Introduction
The incidence of congenital heart disease (CHD)
varies between 4 and 8 in every 1,000 live births globally
(1). However, it is considerably
higher in the prenatal population; the percentage of miscarriages
and elective abortions in pregnant women with structural CHD is
reportedly 15 and 5%, respectively (2,3). The
development and formation of the human heart is an intricate
process. Unfavorable environmental and embryotoxic factors, genetic
variations, numerical and structural chromosomal aberrations (e.g.
trisomy of chromosome 21), as well as chromosomal microdeletions
(e.g. DiGeorge syndrome), may all interfere with this process, thus
leading to CHD or even miscarriage in some cases (4–6). The
genetic etiology of CHD has been studied extensively over the last
decade; a number of germ line mutations in cardiac transcription
factors (7–13), including NK2 homeobox 5
(NKX2-5) (11,14), GATA binding protein 4
(GATA4) (15–17), T-box 20 (18), and Notch1 (19) have been validated. However, further
research is required to better understand the underlying mechanisms
of CHD. Next generation sequencing (NGS), in addition to its
advantageous cost, accuracy and efficiency, has proven to be
successful in identifying concordant variants in patients with the
same disease (20). Genome-wide
coverage may allow for a nonbiased approach, as it is not
restricted to certain pre-selected regions. The conventional Sanger
sequencing approach has been used to validate the candidate
discordant variants obtained from NGS (21,22).
In order to obtain a comprehensive understanding of
the effect of genetic variants on CHD, the present study used NGS
to sequence the whole exome of a stillborn fetus diagnosed with
CHD. In addition to a number of known CHD-inducing genes, genes
with a poor association were additionally identified. The results
provide a more complete understanding of the effect of specific
genetic variants on CHD.
Materials and methods
Study population
A 0.5×0.5 cm section of tissue from the left
ventricular of a male stillborn fetus (gestational age, 37 weeks),
and 464 peripheral blood samples from 215 non-syndromic patients
with CHD (101 males and 114 females; mean age 8.84±12.98 years old)
and 249 healthy control subjects (118 males and 131 females; mean
age 47.56±16.62) were included in the present study (Table I). All the samples were obtained
from individuals from Fujian Medical University (Fuzhou, China) and
Shengjing Hospital of China Medical University (Shenyang, China)
between 2009 and 2012. The stillborn fetus was diagnosed with
tricuspid atresia and complete transposition of the great arteries
(TGA) as confirmed by autopsy. Written informed consent was
obtained from the parents and guardians of the patient and from the
464 additional subjects. The present study was approved by the
ethics committee of Fujian Medical University (Fuzhou, China), and
adhered to the tenets of the Declaration of Helsinki. Patients with
CHD were routinely screened by performing clinical examinations,
chest X-rays, electrocardiographs and ultrasonic echocardiograms.
The pathological diagnosis of CHD was confirmed by open-heart
surgery. The healthy control subjects were non-CHD adult
outpatients from the same geographic area. Control subjects with
congenital anomalies were excluded from the study.
 | Table I.Phenotype of 215 patients with
non-syndromic congenital heart disease included in the present
study. |
Table I.
Phenotype of 215 patients with
non-syndromic congenital heart disease included in the present
study.
| Phenotype | No. of patients
(%) |
|---|
| Atrial septal
defect | 48 (22.3) |
| Ventricular septal
defect | 52 (24.2) |
| Tetralogy of
Fallot | 24 (11.2) |
| Patent ductus
arteriosus | 24 (11.2) |
| Pulmonary
stenosis | 10 (4.7) |
| Other complex
cardiac malformations | 57 (26.5) |
Whole exome sequencing and data
analysis
Genomic DNA (gDNA) was extracted and purified using
the FlexGen Blood DNA kit (CW0544A; CWBio Technology, Beijing,
China). Purified gDNA (3 µg) was fragmented into 200 bp sequences.
End repair, adenylation and adapter ligation were performed for
library preparation using the NGS Fast DNA Library Prep set and
following the manufacturer's protocol (CWBio Technology, Beijing,
China). Library samples were pooled and hybridized to a customized
capture array, including exons, splicing sites and immediate
flanking intron sequences (5190–6216; SureSelectXT2 Human All Exon
V5, 16; Agilent Technologies, Inc., Santa Clara, CA, USA).
