Introduction
Medical interventions, including surgical resection,
radiotherapy and chemotherapy, have provided therapeutic benefits
for various lung cancer patients. However, tumor recurrence may
occur due to the development of multidrug resistance (1). Therefore, identification of the
molecular pathways involved in the proliferation and apoptosis of
lung cancer cells is of primary concern, as this may facilitate the
development of inhibitors of these signaling pathways (2).
Cell migration, cell-to-cell adhesion, actomyosin
contraction, mitosis and cytokinesis involve the Ras homolog
(Rho)/Rho-kinase signaling pathway (3). In addition, numerous studies have
demonstrated that the Rho/Rho-kinase pathway is prominently
involved in cancer invasion, growth and metastasis, and Rho
guanosine triphosphate hydrolases (GTPase) are involved in
Ras-mediated oncogenic transformation (4,5).
Numerous cancers exhibit overexpression of members of the small
GTPase Rho family, including Rho family members A, C, H,
Ras-related C3 botulinum toxin substrate 1 and cell division
control protein 42 (6–8). Therefore, the aim of the present
study was to investigate whether the Rho/Rho-kinase signaling
pathway may be involved in the proliferation and apoptosis of lung
cancer cells (9,10). A previous study demonstrated that
the Rho-kinase inhibitor Fasudil, inhibited the Rho/Rho-kinase
pathway, which subsequently inhibited the proliferation, invasion,
adhesion and migration of lung cancer cells and induced apoptosis
(11,12).
The aim of the present study was to examine the role
of RhoA in the proliferation and apoptosis of lung cancer cells.
Loss of RhoA expression was achieved using RNA interference (RNAi)
in SPCA1 lung carcinoma cells, and the consequences on cell
proliferation and apoptosis were subsequently analyzed. The use of
RNAi involves double-stranded RNA (dsRNA) sequences that are
homologous to the target gene, which results in sequence-specific,
post-transcriptional gene silencing (13). Stable SPCA1 cell lines with
silenced RhoA expression were isolated following Geneticin (G418)
screening. The authors then tested the hypothesis that RhoA affects
lung cancer proliferation and apoptosis. A secondary aim was to
identify the underlying molecular pathways involved in these
processes.
Materials and methods
Cell culture
SPCA1 human lung carcinoma cells were purchased from
the Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 15% heat-inactivated fetal bovine serum (Thermo
Fisher Scientific, Inc.), 100 mg/ml streptomycin and 100 U/ml
penicillin (Beyotime Institute of Biotechnology, Haimen, China) at
37°C in a humidified atmosphere with 5% CO2.
Vector synthesis
The authors used a BLAST program to design
oligonucleotides based on a RhoA cDNA sequence in Genbank, which
were produced by Takara Biotechnology Co., Ltd. (Dalian, China).
The sequences were as follows: Small interfering (si) RhoA-B,
′5-GCTAATACTAGCGGACTCCGATCTGCCGGAGTCCGCTAGTATTAGAAAAAACAGCTGTTCGA-5′,
and siRhoA-,
5′-GATCCGATTATGATCGCCTGAGGCTCAAGACGGCCTCAGGCGATCATAATCTTTTTGTCGACA-3′.
The oligonucleotides and pRNAT-U6.1/Neo-SiRNA vector (Takara
Biotechnology Co., Ltd.) were digested by BamHI and HindIII (New
England BioLabs, Inc., Ipswich, MA, USA) overnight at 37°C, then
connected by T4 ligase (Thermo Fisher Scientific, Inc.) for 1 h at
room temperature. Following this, the recombinant plasmid was
transfected into DH5α (Takara Biotechnology Co., Ltd.) and screened
overnight at 37°C. The recombinant vector was selected from single
colonies and amplified, finally confirmed by electrophoresis and
DNA sequencing (14).
Determination of G418 screening
concentration
SPCA1 cells during the logarithmic phase of growth
were digested with 0.25% pancreatin (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) and seeded in 96-well plates. The
following day, 11 different concentrations of G418 (Gibco; Thermo
Fisher Scientific, Inc.; 0–1,000 µg/ml in 100 µg/ml increments)
were added to the wells. Six replicate wells were used for each
concentration. In order to avoid the edge effect, the outer wells
of the culture plate were not used. Cells were observed for 1 week,
with the medium refreshed once in the middle of the week. Cells
exhibited various rates of survival for concentrations of G418
between 0 and 400 µg/ml, however a G418 concentration of between
500 and 1000 µg/ml resulted in comprehensive cell death by day 7. A
concentration of 500 µg/ml G418 was therefore selected as the
screening concentration, and 200 µg/ml G418 as the maintenance
concentration.
