in OVX rats.
Radiography, micro-CT and mechanical
analysis of fracture callus
Following dissection of soft tissue from the bone,
visual inspection revealed that AdBMP9-treated bone calluses
appeared stronger and larger compared with those from the
AdGFP-treated group. X-rays of the fractured femora demonstrated
thicker calluses, suggesting an increased bone mass in
AdBMP9-treated animals after 4 weeks (Fig. 3Aa). Ectopic bone formation was
observed in one rat from the AdBMP9 group (data not shown). The 3D
reconstructions of fractured femurs revealed that fracture gaps
contained numerous calluses in the AdBMP9 group; however, these
were almost undetectable in the AdGFP group. The transverse,
sagittal and coronal micro-CT slices through the center of the
fracture plate revealed that the newly-formed bone calluses were
larger and denser in AdBMP9-treated animals compared with
AdGFP-treated controls (Fig. 3Ab).
The quantitative results of BV, BV/TV, BMC and BMD are presented in
Fig. 3B. AdBMP9 treatment
significantly increased BV by 23.9% and BV/TV by 25.0%
(P<0.001), compared with the AdGFP group. Compared with the
AdGFP group, BMC and BMD in the AdBMP9 group increased by 34.5 and
36.1% of the AdGFP group, respectively (P<0.001).
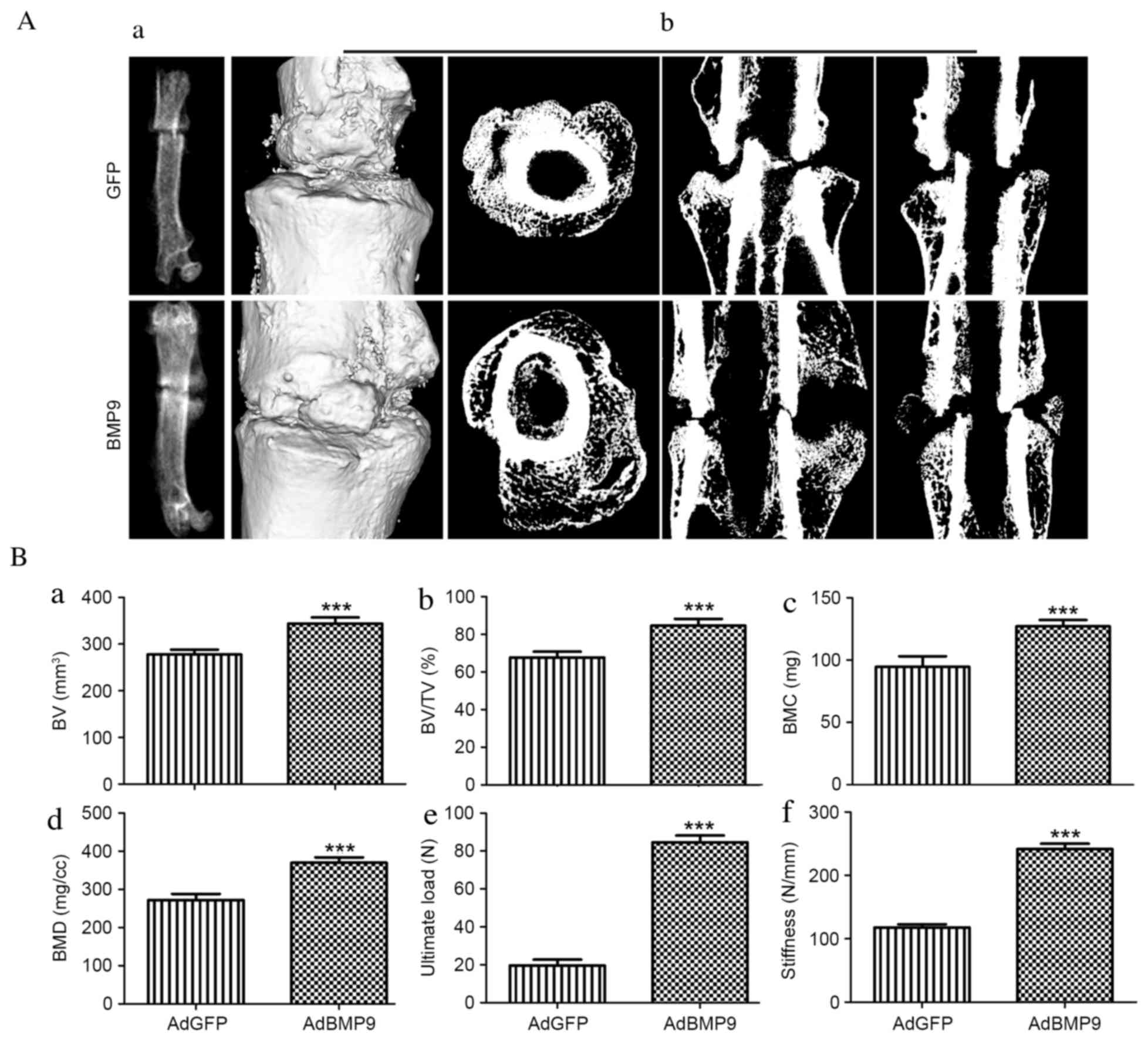 | Figure 3.Morphological and micro-CT analysis
of fracture healing 4 weeks after surgery. (A) The morphology of
the fractured femurs was determined by (a) radiography and (b)
transverse, sagittal and coronal micro-CT 3D reconstructions of the
fracture. (B) Quantitative results of micro-CT analysis presented
as (a) BV, (b) BV/TV, (c) BMC and (d) BMD, and biomechanical
results demonstrating (e) ultimate load force and (f) stiffness.
Data are presented as the mean ± standard error (n=5).
