Introduction
Myocarditis is defined as an inflammatory
infiltration of the myocardium with necrosis and/or degeneration of
cardiomyocytes (1). Viruses are
the primary cause of myocarditis; however, there are additional
infectious causes of myocarditis, including Borrelia
burgdorferi and Trypanosoma cruzi (2). There are a wide range of myocarditis
symptoms, from mild dyspnea, arrhythmias and chest pain that
resolves itself without specific therapy, to cardiogenic death
(2). The major long-term
consequence is dilated cardiomyopathy with chronic heart failure,
for which appropriate treatment remains a significant clinical
challenge.
A rat model of experimental autoimmune myocarditis
(EAM) resembles human giant cell myocarditis and has been widely
used in previous studies (3,4).
Previous reports suggested that oxidative stress results in
myocardial apoptosis, which serves an important role in the
progression of EAM (5,6). Oxidative stress may activate
mitogen-activated protein kinase (MAPK) signaling pathways and
endoplasmic reticulum (ER) stress, both of which lead to myocardial
apoptosis and myocardial damage (7). A previous study demonstrated that
intravenous injection of bone marrow mesenchymal stem cells (BMSCs)
may alleviate myosin-induced myocarditis (8). However, the invasiveness of obtaining
bone marrow and the low numbers of MSCs yielded following
processing limits its clinical potential. In previous years, human
umbilical cord-derived mesenchymal stem cells (HuMSCs), which are
generally discarded as medical waste following delivery, have
become an alternate source of MSCs (9). Cluster of differentiation (CD)29,
CD44, CD59, CD90 and CD105 are expressed in HuMSCs. Markers of
hematopoietic cells, including CD14, CD33, CD34, CD28, CD45 and
CD117, and important graft-vs.-host disease (GVHD) markers,
including CD80, CD86 and CD40, are not detectable or only weakly
expressed in HuMSCs (10).
Therefore, it has been hypothesized that HuMSCs may be broadly used
in regenerative medicine without graft rejection reactions
(11). However, the capacity of
HuMSCs during EAM remains undetermined.
The current study investigated whether intravenous
HuMSCs may improve cardiac function and alleviate myocardial
inflammation in rats with myosin-induced myocarditis, as well as
evaluating the potential underlying mechanisms.
Materials and methods
Animals
Male Lewis rats (n=24; aged 8 weeks; weight 180–200
g) were purchased from Vital River Laboratories Co., Ltd. (Beijing,
China) and were maintained in our animal facilities with
air-conditioning at Shantou University Medical College, under
constant temperature and humidity conditions with a 12:12-h
light-dark cycle. All rats had free access to food and water.
Throughout the studies, all animals were treated in accordance with
the institutional guidelines for animal experiments. Ethical
approval was obtained from the Institutional Review Board of
Shantou University Medical College (Shantou, China).
Preparation of HuMSCs
Ethical approval was obtained from the Institutional
Review Board of Shantou University Medical College (Shantou,
China). HuMSCs were prepared as previously described (10). A total of 5 patients were involved
in the study, and written informed consent was obtained from all
patients. Human umbilical cords from consenting patients who
underwent full-term Caesarian sections were collected immediately
into a sterilized 50 ml tubes, washed with phosphate-buffered
saline (PBS), and cut into 2- to 3-cm thick sections. Following
dissection of the arteries and veins, the remaining tissue, the
Wharton's jelly, was sectioned into smaller fragments and
transferred to 75 cm2 flasks containing Dulbecco's
Modified Eagle's medium/F12 media (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
µg/ml penicillin/streptomycin (Beyotime Institute of Biotechnology,
Haimen, China), 1 g/ml amphotericin B (Gilead Sciences, Inc.,
Foster City, CA, USA), 5 ng/ml epidermal growth factor (EGF;
Invitrogen; Thermo Fisher Scientific, Inc.), and 5 ng/ml basic
fibroblast growth factor (bFGF; Sigma-Aldrich; Merck KGaA).
Cultures remained undisturbed for 5–7 days at 37°C in 5%
CO2 to allow migration of cells from the explants.
Subsequently, the media was replaced.
EAM induction
Purified porcine cardiac myosin (Sigma-Aldrich;
Merck KGaA) was dissolved in 0.01 M PBS and emulsified with an
equal volume of complete Freund's adjuvant supplemented with10
mg/ml Mycobacterium tuberculosis (Sigma-Aldrich; Merck
KGaA). On day 0, rats received a single immunization at two
subcutaneous sites (both footpads) with a total of 0.2 ml emulsion
per rat. Rats not subjected to immunization were included as a
controlgroup (n=8).