RhoA siRNA transfection and
establishment of stable cell lines
SPCA1 cells in the logarithmic phase of growth, and
at passage number <10, were transfected with RhoA siRNA. Each
transfection included a control group (transfected with empty
vectors). SPCA1 cells (2×105 cells/well) were seeded in
6-well plates. Following incubation for 24 h, cells were
transfected with RhoA siRNA in serum-free medium using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol. A
mixture containing 4 µg vector and 10 µl Lipofectamine 2000™ was
incubated for 20 min at room temperature. SPCA1 cells were then
washed and incubated with the vector-Lipofectamine mixture for 24 h
in a CO2 incubator at 37°C. A total of 500 and 200 µg/ml
G418 was then added to wells for three weeks in order to screen for
and maintain the growth of stable RhoA siRNA-transfected cells,
respectively. The recombinant plasmid RhoA-pRNAT-U6.1/Neo-SiRNA
were selected from single colonies and amplified, so the SPCA1 cell
lines expressing RhoA-pRNAT-U6.1/Neo-SiRNA could emit GFP
continuously. Transfection was visualized using fluorescence
microscopy (DFC365FX; Leica Microsystems GmbH, Wetzlar,
Germany).
Western blot analysis
Stable RhoA siRNA-transfected cells and controls
were subject to western blot analysis. To do this, cells were first
washed three times with ice-cold PBS, before they were lysed in
lysis buffer for 30 min in an ice-bath. Following electrophoresis
(30 µg/well) on 10% SDS-PAGE gels, separated protein samples were
transferred to a nitrocellulose membrane. The membrane was then
blocked in 5% non-fat dry milk in TBS with 0.05% Tween-20 (TBST)
for 1 h at room temperature, to prevent nonspecific antibody
binding. Following incubation with rabbit anti-RhoA antibody
(dilution, 1:250; Jingmei Biotech Co., Ltd., Beijing, China) at 4°C
overnight, the membrane was incubated with goat anti-rabbit
antibody (dilution, 1:500; Jingmei Biotech Co., Ltd.). An antibody
to β-actin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
served as a loading control. For the detection of caspase-3 and
signal transducer and activator of transcription (STAT) 3, cell
lysates (40 µg each of protein) from stable RhoA siRNA-transfected
and control cells were added to 2X Laemmli sample buffer (Hyclone;
GE Healthcare Life Sciences), boiled for 5 min and electrophoresed
under the aforementioned conditions with 10% SDS-PAGE gels.
Proteins were transferred to polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), rinsed with TBST
(pH 7.5), and blocked in 5% non-fat dry milk in TBST overnight at
4°C. Membranes were incubated in polyclonal anti-STAT3,
anti-caspase or anti-phosphorylated (phospho)-STAT3 (Tyr-705)
primary antibodies for 3 h. Following rinsing in TBST, the membrane
was incubated with horseradish peroxidase-conjugated goat
anti-rabbit antibody (EMD Millipore, Billerica, MA, USA) for 50
min. Protein bands were detected by enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific, Inc.). Densitometry was used to
semi-quantify band intensities.
Cell proliferation assay
An MTS tetrazolium assay was used to measure cell
proliferation, as previously described (15). Briefly, RhoA siRNA-transfected
cells or controls were seeded into 96-well plates (1,000
cells/well) in 100 µl/well of RPMI 1640 medium. Five replicates for
each experimental group and time points (0, 24, 48 and 96 h) were
used. Following 24 h, 20 µl CellTiter 96 AQueous One Solution
(Promega Corporation, Madison, WI, USA) was added to each well, and
cells were incubated at 37°C for a further 4 h. Absorbance was
measured at a wavelength of 490 nm each day for 7 days.
Fluorescence-activated cell sorting
(FACS) analysis
Cell apoptosis in treated and untreated cells was
measured by FACS or flow cytometry analysis, using the methods
described previously (16). The
FITC-Annexin V Apoptosis Detection kit (BD Pharmingen, San Diego,
CA, USA) was used according to the manufacturer's protocol.
Briefly, fluorescein isothiocyanate (FITC)-Annexin V and propidium
iodide (PI) were used to stain siRNA RhoA-transfected and control
cells for 5 min at room temperature. Annexin
V−PI− (viable cells) and Annexin
V+ (apoptotic) cells were evaluated by flow cytometry. A
BD FACSCalibur flow cytometer (BD Biosciences, Franklin lakes, NJ,
USA) was used to collect data that was then analyzed using BD
CellQuest software (BD Biosciences).
Statistical analysis
Data are expressed as the mean ± standard error.
Variables were compared using the Student's t-test, the
χ2 test and analysis of variance or Fisher's exact test.