***P<0.001 vs. AdGFP control group. CT, computed tomography; B,
bone; V, volume; T, total; BM, bone mineral; C, content; D,
density; GFP, green fluorescent protein; Ad, adenoviral; BMP9, bone
morphogenic protein 9. |
Results of biomechanical tests of fractured femora
are presented as stiffness (N/mm) and ultimate load (N; Fig. 3Be and f). In the AdBMP9 group, a
marked 197.8% increase in mechanical stiffness was observed
compared with the AdGFP group (P<0.001). Additionally, BMP9
significantly increased the ultimate load of the callus by 357.1%
compared with the AdGFP group (P<0.001).
Histological analysis
In longitudinal sections stained with H&E and
Masson's trichrome, periosteal activation was present resulting in
bony callus formation at the fracture ends, and the soft callus was
present consisting of fibrous tissue with chondrogenic
differentiation in and around the fracture gap (Fig. 4A and B). The AdBMP9 group
demonstrated a cartilaginous tissue bridge (Fig. 4Ad and Bd); however, the AdGFP group
revealed fibrous contact or no bridging (Fig. 4Ac and Bc). Consistent with the BMD
of the fractured femur, as determined by micro-CT, the decalcified
callus appeared more compact in the AdBMP9 treated group compared
with the AdGFP group (Fig. 4Ad and f
and Bd and f). The AdBMP9 group demonstrated bone ossification
and the presence of chondrocytes, indicating previous cellular
differentiation (Fig. 4Ad and Bd).
In the AdBMP9 group, the trabecular bone was more mature compared
with the AdGFP group, as indicated by areas stained red, suggesting
that BMP9 accelerated callus remodeling (Fig. 4Bb and f). Quantitative analysis of
the trabecular area percentage of the total area revealed an
increase in the AdBMP9 group compared with the AdGFP group
(P=0.001; Fig. 4C).
