Introduction
Hypoxic-ischemic brain damage (HIBD) is a primary
cause of neonatal death. Asphyxia-induced hypoxia is the
pathological basis of damage to various organs. Myocardial tissue
is highly aerobic, and the accumulation of acidic metabolites
generated by anaerobic glycolysis during asphyxia causes myocardial
energy dysmetabolism and adenosine triphosphate (ATP) reduction,
resulting in myocardial damage. The greater the degree of asphyxia,
the greater the involvement of myocardial cells; thus, the more
severe the myocardial damage (1).
A previous study demonstrated that, during the process of
ischemia-hypoxia, apoptosis and necrosis of myocardial cells
occurs, with an increased number of apoptotic compared with
necrotic cells in the early stage of ischemia-hypoxia (2).
In hypoxia-ischemia, cells of all tissues exhibit
intracellular calcium (Ca2+) overload, thus activating
numerous Ca2+-dependent proteases. Calpain-1 is a
primary protease of the Ca2+-dependent cysteine protease
family, and is closely associated with cytoskeleton remodeling,
apoptosis and necrosis (1–3). Caspase-3 is an important apoptosis
mediator of the caspase family; activated caspase-3 cleaves and
degrades downstream substrates, thus serving a key role in
apoptosis. To date, investigations into myocardial cell apoptosis
caused by HIBD have been limited. The present study established an
HIBD model of 7-day-old neonatal rats, and observed alterations in
mRNA and protein expression of calpain-1 and mRNA expression of
caspase-3 in the myocardial cells of HIBD rats. The aim was to
analyze the associations between calpain-1, caspase-3 and apoptosis
of myocardial cells in HIBD rats, and to investigate the roles of
calpain-1 and caspase-3 in the apoptosis of myocardial cells.
Materials and methods
Animal model
A total of 64 newborn healthy male and female Wistar
rats (weight, 12–18 g; age, 7 days) were provided by the Ethics
Committee, Laboratory Animal Center, Institute of Radiation
Medicine, Chinese Academy of Medical Sciences (Beijing, China).
Rats were housed at 21±2°C and a relative humidity of 30–70% in a
12-h light/dark cycle with free access to food and water. Mean body
masses in the experimental groups were not statistically different
(P>0.05). The modified Rice-Vannucci method (4) was used to construct the HIBD model in
neonatal rats. Rats were randomly divided into control and HIBD
groups. The HIBD group was additionally divided into seven
subgroups of 8 rats each. Rats were anesthetized with ether
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), and a median
neck incision was performed. In HIBD groups, the left common
carotid artery was separated, followed by dual-ligation. The blood
vessels were cut between the ligatures. Following surgery, the rats
were placed into an anoxic tank supplied with constant 8%
O2 + 92% N2 for 2 h (gas flow 1 l/min). When
the rats had recovered, they were returned to the maternal rats for
continuous feeding in a consistent environment. A total of eight
rats per HIBD group were anaesthetized with 2 ml diethyl ether
(Sigma-Aldrich; Merck Millipore) and sacrificed by decapitation at
the following time points: 2, 12 or 24 h, or 2, 3, 5 or 7 days. The
heart was removed quickly and cut into two parts, for storing in
liquid nitrogen, or fixing in 10% neutral formalin (Sigma-Aldrich;
Merck Millipore) and embedding in paraffin (Sigma-Aldrich; Merck
Millipore) for preparation of sections. In the control group, the
common carotid artery was not ligated following separation, and the
rats were sacrificed after 2 h.
Detection of myocardial cell apoptosis
by terminal deoxynucleotidyl transferase dUTP nick-end labeling
(TUNEL) assay
The paraffin sections of heart tissue were dewaxed
in xylene (Sigma-Aldrich; Merck Millipore), hydrated with gradient
ethanol, and subsequently washed in PBS (pH 7.4; Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China) for 15 min. Following fixation
with blocking solution (3% H2O2 dissolved in
methanol; Fuzhou Maixin Biotech Co., Ltd.) for 30 min, the sections
were rinsed with PBS for 15 min and soaked in 0.5% Triton X-100 in
PBS (Sangon Biotech Co., Ltd., Shanghai, China) for 5 min, followed
by rinsing with PBS for 5 min. TUNEL reaction mixture was added,
followed by incubation at 37°C for 1 h. Hoescht stain solution
(1:1,000; Sigma-Aldrich; Merck Millipore) was added, followed by
incubation at room temperature for 10 min. Following three washes
with PBS for 5 min, the cells were observed using a BX61
fluorescent microscope (Olympus Corporation, Tokyo, Japan).
