Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
and inflammatory disease characterized by persistent synovitis;
symptoms include joint pain, stiffness and swelling. In RA, chronic
inflammation leads to synovial hyperplasia termed ‘pannus’, which
erodes bone and cartilage, thereby destroying articular cartilage
(1).
Synovial hyperplasia is caused by alterations in
cell proliferation and cell death, involving increased
proliferation and insufficient apoptosis of synovial cells
(2–4). The proliferation of synovial cells is
regulated by growth factors and cytokines produced in the local
milieu (5,6). The insufficient apoptosis of synovial
cells is caused by the overexpression of transformation-associated
proteins, such as tumor protein p53 (7,8).
Therefore, inhibiting proliferation and inducing apoptosis of
synovial cells may constitute a promising strategy for the
treatment of RA (3).
Methotrexate (MTX), an antifolate derivative, which
inhibits DNA synthesis and induces apoptosis of tumor cells, is
widely used as a therapeutic agent for malignant tumors. MTX is
additionally utilized as a first-line RA treatment, as it exerts
anti-inflammatory and antiproliferative effects on lymphocytes and
synovial cells (9).
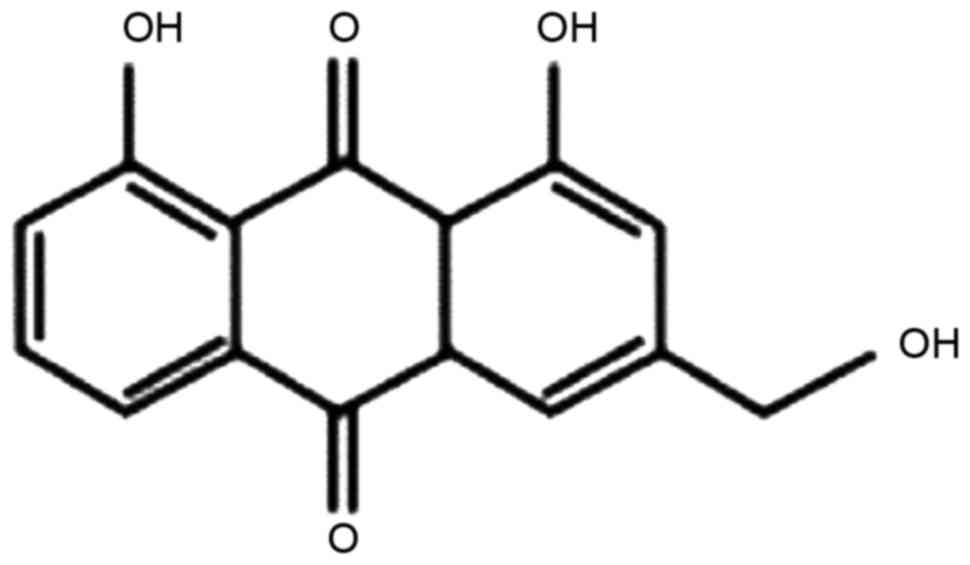
Aloe-emodin (AE; the chemical structure of which is
presented in Fig. 1) is a primary
bioactive component of Aloe vera, Aloe arborescens
and certain Chinese herbs, including Rheum officinale. AE
has been demonstrated to modulate various functions of host cells;
AE exerts anti-inflammatory effects by inhibiting the activation of
macrophages (10,11) and antiproliferative effects on
human tumor cells, including neuroblastoma, hepatoma, leukemia,
tongue squamous cancer and colon cancer cells (12–17).
In HL-60 human leukemia cells, AE exerts antitumor effects by
inhibiting cell proliferation, and inducing apoptosis and cell
cycle arrest (14). Based on these
findings, the present study aimed to investigate whether AE may
inhibit proliferation and induce apoptosis in synovial cells, which
are important in the pathogenesis of RA. The effect of AE on cell
growth, apoptosis and cell cycle distribution of synovial cells was
evaluated using MH7A human RA synovial cells. In addition, the
effect of AE was compared with the effect of MTX, an established
first-line RA treatment.
Materials and methods
Reagents
AE and MTX were purchased from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany). An 100 mM AE stock solution was prepared
by dissolving AE in dimethyl sulfoxide (DMSO), and was stored at
−20°C. The AE stock solution was further diluted to the indicated
concentrations in culture media immediately prior to each
experiment. The final concentrations of AE in the culture media
were 5, 10, 20 and 40 µM. These concentrations were selected since
AE at a concentration range of 6.25–50 µM has been reported to
induce G2/M cell cycle arrest and apoptosis through the
activation of caspase-6 in human colon cancer cells (16). MTX was dissolved in DMSO at 100 mM,
and was further diluted in culture media. The final concentrations
of MTX in the media were 0.01, 0.05, 0.1 and 1 µM.
Cell culture
MH7A human RA synovial fibroblast-like cells
(18) were provided by the RIKEN
BioResource Center (Tsukuba, Japan) through the National
Bio-Resource Project of the Ministry of Education, Culture, Sports
Science and Technology Japan, and maintained in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Nichirei Biosciences,
Inc., Tokyo, Japan), 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C in an atmosphere of 5% CO2. Cells were passaged every 3–4
days.