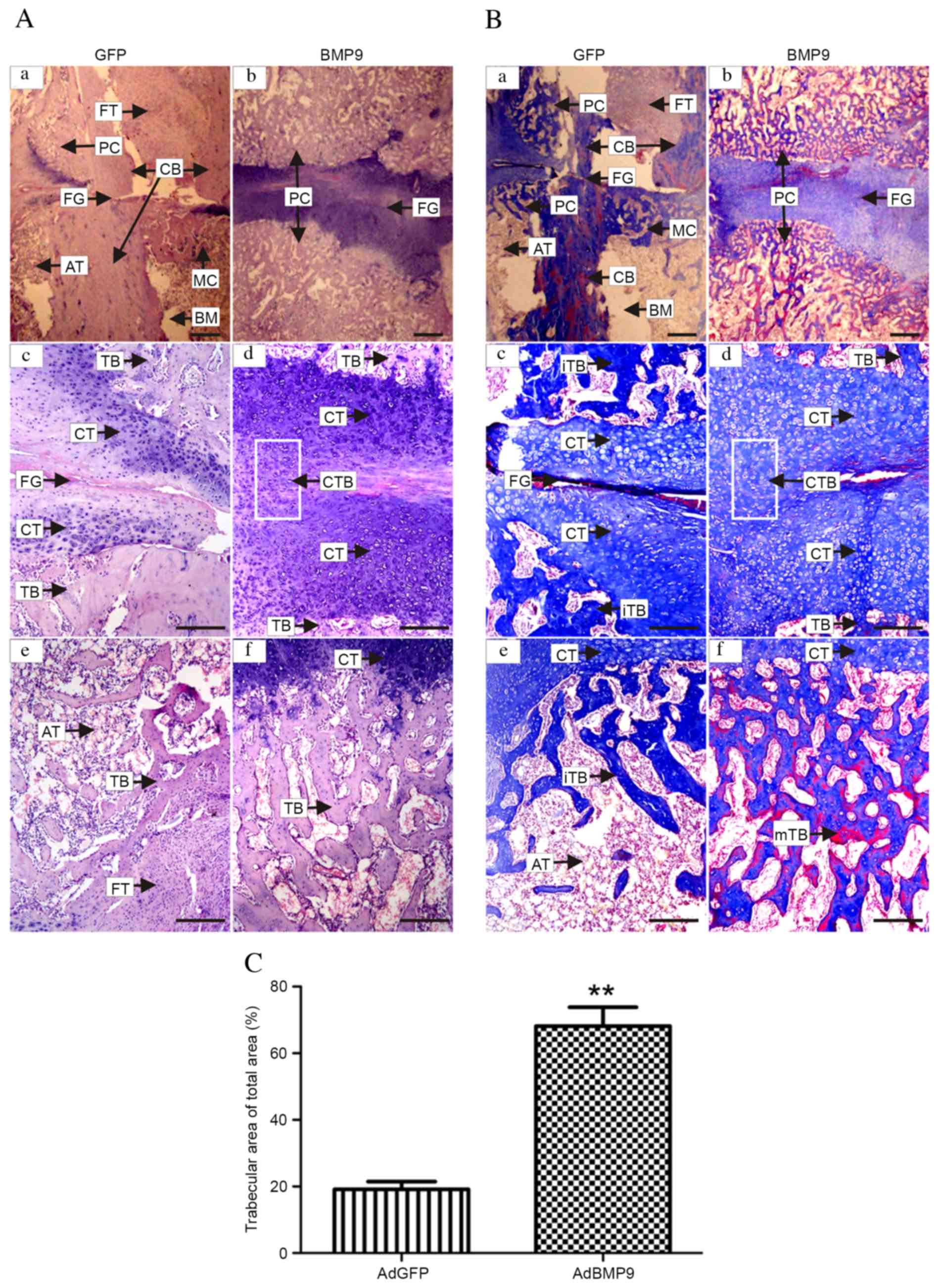 | Figure 4.Histological analysis of fracture
healing 4 weeks post-surgery. Longitudinal sections of the
fractured femora stained with (A) hematoxylin and eosin and (B)
Masson's trichrome. Representative images in (a-d) reveal the
fracture gap and in (e and f) the local trabecular bone at the
proximal end of fracture site. (a, c and e) Representative images
from the GFP group reveal the PC, FG, AT, CB, MC, BM, less CT,
fewer TB and iTB, and that the distribution of FT was prominent in
the nonunion bridge area. (b, d and f) The BMP9 group demonstrated
fusion of the CTB and a larger mTB around the fracture gap. (C)
Quantitative analysis of trabecular area percentage of the total
area. Data are expressed as the mean ± standard error. **P<0.01
vs. AdGFP control group. (a and b) Magnification, ×12.5; scale
bar=1000 µm. (c-f) Magnification, ×40; scale bar=250 µm. PC,
periosteal callus; FG, fracture gap; AT, adipose tissue; CB,
cortical bone; MC, marrow callus; BM, bone marrow; CT,
cartilaginous tissue; TB, trabecular bones, i, immature; m, mature;
FT, fibrous tissue; CTB, cartilaginous tissue bridge; GFP, green
fluorescent protein. |
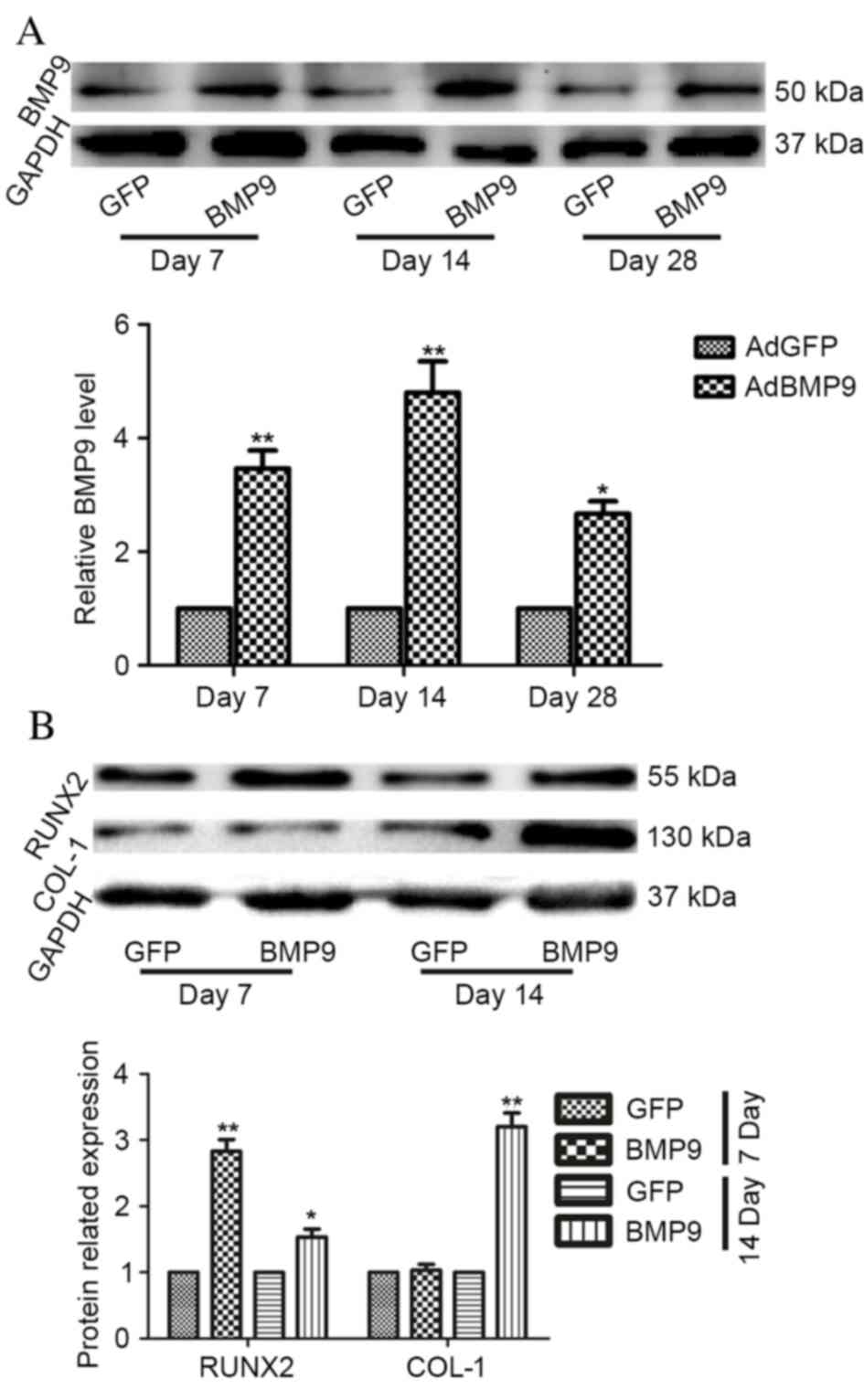
AdBMP9 induces callus formation by
promoting osteoblastic differentiation in vivo
Protein expression levels of BMP9 were detected in
the fracture callus at days 7, 14 and 28 post-surgery. BMP9
expression levels in the AdBMP9 group were significantly increased
compared with the AdGFP group (P=0.001). Relative BMP9 expression
levels in bone callus from the AdBMP9 group peaked at day 14
post-surgery (Fig. 5A). Although
BMP9 expression levels in the AdBMP9 group declined at day 28, they
remained significantly greater compared with the AdGFP group
(P=0.017).
Protein expression levels of RUNX2 and COL-1 were
examined by western blotting at days 7 and 14 post-surgery. RUNX2
expression levels in the AdBMP9 group were significantly increased
