Introduction
Arterial stiffness is a vascular biomarker and an
intermediate endpoint for cardiovascular disease (1). Similarly, pollution is an emerging
cause of cardiovascular disease and mortality (2,3). We
have recently reported in patients with inflammatory bowel disease,
a model of a concomitant chronic inflammation and low burden of
traditional cardiovascular risk factors (4), that arterial stiffness and reflected
waves are increased (5–7), dependent upon inflammation and
reduced by immunomodulatory drugs (8) but not by salicylates (9). Despite some methodology issues
(10), these results were
confirmed by an independent group (11). Several possible pathways exist that
could link inflammation and arterial stiffening (12). Because pollution is associated with
elevated levels of serum biomarkers of inflammation (13,14)
and endothelial dysfunction (15–18),
a condition associated with functional arterial stiffening
(12), it is reasonable that
inflammation could be a potential link between pollution and
arterial stiffening and the consequent increase of the
cardiovascular risk.
Pulse wave velocity (PWV) represents the speed at
which the pressure wave generated by left-ventricular ejection is
propagated within the arterial tree and is considered the gold
standard for assessing regional arterial stiffness in clinical
practice. Augmentation pressure and augmentation index are two
measures of reflected pulse waves.
In this work, we aimed to perform a systematic
review to investigate the role of air pollution on arterial
stiffness and wave reflection.
Materials and methods
Review criteria
According to PRISMA guidelines, a systematic
literature search of original studies in humans was performed using
PubMed database (last accessed on January 31, 2017) without
restrictions on the year of publication. The search terms were
‘arterial stiffness’ or ‘pulse wave velocity’ or ‘augmentation
index’ or ‘augmentation pressure’ in combination with
‘PM2.5’ or ‘PM10’ or ‘nitrogen dioxide’ or
‘nitrogen oxides’ or ‘sulfur dioxide’ or ‘NO2’ or
‘NOx’ or ‘SO2’ or ‘pollution’. The inclusion
criteria included i) peer-reviewed publications reporting original
data; ii) a minimum of 10 adult subjects tested to maximize
reliability; and iii) arterial stiffness measured with a
well-accepted and validated technique [pulse wave velocity (PWV)];
reflected waves estimated by augmentation index or augmentation
pressure. Two independent reviewers (L.Z. and P.L.) selected the
studies for inclusion in this systematic review. First, the titles
of the studies were screened for relevance. In case of doubt,
conflict or discussion between the two independent reviewers, the
article was retained. Second, publications with titles or abstracts
appearing to meet these inclusion criteria were selected for
detailed review. In cases of doubt on the inclusion of an article,
a decision was achieved by consensus. The reference lists of the
analysed studies were also searched. These studies were subjected
to the same selection procedures. A narrative of the collected data
has been reported. Because of the high heterogeneity of the
studies, a meta-analysis was not performed. Whenever possible, data
were presented as the mean ± standard deviation and
percentages.