Sequencing was performed on an Illumina HiSeq 2500 instrument
(Illumina, Inc., San Diego, CA, USA) to generate paired end reads.
Adapter and low quality sequences (quality score ≤20 and sequencing
depth ≤5) in the raw data were then removed using the
Burrows-Wheeler Aligner (http://bio-bwa.sourceforge.net/bwa.shtml) (23). The sequencing reads were mapped to
the human reference genome (hg19, http://genome.ucsc.edu) using the short
oligonucleotide alignment program (SOAP) (http://soap.genomics.org.cn/soapsnp.html) and the
Burrows-Wheeler Aligner (24,25).
Single nucleotide polymorphisms (SNPs) and indels were detected
using the Genome Analysis Toolkit and the SOAPsnp algorithm
(http://soap.genomics.org.cn/soapsnp.html) (26), while annotation was performed
according to the Consensus Coding Sequence of human GRCh37/hg19
(http://genome.ucsc.edu/cgi-bin/hgTracks?db=hg19), the
Human Genome Project (HGP, human genome build, 36.3), the Single
Nucleotide Polymorphism Database (dbSNP; version, 130; www.ncbi.nlm.nih.gov/snp), the Haplotype Map
Project (https://www.broadinstitute.org/data-software-and-tools)
and the Sorting Intolerant From Tolerant prediction tool (27–29).
Sanger sequencing and protein
structure prediction
Exon 2 of iroquois homeobox 1 (IRX1) was amplified
in CHD patients and healthy controls by polymerase chain reaction
(PCR), PCR reactions consisted of 20–30 ng of genomic DNA, 3 µl of
PCR buffer, 3 µl of dNTPs, 0.3 µl of Hotstar Taq (Qiagen), 1.5 µl
(20 pmol/µl) of each primer pair (forward:
5′-GGGTGACTTCCTGATCTGCC-3′; reverse: 5′-GAAGCAGGGATTAAGCGCAG3′), to
a volume of 30 µl with distilled water. Reactions started with 15
min at 95°C, followed by 30 cycles of 45 sec at 95°C, 30 sec at
60°C, 45 sec at 72°C and finished with a 10 min extension period at
72°C. using the forward primer, 5′-GGGTGACTTCCTGATCTGCC-3′ and
reverse primer, 5′-GAAGCAGGGATTAAGCGCAG3′. The PCR products were
sequenced using the automated ABI 3730XL sequencer (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
the forward primer, 5′-TCGAGTCCATTGAAGCGG-3′ and reverse primer,
5′-TACCCTCCCGGCTCATGC-3′. Amino acid sequences of IRX1 in
additional mammalian species were obtained from NCBI GenBank
(www.ncbi.nlm.nih.gov/genebank), and
sequence conservation analysis was performed using CLC Main
Workbench version 7.7.3 (CLCbio; Qiagen Bioinformatics, Aarhus,
Denmark).
Results
To comprehensively investigate the association
between germline mutations and CHD susceptibility, the present
study was completed in two consecutive steps. Whole exome
sequencing of the stillborn fetus discovered 17,601 SNP sites,
spanning 98% of the target region (Table II). These variants were
subsequently annotated according to the dbSNP and HGP databases.
17,302 missense sites and 309 indel sites were then selected as the
candidate genetic variants (Table
II). In the subsequent analysis, genes associated with heart
formation, development and cardiovascular disease were selected for
further consideration. A number of known causative genes for
congenital heart malformations, including cysteine rich with EGF
like domains 1 (CRELD1) (30), tolloid like 1 (TLL1)
(31), cbp/P300 interacting
transactivator with Glu/Asp rich carboxy-terminal domain 2
(CITED2) (32,33) and myocardin (MYOCD)
(33), were detected in the
present study. However, no mutations in the exons of additional
pivotal genes, including GATA4, GATA6, NKX2-5, T-box
transcription factor and heart and neural crest derivatives
expressed 2, were identified (Table
III).
 | Table II.Main features of whole exome
sequencing results. |
Table II.
