Introduction
Cancer is a global health problem, and is gradually
becoming the primary contributor to mortality rates in women and
men in developing and developed countries (1). In previous years, chemotherapy,
radiotherapy, surgery and immunotherapy have been the primary
treatment methods for cancer (2).
Chemotherapy has always been considered the principle and most
common method, however, it exhibits drawbacks, including the low
concentration of chemotherapeutic drug at the tumor site, and
serious side effects due to chemotherapeutic drugs not specifically
targeting tumors and always affecting normal cells/tissues.
Furthermore, chemotherapeutic drugs only possess a limited capacity
to penetrate into the parenchyma of solid tumors. Due to these
limitations, improving the efficiency of chemotherapeutic drugs is
an urgent requirement (3,4).
As drugs for the treatment of solid tumors can only
penetrate 3–5 cell diameters from blood vessels (5), the low concentration of anticancer
drugs at the tumor site is a substantial obstacle for tumor
treatment, which limits the anticancer efficacy and suggests that
the effective drug concentration at the tumor site is markedly
lower, compared with the dose of exposure. In previous decades,
cancer studies have focused on tumor-targeting peptides, which
support drug penetration. Conventional RGD-peptides bind
selectively to integrins αvβ3 and αvβ5, which are excessively
expressed on several types of tumor (6). These peptides successfully deliver
drugs, nanoparticles, viruses and biologicals to the blood vessels
(7), and marginally increase
accumulation within the tumor, however, their limited loading
capacity in the tumor parenchyma remains a challenge for cancer
therapy.
In previous years, improved novel peptides aimed at
identifying tumor blood vessels and tumor cells appear to be more
effective in the treatment of tumors, compared with traditional
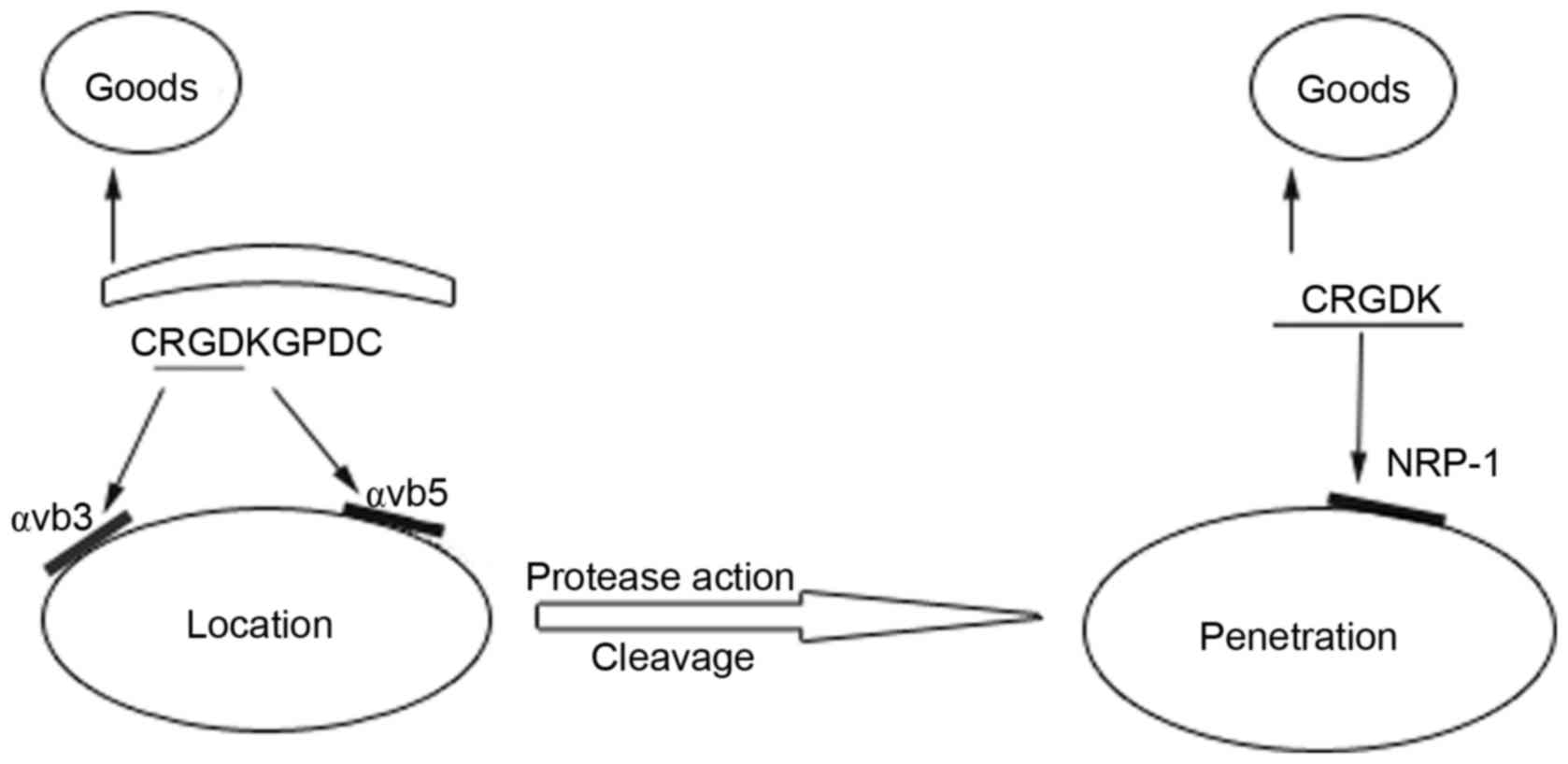
penetrating peptides (7,8). The iRGD (CRGDKGPDC) peptide has been
developed on the basis of RGD peptides and is composed of nine
amino acids. It first binds to αv integrins, which are expressed on
tumor cells and vessels. Subsequently, it is cleaved by proteases
to expose the neuropilin-1 (NRP-1)-binding CRGDK/R, which
effectively triggers the tumor penetration process (7,9). The
peptide is used to increase the pore diameter and surface area of
tumor blood vessels, and to reduce the pressure effect in the tumor
interstitium, increasing the rate of diffusion of small molecule
drugs (10–12). The scientific interest in iRGD has
resulted from its binding to NRP-1 in particular, as this triggers
extravasation (13). Furthermore,
iRGD specifically penetrates into angiogenic vessels and tumor
tissues. Due to this novel delivery system and the low toxicity to
normal cells, iRGD has attracted significant attention (14). Further advantages of iRGD arise
from its simple and low-cost synthesis. Coupled with iRGD, drugs,
nanoparticles and proteins can be effectively delivered to the
tumor site, which reduces side effects. At present, the iRGD