Results
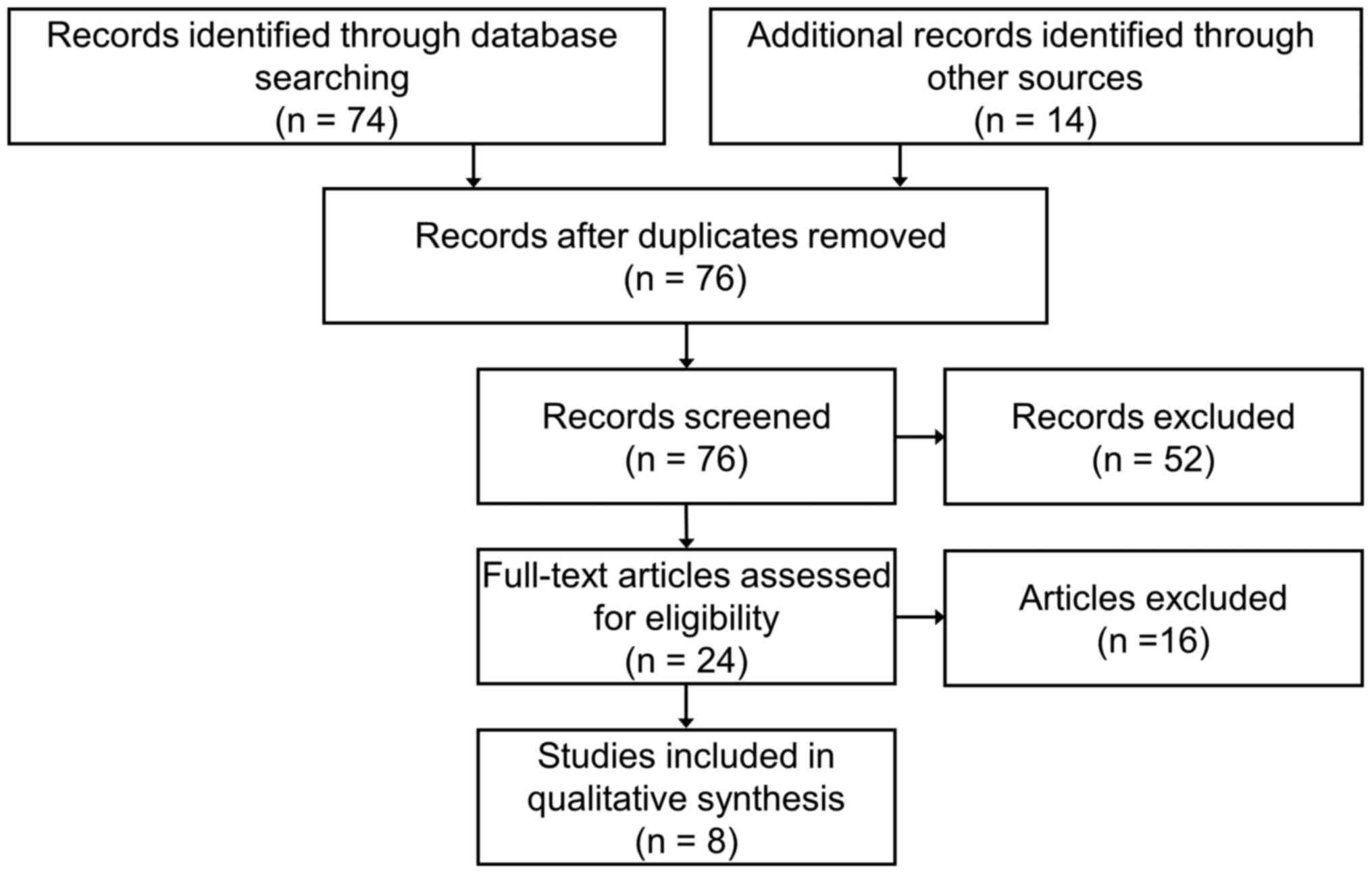
The PRISMA flow diagram is reported in Fig. 1, summarizing the number of studies
included for analysis based on the search criteria of this
systematic review. Using the search terms, a total of 76 studies
were identified. In total, 52 studies were excluded based on a
review of the title and the abstract only; 16 studies were excluded
after the reading of the full text. The remaining 8 studies,
published within the last 8 years, reported carotid-femoral PWV (2
studies), brachial-ankle PWV (2 studies), augmentation index and/or
augmentation pressure measurements (6 studies) and were considered
for the review (19–26). The main results of the studies
included in this review are reported in Table I.
 | Table I.Studies that have evaluated the effect
of gaseous or particulate matter pollutants on arterial
stiffness. |
Table I.