Measurement of viable cells
MH7A cells (5×104 cells/well) were seeded
into 12-well plates and cultured for 24 h. Cells were subsequently
incubated with 5, 10, 20 or 40 µM AE, 0.01, 0.05, 0.1 or 1 µM MTX,
or DMSO as a vehicle control (0.04% for AE and 0.001% for MTX), for
3 days. Following incubation, cells were harvested with
trypsin/EDTA, stained with 0.25% trypan blue and counted using an
automated cell counter (Thermo Fisher Scientific, Inc.).
Alternatively, MH7A cells (1×104
cells/well) were seeded into 48-well plates and cultured for 24 h.
Cells were subsequently incubated with AE, MTX or DMSO, as
aforementioned. Following incubation, live cells were evaluated
using Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) according to the manufacturer's protocol.
The absorbance of each well was measured at a wavelength of 450 nm
using a microplate reader (xMark™; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Analysis of cell death
MH7A cells (2×105 cells/well) were seeded
into 6-well plates and cultured for 24 h. Cells were subsequently
incubated with AE, MTX or DMSO for 2 days. Following incubation,
cells were harvested with trypsin/EDTA, and apoptosis was measured
using an Annexin V-fluorescein isothiocyanate/propidium iodide (PI)
assay kit (Medical & Biological Laboratories Co., Ltd., Nagoya,
Japan). Cells were analyzed for early and late apoptosis, and
necrosis, using a flow cytometer (FACSCalibur; BD Biosciences, San
Jose, CA, USA) and the software BD CellQuest™ Pro
version 6.0 (BD Biosciences).
Analysis of cell cycle phase
distribution
MH7A cells (2×105 cells/well) were seeded
into 6-well plates and cultured for 24 h. Cells were subsequently
incubated with AE, MTX or DMSO for 2 days. Following incubation,
cells were harvested with trypsin/EDTA, fixed and permeabilized
with 100% ethanol, stained with PI (Cell Cycle Phase Determination
kit; Cayman Chemical Company, Ann Arbor, MI, USA) and analyzed by
flow cytometry.
Statistical analysis
Data are presented as the mean ± standard deviation.
The statistical significance of the differences between groups was
assessed using one-way analysis of variance followed by a post hoc
Bonferroni test for multiple comparisons. Statistical analysis was
performed using GraphPad Prism software version 5 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of AE and MTX treatment on the
proliferation of MH7A cells
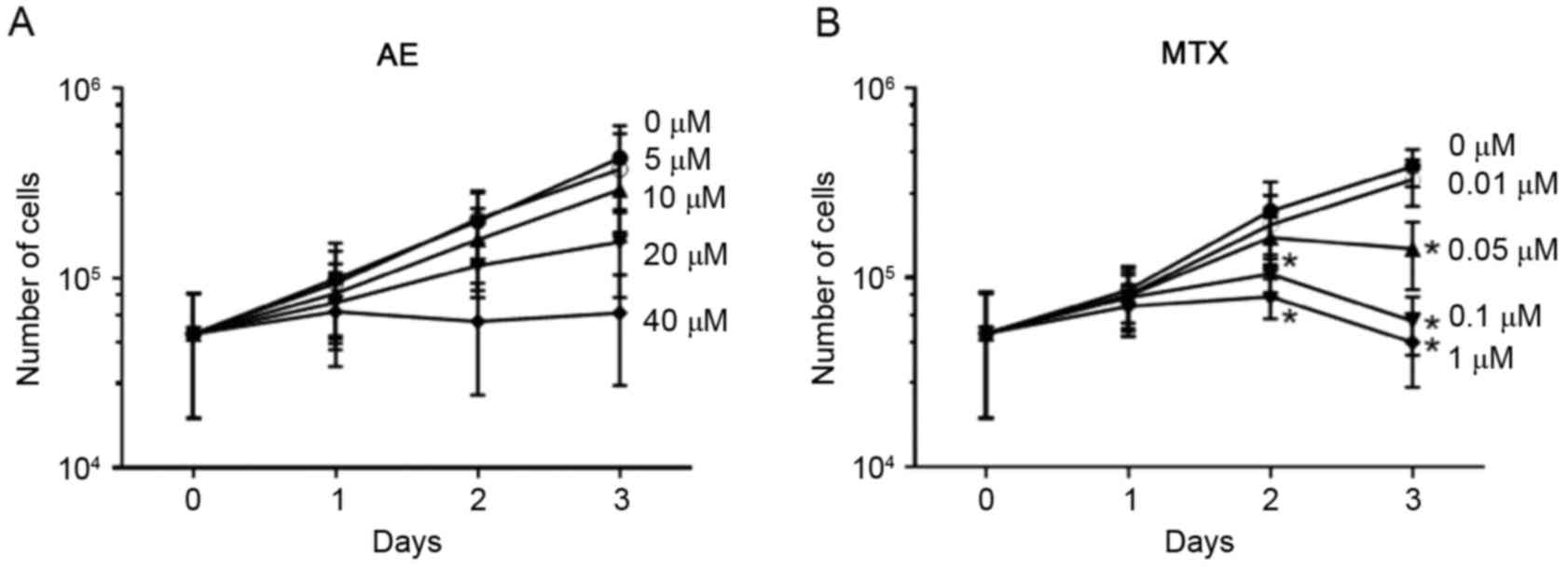
To evaluate the effect of AE and MTX on the
proliferation of synovial cells, MH7A cells were cultured for 3
days in the absence or presence of 5–40 µM AE or 0.01–1 µM MTX, and
the number of viable cells was measured by trypan blue exclusion
staining. As presented in Fig. 2,
the total number of live MH7A cells increased ~8-fold in 3 days of
culture in the absence of AE and MTX. AE treatment inhibited the
increase in cell number in a dose-dependent manner (Fig. 2A); however, this effect was not
significant. MTX treatment significantly inhibited the increase in
MH7A cell numbers at concentrations ≥0.05 µM (P<0.05; Fig. 2B).