peptide is widely used in the diagnosis and treatment of
tumors.
Initial peptide identification
The tumor vasculature is indispensable in the
process of tumor growth and metastasis, as it not only provides the
tumor with the necessary nutrients and oxygen, but also assists in
the transportation of cancer cells to adjacent or distant organs
for tumor metastasis (15).
Therefore, the tumor vasculature can be considered as the target of
tumor diagnosis and treatment. Phage peptides, first identified by
Pasqualini and Ruoslahti in 1996, have been widely used (16). The homing peptide on the phage coat
protein combines with target molecules in vascular endothelial
cells. A mass screening of the phage allows for the selective
detection of targeted phage peptides, which combine into tumor
blood vessels (17–19). Using this method, an increasing
number of peptides targeting the tumor vasculature can be
identified. Substantial experimental data have shown that these
peptides can deliver drugs to tumors and act as effective image
contrast agents (20,21), thus are important in tumor
diagnosis and treatment. RGD and NGR are the two most well-known
short peptides targeting tumor vasculature in phages. iRGD has been
developed as a novel type of tumor-penetrating peptide, which not
only targets tumor blood vessels, but can also deliver drugs deep
into the tumors. The iRGD peptide results from the connecting of
RGD with NRP-1 peptide ligands. iNGR was designed based on known
sequence elements (22).
Mechanism of iRGD penetration
The RGD sequence (Arg-Gly-Asp) selectively binds to
αvβ3 and αvβ5 integrins which are overexpressed in the tumor
vasculature (23). Further studies
have shown that connecting the peptide ligands of NRP-1 and RGD
improves drug delivery to the extravascular tissues in addition to
the tumor blood vessels. The target peptide CendR, can only be
activated when the C-terminus of the motif is exposed.
Subsequently, the activated CendR can bind to NRP-1, which is
expressed at high levels on tumor tissues, to initiate deep tissue
penetration (8). In general, iRGD
acts through the following steps: RGD peptides bind to αvβ3 and
αvβ5 integrins on tumor cells and tumor blood vessel endothelial
cells. Secondly, under the action of cellular proteases, the
peptide is cleaved to expose the activated CendR motif at the
C-terminus. Finally, CendR binds to NRP-1, which triggers tumor
tissue penetration carrying the cargo at the N-terminus(Fig. 1).
iRGD for tumor diagnosis and treatment
iRGD in tumor preclinical
diagnosis
In 2009, Sugahara et al intravenously infused
iRGD-linked iron oxide nanoworms into tumor-burdened mice. A low
MRI signal intensity was detected in the entire tumor, which was
confirmed using MRI imaging. Therefore, the detection methods
consistently indicated the superiority of iRGD over the RGD peptide
in terms of its ability to transfer diagnostic agents to tumors
(7). In 2011, Ye et al
synthesized the two near-infrared fluorescence-labeled iRGD
peptides, Ac-Cys (IRDye800CW)-iRGD and DOTA-Cys (IRDye800CW)-iRGD.
