Introduction
Metabolic syndrome refers to a subset of metabolic
abnormalities, which may increase the likelihood of developing
cardiovascular disease and type II diabetes (1). In recent years, metabolic syndrome
has become an increasing public health concern. It is estimated
that ~250 million people will suffer from type II diabetes by the
year 2020 (2). Therefore,
determining appropriate treatment strategies is particularly
important for public health. Currently, the specific factors that
lead to type II diabetes remain unknown. However, it is clear that
insulin resistance serves a major role in the development of type
II diabetes (3). Metabolic
syndrome is characterized by insulin resistance, which is defined
as impaired insulin function in target organs (4). The liver is an important
insulin-sensitive organ, particularly in the regulation of glucose
homeostasis. Hepatic insulin resistance is believed to induce a
series of systematic consequences, and clinical observations have
confirmed that insulin resistance is closely associated with
hepatic pathologies (5).
Tea is a popular beverage consumed worldwide.
Compared with other types of tea, green tea has been reported to
exert various beneficial effects on metabolic syndrome (6). For instance, green tea consumption
has been demonstrated to improve fat oxidation, which prevents
obesity and insulin resistance in healthy individuals (7). In a previous clinical study, frequent
consumption of green tea was associated with a decreased risk of
type II diabetes, and it improved metabolic syndrome by decreasing
insulin resistance and increasing glucose tolerance (6).
Polyphenolic compounds extracted from green tea have
been demonstrated to increase insulin sensitivity (8–11).
Among these polyphenolic compounds, epigallocatechin-3-gallate
(EGCG) reduces the fecal lipid content and prompts fecal
cholesterol and fat excretion in rats fed on a high fat diet
(12). In addition, a considerable
number of studies have demonstrated that tea polyphenols decrease
obesity and hyperlipidemia, primarily by inhibiting lipolysis and
enhancing fat emulsification (13,14).
However, a limited number of studies have investigated the
mechanisms associated with green tea consumption and glucose
metabolism in insulin-sensitive organs.
In order to investigate the specific effects of tea
polyphenols on insulin resistance, the present study examined the
effect of EGCG on insulin resistance in human HepG2 cells exposed
to high glucose.
Materials and methods
EGCG
EGCG was purchased from Shanghai SolarBio Bioscience
and Technology Co., Ltd. (Shanghai, China). To determine the
dose-response association of EGCG on glycogen synthesis and
lipogenesis, a series of molar concentrations (0.01, 0.1, 1.0 and
10 µM) of EGCG were prepared as previously reported (15).
Cell culture and treatments
The human hepatocyte cell line HepG2 (ref. no.
ATCCHB8065; American Type Culture Collection, Manassas, VA, USA)
were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing normal
glucose (5 mM), supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin (Thermo
Fisher Scientific, Inc.), in an incubator at 37°C and 5%
CO2. HepG2 cells were cultured in complete media (CM)
with 10% FBS until 70% confluence was reached. At 24 h prior to all
experimental procedures, 5 or 30 mM D-glucose (Thermo Fisher
Scientific, Inc.), termed low glucose (LG) and high glucose (HG)
respectively, were added to cells.
Cells (4×103) were seeded on 24-well
plates for all assays. When the cells reached 90% confluence, the
CM was discarded and starvation medium (SM) containing 0.5% FBS was
added. Following incubation at 37°C with SM for 6 h, 100 nM insulin
(Eli Lilly Australia Pty, Ltd., Melrose Park, NSW, Australia) was
added to each well, followed by the addition of 0.01–10 µM EGCG to
the appropriate wells in duplicate. Plates were then maintained for
24 h in 5% CO2 at 37°C. This treatment procedure was
used for the purposes of all experiments in the present study.
Isolation of mouse primary
hepatocytes
A single male C57BL/6J mouse (weight, 20–25 g; age,
12 weeks) was obtained from the Peking University Health Science
Center (Beijing, China). It was maintained in a constructed shelter
at 20–26°C, 30–60% humidity, 12:12 light/dark cycle, and provided
with adequate supplies of food and fresh water. All experiments
were approved by the Institutional Animal Care and Use Committee at
the Fourth Military Medical University (Xi'an, China; no.
20150302). The liver was removed and primary hepatocytes were
isolated using a two-step collagenase perfusion method (0.2 mg/ml
type IV collagenase in Hanks' balanced salt solution;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) as described
previously (15). The hepatocytes
were collected by centrifugation at 100 × g for 8 min at room
temperature. The cells were immediately re-suspended in pre-warmed
William's E medium (Merck Millipore) supplemented with 10% FBS, 20
ng/ml dexamethasone (Merck Millipore), an insulin (5
mg/l)-transferrin (5 mg/l)-sodium selenite (5 g/l) solution (Merck
Millipore), and 10 g/ml gentamicin (Invitrogen; Thermo Fisher
Scientific, Inc.). The hepatocytes were then plated in
collagen-coated 25 cm2 flasks at 1×106
cells/flask.
Measurement of glycogen content
Following pretreatment with 5 and 30 mM D-glucose,
glycogen levels were measured in the HepG2 cells and primary
hepatocytes at 37°C for 3 h in the presence of 100 nmol/l insulin
(US Biological, Salem, MA, USA), using a Glycogen Assay kit
(BioVision, Inc., Milpitas, CA, USA), according to the
manufacturer's protocol. Briefly, for hydrolysis, the Enzyme
Mixture was added and the plates were incubated for 30 min at 37°C;
then the Glycogen Fluorometric Detector solution, which contains
10-acetyl-3,7-dihydroxyphenoxazine, was added and the fluorescence
signal was monitored with an excitation wavelength of 530–540 nm
and an emission wavelength of 585–595 nm.