In addition, the effect of AE and MTX on MH7A cells
was analyzed by CCK-8 assay. CCK-8 assay is based on reduction of
tetrazolium salt by live and metabolically active cells, and is
therefore utilized for the quantification of living cells in
culture. As presented in Fig. 3,
the number of viable MH7A cells increased ~6-fold in 3 days in the
absence of AE and MTX. When measured using the CCK-8 assay, 10–40
µM AE (Fig. 3A) and 0.05–1 µM MTX
(Fig. 3B) significantly inhibited
the increase in the number of live cells in a dose-dependent manner
(P<0.05). These observations indicate that AE and MTX inhibit
the proliferation of MH7A cells.
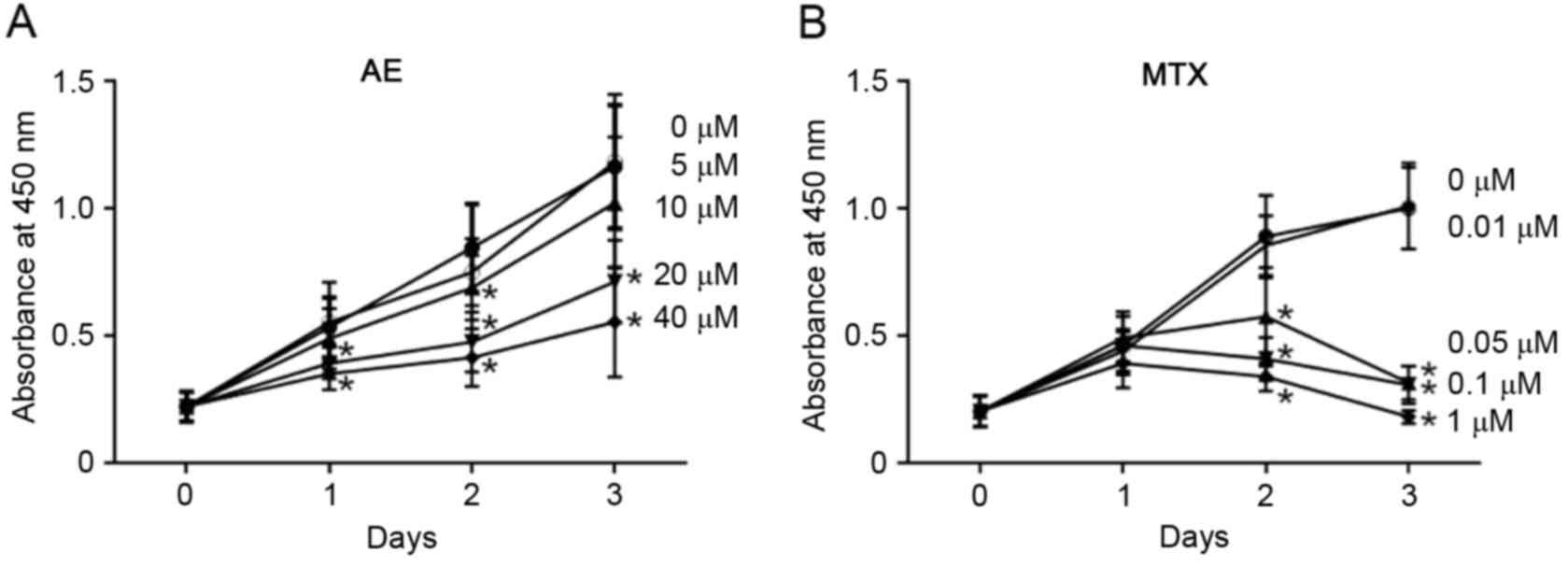 | Figure 3.Evaluation of the effect of AE and MTX
on MH7A cell proliferation by CCK-8 assay. MH7A cells
(1×104 cells/well) were seeded into 48-well plates for
24 h, and treated with (A) AE (5, 10, 20 or 40 µM), or (B) MTX
(0.01, 0.05, 0.1 or 1 µM) for 1, 2 and 3 days. Cells treated with
the vehicle dimethyl sulfoxide served as a control (0 µM). Live
cell numbers were determined by CCK-8 assay. Data are presented as
the mean ± standard deviation of 3 (AE) or 4 (MTX) independent
experiments. *P<0.05 vs. 0 µM. AE, aloe-emodin; MTX,
methotrexate; CCK-8, Cell Counting kit-8. |
Effect of AE and MTX treatment on MH7A
cell death
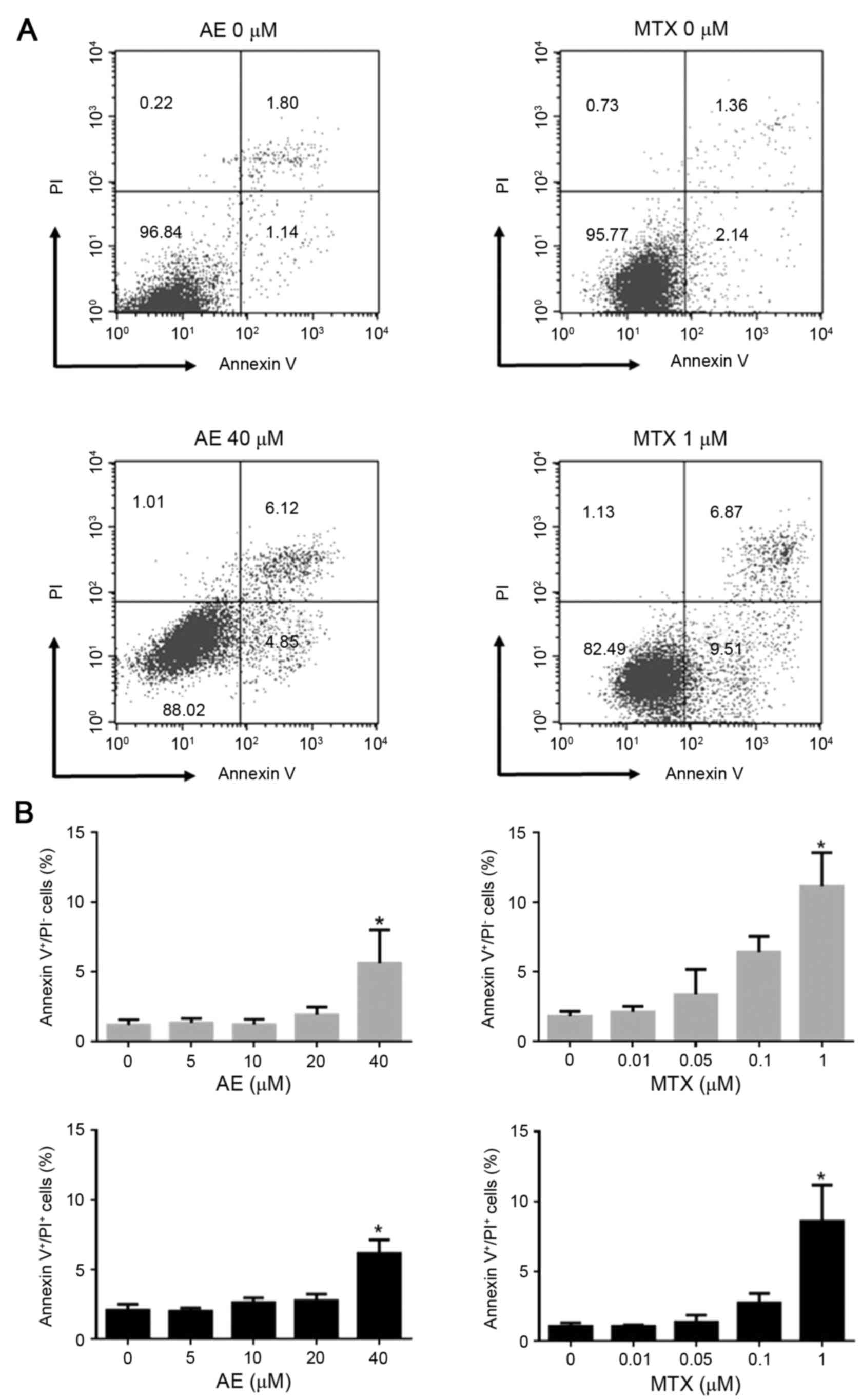
The effect of AE and MTX treatment on MH7A cell
death was examined. MH7A cells were incubated in the absence or
presence of 5–40 µM AE or 0.01–1 µM MTX for 2 days, and apoptosis
and necrosis were analyzed by Annexin V/PI staining (Fig. 4A). Flow cytometric analysis