Apoptosis-positive nuclei appeared green and apoptosis-negative
cells appeared blue. The terminal deoxynucleotidyl transferase-free
TUNEL mixture served as a negative control. A total of 10 fields of
vision were randomly selected from each section. The number of
positive cells per 500–1,000 cells was counted under a high
magnification, and the percentage of positive cells was calculated
as the apoptosis index (AI).
Detection of calpain-1 and caspase-3
mRNA expression levels by reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from myocardial tissue using
TRIzol® (Sangon Biotech Co., Ltd.) according to the
manufacturer's protocol, and RT was performed to obtain cDNA for
PCR amplification. The RT and PCR kits were purchased from Sangon
Biotech Co., Ltd. The RT reaction system was as follows: 4 µl
PrimeScript Buffer (5X), 1 µl PrimeScript RT Enzyme Mix I, 1 µl 50
µmol/l Oligo Dt Primer, 1 µl 100 µmol/l Random 6 mers, and 13 µl
total RNA. A Premix Ex Taq PCR system was used. The primers were
designed and synthesized by Sangon Biotech Co., Ltd. β-actin served
as an internal control. The primer sequences were as follows:
Forward, 5′-GGAGATTACTGCCCTGGCTCCTA and reverse,
5′-GACTCATCGTACTCCTGCTTGCTG for β-actin (amplified fragment, 150
bp); forward, 5′-GGGGTGAAGTGGAGTGGAAAG and reverse,
5′-TTAAGGGCGTCAGGTGTAAGG for calpain-1 (amplified fragment, 184
bp); forward, 5′-GAGACAGACAGTGGAACTGACGATG and reverse,
5′-CACGGATCTGTTTCTTTGC for caspase-3 (amplified fragment, 147 bp).
The parameters of the PCR reaction are presented in Table I. Agarose gel electrophoresis (2%;
Sangon Biotech Co., Ltd.) was performed on PCR products, which
generated bands with 213, 298 and 749 bp, respectively. GelDoc 2000
gel imaging system and Quantity One software version 4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) were used to detect the band
optical density (OD) ratio of the target fragment to β-actin
fragment for semi-quantitative analysis.
 | Table I.Polymerase chain reaction
conditions. |
Table I.
Polymerase chain reaction
conditions.
| Thermocycling
step | Calpain-1 | Caspase-3 | β-actin |
|---|
| Pre-denaturation | 94°C ×5 min | 94°C ×12 min | 94°C ×5 min |
| Denaturation | 94°C ×30 sec | 94°C ×30 sec | 94°C ×30 sec |
| Annealing | 54°C ×30 sec | 54°C ×30 sec | 54°C ×30 sec |
| Extension | 72°C ×30 sec | 72°C ×30 sec | 72°C ×30 sec |
| No. of cycles | 35 | 35 | 27 |
| Re-extension | 72°C ×5 min | 72°C ×8 min | 72°C ×8 min |
Detection of calpain-1 protein
expression levels by western blot analysis
The frozen myocardial tissue was ground in liquid
nitrogen (Fuzhou Maixin Biotechnology Development Co., Ltd.) and
cell lysis buffer (Fuzhou Maixin Biotechnology Development Co.,
Ltd.) was added to prepare cell lysates. Following centrifugation
at 4°C at a speed of 256 × g for 10 min, the supernatant was
collected for further experiments. The same quantity of protein was
collected per group and denatured for 10 min. Proteins (5 µg)
underwent 10% SDS-PAGE (Sangon Biotech Co., Ltd.), following which
separated proteins were transferred onto polyvinylidene difluoride
(PVDF) membranes (Sigma-Aldrich; Merck Millipore) and blocked with
5% bovine serum albumin solution (Sangon Biotech Co., Ltd.) at 4°C
overnight. Membranes were incubated with a rabbit anti-rat
calpain-1 polyclonal antibody (1:200; catalog no. 3189-100;
BioVision, Inc., Milpitas, CA, USA) at 37°C for 2 h. Following
washing with PBS, the membranes were incubated with a horseradish
peroxidase-labeled goat anti-rabbit IgG secondary antibody (1:200;
catalog no. 6905-250; BioVision, Inc.) at 37°C for 1 h. An Enhanced
Chemiluminescence substrate (Fuzhou Maixin Biotechnology
Development Co., Ltd.) was added and proteins were imaged using a
Gel Imaging system. Proteins with a molecular weight of 76 and 80
kDa were visualized on the PVDF membrane. Quantity One software
version 4.1.0 (Bio-Rad Laboratories, Inc.) was used to analyze the
OD of protein bands. The OD value of the active segment of calpain
was normalized against the value of the total protein to calculate
the ratio of the two proteins (76:80 kDa). The greater the ratio,
the greater the degree of positive reaction and target protein
activity, whereas a small ratio represented a weaker interaction
with the target protein.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA) was used
for the analysis. A one-way analysis of variance A one-way analysis
of variance followed by Tukey's post hoc test was used for multiple
comparisons between groups, and Dixon's Q-test was used for paired
comparisons. Pearson's correlation coefficient was performed among
different indicators. P<0.05 was considered to indicate a
statistically significant difference.