A total of 16immunized rats were randomly divided
into two groups. Rats were randomly assigned into the following two
groups: Control group, 0.2 ml PBS only (n=8);HuMSCs group, 0.2 ml
HuMSCs (1×106 cells/animal; n=8). A total of 10 days
following myosin injection, HuMSCs or vehicle (PBS) were
administered intravenously via the tail vein.
Echocardiographic studies
Echocardiography was performed 21 days following the
myosin injection. Rats were anesthetized with 1.5–2.0% volume of
isoflurane in air (Sigma-Aldrich; Merck KGaA). A 13-MHz probe was
placed at the left fourth intercostal space for imaging using
two-dimensional echocardiography (AcusonAntares, Siemens AG,
Munich, Germany). Left ventricular systolic dimension (LVDs), left
ventricular diastolic dimension (LVDd), interventricular septal
thickness (IVS), left ventricular posterior wall thickness (LVPW)
and fractional shortening (FS%) were measured and recorded as the
mean for three beats. Fractional shortening (%) was calculated as
[(LVDd-LVDs)/LVDd]x100%. Investigators blinded to the treatment
group performed the echocardiography studies and all analyses were
performed offline.
Histopathological studies
Following echocardiographic analyses, rats were
sacrificed by cervical dislocation. Hearts were excised above the
origin of the great vessels 21 days after the myosin injection.
Hearts were fixed in 4% paraformaldehyde for 6 h at 4°C, embedded
in paraffin, sectioned to 4-µm thickness, and stained with
hematoxylin and eosin (H&E). A cardiovascular pathologist with
no knowledge of the experimental groups evaluated H&E-stained
sections. Myocardial injury and inflammation were characterized by
assigning histoscores to every fifth cross section, according to a
previously published 6-tier scoring system (grade 0, no
inflammation; grade 1, cardiac infiltration in <5% of the
cardiac sections; grade 2, 6–10% infiltration; grade
3,11–30%infiltration; grade 4, 31–50% infiltration; and grade 5,
infiltration in >50% of cardiac sections) (12,13).
Western blotting
Heart tissues were homogenized in ice-cold
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Following centrifugation (12,000 ×
g for 10 min at 4°C), supernatants were collected and the
total protein concentration in samples was measured by use of a BCA
Protein Assay kit (Beyotime Institute of Biotechnology), according
to the manufacturer's protocol. For western blotting assays, 30 µg
of total protein was separated by 7.5% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto
polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA). Filters were blocked with 5% non-fat dry milk
in TBST (20 mM Tris, pH 6.8, 137 mM NaCl, 0.1% Tween-20) overnight
at 4°C, washed, and incubated overnight at 4°C with a 1:1,000
dilution of primary antibodies in blocking solution. Washing was
conducted four times with TBST, for 10 min each, with constant
shaking. The following primary antibodies were used: GAPDH (catalog
no. D4C6R), extracellular signal-regulated kinase (ERK)-1/2
(catalog no. 9258), phosphorylated (p)-ERK-1/2 (catalog no. 4668),
p38 MAPK (catalog no. 8690) and p-p38 MAPK (catalog no. 4511),
which were all purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Glucose-regulated protein 78 (GRP78) (catalog
no. SC-13968) and caspase 12 (catalog no. SC-5627) were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Membranes
were subsequently washed and incubated for 1 h at room temperature
with a 1:2,000 dilution of horseradish peroxidase-labeled goat
anti-rabbit IgG secondary antibody (catalog no. 4050-05; Southern
Biotechnology Associates, Inc. USA). Protein bands were visualized
using the ECL Plus chemiluminescence kit (Amersham Biosciences,
Uppsala, Sweden).
Detection of apoptosis
Paraffin-embedded heart tissues were cut into 4-µm
thick sections at room temperature. Terminal deoxynucleotidyl
transferase dUTP nick end labeling (TUNEL) assays were performed
using an in situ apoptosis detection kit according to the
manufacturer's protocol (Beyotime Institute of Biotechnology).