Western blotting
Cellular proteins were extracted using
radioimmunoprecipitation assay buffer [50 mM Tris/HCl, pH 7.4; 150
mM NaCl; 1% (v/v) nonidet P-40; 0.1% (w/v) SDS; Shanghai SolarBio
Bioscience and Technology, Co., Ltd.] containing 1% (v/v)
phenylmethanesulfonyl fluoride (Shanghai SolarBio Bioscience and
Technology, Co., Ltd.), 0.3% (v/v) protease inhibitor (Merck
Millipore) and 0.1% (v/v) phosphorylated proteinase inhibitor
(Merck Millipore). Lysates were centrifuged at 1,000 × g and 4°C
for 15 min, and the supernatant was collected to obtain total
protein. A Bicinchoninic Acid Protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.) was used to determine the protein
concentration. Equal amounts of protein (15 µg) were loaded onto a
SDS-PAGE gel [10% (v/v) polyacrylamide] and transferred onto a
polyvinylidene difluoride membrane. Nonspecific binding was blocked
using 8% (w/v) milk in TBST (5% Tween-20) for 2 h at room
temperature. The membranes were then incubated with primary
antibodies against phosphorylated (p)-insulin receptor substrate 1
(IRS1, Ser307; ab1194; 1:250), IRS1 (ab52167; 1:500), p-glycogen
synthase kinase (p-GSK3β, Ser9; ab131097; 1:500), GSK3β (ab170191;
1:1,000), p-protein kinase B (p-AKT; ab8933; 1:500), AKT (ab64148;
1:1,000) (Abcam, Shanghai, China) and β-actin (sc130300; 1:1,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Following several washes with TBST, the membranes were incubated in
horseradish peroxidase (HRP)-conjugated goat anti-rabbit (ab97051)
or goat anti-mouse immunoglobulin G (ab6789; Abcam) at a 1:5,000
dilution for 2 h at room temperature and then washed with
phosphate-buffered saline (PBS). The target proteins were
visualized using enhanced chemiluminescence (Merck Millipore)
according to the manufacturer's recommendations, quantified using
densitometry analysis and normalized against β-actin. Data are
expressed as the fold-change compared to the β-actin.
To further determine whether the EGCG-associated
enhancement of insulin signaling was due to scavenging reactive
oxygen species (ROSs), the antioxidant N-acetyl-cysteine (NAC; 10
mM; Beyotime Institute of Biotechnology, Jiangsu, China) was added
either with or without EGCG, and the cells were incubated for 1 h.
Then the whole proteins were extracted and western blotting was
performed as aforementioned.
Determination of ROS
HepG2 cells cultured on 6-well chamber slides
(1×106) were washed with PBS three times for 5 min/wash,
and the slides were incubated with the ROS fluorescent probe
dihydroethidium (DHE; Vigorous Biotechnology Beijing Co., Ltd.,
Beijing, China) in serum-free DMEM/F-12 medium (Thermo Fisher
Scientific, Inc.) for 30 min at 37°C in the dark. The slides were
then fixed in 4% paraformaldehyde for 30 min at room temperature.
The slides were washed with PBS and mounted. Immunofluorescence
images were captured by fluorescence microscopy.
ROS production
Intracellular ROS generation was monitored by flow
cytometry using the peroxide-sensitive fluorescent probe,
2′,7′-dichlorofluorescin diacetate (DCFH-DA; Molecular Probes;
Thermo Fisher Scientific, Inc.) as described previously (16). DCFH-DA is converted by
intracellular esterases to DCFH, which is oxidized to the highly
fluorescent DCF in the presence of an oxidant.
HepG2 cells (50–60% confluent) were pre-incubated
with LG (5 mM D-glucose) or HG (30 mM D-glucose) for 24 h at 37°C,
then treated with 5 µM EGCG. Finally, cells were washed twice with
1X PBS and diluted to 2–3×106 cells/ml in 10 mmol/l
DCHF-DA dyes. Cells were then incubated at room temperature for 30
min in the dark. The cells were subsequently analyzed by flow
cytometry (Becton Dickinson; BD Biosciences, Franklin Lakes, NJ,
USA).
Statistical analysis
All experiments were repeated at least three times.