Main features of whole exome
sequencing results.
| Feature | Data |
|---|
| Raw data (Mb) | 5,597.55 |
| Clean data
(Mb) | 5,571.7 |
| Aligned (%) | 99.62 |
| Initial bases on
target | 55,336,911 |
| Bases covered on
target | 49,342,125 |
| Coverage of target
region (%) | 98.00 |
| Total effective
yield (Mb) | 5,133.05 |
| Effective sequence
on target (Mb) | 2,756.55 |
| Fraction of
effective bases on target (%) | 53.70 |
| Average sequencing
depth on target (%) | 54.76 |
| Fraction of target
with at least 4x coverage (%) | 96.30 |
| Fraction of target
with at least 10x coverage (%) | 93.20 |
| Fraction of target
with at least 20x coverage (%) | 84.50 |
| Duplication rate
(%) | 6.1949 |
| Total SNP | 17,601 |
| Missense sites | 17,302 |
| Indel sites | 309 |
 | Table III.Mutation sites of the congenital
heart disease-associated genes identified in the present study. |
Table III.
Mutation sites of the congenital
heart disease-associated genes identified in the present study.
| Gene | Accession no. | Exon | Position | Protein | Effect |
|---|
| CRELD1 | NM_015513 | 1 | c.37A>G | p.M13V | Nonsynonymous |
| TMEM43 | NM_024334 | 7 | c.536T>C | p.M179T | Nonsynonymous |
| TLL1 | NM_012464 | 20 | c.2872A>G | p.T958A | Nonsynonymous |
| CITED2 | NM_001168388 | 2 | c.148G>A | p.A50T | Nonsynonymous |
| IRX3 | NM_024336 | 2 | c.1265T>C | p.L422P | Nonsynonymous |
| IRX5 | NM_005853 | 3 | c.763C>A | p.P255T | Nonsynonymous |
| MYOCD | NM_153604 | 10 | c.1941G>C | p.Q647H | Nonsynonymous |
| IRX1 | NM_024337 | 2 | c.718C>G | p.Q240E | Nonsynonymous |
| IRX4 | NM_016358 | 3 | c.381A>G | p.P127P | Synonymous |