Sections were mounted and examined using light microscopy. For each
animal, five sections were scored for apoptotic nuclei. Only nuclei
that were clearly located in cardiac myocytes were considered.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Analyses of differences between groups were performed using one-way
analyses of variance, followed by Tukey's multiple comparison tests
using SPSS software version 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
HuMSC treatment improves cardiac
structure and function, and cardiomyocyte apoptosis
A significant suppression of cardiac function in EAM
rats 21 days following cardiac myosin injection was observed. There
was significant impairment in the systolic and diastolic components
of cardiac contraction as presented in echocardiographic analyses
compared with normal rats (P<0.01; Fig. 1A). HuMSC treatment significantly
reversed cardiac remodeling with reduced LVDs and LVDd, and
increased FS compared with vehicle-treated EAM rats (Table I). There was marked inflammatory
cellular infiltration in the control group as identified by H&E
staining, whereas HuMSCs-treated rats exhibited much less
inflammatory cellular infiltration (Fig. 1A and B). Cardiomyocyte apoptosis
was confirmed by TUNEL staining of myocardial tissue slices.
Control animals demonstrated numerous TUNEL-positive apoptotic
nuclei whereas HuMSCs-treated animals exhibited fewer apoptotic
cells (Fig. 1A and C).
Additionally, control rats demonstrated LV remodeling with
increased LVDd and LVDs, and reduced FS in vehicle-treated EAM
rats, when compared with untreated rats (normal group), indicating
impaired myocardial function (Fig.
1D).
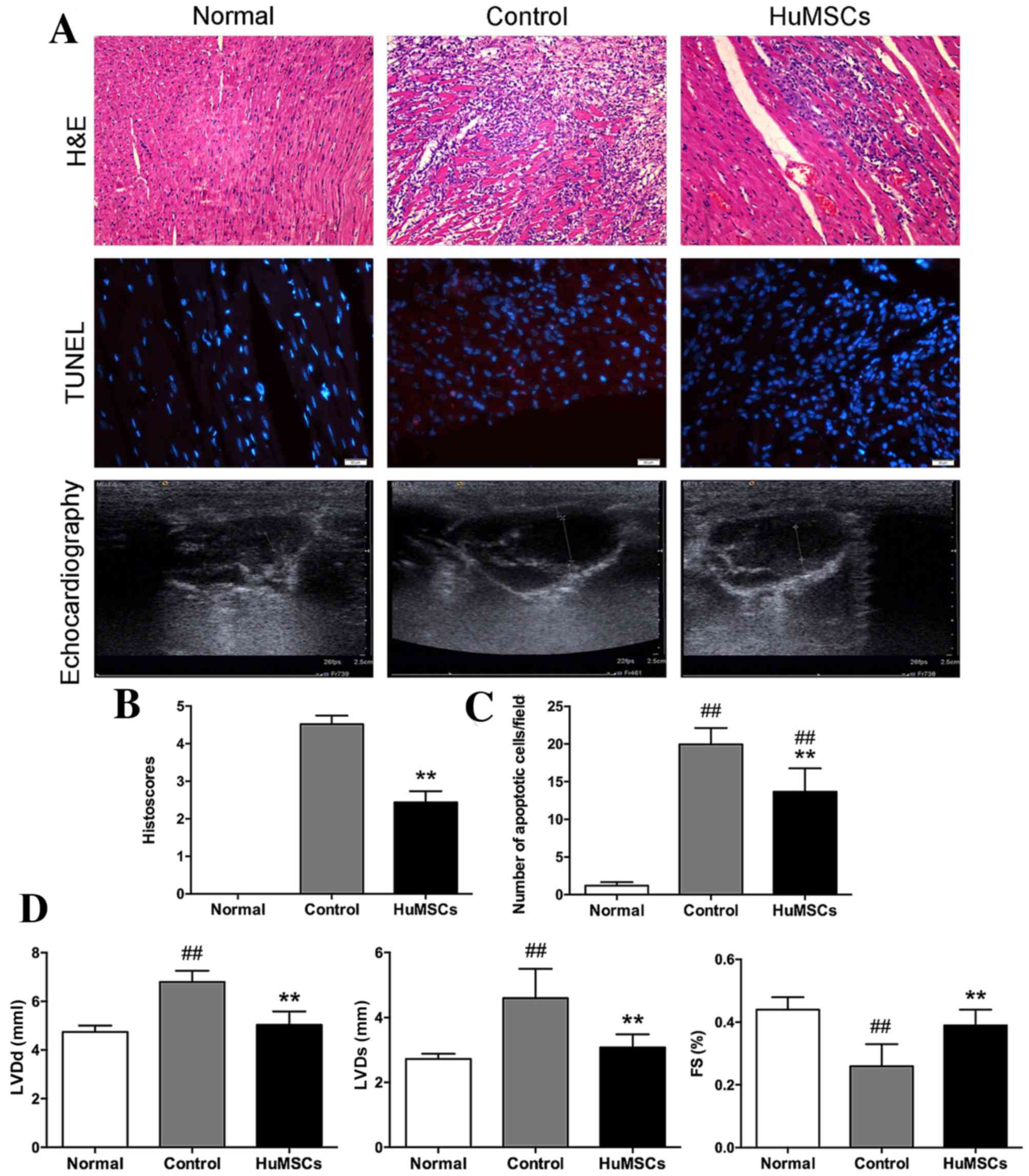 | Figure 1.Experiments comparing cellular
infiltration, levels of apoptosis and systolic and diastolic
components in normal cells, control cells and HuMSCs. (A) H&E
staining of left ventricular tissue slices (magnification, ×100).