Studies that have evaluated the effect
of gaseous or particulate matter pollutants on arterial
stiffness.
| Authors (refs.) | Year of
publication | Subjects, n | Population | Measure of arterial
stiffness | Inflammatory
biomarkers | Measure of
pollution | Main results |
|---|
| Fang et al
(19) | 2008 | 26 | Welders | AIx |
|
PM2.5 | 6 h
PM2.5 exposure (median 390 µg/m3, range
30–2620 µg/m3) was associated with a slight 2.8%
increase in AIx (95%CI −1.4 to 7.0%). Additional exposure the day
before the monitoring was associated with a significant increase
(5.1%; 95%CI 0.8–9.5%). |
| Lenters et
al (20) | 2010 | 745 | Young adults, urban
population | cf-PWV, AIx |
|
PM2.5 | Long-term
PM2.5 exposure (21.4±1.1 µg/m3) was not
associated with either carotid-femoral PWV or AIx. |
| Shan et al
(21) | 2014 | 23 | Rural
population | cf-PWV, AIx | hsCRP |
PM2.5 | Comparable
carotid-femoral PWV, AIx and hsCRP in high (101±37
µg/m3) vs. low (39±11 µg/m3) 1-day
PM2.5 exposure group. |
| Mehta et al
(22) | 2014 | 370 | Elderly men, urban
population | AIx, AP |
|
PM2.5 | Short-term changes
in PM2.5 (3.6 µg/m3) were positively
associated with AIx and AP. |
| Jiang et al
(23) | 2016 | 321 | General population,
urban population | AIx | IL-6 |
PM2.5 | Higher AIx and IL-6
in high PM2.5 group (111 vs. 68 µg/m3
exposure). |
| Wu et al
(24) | 2016 | 89 | Healthy adults | ab-PWV | hsCRP |
PM2.5 | A
10-µg/m3 increase in PM2.5 concentration at a
1-day lag was associated with 2.1% (95%CI 0.7–3.6%) increases in
brachial-ankle PWV for 24 h of exposure. No significant association
was observed between hsCRP and PM2.5 levels. |
| Adamopoulos et
al (25) | 2010 | 1,222 | Patients with
hypertension | AP |
|
PM10 | 24 h mean
PM10 concentrations were positively associated to the AP
(Pearson r=0.06, p=0.04). |
| Weng et al
(26) | 2015 | 127 | Patients undergoing
hemodialysis | ab-PWV | hsCRP |
PM10 | Previous 12-month
average concentration of PM10 (57.9±5.7
µg/m3) was positively correlated with brachial-ankle PWV
(β=0.13, p=0.04). hsCRP was positively correlated with
brachial-ankle PWV (β=0.23, p=0.01). |
| Lenters et
al (20) | 2010 | 745 | Young adults | cf-PWV, AIx |
| NO2,
SO2 | Long-term exposure.
4.1% (95% confidence interval 0.1–8.0%) increase in carotid-femoral
PWV and a 37.6% (2.2–72.9%) increase in AIx for a 25
µg/m3 increase in NO2, and a 5.3% (0.1–10.4%)
increase in carotid-femoral PWV for a 5 µg/m3 increase
in SO2. |
| Mehta et al
(22) | 2014 | 370 | Elderly men, urban
population | AIx, AP |
| NO2,
SO2 | Short-term changes
in SO2 (2.3±1.9 µg/m3) were positively
associated with AIx and AP. No associations were observed between
NO2 (0.02±0.01 ppm) and AIx. |
| Wu et al
(24) | 2016 | 89 | Healthy adults | ab-PWV | hsCRP | NO2 | No significant
association with brachial-ankle PWV was observed for
NO2. A 10 ppb increase in NO2 was associated
with 37% (95% CI 17–61%) increases in hsCRP for 1-day of
exposure. |
Exposure to air pollutants and
arterial stiffness
The exposure to particulate matter pollutants
(PM2.5, PM10) was evaluated in 8 studies
(19–26). In 6 of them (19,22–26),
the exposure was associated with an increased arterial stiffness
and/or wave reflection (estimated by brachial-ankle PWV,
augmentation index and augmentation pressure) whereas in the
remaining two studies (20,21)
the exposure was not associated with either carotid-femoral PWV or
augmentation index. However, in one of these negative studies
(20), the PM2.5
exposure variability within the urban population was very low (mean
PM2.5 exposure: 21.4±1.1 µg/m3); in the
second negative study (21),
despite the augmentation index was slightly increased (25.4±4.8%
vs. 22.6±5.1%) in the group of patients with the higher
PM2.5 exposure, this difference was not significant,
probably for the small sample size (n=23). The exposure to gaseous
pollutants (NO2, NOx, SO2) was
evaluated in 3 studies (20,22,24)
and was associated with increased arterial stiffness and wave
reflection in two large studies (20,22)
whereas no significant association with brachial-ankle PWV was
observed for NO2 in the third, smaller study (24).