at the two time points compared with the AdGFP group (P=0.009 and
P=0.047, respectively), whereas protein expression levels of COL-1
were only markedly enhanced at day 14 (P=0.009; Fig. 5B).
Discussion
In the present study, radiographic, biomechanical
and histomorphometric analysis indicated that BMP9 in gelatin
sponges may mediate callus formation in osteoporotic rats. A total
of four weeks after implantation, BMP9 treatment significantly
increased the formation and microstructure of bone callus, and
improved healing, in rats with osteoporotic fractures, compared
with the AdGFP control group. Furthermore, BMP9 in gelatin sponges
successfully enhanced osteogenesis and upregulated the expression
levels of RUNX2 and COL-1. These results demonstrated that AdBMP9
locally applied via gelatin sponges may improve callus formation
and enhance bone-healing ability in osteoporotic rats.
MSCs are important osteogenic progenitors that are
regulated by osteogenic factors, and may be used for bone tissue
engineering. BMPs are a family of growth factors considered to
serve a pivotal role in bone formation. A previous study
demonstrated that exposure of MSCs to osteogenic BMPs resulted in
increased osteoblastic differentiation, as indicated by upregulated
expression levels of osteoblast-specific markers (25). BMP9, additionally known as growth
differentiation factor 2, is one of the most important bone-forming
BMPs; however, its precise role in the skeletal system remains to
be fully elucidated (4,26). A previous study demonstrated that
BMP9 may induce osteogenic differentiation (27). Additionally, a previous in
vivo study revealed that injection of athymic nude mice with
BMP9 or BMP9-transduced cells induced bone formation (4). Furthermore, BMP-9 has been reported
to successfully induce spinal fusion and repair of nonunion bone
fractures in rat models. In a study by Dumont et al