TUNEL staining of left ventricular tissue slices depicting
apoptotic nuclei (magnification, ×400). Echocardiography in four
chambers. (B) Histoscores of H&E staining reflecting levels of
cardiac infiltration. (C) Bar graphs presenting the average number
of TUNEL-positive cells per field. (D) Quantification of LVDs, LVDd
and FS. Data are expressed as the mean ± standard deviation.
##P<0.01 vs. Normal, **P<0.01 vs. Control. HuMSCs,
human umbilical-derived mesenchymal stem cells; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick end labeling; LVDs, left
ventricular systolic dimension; LVDd, left ventricular diastolic
dimension; FS, fractional shortening. |
 | Table I.Alterations to echocardiographic
parameters 3 weeks following treatment with HuMSCs in EAM rats. |
Table I.
Alterations to echocardiographic
parameters 3 weeks following treatment with HuMSCs in EAM rats.
| Parameter | Normal | Control | HuMSCs |
|---|
| LVDd (mm) | 4.75±0.25 |
6.80±0.46a |
5.04±0.55b |
| LVDs (mm) | 2.72±0.16 |
4.60±0.90a |
3.08±0.40b |
| IVS (mm) | 1.88±0.83 | 1.94±0.18 | 1.74±0.21 |
| LVPW (mm) | 2.0±0.12 | 1.64±0.13 | 1.78±0.20 |
| FS (%) | 0.44±0.04 |
0.26±0.07a |
0.39±0.05b |
HuMSCs treatment modulates the ERK1/2
pathway
As assessed by western blot analysis (Fig. 2A), p-ERK1/2 expression was
significantly increased in control rats compared with normal rats,
suggesting that oxidative stress mediates stimulation of ERK1/2
signaling. HuMSC treatment significantly ameliorated protein
expression levels of ERK 1/2 (##P<0.05 vs. Normal,
*P<0.05 vs. Control) and p-ERK1/2 in EAM rats
(##P<0.05 vs. Normal, *P<0.05 vs. Control)
(Fig. 2B). p38-MAPK and p-p38MAPK
levels did not differ between the three groups (data not
shown).
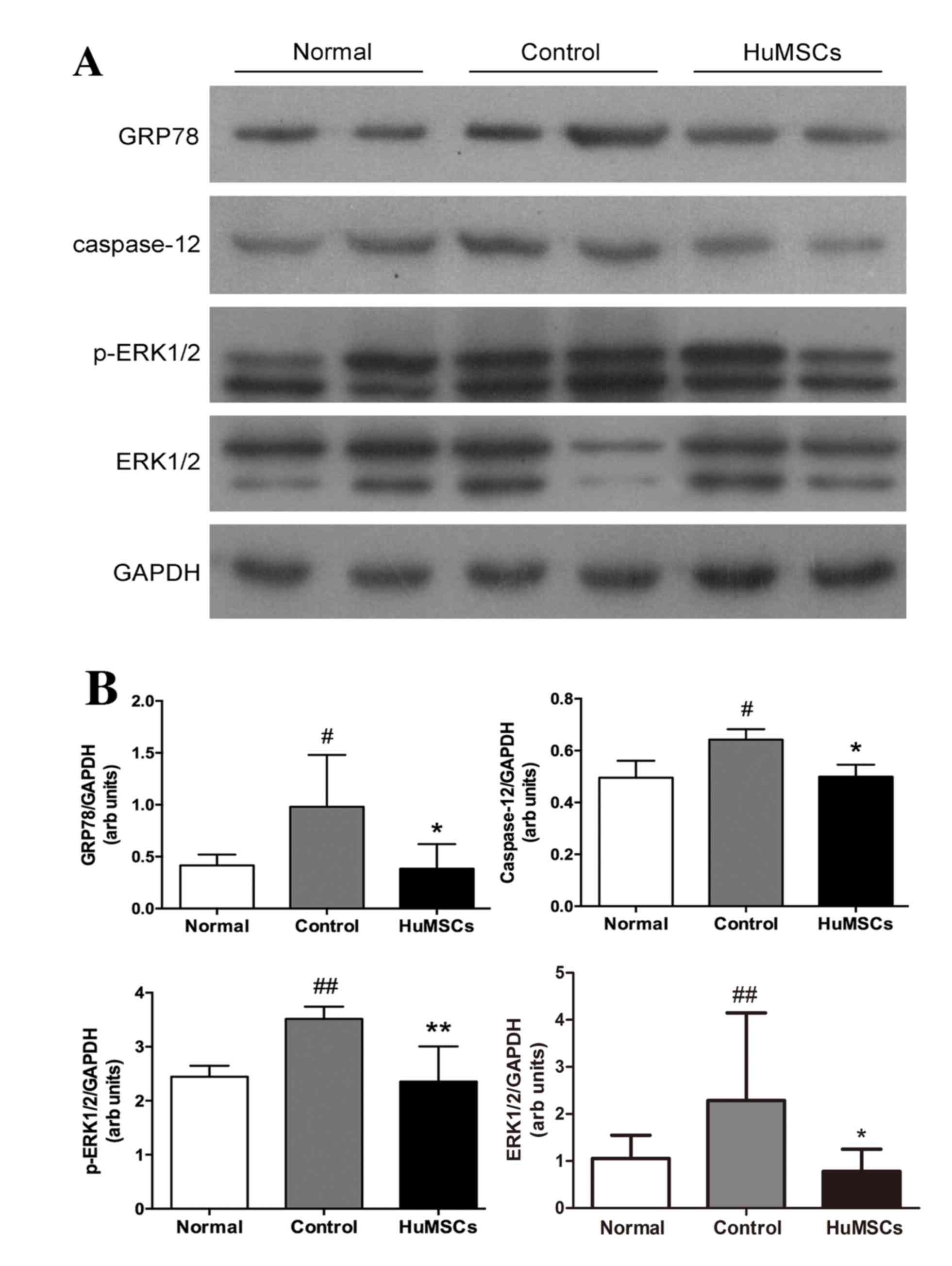 | Figure 2.Protein expression levels of GRP78,
caspase-12, ERK1/2 and p-ERK1/2. (A) Representative western blot