Results
Myocardial cell apoptosis
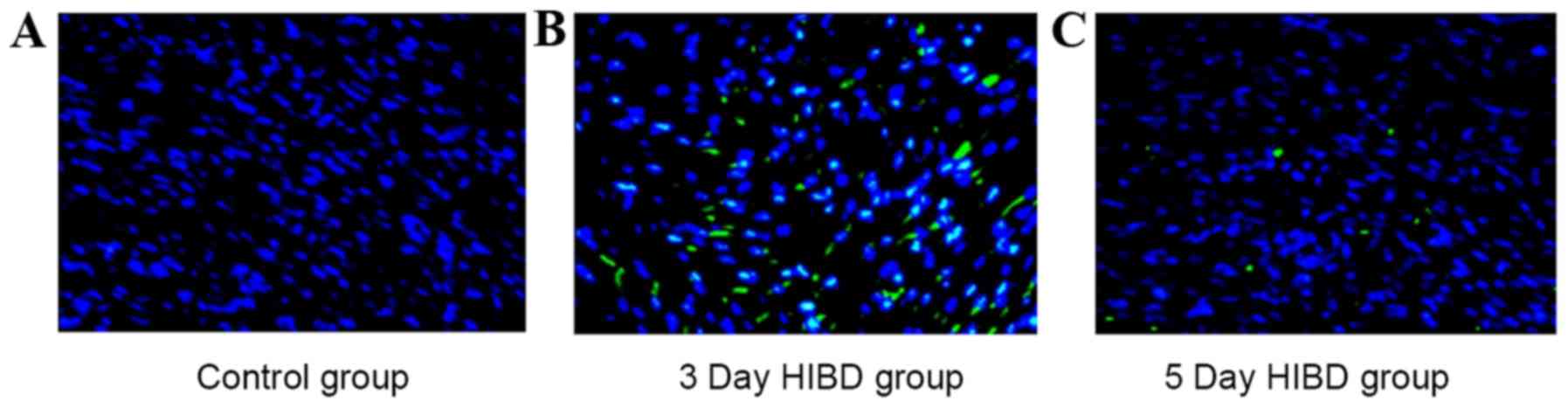
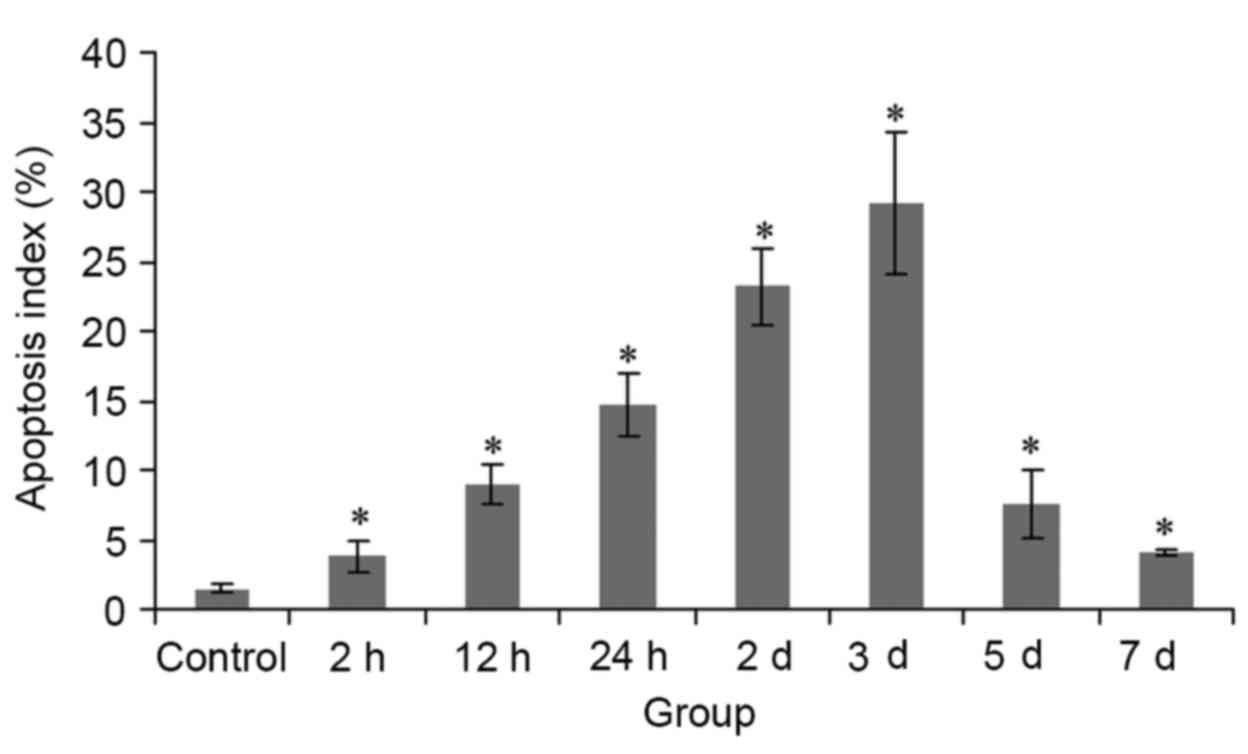
As indicated by green-labeled apoptosis-positive
myocardial cells, apoptosis was low in the control group (Fig. 1A), whereas the 3 (Fig. 1B) and 5 day (Fig. 1C) HIBD groups exhibited increased
levels, particularly in the 3 day HIBD group. Compared with the
control group, AI in HIBD groups was significantly increased
(P<0.05), with the greatest score in the 3 day HIBD group.
Compared with the control group, AI in all HIBD groups was
significantly increased, between 2.5- (2 h group) and 19.1- (3 day
group) fold (Fig. 2).
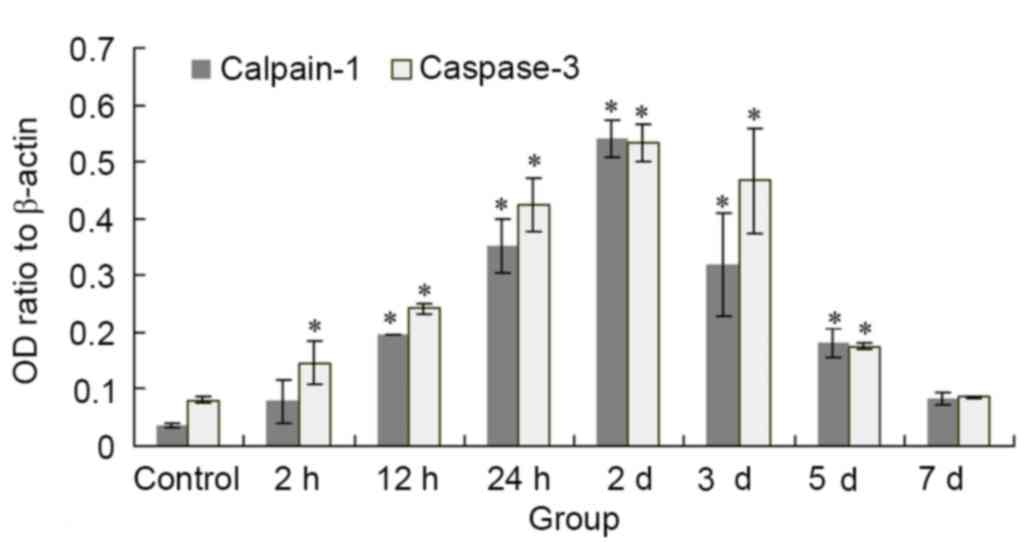
Alterations of calpain-1 and caspase-3
mRNA and protein expression levels in myocardial tissue
Compared with the control group, mRNA expression
levels of calpain-1 were significantly increased in the 12 and 24
h, and 2, 3 and 5 day HIBD groups (P<0.05), with the greatest
expression levels in the 2 day group. Compared with the control
group, the mRNA expression levels of caspase-3 were significantly