These peptides were injected intravenously into mice bearing
MDA-MB-435 tumors, which revealed the tumor locations (24). In 2015, iRGD-modified porous
silicon nanoparticles were verified for cancer theranostics through
in vitro and in vivo experiments (25). This revealed the potential of the
iRGD peptide as a diagnostic reagent in clinical practice.
iRGD mediates drug delivery for cancer
treatment
The mode of action of iRGD is well understood and
its preclinical application is developing widely. Substantial
investigations have been performed to investigate the application
of iRGD as an antitumor agent in vivo and in vitro,
which are briefly summarized in the following.
Sugahara et al (26) reported that co-injection with iRGD
enhances the antitumor effect of free drugs without a chemical
entity, and reduced the side effects of drugs in mice bearing five
tumors, including human breast cancer, prostate cancer and
pancreatic adenocarcinoma. Investigations have revealed the
simplicity and effectiveness of the co-injection of iRGD and
tumor-targeting drugs. This combination does not alter the chemical
structure of the drugs, thus avoiding structural alterations, which
may reduce the activity of the functional drugs. These findings
also encourage the co-administration of iRGD in various forms of
drug applications for future investigations in the treatment of
different types of tumor. Sugahara et al also reported that
iRGD effectively improved the curative effect of doxorubicin (DOX)
in inhibiting peritoneal carcinomatosis. It was confirmed that the
intratumoral aggregation of intraperitoneally co-injected DOX in
mice was ~1.5 times higher. Based on this finding, it was concluded
that intraperitoneally co-administered iRGD can be regarded as a
simple and effective way to inhibit the progression of peritoneal
carcinomatosis and improve the effect of chemotherapy (26).
In studies involving transplantation models,
cisplatin (CDDP) was co-administered with iRGD resulting in an
increase in survival rates by 30% and a substantial reduction in
the toxicity of the chemotherapeutic drugs (27).
In 2013, Gu et al associated iRGD with
paclitaxel-loaded nanoparticles for drug delivery to the C6 glioma
parenchyma. Mice bearing C6 glioma cells, which were treated with
the iRGD-associated nanoparticles, had markedly increased survival
rates, compared with the mice, which had not received the
iRGD-associated treatment (28).
iRGD has also been selected as a targeting ligand
and has been used for the modification of sterically-stabilized
liposomes (SSLs). Chemotherapeutic drugs (CLA-PTX or DOX) were
loaded to these liposomes to yield iRGD-SSL-CLA-PTX or
iRGD-SSL-DOX, which were assessed in C57BL/6 mice bearing B16-F10
tumors, respectively. The results showed that the tumor volumes
were significantly reduced upon iRGD-SSL-CLA-PTX or iRGD-SSL-DOX
treatment, compared with tumors in mice, which had not received the
corresponding iRGD modification (29,30).
For the efficient delivery of the DOX-polymer to
tumor tissues, Wang et al synthesized an iRGD-PPCD
conjugate, which was injected intravenously into mice with
subcutaneously implanted C6-glioma-tumors. These in vivo
investigations showed that iRGD-PPCD has a superior penetrating
capacity, compared with RGD. Statistical analysis revealed that the
median duration of survival of the mice following treatment with
iRGD-mediated drugs was longer, compared with that following
treatment with RGD-mediated drugs, indicating that iRGD improved
the antitumor effect and penetration efficacy (31).
Akashi et al developed pancreatic cancer
models. They treated tumor-burdened nude mice with a combination of
gemcitabine (GEM) and iRGD, and observed prominent tumor reduction,
compared with mice treated with GEM only in the cell line-based
xenografts (32).
Derived from the anti-apoptotic protein, Bfl-1,
amphipathic tail-anchoring peptide (ATAP) is used as a
mitochondrial targeting peptide. The ATAP modifications,
ATAP-iRGD-M8 and ATAP-iRGD exhibit improved stability and
solubility, and improved capacity in selective delivery to tumor
tissues. The two peptides significantly decreased tumor sizes in
nude mice burdened with DU145 and PC3 cells (14).