Increased arterial stiffness and wave reflection
were reported in 4 of the 5 studies that evaluated the short-term
exposure to particulate matter pollutants (19,21,22,24,25)
and in 2 of the 3 studies that evaluated the long-term exposure to
particulate matter pollutants (20,23,26),
whereas an increased wave reflection was reported in 1 of the 2
studies that evaluated the short-term (≤1 day) exposure to gaseous
pollutants (22,24), whereas both increased arterial
stiffness and wave reflection were reported in one article after
long-term exposure to gaseous pollutants (20).
The association between serum biomarkers of vascular
inflammation and air pollutants was evaluated in 3 studies
(21,24). One study (24) evaluated the effect of
NO2 exposure on hsCRP and revealed a positive
association between 1-day NO2 exposure and hsCRP levels.
No significant association was observed between PM2.5
levels and hsCRP in either study (21,24).
The association between serum biomarkers of inflammation and
arterial stiffness was evaluated only in one study (26) in which hsCRP was positively
correlated with brachial-ankle PWV.
Discussion
In the present systematic review of the effect of
air pollution on arterial stiffness and wave reflection, we
included 8 studies for discussion. A total of 6 of the 8 studies
reported an increased arterial stiffness and/or wave reflection
after particulate matter pollutants exposure. The exposure to
gaseous pollutants was associated with increased arterial stiffness
and wave reflection in 2 of the 3 studies with available data.
Increased arterial stiffness and wave
reflection after exposure to air pollutants
Possible underlying mechanisms. During the last
decades, several clinical settings, either physiological or
pathological, has been associated with increased arterial stiffness
and wave reflection. Most of them, such as the elderly and chronic
diseases, are irreversible, other clinical conditions, such as
chronic inflammation, are associated with increased arterial
stiffness (4–12,27)
and potentially reversible, as suggested in recent studies showing
that the reduction of inflammation can be associated with a
reduction of arterial stiffness (6,8,27).
In this review, we reported that arterial stiffness is increased
after exposure of air pollutants. Despite it remains to be
demonstrated whether the reduction of chronic exposure to air
pollution is associated with arterial destiffening, it was reported
that wave reflection increased immediately after 1 h of exposure to
diesel exhaust while there was no effect on arterial stiffness 40
min after exposure (28),
suggesting that acute exposure to pollutants could be associated
with reversible (functional) arterial stiffening.
Several mechanisms involved in the stiffening of
large arteries after exposure to air pollution could be involved.
In this regard, acute and chronic inflammation could play a role in
this process. Despite only one of the studies included in this
review explored the association between air pollution and a
biomarker of vascular inflammation (24), the negative effects of air
pollution on inflammatory state are well known and widely
documented in literature.