(8), percutaneous paraspinal
injection of AdBMP9-transduced MSCs resulted in significant ectopic
bone formation and successful spinal fusion. A study by
Kimelman-Bleich et al (28)
indicated that in vivo electroporation of a BMP9 plasmid, in
combination with recruitment of host progenitor cells, induced
fracture repair in mouse models of nonunion of the radius. However,
to the best of our knowledge, few studies have investigated the
osteogenic effects of AdBMP9 in a rat model of osteoporotic
fracture. A recent study by Shui et al (9) indicated that subcutaneous
implantation of BMP9-transduced preosteoblastic cells with a COL-1
sponge scaffold demonstrated robust and mature cancellous bone
masses, compared with a demineralized bone matrix carrier or direct
subcutaneous injection. Therefore, the present study investigated
healing in a rat osteoporotic fracture model, following the
implantation of gelatin sponges containing AdBMP9. A previous study
demonstrated that the process of fracture healing in rats is
similar to that in other mammals; at day 21, endochondral bone
formation is almost complete and bone remodeling begins (29). Scaffolds containing AdBMP7 have
been revealed to maintain a stable release of BMP7 for >21 days
in vitro, and BMP7 concentrations peaked after 14 days
(30). Therefore, protein
expression levels of BMP9 were investigated at various time points
in AdBMP9-transfected MSCs and in rats with osteoporotic fractures
implanted with sponges containing AdBMP9. The present study
demonstrated that protein expression levels of BMP9 were
significantly upregulated by AdBMP9, peaking at 14 days
post-implantation. In addition, the effects of AdBMP9 on the
microstructure, histology and biomechanics of bony callus were
evaluated 28 days post-implantation. AdBMP9 in gelatin sponges was
identified to increase callus formation and improve its mechanical
properties during the early stages of fracture healing.