Puig-Saus et al (33) inserted iRGD into an oncolytic
adenovirus to increase adenovirus penetration to the tumor mass. By
inserting the peptide, nude mice bearing subcutaneous A549 and MIA
PaCa-2 xenograft tumors showed improved tumor growth control,
compared with mice without iRGD, and extended mean survival rates.
These findings effectively demonstrated that the iRGD peptide
enhances transduction, intratumoral dissemination and adenovirus
infiltration into the tumor parenchyma to exert an antineoplastic
effect (26,33).
Porous silicon (PSi) has been coupled with iRGD as a
drug delivery carrier. The final sorafenib-loaded PSi-iRGD has
shown a more marked antitumor effect in vitro, which again
confirmed the above-described effect of iRGD (34).
Epidermal growth factor receptors (EGFRs), which are
closely associated with the prognosis of cancer, are expressed at
high levels on the surfaces of different human tumor cells,
including gastric cancer and gastric lung cancer. It has been
reported that anti-EGFR-iRGD, a composite protein targeting αvβ3,
αvβ5, NRP-1 and EGFRs, shows potent tumor tissue penetration
ability. In vivo investigations of mice bearing subcutaneous
BGC-823, which received intraperitoneal injections, showed that the
combination of DOX with anti-EGFR-iRGD was more effective at
inhibiting BGC-823 MCS growth, compared with chemotherapeutic drugs
(35).
A report in 2015 described the co-administration of
DOX-loaded, CDDP-crosslinked and polysaccharide-based nanoparticles
(Dex-SA-DOX-CDDP) with iRGD, afford marked advantages in inhibiting
the tumor growth and metastasis of murine colorectal carcinoma and
metastatic mammary carcinoma, compared with the corresponding
nanoparticles without iRGD co-administration (36). Peng and Kopeček conjugated matrix
metalloproteinase-2 with iRGD, and the resulting novel
tumor-penetrating peptide conjugates exhibited the highest
cytotoxicity towards DU-145 cells, which prompted further
investigation (37). Zhang et
al established a human non-small cell lung cancer xenograft
nude mice model with A549 cells. Treatment comprising a combination
of GEM and iRGD was applied, which inhibited the growth of tumors,
which were not sensitive to the same dose of GEM alone. Apoptotic
cells in the tumor tissues were detected using a TUNEL assay and
the statistical analysis revealed that the highest apoptotic index
was for the groups treated with the GEM/iRGD combination (38).
Wang et al demonstrated that iRGD-modified
nanoparticles improved the tumor absorption of nanoparticles when
injected intravenously. Intratumor injection confined the
nanoparticles to the tumor to a greater degree, compared with the
free drug (25). This study
revealed another application of the iRGD peptide.
iRGD mediates biological product
delivery for cancer treatment
In addition to chemotherapeutic drugs, Lao et
al introduced the iRGD peptide sequence into the C-terminus of
the thymopoietin pentapeptide, TP5, to improve the poor penetration
ability of TP5. The findings suggested that the injections of
TP5-iRGD inhibited melanoma progression more effectively, compared
with the native peptide. In addition, computational observations of
the mechanism of activity confirmed the potential of the peptide
for tumor therapy (39).
Chen et al reported that the cell death
domain (CDD) was effective for inducing cell apoptosis, therefore,
CDD was fused to iRGD to obtain a tissue-penetrating protein. By
injecting the protein intratumorally into mice bearing
orthotopically-implanted MCF-10CA1a breast tumors, CDD-iRGD
inhibited the growth of tumors, with a decrease in the tumor
volumes by 77% (40). These
studies reflect potential applications of iRGD coupled with
biological products. The conjugation of iRGD with DSPE-PEG (2000)
nanomicelles-salinomycin (M-SAL) to yield M-SAL-iRGD also exhibited
a prominent increase in cytotoxicity in cancer stem cells and liver
cancer cells (41) (Table I).
 | Table I.iRGD as a carrier applied in tumor
treatment. |
Table I.
iRGD as a carrier applied in tumor
treatment.