increased in all HIBD groups (P<0.05), with the exception of the
7 day group, with the greatest expression levels in the 2 day
group. Calpain-1 and caspase-3 mRNA expression levels were
decreased in the 3 and 5 day groups compared with the 2 day group;
however, levels remained greater than those of the control group
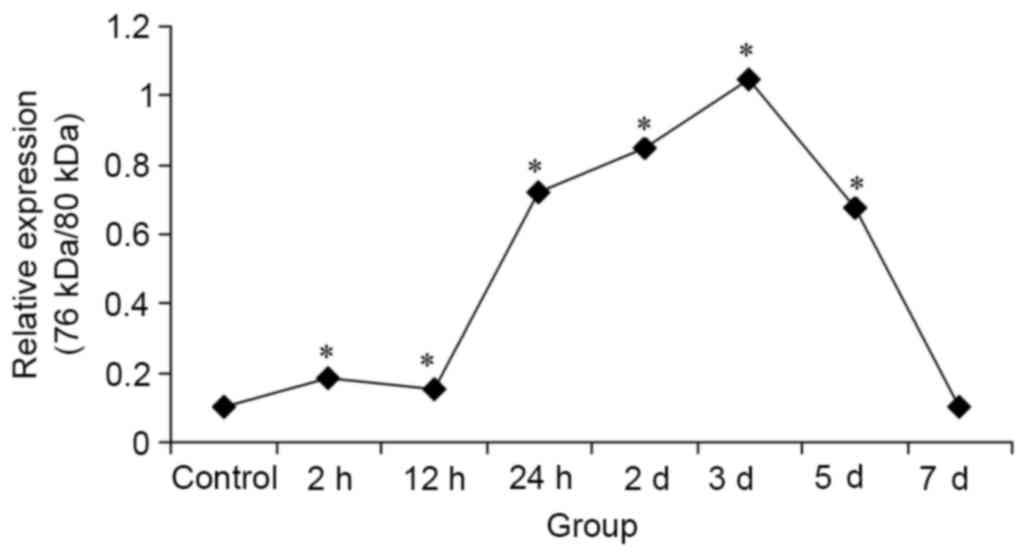
(P<0.05; Fig. 3). Protein
expression levels of calpain-1 (76/80 kDa) in the HIBD groups were
significantly increased compared with the control group, with the
exception of the 7 day group, and peaked at 3 days (P<0.05;
Fig. 4).
Correlations between myocardial cell
AI and calpain-1 and caspase-3 expression
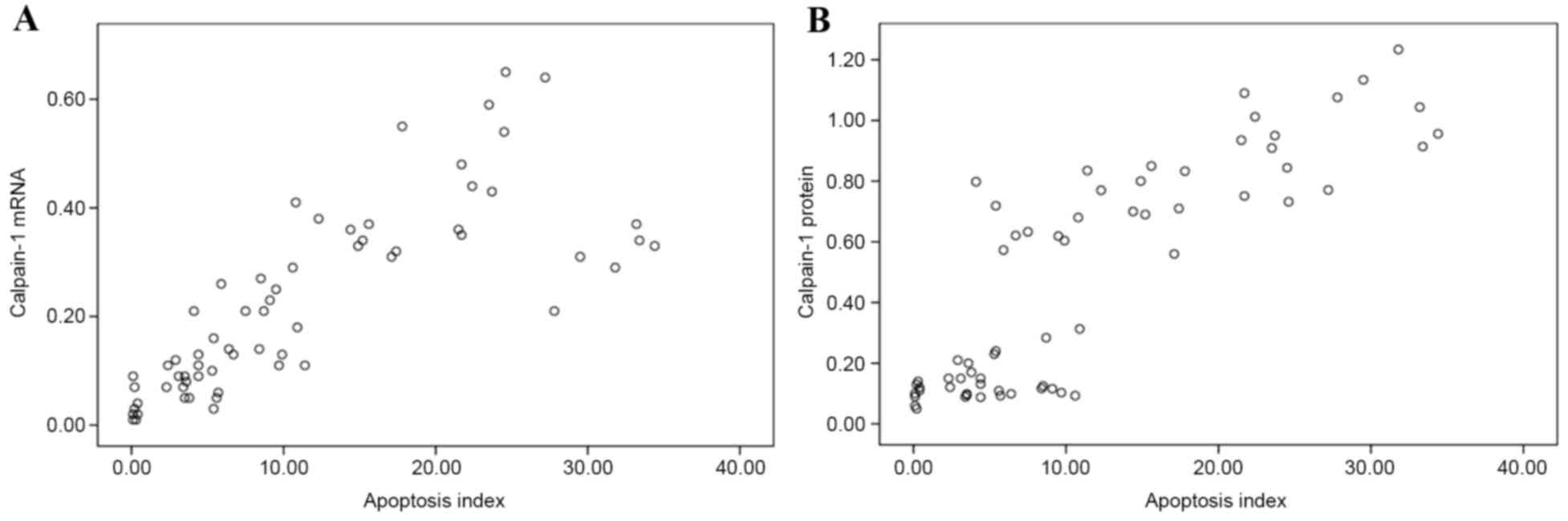
Pearson's correlation analysis revealed that
calpain-1 mRNA (Fig. 5A) and
protein (76/80 kDa; Fig. 5B)
expression levels had positive linear correlations with AI
(r=0.786, P=0.001; and r=0.853, P=0.001, respectively). This
indicated that calpain-1 may be involved in hypoxic-ischemic
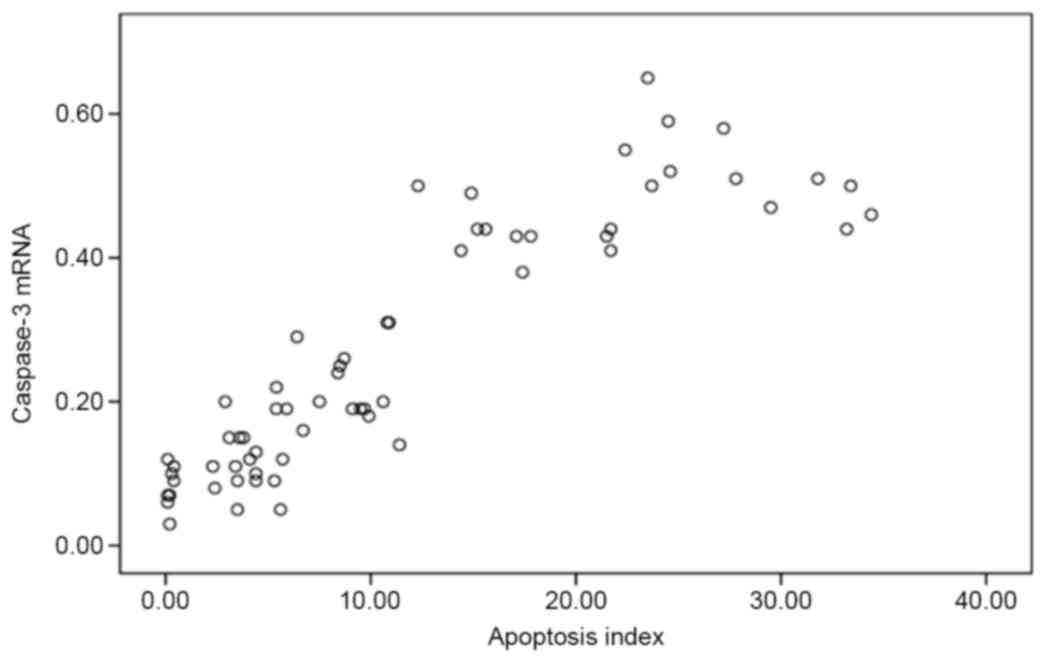