| IRX4 | NM_016358 | 2 | c.90A>C | p.G30G | Synonymous |
| IRX1 | NM_024337 | 2 | c.1272T>C | p.N424N | Synonymous |
| NKX2-5 | NM_001166176 | 1 | c.63A>G | p.E21E | Synonymous |
| TBX20 | NM_001166220 | 1 | c.39T>C | p.S13S | Synonymous |
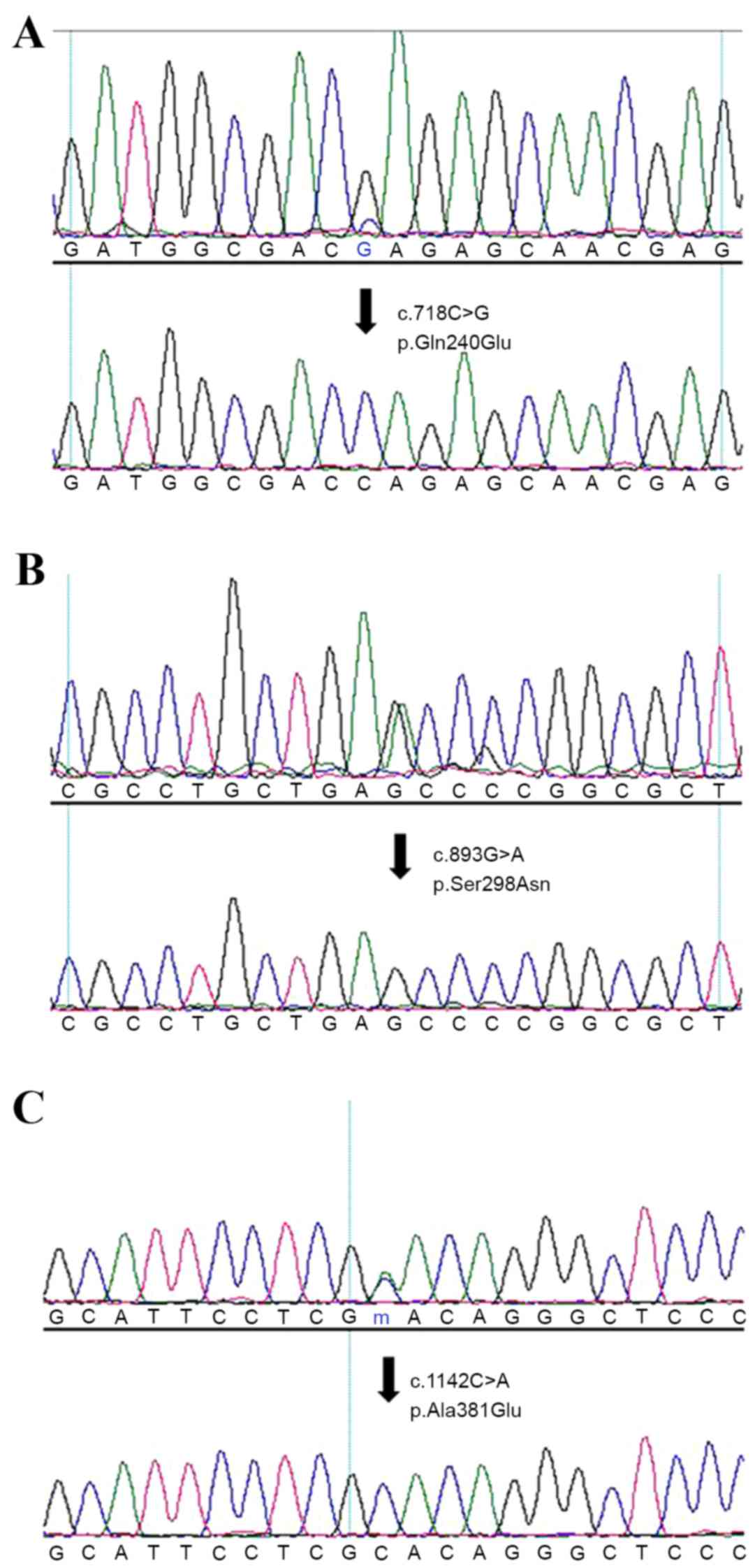
Out of the candidate genes identified, a variant of
IRX1 (c.718C>G, p.Gln240Glu), which is an important gene
involved in early heart development and limb formation (34), was selected for further
investigation. The c.718C>G variant and whole exon 2 of the
IRX1 gene were screened in 215 non-syndromic patients with
CHD and 249 healthy control subjects by PCR and Sanger sequencing.
The c.718C>G variant was detected in a male (age, 5 years) with
TGA and an atrial septal defect (Fig.
1, Table IV). In addition, a
novel variant, c893G>A, p.S298N, was detected in a 1-year-old
male with total anomalous pulmonary venous drainage (Fig. 1; Table IV). An additional previously
identified variant, c.1142C>A (p.A381E; dpSNP cluster ID,
rs530506520) was identified in a 3-year-old male with a ventricular
septal defect phenotype (Fig. 1;
Table IV). However, no
non-synonymous variant was detected in the 249 healthy control
subjects. Conservation analysis demonstrated that glutamine 240 and
serine 298 residues are highly conserved among different mammalian
species, while alanine 381was only moderately conserved (Fig. 2).
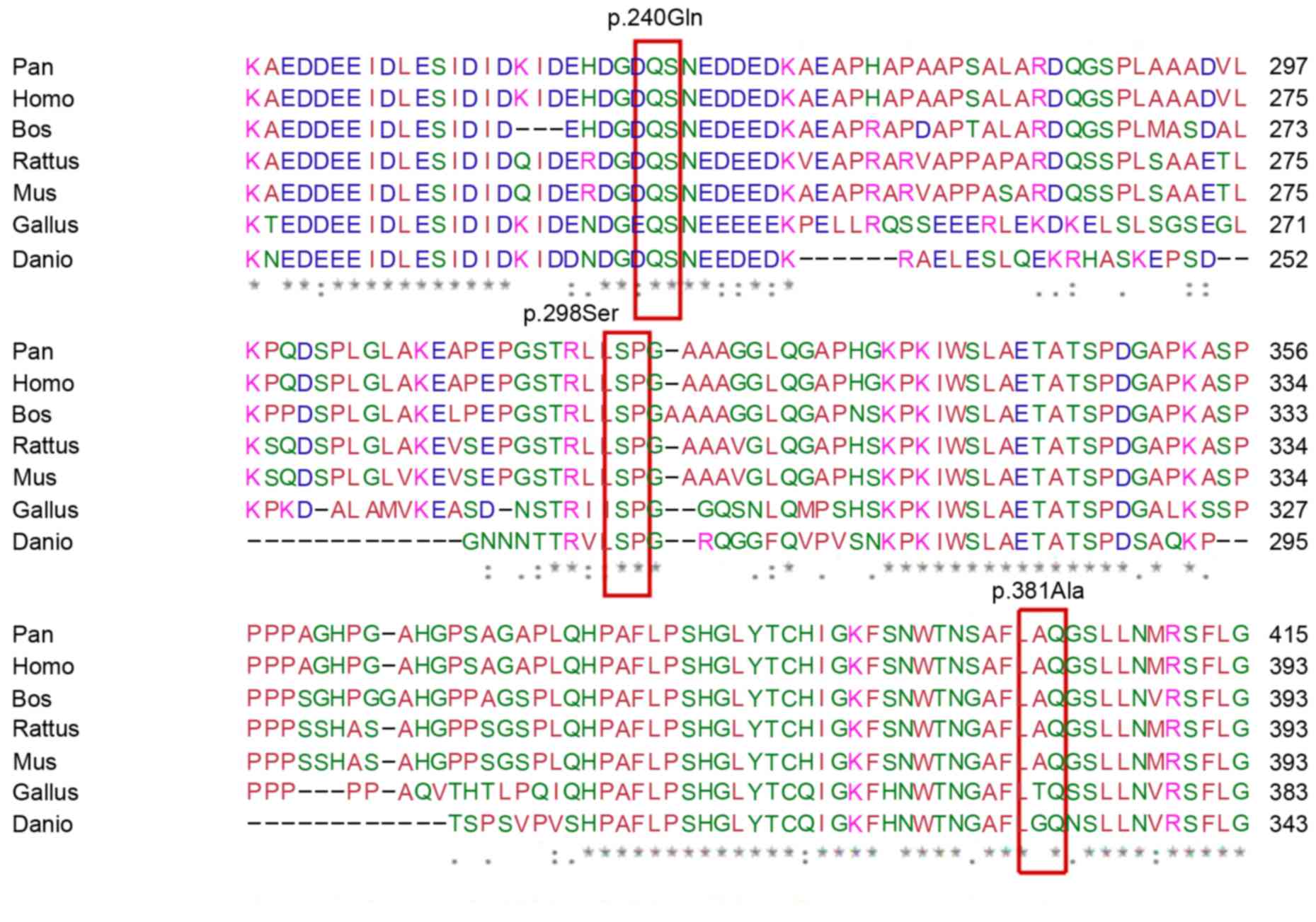 | Figure 2.Conservation analysis of iroquois
homeobox 1 among different mammalian species performed using CLC
Main Workbench software. P.240Gln and p.298Ser were highly
conserved in mammalian species whereas p.381Ala was conserved only