Statistical analyses were performed using SPSS software, version
22.0 (IBM SPSS, Armonk, NY, USA). The data are expressed as the
mean ± standard deviation and were analyzed using one-way analysis
of variance followed by the Tukey-Kramer multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
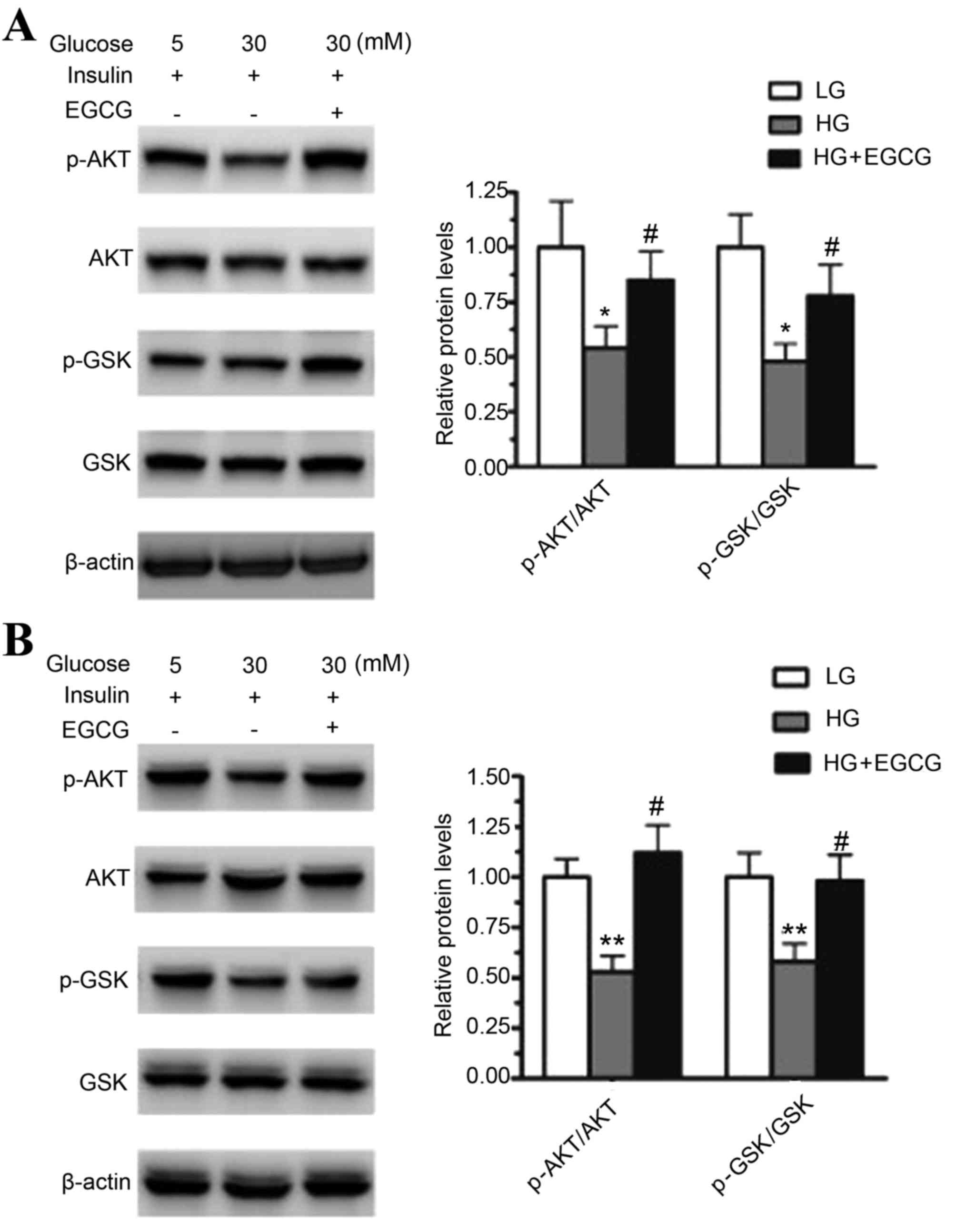
EGCG activates the AKT/GSK pathway in
HepG2 cells and primary hepatocytes
To determine the effect of HG on the AKT/GSK
signaling pathway, HepG2 cells and mouse primary hepatocytes were
treated with 30 or 5 mM glucose for 24 h. HG treatment
significantly decreased the protein expression levels of
phosphorylated AKT and GSK when compared to that of the LG
treatment in HepG2 cells (p-AKT, P<0.05; p-GSK, P<0.05) and
mouse primary hepatocytes (p-AKT, P<0.01; p-GSK, P<0.01;
Fig. 1). The effect of EGCG on the
levels of phosphorylated AKT and GSK in HG-treated HepG2 cells and
primary hepatocytes was then analyzed. Pretreatment with EGCG
significantly restored the levels of p-AKT and p-GSK in HepG2 cells
(p-AKT, P<0.05; p-GSK, P<0.05) and primary hepatocytes
(p-AKT, P<0.05; p-GSK, P<0.05) when compared to cells exposed
to HG alone (Fig. 1). These
results indicated that HG impaired insulin signaling in HepG2 cells
and mouse primary hepatocytes, and EGCG improved HG-induced insulin
resistance in the two cell types.