images and (B) quantification of GRP78, caspase-12 and p-ERK1/2
protein expression levels. GAPDH served as an internal control.
Data are expressed as the mean ± standard deviation.
#P<0.05 vs. Normal, ##P<0.01 vs.
Normal, *P<0.05 vs. Control, **P<0.01 vs. Control. Normal,
age-matched untreated rats; Control, immunized rats treated with
vehicle; HuMSCs, immunized rats treated with HuMSCs; GRP78,
glucose-regulated protein 78; ERK, extracellular-signal regulated
kinase; HuMSCs, human umbilical cord-derived mesenchymal stem
cells; p, phosphorylated. |
HuMSCs protect against ER stress
GRP78 is a marker of ER stress. ER stress leads to
activation of caspase 12, resulting in cardiomyocyte apoptosis.
Significant increases were observed in myocardial GRP78 and caspase
12 expression in EAM animals, indicating the involvement of ER
stress. HuMSC-treated animals exhibited significant attenuation of
these ER stress markers compared with the control group (Fig. 2B).
Discussion
In the present study, the therapeutic potential of
HuMSCs in the acute phase of myocarditis was investigated. In a rat
model of acute myocarditis, intravenous administration of HuMSCs 10
days following myosin injection significantly improved cardiac
function. Echocardiographic analyses demonstrated LV remodeling
with increased LVDs, LVDd and reduced FS in vehicle-treated EAM
rats compared with normal rats, indicating impaired systolic and
diastolic function of the myocardium. HuMSC treatment positively
affected LV remodeling by significantly reversing the increased
LVDs and LVDd, and reducing FS. Pathological findings in the heart
21 days following myosin injection indicated that EAM rats in the
control group suffered extensive inflammatory cellular
infiltration, whereas rats treated with HuMSCs exhibited
significantly less infiltration. Based on these results, HuMSCs
demonstrated protective effects in myocardial inflammation.