Additionally, AdBMP9 was demonstrated to induce ectopic bone
formation, consistent with a previous study, which indicated that
BMP9 initiated the process of bone formation following percutaneous
injection into the thigh musculature (31).
BMP9 has been suggested to mediate osteogenesis via
signaling pathways unique from other BMPs. A previous study
reported that BMP9 activated the expression of osteogenic genes,
including RUNX2, and a master regulatory gene in MSC osteoblast
differentiation, COL-1 (32).
RUNX2 has been demonstrated to contribute to BMP9-induced ectopic
bone formation (33). The findings
of the present study revealed that overexpression of BMP9
upregulated RUNX2 and COL-1 expression levels in fractured bone
callus. These characteristics corresponded with the histological
appearance of trabecular bones in the AdBMP9 group. These results
indicated that endochondral and intramembranous bone formation were
promoted by BMP9 during early fracture healing.
However, the present study had various limitations.
Animals were only assessed at 4 weeks post-fracture, consistent
with our and others previous studies (9,30);
however, the fracture repair effect of BMP9 was demonstrated by the
present study. In future studies, the release of BMP9 by AdBMP9 and
fracture healing should be examined at additional time points.
Furthermore, the cortices were not clearly bridged with bone callus
in the two groups. Osteoporotic and unstable fracture factors may
be primary reasons for improper healing. Nevertheless, the callus
bridge gradually formed in the AdBMP9 group, as demonstrated by
histology. In the control group, the fracture line was visible and
fibrous tissue filled the fracture gap. Therefore, BMP9 may improve
the maturation and formation of bone and cartilage tissue, and
mediate callus formation and remodeling.
In conclusion, the results of the present study
demonstrated that after 4 weeks, AdBMP9 in gelatin sponges directly
mediated callus formation, and improved bone mass and strength in
osteoporotic rats with femora fractures. However, the efficacy and
safety of BMP9 administration in large animals and humans remains
unclear and requires further investigation. Despite the limitations
of the present study, the effects of BMP9 implicate it as a
potential novel therapeutic target for fracture healing.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272005 to Z.-L.D
and 31000434 to L.C.) and the Nature Science Foundation of
Chongqing (grant no. 2013jjB10021 to Z.-L.D.).
References
|
1
|
Johnell O and Kanis J: Epidemiology of
osteoporotic fractures. Osteoporos Int. 16:(Suppl 2). S3–S7. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song T, Wang W, Xu J, Zhao D, Dong Q, Li
L, Yang X, Duan X, Liang Y, Xiao Y, et al: Fibroblast growth factor
2 inhibits bone morphogenetic protein 9-induced osteogenic
differentiation of mesenchymal stem cells by repressing Smads
signaling and subsequently reducing Smads dependent up-regulation
of ALK1 and ALK2. Int J Biochem Cell Biol. 45:1639–1646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamplot JD, Qin J, Nan G, Wang J, Liu X,
Yin L, Tomal J, Li R, Shui W, Zhang H, et al: BMP9 signaling in
stem cell differentiation and osteogenesis. Am J Stem Cells.