myocardial damage. In addition, the caspase-3 mRNA expression
levels were positively correlated with AI (r=0.894; P=0.001;
Fig. 6), suggesting that the
mitochondrial-dependent caspase-3-activated signaling pathway
served an important role in hypoxic-ischemic myocardial cell
apoptosis.
Discussion
The calpain protease family is widely distributed in
the majority of mammalian tissues, taking the form of zymogens in
their resting state, and is primarily activated by Ca2+
in vivo. Once activated, calpains may hydrolyze a large
number of intracellular signaling and structural proteins, involved
in pathophysiological processes including cell movement and
apoptosis, and cell cycle and gene regulation (1). Calpain-1 primarily exists in the
Z-line of sarcomere of myocardial cells, and co-localizes with its
endogenous inhibitor calpastatin. Under physiological conditions,
intracellular Ca2+ concentrations are maintained at low
levels. When stimulated by hypoxia and ischemia, cells instantly
increase partial or entire intracellular Ca2+
concentrations via a range of underlying mechanisms, thus
overloading intracellular Ca2+. When intracellular
Ca2+ concentration is increased, the large subunit of
calpain-1 (80 kDa), which has catalytic activity, is hydrolyzed to
76 kDa, and the zymogen is activated. A previous study demonstrated
that activation of calpain-1 is associated with increasing
intracellular free Ca2+ concentrations (5). Excessive activation is involved in
the occurrence of ischemic hypoxic cardiac tissue damage (3,6,7). The
primary roles of calpain in apoptosis involve regulation of the
apoptotic pathways. A previous study on HL-1 cardiomyocytes
revealed that when Ca2+ is overloaded, the mitochondrial
membrane potential is reduced and calpain activity is upregulated
(8). Calpain has been demonstrated
to localize inside mitochondria, suggesting that it may cause
abnormalities in mitochondrial function (8). During the differentiation of
myocardial cells, the death receptor-dependent extrinsic apoptotic
signaling pathway is inhibited; therefore, mitochondria serve
important roles in the apoptosis of myocardial cells.