In another model of chronic inflammation it was
reported that inflammation may be associated with functional or
structural arterial stiffening (12). Functional arterial stiffening could
be the consequence of endothelial dysfunction, reduced
production/increased inactivation of nitric oxide and reduced
vasodilation whereas structural arterial stiffening can be
associated with the production of uncoiled stiff collagen,
degradation of elastin, smooth muscle cell migration and intima
proliferation, vascular calcification and stiffening of the
extracellular matrix. The increased arterial stiffness after
short-term exposure to air pollutants is in agreement with the
finding that even an acute, mild and transitory inflammatory
stimulus is associated with arterial stiffening (29) and suggests a functional arterial
stiffening. In support of this hypothesis, there is evidence that
particulate matter pollutants exposure is associated with acute
arterial vasoconstriction (16)
and endothelial dysfunction (14,16–18).
Moreover, the increase in augmentation index and augmentation
pressure, two indices of increased backward reflected waves, after
exposure to air pollution (19,22,23,25)
may be the consequence of the vasoconstriction at the level of the
microcirculation (25). Whether
the long-term exposure to air pollutants is associated with
functional or structural arterial stiffening is not known because
none of the studies included in this review explored the effect of
the reduction of long-term exposure to air pollution.
In presence of increased arterial stiffness, the
destiffening of large arteries is an objective with important
implications on the cardiovascular risk, as suggested by the
increased survival after the reduction of arterial stiffness
(30). To obtain an arterial
destiffening, three levels of treatment can be used. The first is
the prescription of a drug that reduces the arterial stiffness,
such as an antihypertensive with an independent effect on arterial
stiffness. Unfortunately, using this option the causal factors of
the arterial stiffening remain active. The second level of
treatment consists in the reduction, most probably, or elimination,
hardly, of a known causal factor of arterial stiffening, i.e. the
chronic inflammation (6,8,27).
This approach, acting in a step before the arterial wall
stiffening, can be potentially highly effective in single patients.
Regarding this point, interventional multicentre studies are
ongoing. The model studied in this review has a great clinical
importance because, reducing the air pollution, we can reduce the
arterial stiffening already occurred and also prevent the
development of chronic inflammation, a causal factor of arterial
stiffening and several cardiovascular complications, acting at a
population-based level of treatment. Consequently, the evidence
that air pollution is associated with increased arterial stiffness
has also a potential great epidemiological relevance and could
represent a warning for the lawmakers and the occupational
physicians.
Methodological issues
The present study has several strengths. First, to
the best of our knowledge, no systematic review studies have
determined whether arterial stiffness is increased after air
pollution. Second, we used widely accepted measures of arterial
stiffness and wave reflection. PWV represents the speed at which
the pressure wave is generated by left-ventricular contraction is
transmitted within the arterial tree. The carotid-femoral PWV
represents the gold standard for arterial stiffness assessments in
daily practice whereas ankle-brachial PWV represents the stiffness
of muscular and elastic arteries considered as a whole.
Augmentation index and augmentation pressure are measures of wave
reflection. This study may have also a number of potential
limitations. First, all of the studies included in this review did
not explore the effect of the reduction of exposure to air
pollutants on arterial stiffness and reflection waves. Future
prospective studies need to be performed to confirm the hypothesis
of a destiffening effect of a population-based reduction of the air
pollution. Second, due to the high heterogeneity of the time of
exposure and the concentration of air pollutants, outcomes and
population studied included in this systematic review, a
meta-analysis was not performed. Third, we con not exclude that the
presence of comorbidities could have influenced the results of the
studies included in this review. Adamopoulos et al (25) included patients with hypertension,
Weng et al included patients undergoing haemodialysis
(26), whereas other authors
(19–23) included patients from the general
population, and did not report any exclusion criteria or included
patients with several cardiovascular risk factors.
In conclusion, available evidence supports an
association of gaseous and particulate matter pollutants with an
increased arterial stiffness and wave reflection. These findings
may have important clinical implications for population-based
strategies aimed at the reduction of arterial stiffness, an
intermediate end-point of cardiovascular diseases.
References
|
1
|
Vlachopoulos C, Xaplanteris P, Aboyans V,
Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A,
Landmesser U, Laurent S, et al: The role of vascular biomarkers for
primary and secondary prevention. A position paper from the
European Society of Cardiology Working Group on peripheral
circulation: Endorsed by the Association for Research into Arterial
Structure and Physiology (ARTERY) Society. Atherosclerosis.