2:1–21. 2013.PubMed/NCBI
|
|
4
|
Kang Q, Sun MH, Cheng H, Peng Y, Montag
AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, et al:
Characterization of the distinct orthotopic bone-forming activity
of 14 BMPs using recombinant adenovirus-mediated gene delivery.
Gene Ther. 11:1312–1320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu N, Jiang D, Huang E, Liu X, Li R, Liang
X, Kim SH, Chen X, Gao JL, Zhang H, et al: BMP9-regulated
angiogenic signaling plays an important role in the osteogenic
differentiation of mesenchymal progenitor cells. J Cell Sci.
126:532–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zhang H, Zhang W, Huang E, Wang N,
Wu N, Wen S, Chen X, Liao Z, Deng F, et al: Bone morphogenetic
protein-9 effectively induces osteo/odontoblastic differentiation
of the reversibly immortalized stem cells of dental apical papilla.
Stem Cells Dev. 23:1405–1416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Helm GA, Alden TD, Beres EJ, Hudson SB,
Das S, Engh JA, Pittman DD, Kerns KM and Kallmes DF: Use of bone
morphogenetic protein-9 gene therapy to induce spinal arthrodesis
in the rodent. J Neurosurg. 92:(Suppl 2). S191–S196. 2000.
|
|
8
|
Dumont RJ, Dayoub H, Li JZ, Dumont AS,
Kallmes DF, Hankins GR and Helm GA: Ex vivo bone morphogenetic
protein-9 gene therapy using human mesenchymal stem cells induces
spinal fusion in rodents. Neurosurgery. 51:1239–1245. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shui W, Zhang W, Yin L, Nan G, Liao Z,
Zhang H, Wang N, Wu N, Chen X, Wen S, et al: Characterization of
scaffold carriers for BMP9-transduced osteoblastic progenitor cells
in bone regeneration. J Biomed Mater Res A. 102:3429–3438. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuroda S, Sumner DR and Virdi AS: Effects
of TGF-β1 and VEGF-A transgenes on the osteogenic potential of bone
marrow stromal cells in vitro and in vivo. J Tissue Eng.
3:20417314124597452012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC and Kinzler KW: A protocol for
rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sosa I, Cvijanovic O, Celic T, Cuculic D,
Crncevic-Orlic Z, Vukelic L, Cvek Zoricic S, Dudaric L, Bosnar A
and Bobinac D: Hepatoregenerative role of bone morphogenetic
protein-9. Med Sci Monit. 17:HY33–HY35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang N, Song WX, Luo J, Luo X, Chen J,
Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, et al: BMP-9-induced
osteogenic differentiation of mesenchymal progenitors requires
functional canonical Wnt/beta-catenin signalling. J Cell Mol Med.
13:2448–2464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo J, Tang M, Huang J, He BC, Gao JL,
Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, et al: TGFbeta/BMP type I
receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic
signaling in mesenchymal stem cells. J Biol Chem. 285:29588–29598.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Jiang W, Huang J, He BC, Zuo GW,
Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, et al: Insulin-like
growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic
differentiation and bone formation. J Bone Miner Res. 25:2447–2459.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li RD, Deng ZL, Hu N, Liang X, Liu B, Luo
J, Chen L, Yin L, Luo X, Shui W, et al: Biphasic effects of TGFβ1
on BMP9-induced osteogenic differentiation of mesenchymal stem
cells. BMB Rep. 45:509–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Yuan SX, Wang DX, Wu QX, Wang X,
Pi CJ, Zou X, Chen L, Ying LJ, Wu K, et al: The role of COX-2 in
mediating the effect of PTEN on BMP9 induced osteogenic
differentiation in mouse embryonic fibroblasts. Biomaterials.