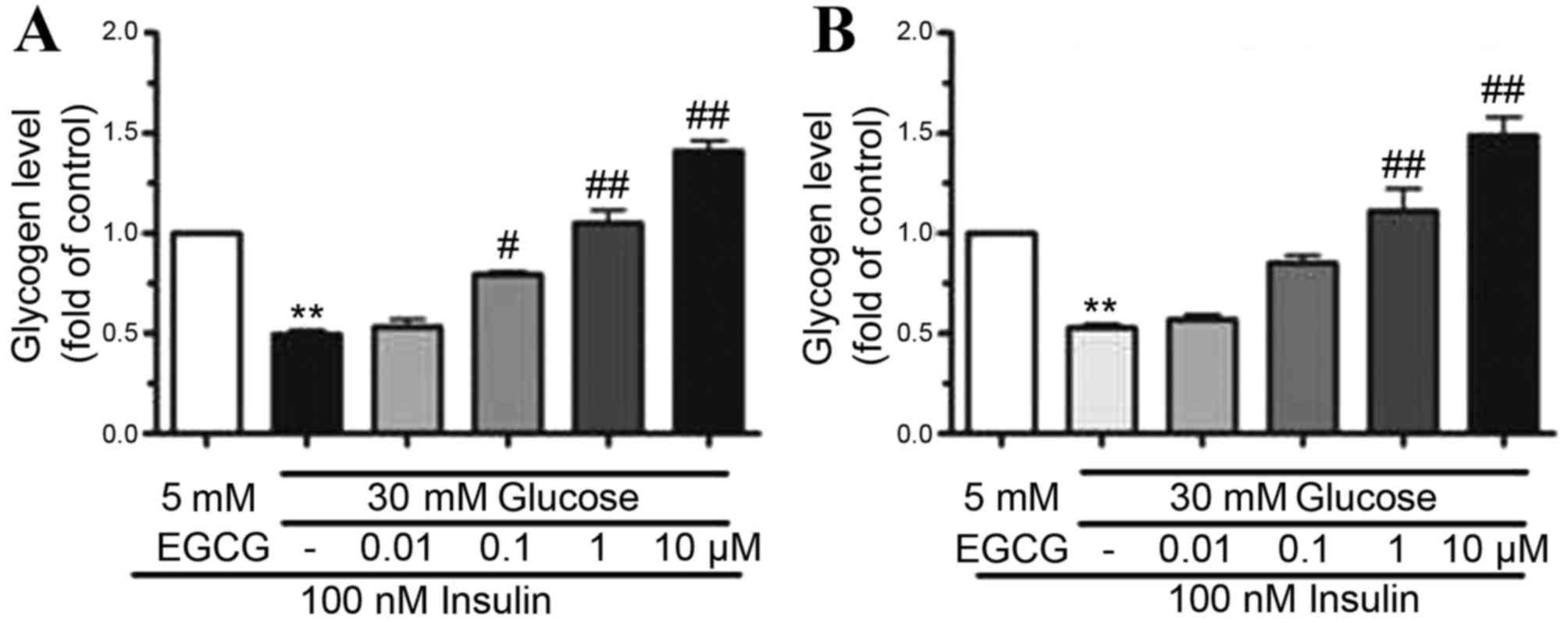
EGCG increased glycogen synthesis in
HepG2 cells and primary hepatocytes
To determine the effect of EGCG on glycogen
synthesis, the glycogen content in HepG2 cells and hepatocytes
pretreated with HG (30 mM) was determined. EGCG was added to the
cell cultures at concentrations of 0.01–10 µM. Following
stimulation of cells with 100 nM insulin, glycogen synthesis
decreased by ~50% in HepG2 cells (P<0.01) and primary
hepatocytes (P<0.01) exposed to HG when compared to those
treated with 100 nM insulin plus LG, indicating that HG treatment
may lead to insulin resistance (Fig.
2). By contrast, when cells were pre-treated with EGCG (0.01–10
µM), glycogen synthesis was gradually restored in HepG2 cells and
primary hepatocytes (Fig. 2).
Treatment of cells with 10 µM EGCG resulted in a two-fold increase
in glycogen levels in HepG2 (P<0.01) and primary hepatocytes
(P<0.01) when compared with HG-only treated cells (Fig. 2). These results indicated that
glycogen synthesis was improved by EGCG in a dose-dependent
manner.
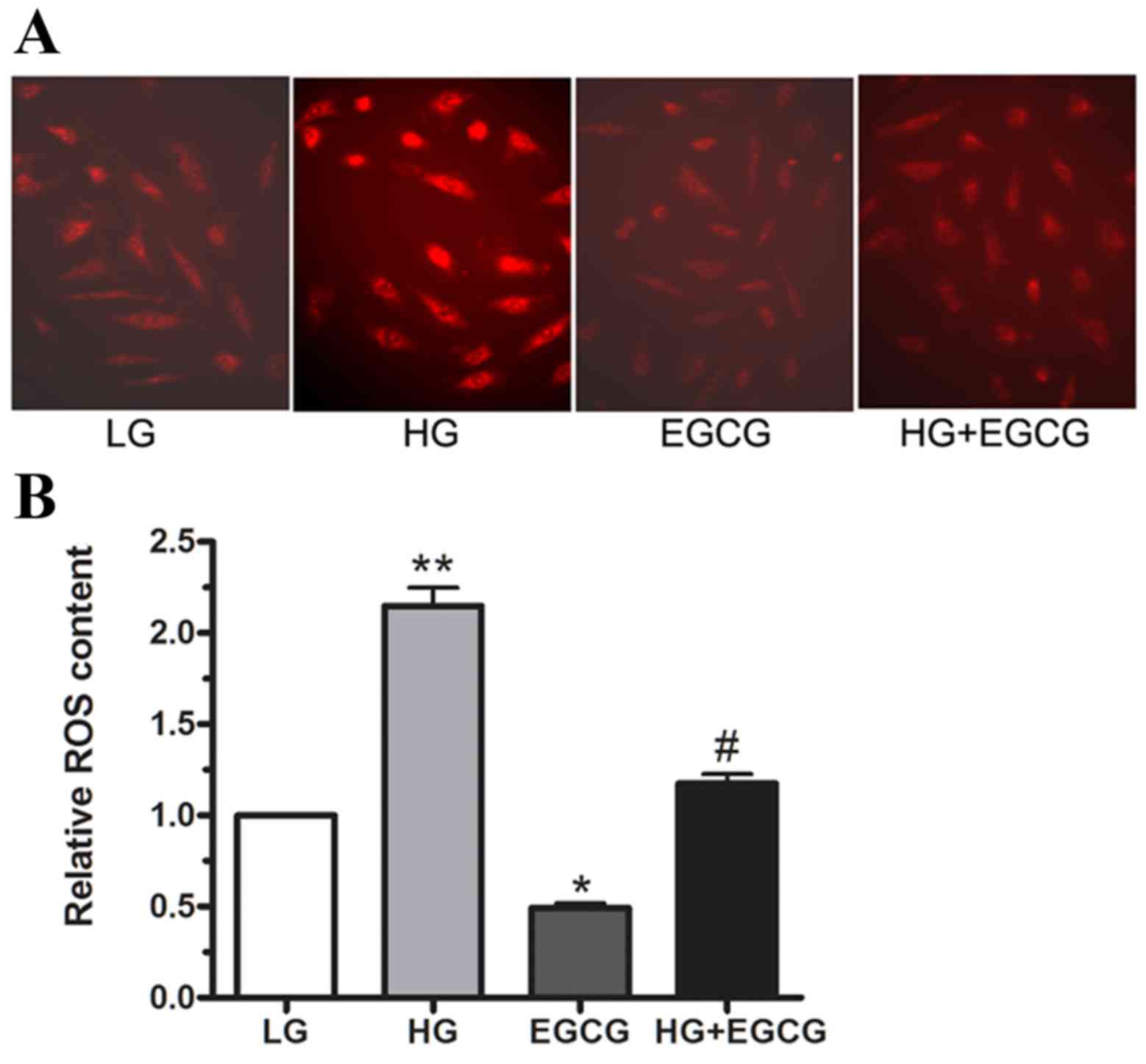
ROS are involved in HG-induced insulin
resistance
To determine whether HG treatment induced ROS