Apoptosis serves an important role in maintaining
the physiological homeostasis of the body, and growth and
development. Additionally, it is involved in the pathogenesis of
numerous diseases. A previous study demonstrated that significant
apoptosis of myocardial cells in neonatal rats occurs following
HIBD occurrence, and that this abnormal apoptosis remains 7 days
later (9). The present study
selected 7-day-old Wistar rats to establish an experimental animal
model; this avoids shortcomings including single influencing
factors in in vitro studies and contamination of cells, and
reflects the impact of systemic nerves and body fluids on
myocardial tissues following HIBD. The results demonstrated that
following HIBD, apoptosis of myocardial cells significantly
increased, with the greatest rates at 3 days, and remained
significantly increased compared with the control group at 7 days.
Furthermore, the present study revealed that following HIBD, the
expression levels of myocardial calpain-1 and caspase 3 were
significantly increased compared with the control group, and were
significantly positively correlated with AI. This suggested that
hypoxia and ischemia are associated and may induce apoptosis of
myocardial cells and upregulate calpain-1 and caspase-3 expression
levels. Therefore, calpain-1 and caspase-3 may co-mediate apoptosis
in myocardial cells.
Calpain was previously considered to be closely
associated with cell death; however, studies have demonstrated that
it may be involved in apoptosis (10,11).
Calpain is hypothesized to induce apoptosis in cells under specific
stimuli; however, its exact role remains unknown. Calpain-1 may
regulate apoptosis of myocardial cells. Ischemia and hypoxia may
stimulate the release of intracellular Ca2+ from the
endoplasmic reticulum and mitochondria, inducing intracellular
Ca2+ overload and triggering a series of damaging
reactions, known as the ‘final common pathway’ leading to the death
of myocardial cells. Activated calpain has been demonstrated to act
on cytoskeletal and membrane proteins, calspectins, and ATP
enzymes, altering their structure and function and inducing cell
death (12). Caspase-3 is an
important protease during the implementation phase of apoptosis. It
hydrolyzes calpastatin, the endogenous inhibitor of calpain, and
impedes the inhibitory effects of calpastatin towards calpain,
thereby increasing calpain activity, forming a positive feedback
loop and further promoting apoptosis (13,14).
Gafni et al (15)
demonstrated that calpain hydrolyzes caspase-7, −10 and −12,
further promoting caspase-induced apoptosis. It is hypothesized
that the calpain and caspase families co-mediate apoptosis.
Previous studies have demonstrated that calpain may belong to the
apoptosis-associated B-cell lymphoma 2 family, and activate
additional pro-apoptotic substances including cyclin-dependent
kinase 5, apoptotic-protease-activating factor 1, c-Jun N-terminal
kinase, transcription factor AP-1 and fos proto-oncogene, AP-1
transcription factor subunit via the hydrolysis signaling pathway,
thus mediating apoptosis (16,17).
Guo et al (18) revealed
that hypoxia induces apoptosis of cardiomyocytes and causes
mitochondria to release cytochrome C into the cytoplasm,
upregulating expression levels of upstream caspase-9 and its
downstream effector caspase-3. Therefore, activation of the
mitochondria-dependent caspase-3 signaling pathway serves an
important role in hypoxia-induced apoptosis of myocardial cells. In
addition, when myocardial cell apoptosis occurs, increased content
and activity of calpain-1 inside nuclei further promotes apoptosis
(19). Increased activity and
expression levels of caspase-3 may directly or indirectly inhibit
calpastatin expression levels and activity, thus activating and
upregulating calpain-1. Once activated, calpain damages the
lysosomal membrane and releases cathepsin, which in turn activates
caspase-3, thus forming a pro-apoptotic feedback mechanism.
Overactivation of calpain-1 maintains and aggravates pathological
conditions; however, it interacts with exogenous and endogenous
apoptotic signal transduction pathways, resulting in apoptosis of
myocardial cells (20). Calpain-1,
calpastatin and caspase-3 are closely associated; however, their
specific roles in the apoptosis of myocardial cells require further
investigation.
In conclusion, the present study demonstrated that
in a rat model of HIBD, myocardial cell apoptosis increased, which
positively correlated with increased expression levels of calpain-1
and caspase-3. The role of calpain-1 has received increasing
attention; however, its exact underlying molecular mechanisms in
the apoptosis of myocardial cells remain to be elucidated. Further
investigation is required into apoptosis genes and the underlying
mechanisms, using gene transfection and drug studies, to provide
novel strategies for the prevention and treatment of HIBD.