in Pan, Homo, Bos, Rattus and Mus, but not in Gallus and Danio.
Pan, Pan troglodytes; Homo, Homo sapiens; Bos, Bos taurus; Rattus,
Rattus norvegicus; Mus, Mus musculus; Gallus, Gallus gallus; Danio,
Danio rerio. |
 | Table IV.Missense mutation sites in
iroquois homeobox 1 identified in 215 sporadic cases of
CHD. |
Table IV.
Missense mutation sites in
iroquois homeobox 1 identified in 215 sporadic cases of
CHD.
| Position | Protein | Ref SNP number | Gender | Age (years) | Phenotype |
|---|
| c.718C>G | p.Gln240Glu | Novel | Male | 5 | TGA+ASD |
| c.893G>A | p.Ser298Asn | Novel | Male | 1 | TAPVD |
| c.1142C>A | p.Ala381Glu | rs530506520 | Male | 3 | VSD |
Discussion
NGS has become a powerful tool for identifying
concordant variants in patients with the same disease. In previous
studies it has successfully identified the causative gene of
monogenic diseases (35), as well
as a number of cancers, autoimmune diseases (36) and neurodegenerative diseases
(37). In the present study, NGS
was used to sequence the exome of a stillborn fetus with tricuspid
atresia and complete TGA. In total, 17,302 missense sites and 309
indel sites were selected as candidate genetic variants. Out of
these, a number of known cardiovascular disease-associated variants
were identified, including CRELD1 (30), CITED2 (32), MYOCD (33), transmembrane protein 43 (38), TLL1 (31), IRX-1, IRX-3 and
IRX-5. Therefore, the authors hypothesize that CRELD1,
CITED2 and TLL1 genetic variants may have been
responsible for the development of CHD in the fetus. It is possible
that the IRX-1, IRX-3 and IRX-5 variants may have
additionally contributed to CHD development.