production in cultured HepG2 cells, ROS levels were measured using
DHE staining. Following 24 h incubation with LG and HG, 10 µM EGCG
was added, and ROS production was determined using
immunofluorescence and flow cytometry (Fig. 3A and B, respectively). The
microimages in Fig. 3A demonstrate
that ROS content was markedly reduced in HG + EGCG-treated cells
compared with HG-only treated cells. As shown in Fig. 3B, HG treatment enhanced ROS
production by ~1-fold relative to LG-treated cells (P<0.01).
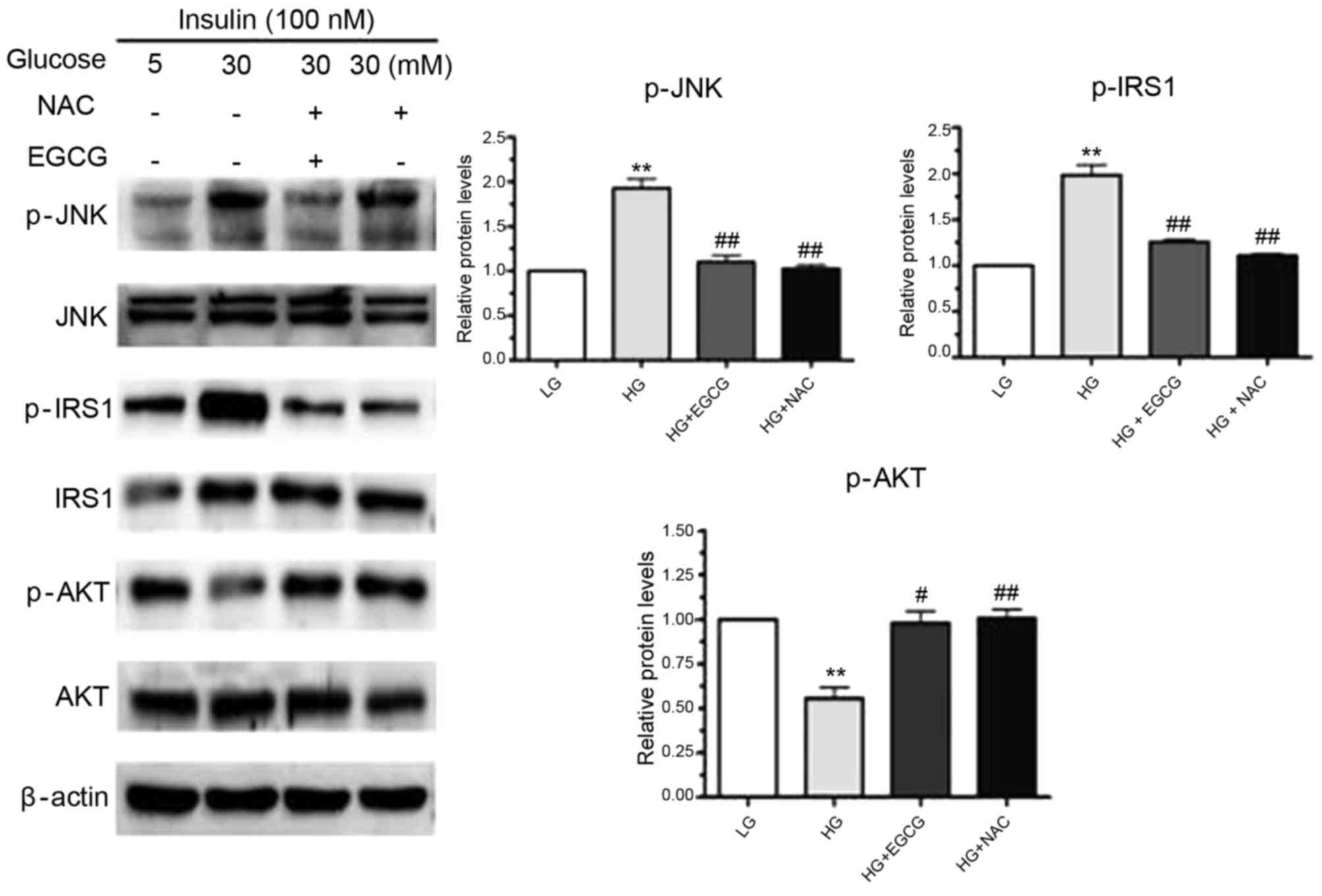
EGCG alters the insulin-signaling
pathway in hepatocytes treated with HG
Owing to the vital role the insulin-signaling
pathway serves in glycogen synthesis (17), the authors investigated whether
EGCG affects the insulin-signaling pathway in hepatocytes treated
with EGCG. As shown in Fig. 4, a
significant increase in p-JNK expression was observed in response
to HG-treatment of HepG2 cells (P<0.01). In addition to
increased JNK phosphorylation, phosphorylation of the residue
Ser307 in IRS-1 was significantly enhanced by HG treatment
(P<0.01; Fig. 4). HG-induced
activation of JNK may have been responsible for the impaired
phosphorylation of AKT and GSK (Figs.