| Author, year | Load carried by
iRGD | Tumor | (Refs.) |
|---|
|
| Drug |
|
|
| Sugahara et
al, 2010 | DOX | Peritoneal
carcinomatosis | (26) |
| Song et al,
2012 | CDDP | A549 | (27) |
| Du et al,
2014 |
iRGD-SSL-CLA-PTX | B16-F10 | (29) |
| Yu et al,
2013 | iRGD-SSL-DOX | B16-F10 | (30) |
| Wang et al,
2014 | Paclitaxel,
iRGD-PPCD | C6 glioma | (31) |
| De et al,
2014 | ATAP-iRGD-M8 | DU145 and PC3 | (14) |
| Sugahara et
al, 2010; | Oncolytic
adenovirus | A549 and MIA
PaCa-2 | (26,33) |
| Puig-Saus et
al, 2014 |
|
|
|
| Sha et al,
2015 | DOX-anti-EGFRs | BGC-823 | (35) |
| Li et al,
2015 |
Dex-SA-DOX-CDDP | Colorectal
carcinoma | (36) |
| Zhang et al,
2015 | Gemcitabine | A549 | (38) |
|
| Biological
product |
| Lao et al,
2014 | Thymopoietin
pentapeptide | Melanoma | (39) |
| Chen et al,
2013 | Cell death
domain | MCF-10CA1a | (40) |
| Mao et al,
2015 | M-SAL | Cancer stem
cells | (41) |
iRGD inhibits metastasis
Metastasis is the primary cause of cancer-associated
mortality, and studies have focused increasingly on the development
of methods for improving resistance and treating cancer metastasis.
The iRGD peptide itself does not affect the survival of cells
(42), however, it has been
reported to inhibit the metastasis of tumors.
As a tissue-penetrating peptide, iRGD efficiently
delivers drugs and biological products to various tumors in rodent
models. In 2015, Sugahara et al suggested that the iRGD
peptide also spontaneously inhibits tumor metastasis, which was
confirmed experimentally. Depending on NRP-1, iRGD inhibits
spontaneous tumor metastasis, but has no effect on the size of
primary tumors. Sugahara et al demonstrated this by
developing nude mice models via the orthotopic transplantation of
GFP-PC-3 and LM-PmC cells, which led to spontaneous metastases in
different organs. The peptide was then injected intravenously into
the tumor-bearing mice and circulated for 1 h. Based on the
analysis of a series of in vivo and in vitro
experiments, it was confirmed that the iRGD peptide inhibited tumor
cells migration (42).
In 2015, Hamilton et al reported that iRGD
nanoparticles significantly inhibited tumor development as long as
they were applied in the early metastatic phase of tumor
progression (43). Ni et al
confirmed that only decorated nanocrystallites achieved complete
intratumoral transfer and accessed cancer stem cells in the murine
model, leading to the inhibition of 4T1 proliferation and
metastasis (44).
Conclusion
iRGD consists of two motifs: The RGD motif, which
binds to αv integrins aiming to target drugs accumulated in tumor
tissues, and the CendR motif, which binds to NRP-1 to effectively
inhibit tumor metastasis. In combination with contrast agents,
chemotherapeutic drugs, nanoparticles or proteins, the iRGD peptide
can be effectively delivered to the tumor site, reduce the side
effects of drugs and improve the curative efficacy of drugs. In
previous years, the application of the iRGD peptide for cancer
diagnosis and therapy was assessed in pre-clinical in vivo
and in vitro experiments, revealing promising results for
tumor treatment. In addition to the progress in phage library
technology and the development of new screening technologies, the
iRGD tumor-tissue-penetrating peptide is likely to be more widely
applied in the clinical diagnosis and treatment of tumors.
It is noteworthy that the iRGD peptide was
investigated in a mice model with subcutaneously transplantated
tumors, whereas tumor xenograft models did not actually imitate the
complex microenvironment of the cancer cells originated from the
organ. Therefore, the interpretation of experimental results can
have significant limitations. The microenvironment is important for
balancing the factors of promoting angiogenesis and
anti-angiogenesis, which determine the heterogeneity of
angiogenesis in tumors (45).
Studies, including a study by Hoffman et al (46–48)
independently confirmed that tumor-penetrating peptides are
important in a nude mouse model with orthotopically-transplanted
tumors.
A variety of inflammatory cells, including
lymphocytes, neutrophils and macrophages, infiltrate the tumor
microenvironment and can secrete different types of cytokines,
including growth factors and chemokines (49). Almost all of these inflammatory
mediators are involved in tumor angiogenesis. Chronic inflammation
is usually accompanied by regeneration and angiogenesis, which may
increase the risk of certain types of cancer. Based on reports that
tumors and inflammatory tissues have certain biomarkers in common,
it is likely that tumor blood vessel-targeted peptides are also
combined in the blood vessels of inflammatory diseases, similar to
inflammation being associated with the formation of blood vessels.
Lahdenranta et al (50)
reported that RGD and NGR can be combined into the vessels of
hypoxia-induced retinopathy and Buehler et al (51) indicated that the two peptides
target the blood vessels of ischemic hearts. These findings further
increase the scope of possible applications of the
tumor-penetrating peptide, iRGD.