The IRX gene is highly conserved among
vertebrates. A total of 6 IRX genes (IRX1-IRX6) are
organized in two cognate clusters of three genes, IRX1, IRX2,
IRX4 and IRX3, IRX5, IRX6, respectively (39,40).
The IRX gene exhibits restricted temporal and spatial
expression patterns during murine neural and cardiac development
(41). IRX4 was the first
cardiac transcription factor identified to be expressed in the
ventricles alone at all stages of heart development (40). In chicken embryos, aberrant
expression of Irx4 affects heart chamber development
(42). In mice, targeted
inactivation of Irx4 led to aberrant ventricular gene
expression, including reduced expression of the basic
helix-loop-helix transcription factor (40). In this previous study, adult
Irx4Dex2/Dex2 mice developed cardiomyopathy characterized by
cardiac hypertrophy and impaired contractile function (40). Cardiac expression of Irx1,
Irx2 and Irx5 may partially compensate for loss of
Irx4 function (41).
In the present study, the coding sequence of the
IRX1 gene was screened in sporadic non-syndromic patients
with CHD and healthy volunteers. The number of missense mutations
identified was higher in CHD patients when compared with healthy
volunteers (3 of the 215 CHD cases vs. 0 of the 249 controls).
These results further support the notion that disrupted IRX1
may be insufficient to induce a CHD phenotype, and that variants of
the IRX1 gene only contribute to an increased susceptibility
of CHD.
In the present study, the whole exome of a stillborn
fetus with tricuspid atresia and complete TGA was sequenced. A
number of missense mutations in known CHD-associated genes,
including CRELD1, CITED2 and TLL1 were detected. In
addition, the missense mutation rate of IRX1 was observed to
be higher in patients with sporadic CHD when compared with normal
healthy volunteers. This suggests that genetic variants of
IRX1 may contribute to the development of CHD.
Acknowledgements
The authors of the present study would like to thank
all of the participants for their contributions to this research.
The present study was supported by the National Key Research and
Development Program (grant no. 2016YFC1000501) and the National
Natural Science Fund (grant no. 81470525).
References
|
1
|
Marelli AJ, Mackie AS, Ionescu-Ittu R,
Rahme E and Pilote L: Congenital heart disease in the general
population: Changing prevalence and age distribution. Circulation.
115:163–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drenthen W, Pieper PG, Roos-Hesselink JW,
van Lottum WA, Voors AA, Mulder BJ, van Dijk AP, Vliegen HW, Yap
SC, Moons P, et al: Outcome of pregnancy in women with congenital
heart disease: A literature review. J Am Coll Cardiol.
49:2303–2311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whittemore R, Hobbins JC and Engle MA:
Pregnancy and its outcome in women with and without surgical
treatment of congenital heart disease. Am J Cardiol. 50:641–651.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marino B and Digilio MC: Congenital heart
disease and genetic syndromes: Specific correlation between cardiac
phenotype and genotype. Cardiovasc Pathol. 9:303–315. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Unolt M, Putotto C and Marino D:
Congenital heart disease, genetic syndromes, and major noncardiac
malformations. Eur J Pediatr. 171:18612012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gelb BD: Genetic basis of syndromes
associated with congenital heart disease. Curr Opin Cardiol.
16:188–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al Turki S, Manickaraj AK, Mercer CL,
Gerety SS, Hitz MP, Lindsay S, D'Alessandro LC, Swaminathan GJ,
Bentham J, Arndt AK, et al: Rare variants in NR2F2 cause congenital
heart defects in humans. Am J Hum Genet. 94:574–585. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu CX, Gong HR, Liu XY, Wang J, Zhao CM,
Huang RT, Xue S and Yang YQ: A novel HAND2 loss-of-function
mutation responsible for tetralogy of Fallot. Int J Mol Med.