Myosin-induced EAM contributes to a model that is
similar to giant cell myocarditis in humans with three phrases:
Antigen-priming (from days 0 to 14), autoimmune response (from days
14 to 21), and a chronic phase featuring cardiac remodeling and
fibrosis (3). Although EAM
pathogenesis has remains to be fully elucidated, MAPK signaling
pathways and ER stress-induced myocardial apoptosis are likely to
be involved.
MAPKs are a group of serine/threonine protein
kinases. There are three MAPK subfamilies: ERK, c-Jun N-terminal
kinase and p38-MAPK. Overexpression of mitogen activated protein
kinase kinase (MEK) 1, the upstream activator of ERK 1/2, results
in cardiomyocyte hypertrophy in vitro (14). The MEK/ERK cascade serves a role in
fibrotic diseases (15,16) and affects lymphocyte activation and
differentiation (17–19). In addition, the MEK/ERK signaling
pathway enhances the production of diverse proinflammatory
cytokines, including interleukin (IL)-β, tumor necrosis factor-α
and IL-6 (20–22). The MEK/ERK signaling pathway is
targeted for the treatment of rheumatoid arthritis (23), and is involved in IL-17-mediated
cardiac fibrosis in EAM (24).
Activation of ERK1/2 serves a role in cardiomyocyte apoptosis
caused by doxorubicin (25).
Proteomic and biochemical analyses further revealed that ERK-1/2
signaling and ER stress mediates cardiomyocyte apoptosis in EAM
(26).
In the present study, p-ERK1/2 expression was
significantly increased in EAM rats treated with the vehicle.
However, HuMSC supplementation protected against cardiac damage as
evidenced by decreased levels of p-ERK1/2 proteins. However,
p38-MAPK levels did not differ among the three groups. These
results confirmed the involvement of ERK1/2 signaling in EAM, as
well as indicating that HuMSC treatment may protect animals from
cardiac damage by avoiding activation of the ERK1/2 signaling
pathway.
The ER is generally defined as an organelle that
participates in folding of membrane and secretory proteins
(27). ER stress, caused by
stimuli such as ischemia, hypoxia, heat shock, genetic mutation and
oxidative stress, can lead to ER dysfunction (27). ER stress is becoming a focus of
research because it causes numerous inflammatory disorders
(27). In response to ER stress,
there is a marked upregulation of ER chaperones including GRP78.
Cell apoptosis may occur via caspase 12, which is localized in the
ER and activated by ER stress (7,28).
Studies have revealed that quercetin and edaravone improve
autoimmune myocarditis and decrease cardiomyoctye apoptosis by
relieving ER stress (29,30). In the present study, HuMSC
treatment was demonstrated to protect EAM rats from ER stress, as
demonstrated by downregulated expression of GRP78 and caspase 12
(Fig. 2B and C).
Identification of TUNEL-positive apoptotic cells in
the myocardium is useful for examining the intensity of damage to
myocardial cells (31). In
addition, the anti-apoptotic role of HuMSCs was confirmed by
reduced numbers of TUNEL-positive apoptotic cells in the myocardial
sections of EAM rats treated with HuMSCs. Based on these data, the
authors suggested that treatment with HuMSCs is effective for the
prevention of myocardial apoptosis in EAM rats and may protect them
from myocardial damage.
In conclusion, the present study demonstrated that
the protective effects of intravenously administered HuMSCs in EAM
result from regulation of ER stress and ERK1/2 signaling-mediated
apoptosis. AsHuMSCs are available in large numbers using
non-invasive procedures, these findings provide a novel perspective
for the treatment of acute myocarditis.
Acknowledgements
The current study was supported by grants from the
National Natural Science Foundation of China (grant no. 81070478),
the Science and Technology Program of Guangdong Province (grant no.
2012B0031800443), the Science and Technology Program Project of
Shantou (grant no. 2012165 and 2013), the Basic and Clinical
Scientific Research Foundation of Shantou University Medical
College (grant no. 201410), the Science and Technology Program of
Shenzhen (grant no. JCYJ20150402092905162) and the Research Project
of Health and Family Planning Commission of Shenzhen Municipality
(grant no. 201501053).