In conclusion, the iRGD peptide interacts with
integrin and its ligand, has promising application prospects, and
identifies tumors early for diagnosis and treatment. iRGD can be
combines with chemical drugs, immune modulators and cytokines to
effectively penetrate tumors, and exert more marked antineoplastic
effects. The effect of the peptide has been confirmed by a
substantial number of animal experiments, confirming significant
effects and minimal side effects. Therefore, iRGD offers promise
for improving the treatment of tumors in humans. However, for final
application in cancer treatment, iRGD requires further
investigation and successful outcomes in clinical trials.
Acknowledgements
This review was supported by grants from the Nature
Science Foundation of China (grant no. 81301946), the China
Postdoctoral Science Foundation Funded Project (grant nos.
2013M540467 and 53470135), the Nature Science Foundation of Jiangsu
Province (grant no. BK2012146) and the Jiangsu Provincial Office of
Education Foundation (grant no. JHB2012-34).
References
|
1
|
Rahimi Z, Kasraei R, Najafi F, Tanhapoor
M, Abdi H, Rahimi Z, Vaisi-Raygani A, Aznab M and Moradi M: Cancer
notification at a referral hospital of Kermanshah, Western Iran
(2006–2009). Asian Pac J Cancer Prev. 16:133–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J, Zhang Q, Li K, Yin H and Zheng JN:
Composite peptide-based vaccines for cancer immunotherapy (Review).
Int J Mol Med. 35:17–23. 2015.PubMed/NCBI
|
|
3
|
Rabbani-Chadegani A, Paydar P, Amirshenava
M and Aramvash A: An in vitro study on the effect of vinca
alkaloid, vinorelbine, on chromatin histone, HMGB proteins and
induction of apoptosis in mice non-adherent bone marrow cells. Drug
Chem Toxicol. 38:220–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hai-Tao Z, Hui-Cheng L, Zheng-Wu L and
Chang-Hong G: A tumor-penetrating peptide modification enhances the
antitumor activity of endostatin in vivo. Anticancer Drugs.
22:409–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minchinton AI and Tannock IF: Drug
penetration in solid tumours. Nat Rev Cancer. 6:583–592. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiong JP, Stehle T, Diefenbach B, Zhang R,
Dunker R, Scott DL, Joachimiak A, Goodman SL and Arnaout MA:
Crystal structure of the extracellular segment of integrin alpha
Vbeta3. Science. 294:339–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugahara KN, Teesalu T, Karmali PP,
Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF and
Ruoslahti E: Tissue-penetrating delivery of compounds and
nanoparticles into tumors. Cancer Cell. 16:510–520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teesalu T, Sugahara KN, Kotamraju VR and
Ruoslahti E: C-end rule peptides mediate neuropilin-1-dependent
cell, vascular, and tissue penetration. Proc Natl Acad Sci USA.
106:16157–16162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Emerich DF, Snodgrass P, Dean RL,
Lafreniere D, Agostino M, Wiens T, Xiong H, Hasler B, Marsh J, Pink
M, et al: Bradykinin modulation of tumor vasculature: I. activation
of B2 receptors increases delivery of chemotherapeutic agents into
solid peripheral tumors, enhancing their efficacy. J Pharmacol Exp
Ther. 296:623–631. 2001.PubMed/NCBI
|
|
11
|
Emerich DF, Dean RL, Snodgrass P,
Lafreniere D, Agostino M, Wiens T, Xiong H, Hasler B, Marsh J, Pink
M, et al: Bradykinin modulation of tumor vasculature: II.
activation of nitric oxide and phospholipase A2/prostaglandin
signaling pathways synergistically modifies vascular physiology and
morphology to enhance delivery of chemotherapeutic agents to
tumors. J Pharmacol Exp Ther. 296:632–641. 2001.PubMed/NCBI
|
|
12
|
Li CJ, Miyamoto Y, Kojima Y and Maeda H:
Augmentation of tumour delivery of macromolecular drugs with
reduced bone marrow delivery by elevating blood pressure. Br J
Cancer. 67:975–980. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kadonosono T, Yamano A, Goto T, Tsubaki T,
Niibori M, Kuchimaru T and Kizaka-Kondoh S: Cell penetrating
peptides improve tumor delivery of cargos through
neuropilin-1-dependent extravasation. J Control Release. 201:14–21.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De G, Ko JK, Tan T, Zhu H, Li H and Ma J:
Amphipathic tail-anchoring peptide is a promising therapeutic agent
for prostate cancer treatment. Oncotarget. 5:7734–7747. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nyberg P, Salo T and Kalluri R: Tumor
microenvironment and angiogenesis. Front Biosci. 13:6537–6553.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pasqualini R and Ruoslahti E: Organ
targeting in vivo using phage display peptide libraries. Nature.