37:445–451. 2016.PubMed/NCBI
|
|
9
|
Pan Y, Geng R, Zhou N, Zheng GF, Zhao H,
Wang J, Zhao CM, Qiu XB, Yang YQ and Liu XY: TBX20 loss-of-function
mutation contributes to double outlet right ventricle. Int J Mol
Med. 35:1058–1066. 2015.PubMed/NCBI
|
|
10
|
Pan Y, Wang ZG, Liu XY, Zhao H, Zhou N,
Zheng GF, Qiu XB, Li RG, Yuan F, Shi HY, et al: A Novel TBX1
Loss-of-function mutation associated with congenital heart disease.
Pediatr Cardiol. 36:1400–1410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu XK, Qiu XB, Yuan F, Wang J, Zhao CM,
Liu XY, Zhang XL, Li RG, Xu YJ, Hou XM, et al: A novel NKX2.5
loss-of-function mutation associated with congenital bicuspid
aortic valve. Am J Cardiol. 114:1891–1895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi LM, Tao JW, Qiu XB, Wang J, Yuan F, Xu
L, Liu H, Li RG, Xu YJ, Wang Q, et al: GATA5 loss-of-function
mutations associated with congenital bicuspid aortic valve. Int J
Mol Med. 33:1219–1226. 2014.PubMed/NCBI
|
|
13
|
Wang J, Mao JH, Ding KK, Xu WJ, Liu XY,
Qiu XB, Li RG, Qu XK, Xu YJ, Huang RT, et al: A novel NKX2.6
mutation associated with congenital ventricular septal defect.
Pediatr Cardiol. 36:646–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reamon-Buettner SM, Hecker H,
Spanel-Borowski K, Craatz S, Kuenzel E and Borlak J: Novel NKX2-5
mutations in diseased heart tissues of patients with cardiac
malformations. Am J Pathol. 164:2117–2125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang R, Fan LL, Huang H, Cao BB, Li XP,
Peng DQ and Xia K: A novel mutation of GATA4 (K319E) is responsible
for familial atrial septal defect and pulmonary valve stenosis.
Gene. 534:320–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao L, Xu JH, Xu WJ, Yu H, Wang Q, Zheng
HZ, Jiang WF, Jiang JF and Yang YQ: A novel GATA4 loss-of-function
mutation responsible for familial dilated cardiomyopathy. Int J Mol
Med. 33:654–660. 2014.PubMed/NCBI
|
|
17
|
Yang YQ, Li L, Wang J, Liu XY, Chen XZ,
Zhang W, Wang XZ, Jiang JQ, Liu X and Fang WY: A novel GATA4
loss-of-function mutation associated with congenital ventricular
septal defect. Pediatr Cardiol. 33:539–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Debenedittis P, Harmelink C, Chen Y, Wang
Q and Jiao K: Characterization of the novel interaction between
muskelin and TBX20, a critical cardiogenic transcription factor.
Biochem Biophys Res Commun. 409:338–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Theis JL, Hrstka SC, Evans JM, O'Byrne MM,
de Andrade M, O'Leary PW, Nelson TJ and Olson TM: Compound
heterozygous NOTCH1 mutations underlie impaired cardiogenesis in a
patient with hypoplastic left heart syndrome. Hum Genet.
134:1003–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swerdlow DI and Humphries SE: Genetics of
CHD in 2016: Common and rare genetic variants and risk of CHD. Nat
Rev Cardiol. 14:73–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuhlenbäumer G, Hullmann J and Appenzeller
S: Novel genomic techniques open new avenues in the analysis of
monogenic disorders. Hum Mutat. 32:144–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imelfort M, Batley J, Grimmond S and
Edwards D: Genome sequencing approaches and successes. Methods Mol
Biol. 513:345–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H: Exploring single-sample SNP and
INDEL calling with whole-genome de novo assembly. Bioinformatics.
28:1838–1844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kent WJ, Hsu F, Karolchik D, Kuhn RM,
Clawson H, Trumbower H and Haussler D: Exploring relationships and
mining data with the UCSC gene sorter. Genome Res. 15:737–741.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raney BJ, Dreszer TR, Barber GP, Clawson
H, Fujita PA, Wang T, Nguyen N, Paten B, Zweig AS, Karolchik D and
Kent WJ: Track data hubs enable visualization of user-defined
genome-wide annotations on the UCSC Genome Browser. Bioinformatics.