demonstrated that treatment with 40 µM AE significantly increased
the percentage of Annexin V+/PI− (early
apoptotic; 5.6±2.4 vs. 1.2±0.4%) and Annexin
V+/PI+ (late apoptotic; 6.2±1.0 vs. 2.1±0.4%)
cells, compared with untreated MH7A cells (P<0.05; Fig. 4B). This indicated that AE treatment
induces early and late apoptosis in MH7A cells. Similarly,
treatment with 1 µM MTX increased the percentage of Annexin
V+/PI− (11.2±2.4 vs. 1.8±0.4%) and Annexin
V+/PI+ (8.6±2.6 vs. 1.0±0.3%) cells, compared
with untreated cells (P<0.05; Fig.
4B). Concentrations ≤20 µM AE and ≤0.1 µM MTX exhibited no
effect on apoptosis (Fig. 4B).
These observations indicated that only 40 µM AE and 1 µM MTX
induced apoptotic cell death in MH7A cells. AE and MTX did not
induce necrotic cell death (Annexin V−/PI+
cells) in MH7A cells even at high concentrations evaluated in the
present study (data not shown).
Effect of AE and MTX treatment on cell
cycle phase distribution of MH7A cells
Cell proliferation is regulated by cell cycle
progression. Thus, to clarify the underlying mechanism involved in
the inhibitory action of AE or MTX on the proliferation of MH7A
cells, the effect of AE and MTX on cell cycle phase distribution
was examined. MH7A cells were cultured for 2 days in the absence or
presence of 5–40 µM AE or 0.01–1 µM MTX, and stained with PI to
determine the DNA content of the cells (Fig. 5A). The results demonstrated that
treatment with 40 µM AE increased the percentage of cells in G2/M
phase and decreased the percentage of cells in G1 phase compared
with untreated cells (P<0.05; Fig.