1A and 4). However, the
alterations in JNK, IRS-1, AKT and GSK expression that were induced
by HG were reversed by EGCG treatment (Figs. 1 and 4). When HepG2 cells were pretreated with
NAC and exposed to HG, JNK activation and phosphorylation of the
Ser307 residue of IRS-1 were significantly reduced in NAC-treated
cells compared with HG-only treated cells (p-JNK, P<0.01;
p-IRS-1, P<0.01; Fig. 4). These
results suggested that EGCG ameliorates HG-induced insulin
resistance in signaling hepatocytes by altering the ROS-induced
JNK/IRS1/AKT/GSK signaling pathway.
Discussion
Type II diabetes mellitus has increasingly become a
worldwide public health problem as it often leads to severe
complications, including coronary disease, heart failure,
retinopathy, peripheral neuropathy and hypertension (4,18).
Owing to the impaired capacity to utilize insulin in target organs,
insulin resistance is recognized as a pathophysiological marker for
type II diabetes. As an important organ in glucose metabolism, the
liver serves a vital role in regulating metabolic processes. During
insulin resistance, hepatic glycogen synthesis is markedly reduced
and insulin signaling is impaired; these events may lead to the
development of hyperglycemia and type II diabetes (19). In addition to genetic factors,
insulin resistance is predominantly caused by environmental
factors, including obesity, a sedentary lifestyle, pregnancy and
the excess hormone production (19). Hyperglycemia is recognized as a
common pathogenic factor involved in a series of additional
complications in patients with type II diabetes. Previous research
has indicated that increased levels of free fatty acids and glucose
content inpatients with type II diabetes is associated with
enhanced ROS production and oxidative stress (20). In addition, ROS has been
demonstrated to severely impair the insulin-signaling pathway,
which promotes the progression of type II diabetes further.
Oxidative stress may lead to further tissue damage, as it is often
the result of an imbalance between ROS production and antioxidant
defenses (21).
At present, antidiabetic agents, such as
α-glucosidase inhibitors, amylin analogs, antidiabetic
combinations, dipeptidyl peptidase 4 inhibitors, incretin mimetics
and insulin, are widely prescribed for patients with type II
diabetes. However, clinicians and researchers are becoming
increasingly concerned with the resulting liver injury. Natural
remedies extracted from medicinal plants have demonstrated their
effectiveness as alternative treatments of hyperglycemia (9,22).
In eastern Asia, green tea is a popular traditional beverage, and
previous research has indicated that regular green tea consumption
reduces the risk of liver disease (23). Green tea has anti-inflammatory,
antioxidative, antimutagenic and anticarcinogenic properties, which
make it highly beneficial for public health (15). Furthermore, a previous
epidemiological study demonstrated that regular green tea
consumption may reduce the risk of developing type II diabetes
(24). In streptozocin-induced
diabetic mice, green tea was reported to improve hyperglycemia
(22). Furthermore, green tea
extracts have been demonstrated to function as effective free
radical scavengers (5). EGCG is
the primary polyphenol extracted from green tea and has been
reported to increase fecal cholesterol excretion in rats fed on a
high-fat diet when compared to controls (25). Due to the anti-hyperglycemic and
antioxidant properties of green tea, the aim of the present study
was to explore the hepatoprotective effects of EGCG in a HG-induced
insulin resistance cell model.
To examine the effect of HG on insulin signaling, 30
mM glucose was applied to stimulate HepG2 cells and primary
hepatocytes. Compared with the LG group, HG significantly reduced
the phosphorylation of AKT and GSK in the two cell types. These
results indicated that HG induced insulin resistance in HepG2 and
primary mouse hepatocytes. In addition, when these cells were
pretreated with EGCG, the phosphorylation levels of AKT and GSK
were restored, indicating the protective effects of EGCG against
insulin resistance. Glycogen levels were measured using a glycogen
assay kit. The results demonstrated that EGCG significantly
restored glycogen synthesis in the two cell types when treated with
HG. These in vitro experiments indicated that EGCG may
protect hepatocytes from HG-induced insulin resistance.