241:507–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heo J, Schauer JJ, Yi O, Paek D, Kim H and
Yi SM: Fine particle air pollution and mortality: Importance of
specific sources and chemical species. Epidemiology. 25:379–388.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pope CA III, Burnett RT, Thurston GD, Thun
MJ, Calle EE, Krewski D and Godleski JJ: Cardiovascular mortality
and long-term exposure to particulate air pollution:
Epidemiological evidence of general pathophysiological pathways of
disease. Circulation. 109:71–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zanoli L, Inserra G and Castellino P:
Increased cardiovascular risk in subjects with a low prevalence of
classic cardiovascular risk factors: The inflammatory bowel disease
paradox. Trends Cardiovasc Med. 25:705–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zanoli L, Cannavò M, Rastelli S, Di Pino
L, Monte I, Di Gangi M, Boutouyrie P, Inserra G, Laurent S and
Castellino P: Arterial stiffness is increased in patients with
inflammatory bowel disease. J Hypertens. 30:1775–1781. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zanoli L, Rastelli S, Granata A, Inserra
G, Empana JP, Boutouyrie P, Laurent S and Castellino P: Arterial
stiffness in inflammatory bowel disease: A systematic review and
meta-analysis. J Hypertens. 34:822–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zanoli L, Granata A, Lentini P, Gaudio A
and Castellino P: Augmentation index is increased in patients with
inflammatory bowel disease, a meta-analysis. Eur J Intern Med. Dec
21–2016.(Epub ahead of print).
https://doi.org/10.1016/j.ejim.2016.12.012.
|
|
8
|
Zanoli L, Rastelli S, Inserra G, Lentini
P, Valvo E, Calcagno E, Boutouyrie P, Laurent S and Castellino P:
Increased arterial stiffness in inflammatory bowel diseases is
dependent upon inflammation and reduced by immunomodulatory drugs.
Atherosclerosis. 234:346–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zanoli L, Boutouyrie P, Lentini P,
Rastelli S and Castellino P: Maintenance therapy with salicylates
is associated with aortic stiffening in patients with inflammatory
bowel disease. J Hypertens. (In press). https://doi.org/10.1097/HJH.0000000000001235
|
|
10
|
Zanoli L, Signorelli SS, Inserra G and
Castellino P: Subclinical atherosclerosis in patients with
inflammatory bowel diseases: A systematic review and meta-analysis.
Angiology. Oct 26–2016.(Epub ahead of print). doi:
10.1177/0003319716675076. PubMed/NCBI
|
|
11
|
Wu GC, Leng RX, Lu Q, Fan YG, Wang DG and
Ye DQ: Subclinical atherosclerosis in patients with inflammatory
bowel diseases: A systematic review and meta-analysis. Angiology.
Jun 1–2016.(Epub ahead of print).
|
|
12
|
Zanoli L, Rastelli S, Inserra G and
Castellino P: Arterial structure and function in inflammatory bowel
disease. World J Gastroenterol. 21:11304–11311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peters A, Fröhlich M, Döring A, Immervoll
T, Wichmann HE, Hutchinson WL, Pepys MB and Koenig W: Particulate
air pollution is associated with an acute phase response in men;
results from the MONICA-Augsburg Study. Eur Heart J. 22:1198–1204.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamagawa E, Bai N, Morimoto K, Gray C, Mui
T, Yatera K, Zhang X, Xing L, Li Y, Laher I, et al: Particulate
matter exposure induces persistent lung inflammation and
endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol.