5B), suggesting that AE induced G2/M phase arrest in
MH7A cells. Conversely, treatment with 1 µM MTX significantly
increased the percentage of cells in S phase and decreased the
percentage of cells in G2/M phases compared with untreated cells
(P<0.05; Fig. 5B). In addition,
1 µM MTX slightly decreased the percentage of cells in G1 phase,
although this effect was not significant. These observations
suggested that MTX induced S phase arrest in MH7A cells. The
effects of AE and MTX on the cell cycle were dose-dependent, with
AE demonstrating significant effects at concentrations ≥20 µM and
MTX demonstrating significant effects at concentrations ≥0.05 µM
(P<0.05; Fig. 5B), compared
with untreated cells. Notably, 20 µM AE and 0.05 and 0.1 µM MTX
significantly induced G2/M and S phase arrest,
respectively, whereas the same concentrations did not induce
apoptotic cell death (Fig. 4).
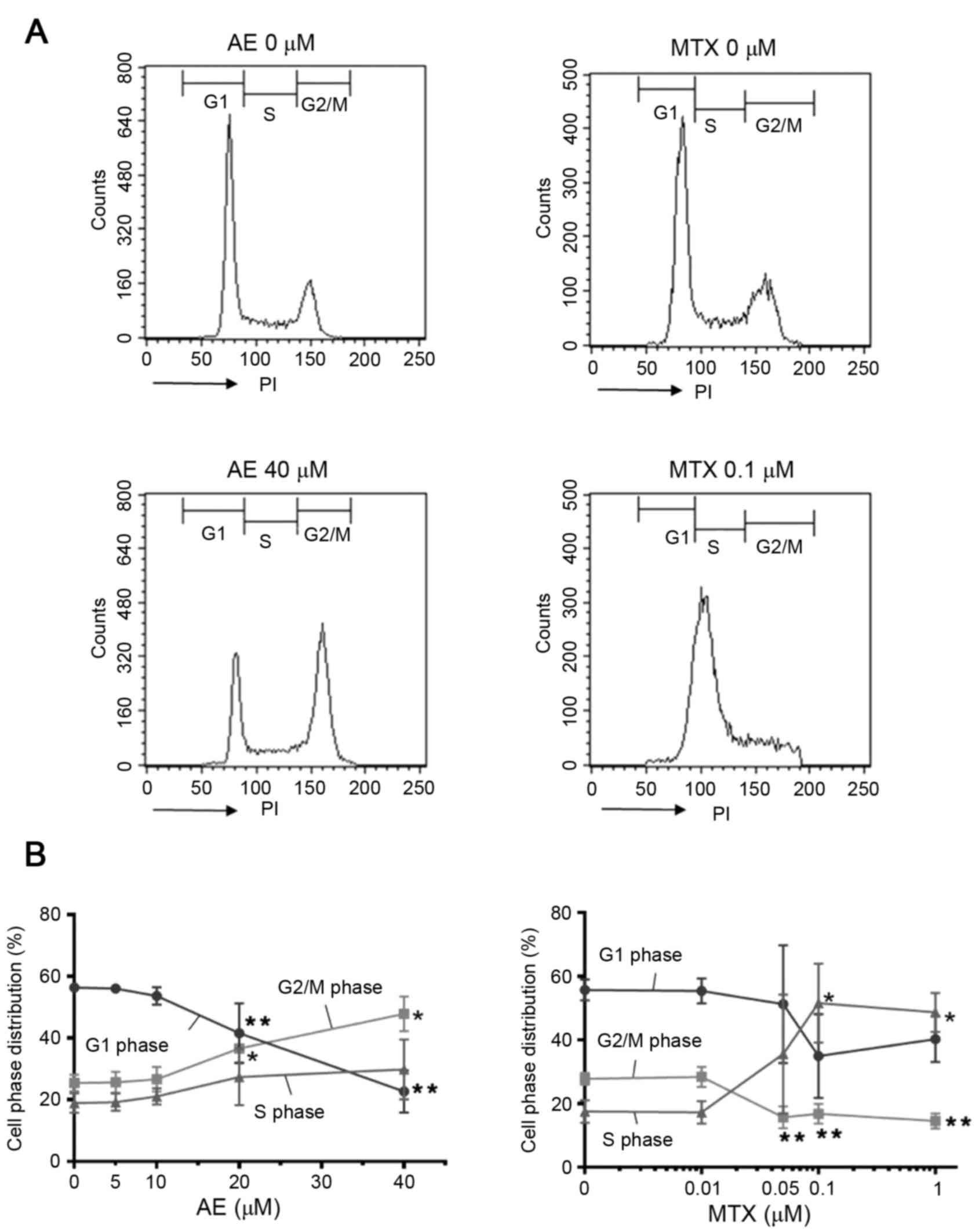 | Figure 5.Evaluation of the effect of AE and MTX
on cell cycle distribution. MH7A cells (2×105
cells/well) were seeded into 6-well plates for 24 h, and treated
with AE (5, 10, 20 or 40 µM), or MTX (0.01, 0.05, 0.1 or 1 µM) for
48 h. Cells treated with the vehicle dimethyl sulfoxide served as a
control (0 µM). Cell cycle distribution was evaluated by PI
staining followed by flow cytometric analysis. (A) Representative
plots of control cells and cells treated with 40 µM AE or 0.1 µM
MTX. (B) Quantification of the percentage of cells in the G1, G2/M
and S phases. Data are presented as the mean ± standard deviation
of 4 independent experiments. *P<0.05 and **P<0.01 vs. 0 µM.