References
|
1
|
Aretz HT, Billingham ME, Edwards WD,
Factor SM, Fallon JT, JJ Jr, Olsen EG Fenoglio and Schoen FJ:
Myocarditis. A histopathologic definition and classification. Am J
Cardiovasc Pathol. 1:3–14. 1987.PubMed/NCBI
|
|
2
|
Cooper LT Jr: Myocarditis. N Engl J Med.
360:1526–1538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kodama M, Hanawa H, Saeki M, Hosono H,
Inomata T, Suzuki K and Shibata A: Rat dilated cardiomyopathy after
autoimmune giant cell myocarditis. Circ Res. 75:278–284. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodama M, Matsumoto Y, Fujiwara M, Masani
F, Izumi T and Shibata A: A novel experimental model of giant cell
myocarditis induced in rats by immunization with cardiac myosin
fraction. Clin Immunol Immunopathol. 57:250–262. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alter P, Jobmann M, Meyer E, Pankuweit S
and Maisch B: Apoptosis in myocarditis and dilated cardiomyopathy:
Does enterovirus genome persistence protect from apoptosis? An
endomyocardial biopsy study. Cardiovasc Pathol. 10:229–234. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lowenstein CJ: Exogenous thioredoxin
reduces inflammation in autoimmune myocarditis. Circulation.
110:1178–1179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arumugam S, Thandavarayan RA, Veeraveedu
PT, Ma M, Giridharan VV, Arozal W, Sari FR, Sukumaran V, Lakshmanan
A, Soetikno V, et al: Modulation of endoplasmic reticulum stress
and cardiomyocyte apoptosis by mulberry leaf diet in experimental
autoimmune myocarditis rats. J Clin Biochem Nutr. 50:139–144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohnishi S, Yanagawa B, Tanaka K, Miyahara
Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K,
Kitamura S and Nagaya N: Transplantation of mesenchymal stem cells
attenuates myocardial injury and dysfunction in a rat model of
acute myocarditis. J Mol Cell Cardiol. 42:88–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McElreavey KD, Irvine AI, Ennis KT and
McLean WH: Isolation, culture and characterisation of
fibroblast-like cells derived from the Wharton's jelly portion of
human umbilical cord. Biochem Soc Trans. 19:29S1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma L, Feng XY, Cui BL, Law F, Jiang XW,
Yang LY, Xie QD and Huang TH: Human umbilical cord Wharton's
Jelly-derived mesenchymal stem cells differentiation into
nerve-like cells. Chin Med J (Engl). 118:1987–1993. 2005.PubMed/NCBI
|
|
11
|
Weiss ML, Mitchell KE, Hix JE, Medicetty
S, El-Zarkouny SZ, Grieger D and Troyer DL: Transplantation of
porcine umbilical cord matrix cells into the rat brain. Exp Neurol.
182:288–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goser S, Ottl R, Brodner A, Dengler TJ,
Torzewski J, Egashira K, Rose NR, Katus HA and Kaya Z: Critical
role for monocyte chemoattractant protein-1 and macrophage
inflammatory protein-1alpha in induction of experimental autoimmune
myocarditis and effective anti-monocyte chemoattractant protein-1
gene therapy. Circulation. 112:3400–3407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zimmermann O, Homann JM, Bangert A, Müller
AM, Hristov G, Goeser S, Wiehe JM, Zittrich S, Rottbauer W,
Torzewski J, et al: Successful use of mRNA-nucleofection for
overexpression of interleukin-10 in murine monocytes/macrophages
for anti-inflammatory therapy in a murine model of autoimmune
myocarditis. J Am Heart Assoc. 1:e0032932012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueyama T, Kawashima S, Sakoda T, Rikitake
Y, Ishida T, Kawai M, Yamashita T, Ishido S, Hotta H and Yokoyama
M: Requirement of activation of the extracellular signal-regulated
kinase cascade in myocardial cell hypertrophy. J Mol Cell Cardiol.
32:947–960. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stratton R, Rajkumar V, Ponticos M,
Nichols B, Shiwen X, Black CM, Abraham DJ and Leask A: Prostacyclin
derivatives prevent the fibrotic response to TGF-beta by inhibiting
the Ras/MEK/ERK pathway. FASEB J. 16:1949–1951. 2002.PubMed/NCBI
|
|
16
|
Stratton R, Shiwen X, Martini G, Holmes A,
Leask A, Haberberger T, Martin GR, Black CM and Abraham D: Iloprost
suppresses connective tissue growth factor production in
fibroblasts and in the skin of scleroderma patients. J Clin Invest.