30:1003–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McKenna A, Hanna M, Banks E, Sivachenko A,
Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly
M and DePristo MA: The genome analysis toolkit: A MapReduce
framework for analyzing next-generation DNA sequencing data. Genome
Res. 20:1297–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar PS, Henikoff S and Ng PC: Predicting
the effects of coding non-synonymous variants on protein function
using the SIFT algorithm. Nat Protoc. 4:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H and Durbin R: Fast and accurate
long-read alignment with Burrows-Wheeler transform. Bioinformatics.
26:589–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
International HapMap 3 Consortium.
Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA,
Peltonen L, Dermitzakis E, Schaffner SF, Yu F, et al: Integrating
common and rare genetic variation in diverse human populations.
Nature. 467:52–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kusuma L, Dinesh SM, Savitha MR,
Krishnamurthy B, Narayanappa D and Ramachandra NB: A maiden report
on CRELD1 single-nucleotide polymorphism association in congenital
heart disease patients of Mysore, South India. Genet Test Mol
Biomarkers. 15:483–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zain M, Awan FR, Cooper JA, Li KW, Palmen
J, Acharya J, Howard P, Baig SM, Elkeles RS, Stephens JW, et al:
Association of TLL1 gene polymorphism (rs1503298, T > C) with
coronary heart disease in PREDICT, UDACS and ED cohorts. J Coll
Physicians Surg Pak. 24:615–619. 2014.PubMed/NCBI
|
|
32
|
Sperling S, Grimm CH, Dunkel I, Mebus S,
Sperling HP, Ebner A, Galli R, Lehrach H, Fusch C, Berger F and
Hammer S: Identification and functional analysis of CITED2
mutations in patients with congenital heart defects. Hum Mutat.
26:575–582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou L, Liu Y, Lu L, Lu X and Dixon RA:
Cardiac gene activation analysis in mammalian non-myoblasic cells
by Nkx2-5, Tbx5, Gata4 and Myocd. PLoS One. 7:e480282012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Joseph EM: Zebrafish IRX1b in the
embryonic cardiac ventricle. Dev Dyn. 231:720–726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dand N, Schulz R, Weale ME, Southgate L,
Oakey RJ, Simpson MA and Schlitt T: Network-informed gene ranking
tackles genetic heterogeneity in exome-sequencing studies of
monogenic disease. Hum Mutat. 36:1135–1144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Y, Shi N, Li M, Chen F and Niu H:
Applications of Next-generation sequencing in systemic autoimmune
diseases. Genomics Proteomics Bioinformatics. 13:242–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu YT, Lee YC and Soong BW: What we have
learned from the next-generation sequencing: Contributions to the
genetic diagnoses and understanding of pathomechanisms of
neurodegenerative diseases. J Neurogenet. 29:103–112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siragam V, Cui X, Masse S, Ackerley C,
Aafaqi S, Strandberg L, Tropak M, Fridman MD, Nanthakumar K, Liu J,
et al: TMEM43 mutation p.S358L alters intercalated disc protein
expression and reduces conduction velocity in arrhythmogenic right
ventricular cardiomyopathy. PLoS One. 9:e1091282014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Houweling AC, Dildrop R, Peters T,
Mummenhoff J, Moorman AF, Rüther U and Christoffels VM: Gene and
cluster-specific expression of the Iroquois family members during
mouse development. Mech Dev. 107:169–174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bruneau BG, Bao ZZ, Fatkin D, Xavier-Neto
J, Georgakopoulos D, Maguire CT, Berul CI, Kass DA, Kuroski-de Bold
ML, de Bold AJ, et al: Cardiomyopathy in Irx4-deficient mice is
preceded by abnormal ventricular gene expression. Mol Cell Biol.
21:1730–1736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Christoffels VM, Keijser AG, Houweling AC,
Clout DE and Moorman AF: Patterning the embryonic heart:
Identification of five mouse Iroquois homeobox genes in the
developing heart. Dev Biol. 224:263–274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bao ZZ, Bruneau BG, Seidman JG, Seidman CE
and Cepko CL: Regulation of chamber-specific gene expression in the
developing heart by Irx4. Science. 283:1161–1164. 1999. View Article : Google Scholar : PubMed/NCBI
|