AE, aloe-emodin; MTX, methotrexate; PI, propidium iodide. |
Discussion
RA is a chronic inflammatory disease characterized
by synovial hyperplasia, in which the proliferation of synovial
cells and infiltrating macrophages and lymphocytes is increased,
and apoptosis of these cells is decreased (3). MTX, an antifolate derivative, is
presently a first-line RA treatment, as it exerts anti-inflammatory
and antiproliferative effects on lymphocytes and synovial cells
(19). However, the administration
of MTX induces a variety of adverse effects, including potentially
life-threatening hepatotoxicity, nephrotoxicity, pulmonary damage
and myelosuppression (20). Thus,
RA patients are utilizing complementary and alternative medicine to
treat RA symptoms (21,22). AE, a bioactive component of Aloe,
is hypothesized to act as an antitumor reagent (17), based on its suppressive action on
the cell growth of various human tumor cells (12). In the present study, the effect of
AE on the growth of synovial cells was examined, and it was
revealed that ≥10 µM AE significantly decreased the numbers of live
MH7A cells, based on the CCK-8 assay. A Trypan blue assay also
revealed that AE decreased the viability of MH7A cells; however,
the deviations of the data were high, and significance was not
detected with regards to the Trypan blue assay.
Previous studies have demonstrated that AE induces
apoptosis in various human tumor cell lines, including SJ-N-KP
neuroblastoma, Hep G2 hepatoma and HL-60 leukemia cells (12–14).
Thus, to elucidate whether AE induces the apoptosis of MH7A cells,
apoptosis analysis was performed in AE-treated MH7A cells using
AnnexinV/PI double staining followed by flow cytometry. The results
indicated that following treatment for 2 days with 40 µM AE, 5.6
and 6.2% of cells were early and late apoptotic, respectively,
whereas the same concentration and duration of treatment inhibited
MH7A cell proliferation by 69%. In addition, AE did not induce
apoptosis at concentrations <40 µM, although AE significantly
inhibited the proliferation of MH7A cells by 25% at 10 µM and 59%
at 20 µM, compared with untreated cells. Therefore, the inhibitory
effects of AE on cell proliferation may not be fully explained by
the induction of apoptosis.
Notably, AE has been reported to modulate the cell
cycle, thereby suppressing the proliferation of cancer cells
(14–16). Thus, to further clarify the
underlying mechanism involved in the inhibitory effect of AE on the
proliferation of MH7A cells, the effect of AE treatment on cell
cycle progression was examined. The majority of cells analyzed in
the untreated group were in G1 phase (in preparation for mitosis).
AE treatment decreased the percentage of cells in G1 phase and
increased the percentage of cells in G2/M phase (prior to/in
mitosis) in a dose-dependent manner compared with the untreated
group, suggesting that AE induced G2/M arrest in MH7A cells. These
observations indicated that AE-treated MH7A cells may not complete
mitosis. AE at a dose of 20 µM induced G2/M arrest, whereas the
same dose was not sufficient to induce apoptotic cell death.
Furthermore, 40 µM AE induced cell cycle arrest in the G2/M phase
by 23% among MH7A cells compared with untreated cells, whereas the
same dose induced apoptosis in only 12% (5.6 and 6.2% of early and
late apoptotic cells) of total cell population. These observations
suggested that the G2/M phase arrest may be more important than the
induction of apoptosis for the inhibition of cell proliferation by
AE.
Consistent with the results of the present study, AE
has been reported to induce G2/M phase arrest in other cells,
including HL-60 and WiDr colon adenocarcinoma cells (14,16).
In WiDr cells, the promoter activity and protein expression levels
of cyclin B1, an essential factor for cell cycle progression from
G2 to M phase, were strongly suppressed by AE treatment (16). Therefore, AE-mediated suppression
of cyclin B1 may be involved in the inhibition of MH7A cell
proliferation. By contrast, AE has been demonstrated to induce G1
phase arrest in Hep G2 human hepatoma cells (13), and S phase arrest in FaDu human
pharyngeal squamous carcinoma, H1299 human lung cancer and MG-63
human osteosarcoma cells (23).
These observations suggested that the cell cycle
progression-associated target molecules of AE may differ in various
cell types (17).
AE is a 1,8-dihydroxyanthraquinone compound, having
an anthraquinone ring and two phenolic hydroxyl groups. Quinones
are highly redox-active molecules that lead to the generation of
reactive oxygen species (ROS). AE has been demonstrated to induce
the loss of mitochondrial membrane potential and caspase-dependent
apoptosis in SCC-4 human tongue squamous cancer cells, through
increased ROS production (15). It
is possible that ROS production may be involved in the AE-induced
apoptosis of MH7A cells observed in the present study. However,
MH7A cells are more resistant to AE compared with the cancer cells
previously examined; 40 µM AE induces 50% apoptosis in SCC-4 cells
(15), and 40% apoptosis in CH27
human lung squamous carcinoma cells (24), whereas 40 µM AE induced only 12%
apoptosis in MH7A cells.