108:241–250. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen D, Heath V, O'Garra A, Johnston J and
McMahon M: Sustained activation of the raf-MEK-ERK pathway elicits
cytokine unresponsiveness in T cells. J Immunol. 163:5796–5805.
1999.PubMed/NCBI
|
|
18
|
DeSilva DR, Jones EA, Favata MF, Jaffee
BD, Magolda RL, Trzaskos JM and Scherle PA: Inhibition of
mitogen-activated protein kinase kinase blocks T cell proliferation
but does not induce or prevent anergy. J Immunol. 160:4175–4181.
1998.PubMed/NCBI
|
|
19
|
Pagès G, Guérin S, Grall D, Bonino F,
Smith A, Anjuere F, Auberger P and Pouysségur J: Defective
thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice.
Science. 286:1374–1377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dumitru CD, Ceci JD, Tsatsanis C,
Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA,
Copeland NG, Kollias G and Tsichlis PN: TNF-alpha induction by LPS
is regulated posttranscriptionally via a Tpl2/ERK-dependent
pathway. Cell. 103:1071–1083. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scherle PA, Jones EA, Favata MF, Daulerio
AJ, Covington MB, Nurnberg SA, Magolda RL and Trzaskos JM:
Inhibition of MAP kinase kinase prevents cytokine and prostaglandin
E2 production in lipopolysaccharide-stimulated monocytes. J
Immunol. 161:5681–5686. 1998.PubMed/NCBI
|
|
22
|
Tuyt LM, Dokter WH, Birkenkamp K, Koopmans
SB, Lummen C, Kruijer W and Vellenga E: Extracellular-regulated
kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in
NF-kappa B-dependent IL-6 expression in human monocytes. J Immunol.
162:4893–4902. 1999.PubMed/NCBI
|
|
23
|
Thiel MJ, Schaefer CJ, Lesch ME, Mobley
JL, Dudley DT, Tecle H, Barrett SD, Schrier DJ and Flory CM:
Central role of the MEK/ERK MAP kinase pathway in a mouse model of
rheumatoid arthritis: Potential proinflammatory mechanisms.
Arthritis Rheum. 56:3347–3357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Zhu H, Su Z, Sun C, Yin J, Yuan H,
Sandoghchian S, Jiao Z, Wang S and Xu H: IL-17 contributes to
cardiac fibrosis following experimental autoimmune myocarditis by a
PKCβ/Erk1/2/NF-κB-dependent signaling pathway. Int Immunol.
24:605–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Mao W, Ding B and Liang CS:
ERKs/p53 signal transduction pathway is involved in
doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am
J Physiol Heart Circ Physiol. 295:H1956–H1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung JH, Choi HJ, Kim SY, Hong KS, Min
SK, Nam MH, Kim CW, Koh YH and Seo JB: Proteomic and biochemical
analyses reveal the activation of unfolded protein response,
ERK-1/2 and ribosomal protein S6 signaling in experimental
autoimmune myocarditis rat model. BMC Genomics. 12:5202011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oyadomari S, Araki E and Mori M:
Endoplasmic reticulum stress-mediated apoptosis in pancreatic
beta-cells. Apoptosis. 7:335–345. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Liu G, Song T, Liu F, Kang W, Zhang
Y and Ge Z: Upregulation of GRP78 and caspase-12 in diastolic
failing heart. Acta Biochim Pol. 55:511–516. 2008.PubMed/NCBI
|
|
29
|
Arumugam S, Thandavarayan RA, Arozal W,
Sari FR, Giridharan VV, Soetikno V, Palaniyandi SS, Harima M,
Suzuki K, Nagata M, et al: Quercetin offers cardioprotection
against progression of experimental autoimmune myocarditis by
suppression of oxidative and endoplasmic reticulum stress via
endothelin-1/MAPK signalling. Free Radic Res. 46:154–163. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arumugam S, Thandavarayan RA, Veeraveedu
PT, Nakamura T, Arozal W, Sari FR, Giridharan VV, Soetikno V,
Palaniyandi SS, Harima M, et al: Beneficial effects of edaravone, a
novel antioxidant, in rats with dilated cardiomyopathy. J Cell Mol
Med. 16:2176–2185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gill C, Mestril R and Samali A: Losing
heart: The role of apoptosis in heart disease-a novel therapeutic
target? FASEB J. 16:135–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
