Hyperglycemia is associated with the development of
vascular and neurological complications, and has been implicated in
their etiologies (26,27). Previous studies have demonstrated
that ROS production serves a major role in insulin resistance
(28,29). In addition, in vitro studies
have revealed that enhanced ROS production activates multiple
serine kinase cascades (30). In
the insulin-signaling pathway, the insulin receptor and IRS are
potential targets of these activated kinases (31,32).
It has been reported that enhanced IRS serine phosphorylation
reduced the level of tyrosine phosphorylation, thereby decreasing
insulin activity (33).
Furthermore, it has been demonstrated that ROS enhances JNK serine
phosphorylation, which increases the serine phosphorylation of the
IRS protein (34). Enhanced IRS-1
serine phosphorylation impairs downstream insulin signaling. AKT
and GSK activation is subsequently reduced, followed by the
reduction of glycogen synthase activity (35).
In the present study, HepG2 cells were pre-treated
with 30 mM glucose and cellular ROS levels were determined by DHE
staining and flow cytometry. The results indicated that HG
treatment significantly enhanced ROS production when compared with
the LG-treated cells. In addition, HG plus EGCG treatment, led to a
significant reduction in ROS production when compared with HG-only
treated cells. The results suggest that HG may increase ROS content
in patients with insulin resistance. EGCG was observed to protect
the cells from abnormal ROS production. To further explore the
protective effect of EGCG on insulin resistance, the expression
levels of key proteins associated with insulin signaling were
analyzed. The results revealed that, in addition to the increased
level of JNK phosphorylation, phosphorylation of the residue Ser307
in IRS-1 was enhanced in HepG2 cells treated with HG. In addition,
HG-induced activation of JNK may have led to impaired
phosphorylation of AKT and GSK. However, the HG-induced alterations
to JNK, IRS-1, AKT and GSK expression were reversed by EGCG
treatment. To further validate the protective effects of EGCG, NAC
(a well-known antioxidant) was applied. When HepG2 cells were
pre-treated with NAC and HG for 1 h, serine phosphorylation of JNK
and IRS1 was significantly decreased in both EGCG and NAC-treated
cells. This was similar to the effect of EGCG exposure. Thus, EGCG
improved insulin signaling potentially through the reduction of ROS
production in hepatocytes.
In conclusion, the results of the present study
demonstrated that HG may induce hepatic insulin resistance. In
addition, ROS may serve a major role in the pathology of insulin
resistance through JNK and IRS1 serine phosphorylation.
Furthermore, EGCG decreased ROS production and affected the
insulin-signaling pathway. Therefore, green tea extracts may be a
promising therapeutic intervention for insulin resistance in
patients with type II diabetes.
References
|
1
|
Ezenwaka CE, Okoye O, Esonwune C, Onuoha
P, Dioka C, Osuji C, Oguejiofor C and Meludu S: High prevalence of
abdominal obesity increases the risk of the metabolic syndrome in
Nigerian type 2 diabetes patients: Using the International diabetes
federation worldwide definition. Metab Syndr Relat Disord.
12:277–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cable JC, Tan GD, Alexander SP and
O'Sullivan SE: The effects of obesity, diabetes and metabolic
syndrome on the hydrolytic enzymes of the endocannabinoid system in
animal and human adipocytes. Lipids Health Dis. 13:432014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CY, Huang CJ, Huang LH, Chen IJ, Chiu
JP and Hsu CH: Effects of green tea extract on insulin resistance
and glucagon-like peptide 1 in patients with type 2 diabetes and
lipid abnormalities: A randomized, double-blinded, and
placebo-controlled trial. PLoS One. 9:e911632014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilbert RE: The endothelium in diabetic
nephropathy. Curr Atheroscler Rep. 16:4102014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crespy V and Williamson G: A review of the
health effects of green tea catechins in in vivo animal models. J
Nutr. 134 Suppl 12:S3431–S3440. 2004.
|
|
6
|
Basu A, Sanchez K, Leyva MJ, Wu M, Betts
NM, Aston CE and Lyons TJ: Green tea supplementation affects body
weight, lipids, and lipid peroxidation in obese subjects with
metabolic syndrome. J Am Coll Nutr. 29:31–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sae-Tan S, Grove KA and Lambert JD: Weight
control and prevention of metabolic syndrome by green tea.
Pharmacol Res. 64:146–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ihm SH, Jang SW, Kim OR, Chang K, Oak MH,
Lee JO, Lim DY and Kim JH: Decaffeinated green tea extract improves
hypertension and insulin resistance in a rat model of metabolic
syndrome. Atherosclerosis. 224:377–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sae-Tan S, Rogers CJ and Lambert JD:
Voluntary exercise and green tea enhance the expression of genes
related to energy utilization and attenuate metabolic syndrome in
high fat fed mice. Mol Nutr Food Res. 58:1156–1159. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thielecke F and Boschmann M: The potential
role of green tea catechins in the prevention of the metabolic
syndrome-A review. Phytochemistry. 70:11–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Senger AE Vieira, Schwanke CH, Gomes I and
Gottlieb MG Valle: Effect of green tea (Camellia sinensis)
consumption on the components of metabolic syndrome in elderly. J
Nutr Health Aging. 16:738–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cia D, Vergnaud-Gauduchon J, Jacquemot N
and Doly M: Epigallocatechin gallate (EGCG) prevents H2O2-induced
oxidative stress in primary rat retinal pigment epithelial cells.