380:364–366. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li ZJ and Cho CH: Development of peptides
as potential drugs for cancer therapy. Curr Pharm Des.
16:1180–1189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Enbäck J and Laakkonen P: Tumour-homing
peptides: Tools for targeting, imaging and destruction. Biochem Soc
Trans. 35:780–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trepel M, Pasqualini R and Arap W: Chapter
4. Screening phage-display peptide libraries for vascular targeted
peptides. Methods Enzymol. 445:83–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruoslahti E, Bhatia SN and Sailor MJ:
Targeting of drugs and nanoparticles to tumors. J Cell Biol.
188:759–768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laakkonen P and Vuorinen K: Homing
peptides as targeted delivery vehicles. Integr Biol (Camb).
2:326–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Myrberg H, Zhang L, Mäe M and Langel U:
Design of a tumor-homing cell-penetrating peptide. Bioconjug Chem.
19:70–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Assa-Munt N, Jia X, Laakkonen P and
Ruoslahti E: Solution structures and integrin binding activities of
an RGD peptide with two isomers. Biochemistry. 40:2373–2378. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye Y, Zhu L, Ma Y, Niu G and Chen X:
Synthesis and evaluation of new iRGD peptide analogs for tumor
optical imaging. Bioorg Med Chem Lett. 21:1146–1150. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CF, Sarparanta MP, Mäkilä EM, Hyvönen
ML, Laakkonen PM, Salonen JJ, Hirvonen JT, Airaksinen AJ and Santos
HA: Multifunctional porous silicon nanoparticles for cancer
theranostics. Biomaterials. 48:108–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugahara KN, Teesalu T, Karmali PP,
Kotamraju VR, Agemy L, Greenwald DR and Ruoslahti E:
Coadministration of a tumor-penetrating peptide enhances the
efficacy of cancer drugs. Science. 328:1031–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song W, Li M, Tang Z, Li Q, Yang Y, Liu H,
Duan T, Hong H and Chen X: Methoxypoly(ethylene
glycol)-block-poly(L-glutamic acid)-loaded cisplatin and a
combination with iRGD for the treatment of non-small-cell lung
cancers. Macromol Biosci. 12:1514–1523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu G, Gao X, Hu Q, Kang T, Liu Z, Jiang M,
Miao D, Song Q, Yao L, Tu Y, et al: The influence of the
penetrating peptide iRGD on the effect of paclitaxel-loaded
MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials.
34:5138–5148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du R, Zhong T, Zhang WQ, Song P, Song WD,
Zhao Y, Wang C, Tang YQ, Zhang X and Zhang Q: Antitumor effect of
iRGD-modified liposomes containing conjugated linoleic
acid-paclitaxel (CLA-PTX) on B16-F10 melanoma. Int J Nanomedicine.
9:3091–3105. 2014.PubMed/NCBI
|
|
30
|
Yu KF, Zhang WQ, Luo LM, Song P, Li D, Du
R, Ren W, Huang D, Lu WL, Zhang X and Zhang Q: The antitumor
activity of a doxorubicin loaded, iRGD-modified
sterically-stabilized liposome on B16-F10 melanoma cells: In vitro
and in vivo evaluation. Int J Nanomedicine. 8:2473–2485.
2013.PubMed/NCBI
|
|
31
|
Wang K, Zhang X, Liu Y, Liu C, Jiang B and
Jiang Y: Tumor penetrability and anti-angiogenesis using
iRGD-mediated delivery of doxorubicin-polymer conjugates.
Biomaterials. 35:8735–8747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akashi Y, Oda T, Ohara Y, Miyamoto R,
Kurokawa T, Hashimoto S, Enomoto T, Yamada K, Satake M and Ohkohchi
N: Anticancer effects of gemcitabine are enhanced by
co-administered iRGD peptide in murine pancreatic cancer models
that overexpressed neuropilin-1. Br J Cancer. 110:1481–1487. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Puig-Saus C, Rojas LA, Laborda E, Figueras
A, Alba R, Fillat C and Alemany R: iRGD tumor-penetrating
peptide-modified oncolytic adenovirus shows enhanced tumor
transduction, intratumoral dissemination and antitumor efficacy.
Gene Ther. 21:767–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang CF, Mäkilä EM, Kaasalainen MH, Liu D,
Sarparanta MP, Airaksinen AJ, Salonen JJ, Hirvonen JT and Santos
HA: Copper-free azide-alkyne cycloaddition of targeting peptides to
porous silicon nanoparticles for intracellular drug uptake.