The effects of AE treatment were compared throughout
the present study with the effects of 0.01–1 µM MTX. The
concentrations of MTX were selected based on the concentrations
detected in synovial fluids of RA patients treated with low-dose
MTX (~1.3 µM) (25). The results
indicated that ≥0.05 µM MTX inhibited proliferation of MH7A cells,
and 1 µM MTX almost completely inhibited proliferation (~90% in 3
days), compared with untreated cells. These observations are in
agreement with previous reports that 2 µM MTX inhibited cell growth
by ~80% in human synovial fibroblasts (6) and 1 µM MTX inhibited cell growth by
~80% in adherent cells of rheumatic synovial tissue (26).
To understand the mechanism of MTX-induced
inhibition of cell proliferation, the effect of MTX on MH7A cell
death was examined in the present study. The results indicated that
1 µM MTX treatment for 2 days induced early and late apoptosis in
11.2 and 8.6%, respectively, of MH7A cells. By contrast, 1 µM MTX
inhibited the proliferation of MH7A cells by ~80% at the same time
point, suggesting that the induction of apoptosis only partially
contributed to the inhibition of cell proliferation by MTX, similar
to AE.
The effect of MTX on the cell cycle progression of
MH7A cells was examined. In contrast to the action of AE, MTX
decreased the percentage of cells in G2/M phase and increased the
percentage of cells in S phase in a dose-dependent manner,
suggesting that MTX induces S phase arrest in MH7A cells. Notably,
MTX induced cell cycle arrest in the S phase by 34% in MH7A cells
at 0.1 µM, a dose at which apoptotic cell death was not observed. A
similar increase in S phase cells was observed following treatment
with 1 µM, at which dose apoptotic cell death was induced in 20%
(11.2 and 8.6% of early and late apoptotic cells) of cells. These
observations suggested that MTX-induced S phase arrest is more
important than apoptosis in the MTX-mediated inhibition of
proliferation.
As MTX is an antimetabolite that interferes with
folate metabolism and DNA synthesis (6), explaining the induction of cell cycle
arrest at the S phase. Apoptosis is additionally regulated by
molecules involved in cell division and cell cycle progression
(27). Thus, MTX-mediated S phase
arrest may induce apoptosis in MH7A cells. In addition, AE-mediated
G2/M phase arrest may induce apoptosis in MH7A cells.
In conclusion, the results of the present study
revealed that AE, and MTX, treatment inhibited the proliferation of
MH7A synovial cells. Furthermore, AE and MTX treatment induced
apoptosis and cell cycle arrest (at G2/M and S phases,
respectively) compared with untreated MH7A cells. For efficient
therapeutic intervention in RA, it is essential to suppress
proliferation and induce apoptosis of synovial and inflammatory
cells in the RA joints (2,3). Thus, the present results suggested
that AE, a natural component of Aloe, may be a safe and effective
agent for the treatment of RA. Future studies are required to
examine more fully the therapeutic potential of AE, including in
vivo studies in animal models of RA.
Acknowledgements
The authors would like to thank Dr K. Miyazawa
(Kissei Pharmaceutical Co., Ltd., Nagano, Japan) for establishing
the MH7A cell line, and the researchers of the Division of Cell
Biology in BioMedical Research Center and the Department of Host
Defense and Biochemical Research, Juntendo University Graduate
School of Medicine (Tokyo, Japan), for technical assistance and
helpful discussion. This study was supported in part by a grant of
The Strategic Research Foundation Grant-Aided Project for Private
Universities from Ministry of Education, Culture, Sport, Science,
and Technology, Japan (MEXT), 2014–2018 (S1411007).
Glossary
Abbreviations
Abbreviations:
|
RA
|
rheumatoid arthritis
|
|
AE
|
aloe-emodin
|
|
MTX
|
methotrexate
|
|
PI
|
propidium iodide
|
|
DMSO
|
dimethyl sulfoxide
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Müller-Ladner U, Pap T, Gay RE, Neidhart M
and Gay S: Mechanisms of disease: The molecular and cellular basis
of joint destruction in rheumatoid arthritis. Nat Clin Pract
Rheumatol. 1:102–110. 2005. View Article : Google Scholar
|
|
2
|
Nishioka K, Hasunuma T, Kato T, Sumida T
and Kobata T: Apoptosis in rheumatoid arthritis: A novel pathway in
the regulation of synovial tissue. Arthritis Rheum. 41:1–9. 1998.
View Article : Google Scholar
|
|
3
|
Pope RM: Apoptosis as a therapeutic tool
in rheumatoid arthritis. Nat Rev Immunol. 2:527–535. 2002.
View Article : Google Scholar
|
|
4
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar :
|
|
5
|
Arend WP and Dayer JM: Cytokines and
cytokine inhibitors or antagonists in rheumatoid arthritis.
Arthritis Rheum. 33:305–315. 1990. View Article : Google Scholar
|
|
6
|
Meyer FA, Yaron I, Mashiah V and Yaron M:
Methotrexate inhibits proliferation but not interleukin-1
stimulated secretory activities of cultured human synovial
fibroblasts. J Rheumatol. 20:238–242. 1993.
|
|
7
|
Firestein GS, Nguyen K, Aupperle KR, Yeo
M, Boyle DL and Zvaifler NJ: Apoptosis in rheumatoid arthritis: p53
overexpression in rheumatoid arthritis synovium. Am J Pathol.