Curr Eye Res. 39:944–952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang EJ, Lee J, Lee SY, Kim EK, Moon YM,
Jung YO, Park SH and Cho ML: EGCG attenuates autoimmune arthritis
by inhibition of STAT3 and HIF-1α with Th17/Treg control. PLoS One.
9:e860622014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Farah BL, Sinha RA, Wu Y, Singh
BK, Bay BH, Yang CS and Yen PM: Epigallocatechin-3-gallate (EGCG),
a green tea polyphenol, stimulates hepatic autophagy and lipid
clearance. PLoS One. 9:e871612014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benelli R, Venè R, Bisacchi D, Garbisa S
and Albini A: Anti-invasive effects of green tea polyphenol
epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo
and serine proteases. Biol Chem. 383:101–105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao C, She T, Wang L, Su Y, Qu L, Gao Y,
Xu S, Cai S and Shou C: Daucosterol inhibits cancer cell
proliferation by inducing autophagy through reactive oxygen
species-dependent manner. Life Sci. 137:37–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saltiel AR: Insulin signaling in the
control of glucose and lipid homeostasis. Handb Exp Pharmacol.
233:51–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hendriksen PH, Oey PL, Wieneke GH,
Bravenboer B and Banga JD: Subclinical diabetic neuropathy:
Similarities between electrophysiological results of patients with
type 1 (insulin-dependent) and type 2 (non-insulin-dependent)
diabetes mellitus. Diabetologia. 35:690–695. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eddouks M, Maghrani M and Michel JB:
Hypoglycaemic effect of Triticum repens P. Beauv. in normal and
diabetic rats. J Ethnopharmacol. 102:228–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stefano GB, Challenger S and Kream RM:
Hyperglycemia-associated alterations in cellular signaling and
dysregulated mitochondrial bioenergetics in human metabolic
disorders. Eur J Nutr. 55:2339–2345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bukhari SA, Naqvi SA, Nagra SA, Anjum F,
Javed S and Farooq M: Assessing of oxidative stress related
parameters in diabetes mellitus type 2: Cause excessive damaging to
DNA and enhanced homocysteine in diabetic patients. Pak J Pharm
Sci. 28:483–491. 2015.PubMed/NCBI
|
|
22
|
Tsuneki H, Ishizuka M, Terasawa M, Wu JB,
Sasaoka T and Kimura I: Effect of green tea on blood glucose levels
and serum proteomic patterns in diabetic (db/db) mice and on
glucose metabolism in healthy humans. BMC Pharmacol. 4:182004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirsch N, Konstantinov A, Anavi S, Aronis
A, Hagay Z, Madar Z and Tirosh O: Prolonged feeding with green tea
polyphenols exacerbates cholesterol-induced fatty liver disease in
mice. Mol Nutr Food Res. 60:2542–2553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weisburger JH and Chung FL: Mechanisms of
chronic disease causation by nutritional factors and tobacco
products and their prevention by tea polyphenols. Food Chem
Toxicol. 40:1145–1154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raederstorff DG, Schlachter MF, Elste V
and Weber P: Effect of EGCG on lipid absorption and plasma lipid
levels in rats. J Nutr Biochem. 14:326–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rösen P, Nawroth PP, King G, Moller W,
Tritschler HJ and Packer L: The role of oxidative stress in the
onset and progression of diabetes and its complications: A summary
of a congress series sponsored by UNESCO-MCBN, the American
diabetes association and the German diabetes society. Diabetes
Metab Res Rev. 17:189–212. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishikawa T, Edelstein D and Brownlee M:
The missing link: A single unifying mechanism for diabetic
complications. Kidney Int Suppl. 77:S26–S30. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paolisso G and Giugliano D: Oxidative
stress and insulin action: Is there a relationship? Diabetologia.
39:357–363. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rudich A, Kozlovsky N, Potashnik R and
Bashan N: Oxidant stress reduces insulin responsiveness in 3T3-L1
adipocytes. Am J Physiol. 272:E935–E940. 1997.PubMed/NCBI
|
|
30
|
Kyriakis JM and Avruch J: Sounding the
alarm: Protein kinase cascades activated by stress and
inflammation. J Biol Chem. 271:24313–24316. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blair AS, Hajduch E, Litherland GJ and
Hundal HS: Regulation of glucose transport and glycogen synthesis
in L6 muscle cells during oxidative stress. Evidence for cross-talk
between the insulin and SAPK2/p38 mitogen-activated protein kinase
signaling pathways. J Biol Chem. 274:36293–36299. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Birnbaum MJ: Turning down insulin
signaling. J Clin Invest. 108:655–659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu K, Zhao W, Gao X, Huang F, Kou J and
Liu B: Diosgenin ameliorates palmitate-induced endothelial
dysfunction and insulin resistance via blocking IKKβ and IRS-1
pathways. Atherosclerosis. 223:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katakam AK, Chipitsyna G, Gong Q, Vancha
AR, Gabbeta J and Arafat HA: Streptozotocin (STZ) mediates acute
upregulation of serum and pancreatic osteopontin (OPN): A novel
islet-protective effect of OPN through inhibition of STZ-induced
nitric oxide production. J Endocrinol. 187:237–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chu J, Zhang H, Huang X, Lin Y, Shen T,
Chen B, Man Y, Wang S and Li J: Apelin ameliorates TNF-α-induced
reduction of glycogen synthesis in the hepatocytes through G
protein-coupled receptor APJ. PLoS One. 8:e572312013. View Article : Google Scholar : PubMed/NCBI
|