Biomaterials. 35:1257–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sha H, Zou Z, Xin K, Bian X, Cai X, Lu W,
Chen J, Chen G, Huang L, Blair AM, et al: Tumor-penetrating peptide
fused EGFR single-domain antibody enhances cancer drug penetration
into 3D multicellular spheroids and facilitates effective gastric
cancer therapy. J Control Release. 200:188–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Tang Z, Zhang D, Sun H, Liu H, Zhang
Y, Zhang Y and Chen X: Doxorubicin-loaded polysaccharide
nanoparticles suppress the growth of murine colorectal carcinoma
and inhibit the metastasis of murine mammary carcinoma in rodent
models. Biomaterials. 51:161–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng ZH and Kopeček J: Synthesis and
activity of tumor-homing peptide iRGD and histone deacetylase
inhibitor valproic acid conjugate. Bioorg Med Chem Lett.
24:1928–1933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Q, Zhang Y, Li K, Wang H, Li H and
Zheng J: A novel strategy to improve the therapeutic efficacy of
gemcitabine for non-small cell lung cancer by the tumor-penetrating
peptide iRGD. PLoS One. 10:e01298652015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lao X, Li B, Liu M, Chen J, Gao X and
Zheng H: Increased antitumor activity of tumor-specific peptide
modified thymopentin. Biochimie. 107:277–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen R, Braun GB, Luo X, Sugahara KN,
Teesalu T and Ruoslahti E: Application of a proapoptotic peptide to
intratumorally spreading cancer therapy. Cancer Res. 73:1352–1361.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mao X, Liu J, Gong Z, Zhang H, Lu Y, Zou
H, Yu Y, Chen Y, Sun Z, Li W, et al: iRGD-conjugated DSPE-PEG2000
nanomicelles for targeted delivery of salinomycin for treatment of
both liver cancer cells and cancer stem cells. Nanomedicine (Lond).
10:2677–2695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sugahara KN, Braun GB, de Mendoza TH,
Kotamraju VR, French RP, Lowy AM, Teesalu T and Ruoslahti E:
Tumor-penetrating iRGD peptide inhibits metastasis. Mol Cancer
Ther. 14:120–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hamilton AM, Aidoudi-Ahmed S, Sharma S,
Kotamraju VR, Foster PJ, Sugahara KN, Ruoslahti E and Rutt BK:
Nanoparticles coated with the tumor-penetrating peptide iRGD reduce
experimental breast cancer metastasis in the brain. J Mol Med
(Berl). 93:991–1001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ni D, Ding H, Liu S, Yue H, Bao Y, Wang Z,
Su Z, Wei W and Ma G: Superior intratumoral penetration of
paclitaxel nanodots strengthens tumor restriction and metastasis
prevention. Small. 11:2518–2526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cao Y: Angiogenesis: What can it offer for
future medicine? Exp Cell Res. 316:1304–1308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoffman JA, Giraudo E, Singh M, Zhang L,
Inoue M, Porkka K, Hanahan D and Ruoslahti E: Progressive vascular
changes in a transgenic mouse model of squamous cell carcinoma.
Cancer Cell. 4:383–391. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh RK, Bucana CD, Gutman M, Fan D,
Wilson MR and Fidler IJ: Organ site-dependent expression of basic
fibroblast growth factor in human renal cell carcinoma cells. Am J
Pathol. 145:365–374. 1994.PubMed/NCBI
|
|
48
|
Li ZJ, Wu WK, Ng SS, Yu L, Li HT, Wong CC,
Wu YC, Zhang L, Ren SX, Sun XG, et al: A novel peptide specifically
targeting the vasculature of orthotopic colorectal cancer for
imaging detection and drug delivery. J Control Release.
148:292–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ariztia EV, Lee CJ, Gogoi R and Fishman
DA: The tumor microenvironment: Key to early detection. Crit Rev
Clin Lab Sci. 43:393–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lahdenranta J, Sidman RL, Pasqualini R and
Arap W: Treatment of hypoxia-induced retinopathy with targeted
proapoptotic peptidomimetic in a mouse model of disease. FASEB J.
21:3272–3278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Buehler A, van Zandvoort MA, Stelt BJ,
Hackeng TM, Schrans-Stassen BH, Bennaghmouch A, Hofstra L,
Cleutjens JP, Duijvestijn A, Smeets MB, et al: cNGR: A novel homing
sequence for CD13/APN targeted molecular imaging of murine cardiac
angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 26:2681–2687.
2006. View Article : Google Scholar : PubMed/NCBI
|