295:L79–L85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Briet M, Collin C, Laurent S, Tan A, Azizi
M, Agharazii M, Jeunemaitre X, Alhenc-Gelas F and Boutouyrie P:
Endothelial function and chronic exposure to air pollution in
normal male subjects. Hypertension. 50:970–976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brook RD, Brook JR, Urch B, Vincent R,
Rajagopalan S and Silverman F: Inhalation of fine particulate air
pollution and ozone causes acute arterial vasoconstriction in
healthy adults. Circulation. 105:1534–1536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urch B, Silverman F, Corey P, Brook JR,
Lukic KZ, Rajagopalan S and Brook RD: Acute blood pressure
responses in healthy adults during controlled air pollution
exposures. Environ Health Perspect. 113:1052–1055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rundell KW, Hoffman JR, Caviston R,
Bulbulian R and Hollenbach AM: Inhalation of ultrafine and fine
particulate matter disrupts systemic vascular function. Inhal
Toxicol. 19:133–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang SC, Eisen EA, Cavallari JM, Mittleman
MA and Christiani DC: Acute changes in vascular function among
welders exposed to metal-rich particulate matter. Epidemiology.
19:217–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lenters V, Uiterwaal CS, Beelen R, Bots
ML, Fischer P, Brunekreef B and Hoek G: Long-term exposure to air
pollution and vascular damage in young adults. Epidemiology.
21:512–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan M, Yang X, Ezzati M, Chaturvedi N,
Coady E, Hughes A, Shi Y, Yang M, Zhang Y and Baumgartner J: A
feasibility study of the association of exposure to biomass smoke
with vascular function, inflammation, and cellular aging. Environ
Res. 135:165–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehta AJ, Zanobetti A, Koutrakis P,
Mittleman MA, Sparrow D, Vokonas P and Schwartz J: Associations
between short-term changes in air pollution and correlates of
arterial stiffness: The Veterans Affairs Normative Aging Study,
2007–2011. Am J Epidemiol. 179:192–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang S, Bo L, Gong C, Du X, Kan H, Xie Y,
Song W and Zhao J: Traffic-related air pollution is associated with
cardio-metabolic biomarkers in general residents. Int Arch Occup
Environ Health. 89:911–921. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu CF, Shen FH, Li YR, Tsao TM, Tsai MJ,
Chen CC, Hwang JS, Hsu SH, Chao H, Chuang KJ, et al: Association of
short-term exposure to fine particulate matter and nitrogen dioxide
with acute cardiovascular effects. Sci Total Environ.
569–570:300–305. 2016. View Article : Google Scholar
|
|
25
|
Adamopoulos D, Vyssoulis G, Karpanou E,
Kyvelou SM, Argacha JF, Cokkinos D, Stefanadis C and van de Borne
P: Environmental determinants of blood pressure, arterial
stiffness, and central hemodynamics. J Hypertens. 28:903–909. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weng CH, Hu CC, Yen TH and Huang WH:
Association between environmental particulate matter and arterial
stiffness in patients undergoing hemodialysis. BMC Cardiovasc
Disord. 15:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mäki-Petäjä KM, Hall FC, Booth AD, Wallace
SM, Yasmin Bearcroft PW, Harish S, Furlong A, McEniery CM, Brown J,
et al: Rheumatoid arthritis is associated with increased aortic
pulse-wave velocity, which is reduced by anti-tumor necrosis
factor-alpha therapy. Circulation. 114:1185–1192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lundbäck M, Mills NL, Lucking A, Barath S,
Donaldson K, Newby DE, Sandström T and Blomberg A: Experimental
exposure to diesel exhaust increases arterial stiffness in man.
Part Fibre Toxicol. 6:72009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vlachopoulos C, Dima I, Aznaouridis K,
Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M and Stefanadis C:
Acute systemic inflammation increases arterial stiffness and
decreases wave reflections in healthy individuals. Circulation.
112:2193–2200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guerin AP, Blacher J, Pannier B, Marchais
SJ, Safar ME and London GM: Impact of aortic stiffness attenuation
on survival of patients in end-stage renal failure. Circulation.
103:987–992. 2001. View Article : Google Scholar : PubMed/NCBI
|