35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen J, Leslie WD, Nielson CM, Majumdar
SR, Morin SN and Orwoll ES: Associations of body mass index with
incident fractures and hip structural parameters in a large
canadian cohort. J Clin Endocrinol Metab. 101:476–484. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu SF and Du XJ: Body mass index may
positively correlate with bone mineral density of lumbar vertebra
and femoral neck in postmenopausal females. Med Sci Monit.
22:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dostal AM, Arikawa A, Espejo L and Kurzer
MS: Long-term supplementation of green tea extract does not modify
adiposity or bone mineral density in a randomized trial of
overweight and obese postmenopausal women. J Nutr. 146:256–264.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng Y, Wang C, Zhang H, Shao C, Gao LH,
Li SS, Yu WJ, He JW, Fu WZ, Hu YQ, et al: Polymorphisms in Wnt
signaling pathway genes are associated with peak bone mineral
density, lean mass, and fat mass in Chinese male nuclear families.
Osteoporos Int. 27:1805–1815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poiana C, Carsote M, Radoi V, Mihai A and
Capatina C: Prevalent osteoporotic fractures in 622 obese and
non-obese menopausal women. J Med Life. 8:462–466. 2015.PubMed/NCBI
|
|
23
|
Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng
G and Hu J: The effects of combined human parathyroid hormone
(1–34) and zoledronic acid treatment on fracture healing in
osteoporotic rats. Osteoporos Int. 23:1463–1474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao X, Wu ZX, Zhang Y, Gao MX, Yan YB,
Cao PC, Zang Y and Lei W: Locally administrated perindopril
improves healing in an ovariectomized rat tibial osteotomy model.
PLoS One. 7:e332282012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luu HH, Song WX, Luo X, Manning D, Luo J,
Deng ZL, Sharff KA, Montag AG, Haydon RC and He TC: Distinct roles
of bone morphogenetic proteins in osteogenic differentiation of
mesenchymal stem cells. J Orthop Res. 25:665–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng H, Jiang W, Phillips FM, Haydon RC,
Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, et al:
Osteogenic activity of the fourteen types of human bone
morphogenetic proteins (BMPs). J Bone Joint Surg Am.
85-A:1544–1552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luther G, Wagner ER, Zhu G, Kang Q, Luo Q,
Lamplot J, Bi Y, Luo X, Luo J, Teven C, et al: BMP-9 induced
osteogenic differentiation of mesenchymal stem cells: Molecular
mechanism and therapeutic potential. Curr Gene Ther. 11:229–240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimelman-Bleich N, Pelled G, Zilberman Y,
Kallai I, Mizrahi O, Tawackoli W, Gazit Z and Gazit D: Targeted
gene-and-host progenitor cell therapy for nonunion bone fracture
repair. Mol Ther. 19:53–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmidmaier G, Wildemann B, Melis B,
Krummrey G, Einhorn TA, Haas NP and Raschke M: Development and
characterization of a standard closed tibial fracture model in the
rat. Eur J Trauma. 30:35–42. 2004. View Article : Google Scholar
|
|
30
|
Zhang Y, Wu C, Luo T, Li S, Cheng X and
Miron RJ: Synthesis and inflammatory response of a novel silk
fibroin scaffold containing BMP7 adenovirus for bone regeneration.
Bone. 51:704–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li JZ, Hankins GR, Kao C, Li H, Kammauff J
and Helm GA: Osteogenesis in rats induced by a novel recombinant
helper-dependent bone morphogenetic protein-9 (BMP-9) adenovirus. J
Gene Med. 5:748–756. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bergeron E, Senta H, Mailloux A, Park H,
Lord E and Faucheux N: Murine preosteoblast differentiation induced
by a peptide derived from bone morphogenetic proteins-9. Tissue Eng
Part A. 15:3341–3349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo
X, Chen J, Bi Y, He BC, Park JK, et al: A comprehensive analysis of
the dual roles of BMPs in regulating adipogenic and osteogenic
differentiation of mesenchymal progenitor cells. Stem Cells Dev.
18:545–559. 2008. View Article : Google Scholar :
|