References
|
1
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qian W, Xiong X, Fang Z, Lu H and Wang Z:
Protective effect of tetramethylpyrazine on myocardial
ischemia-reperfusion injury. Evid Based Complement Alternat Med.
2014:1075012014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen X, Zhang X, Kubo H, Harris DM, Mills
GD, Moyer J, Berretta R, Potts ST, Marsh JD and Houser SR:
Ca2+ influx-induced sarcoplasmic reticulum
Ca2+ overload causes mitochondrial-dependent apoptosis
in ventricular myocytes. Circ Res. 97:1009–1017. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li D, Li X, Wu J, Li J, Zhang L, Xiong T,
Tang J, Qu Y and Mu D: Involvement of the JNK/FOXO3a/Bim pathway in
neuronal apoptosis after hypoxic-ischemic brain damage in neonatal
rats. PLoS One. 10:e01329982015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spencer MJ and Tidball JG: Calpain
concentration is elevated although net calcium-dependent
proteolysis is suppressed in dystrophin-deficient muscle. Exp Cell
Res. 203:107–114. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zucchi R, Ronca F and Ronca-Testoni S:
Modulation of sarcoplamic reticulum function: A new strategy in
cardioprotection? Pharmacol Ther. 89:47–65. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bano D, Munarriz E, Chen HL, Ziviani E,
Lippi G, Young KW and Nicotera P: The plasma membrane
Na+/Ca2+exchanger is cleaved by distinct
protease families in neuronal cell death. Ann N Y Acad Sci.
1099:451–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carpi A, Venerando R, Miotto G, Bertaggia
D and Di Lisa F: Calpain and mitochondrial dysfunction in
Ca2+ overloaded cardiomyocytes. J Cell Cardiol.
42:S1052007. View Article : Google Scholar
|
|
9
|
Doycheva D, Shih G, Chen H, Applegate R,
Zhang JH and Tang J: Granulocyte-colony stimulating factor in
combination with stem cell factor confers greater neuroprotection
after hypoxic-ischemic brain damage in the neonatal rats than a
solitary treatment. Transl Stroke Res. 4:171–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu H, Li X, Li Y, Wang L, Mehta S, Feng Q,
Chen R and Peng T: Calpain-l induces apoptosis in pulmonary
microvascular endothelial cells under septic conditions. Microvasc
Res. 78:33–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blomgreni K, Leist M and Groc L:
Pathological apoptosis in the developing brain. Apoptosis.
12:993–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakamoto YR, Nakajima TR, Fukiage CR,
Sakai OR, Yoshida YR, Azuma MR and Shearer TR: Involvement of
calpain isoforms in ischemia-reperfusion injury in rat retina. Curr
Eye Res. 21:571–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang KK, Posmantur R, Nadimpalli R, Nath
R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L and Allen H:
Caspase-mediated fragmentation of calpain inhibitor protein
calpastatin during apoptosis. Arch Biochem Biophys. 356:187–196.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kar P, Samanta K, Shaikh S, Chowdhury A,
Chakraborti T and Chakraborti S: Mitochondrial calpain system: An
overview. Arch Biochem Biophys. 495:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gafni J, Cong X, Chen SF, Gibson BW and
Ellerby LM: Calpain-1 cleaves and activates caspase-7. J Biol Chem.
284:25441–25449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan Y, Dourdin N, Wu C, De Veyra T, Elce
JS and Greer PA: Ubiquitous calpains promote caspase-12 and JNK
activation during endoplasmic reticulum stress-induced apoptosis. J
Biol Chem. 281:16016–16024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin L, Ye Y and Zakeri Z: p53, Apaf-1,
caspase-3, and −9 are dispensable for Cdk5 activation during cell
death. Cell Death Differ. 13:141–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo Z, Liao Z, Huang L, Liu D, Yin D and
He M: Kaempferol protects cardiomyocytes against
anoxia/reoxygenation injury via mitochondrial pathway mediated by
SIRT1. Eur J Pharmacol. 761:245–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng D, Wang G, Li S, Fan GC and Peng T:
Calpain-1 induces endoplasmic reticulum stress in promoting
cardiomyocyte apoptosis following hypoxia/reoxygenation. Biochim
Biophys Acta. 1852:882–892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith MA and Schnellmann RG: Calpains,
mitochondria, and apoptosis. Cardiovasc Res. 96:32–37. 2012.
View Article : Google Scholar : PubMed/NCBI
|