149:2143–2151. 1996.
|
|
8
|
Firestein GS, Echeverri F, Yeo M, Zvaifler
NJ and Green DR: Somatic mutations in the p53 tumor suppressor gene
in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 94:pp.
10895–10900. 1997; View Article : Google Scholar :
|
|
9
|
Cutolo M, Sulli A, Pizzorni C, Seriolo B
and Straub RH: Anti-inflammatory mechanisms of methotrexate in
rheumatoid arthritis. Ann Rheum Dis. 60:729–735. 2001. View Article : Google Scholar :
|
|
10
|
Park MY, Kwon HJ and Sung MK: Evaluation
of aloin and aloe-emodin as anti-inflammatory agents in aloe by
using murine macrophages. Biosci Biotechnol Biochem. 73:828–832.
2009. View Article : Google Scholar
|
|
11
|
Hu B, Zhang H, Meng X, Wang F and Wang P:
Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits
lipopolysaccharide-induced inflammatory responses in RAW264.7
macrophages. J Ethnopharmacol. 153:846–853. 2014. View Article : Google Scholar
|
|
12
|
Pecere T, Gazzola MV, Mucignat C, Parolin
C, Vecchia FD, Cavaggioni A, Basso G, Diaspro A, Salvato B, Carli M
and Palù G: Aloe-emodin is a new type of anticancer agent with
selective activity against neuroectodermal tumors. Cancer Res.
60:2800–2804. 2000.
|
|
13
|
Kuo PL, Lin TC and Lin CC: The
antiproliferative activity of aloe-emodin is through p53-dependent
and p21-dependent apoptotic pathway in human hepatoma cell lines.
Life Sci. 71:1879–1892. 2002. View Article : Google Scholar
|
|
14
|
Chen HC, Hsieh WT, Chang WC and Chung JG:
Aloe-emodin induced in vitro G2/M arrest of cell cycle in human
promyelocytic leukemia HL-60 cells. Food Chem Toxicol.
42:1251–1257. 2004. View Article : Google Scholar
|
|
15
|
Chiu TH, Lai WW, Hsia TC, Yang JS, Lai TY,
Wu PP, Ma CY, Yeh CC, Ho CC, Lu HF, et al: Aloe-emodin induces cell
death through S-phase arrest and caspase-dependent pathways in
human tongue squamous cancer SCC-4 cells. Anticancer Res.
29:4503–4511. 2009.
|
|
16
|
Suboj P, Babykutty S, Srinivas P and
Gopala S: Aloe emodin induces G2/M cell cycle arrest and apoptosis
via activation of caspase-6 in human colon cancer cells.
Pharmacology. 89:91–98. 2012. View Article : Google Scholar
|
|
17
|
Chen R, Zhang J, Hu Y, Wang S, Chen M and
Wang Y: Potential antineoplastic effects of aloe-emodin: A
comprehensive review. Am J Chin Med. 42:275–288. 2014. View Article : Google Scholar
|
|
18
|
Miyazawa K, Mori A and Okudaira H:
Establishment and characterization of a novel human rheumatoid
fibroblast-like synoviocyte line, MH7A, immortalized with SV40 T
antigen. J Biochem. 124:1153–1162. 1998. View Article : Google Scholar
|
|
19
|
Cronstein BN: Low-dose methotrexate: A
mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev.
57:163–172. 2005. View Article : Google Scholar
|
|
20
|
Kremer JM: Major side effects of low-dose
methotrexateUpToDate. Ravinder NM: UpToDate, Inc.; Waltham, MA:
2014
|
|
21
|
Herman CJ, Allen P, Hunt WC, Prasad A and
Brady TJ: Use of complementary therapies among primary care clinic
patients with arthritis. Prev Chronic Dis. 1:A122004.
|
|
22
|
Ernst E: Herbal medicine in the treatment
of rheumatic diseases. Rheum Dis Clin North Am. 37:95–102. 2011.
View Article : Google Scholar
|
|
23
|
Lin ML, Lu YC, Su HL, Lin HT, Lee CC, Kang
SE, Lai TC, Chung JG and Chen SS: Destabilization of CARP mRNAs by
aloe-emodin contributes to caspase-8-mediated p53-independent
apoptosis of human carcinoma cells. J Cell Biochem. 112:1176–1191.
2011. View Article : Google Scholar
|
|
24
|
Lee HZ, Hsu SL, Liu MC and Wu CH: Effects
and mechanisms of aloe-emodin on cell death in human lung squamous
cell carcinoma. Eur J Pharmacol. 431:287–295. 2001. View Article : Google Scholar
|
|
25
|
Tishler M, Caspi D, Graff E, Segal R,
Peretz H and Yaron M: Synovial and serum levels of methotrexate
during methotrexate therapy of rheumatoid arthritis. Br J
Rheumatol. 28:422–423. 1989. View Article : Google Scholar
|
|
26
|
Nakazawa F, Matsuno H, Yudoh K, Katayama
R, Sawai T, Uzuki M and Kimura T: Methotrexate inhibits rheumatoid
synovitis by inducing apoptosis. J Rheumatol. 28:1800–1808.
2001.
|
|
27
|
Pucci B, Kasten M and Giordano A: Cell
cycle and apoptosis. Neoplasia. 2:291–299. 2000. View Article : Google Scholar :
|