Data were analyzed using SPSS software (version 17.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Successful generation of stable RhoA
siRNA-transfected SPCA1 cell lines and western blot analysis of
RhoA expression
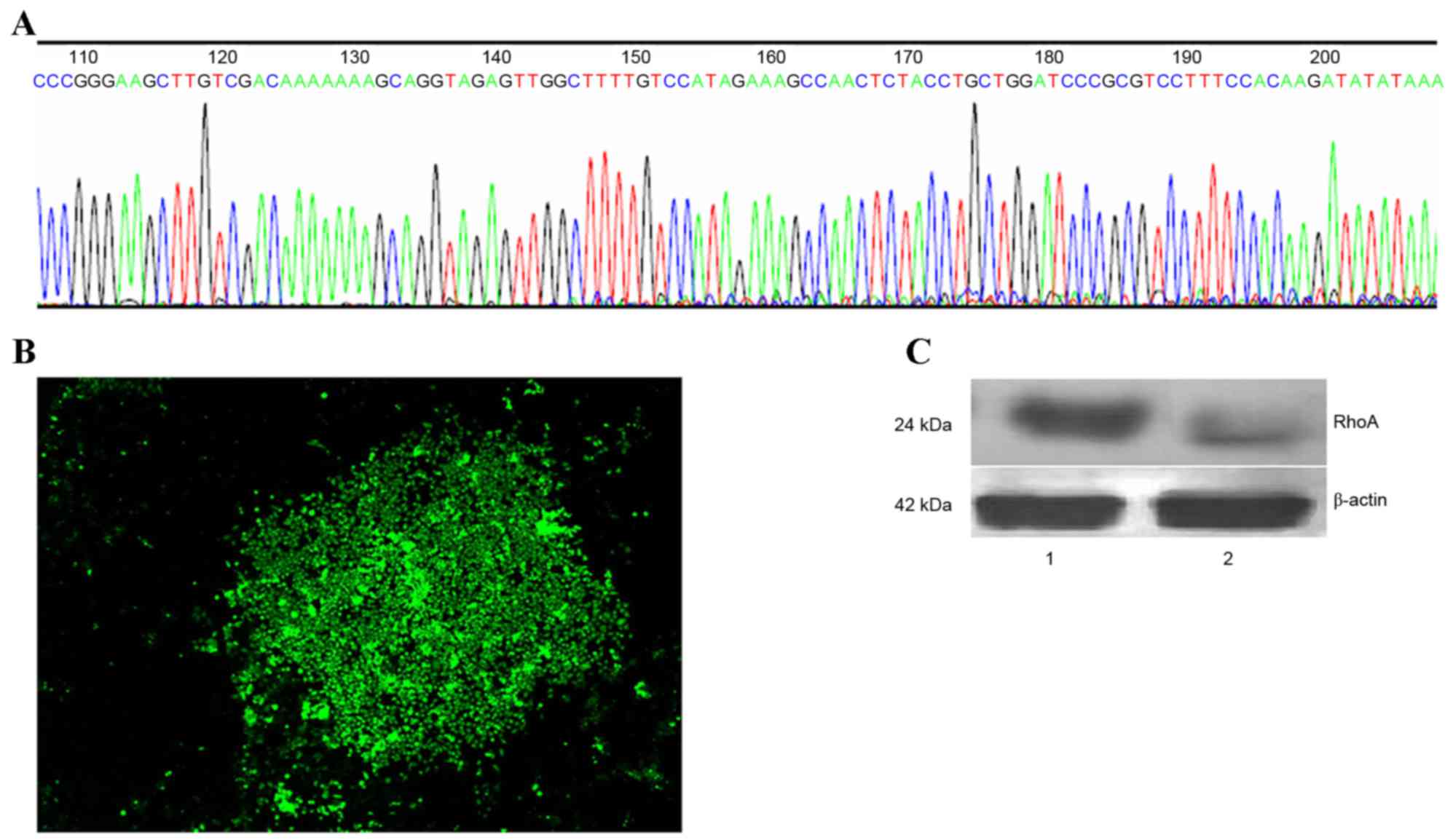
In order to generate a RhoA-pRNAT-U6.1/Neo-SiRNA
vector, oligonucleotide dsRNAs were ligated with pSUPER following
annealing. The sequence of synthesized siRNA oligos and insert
sequences were indistinguishable (Fig.
1A). Stable RhoA siRNA-transfected SPCA1 lung cancer cell lines
were then generated (Fig. 1B).
RhoA protein expression was analyzed by western blotting using an
anti-RhoA antibody. As shown in Fig.
1C, a marked reduction in RhoA protein expression in RhoA
siRNA-transfected cells was observed when compared with the control
cells (Fig. 1C).
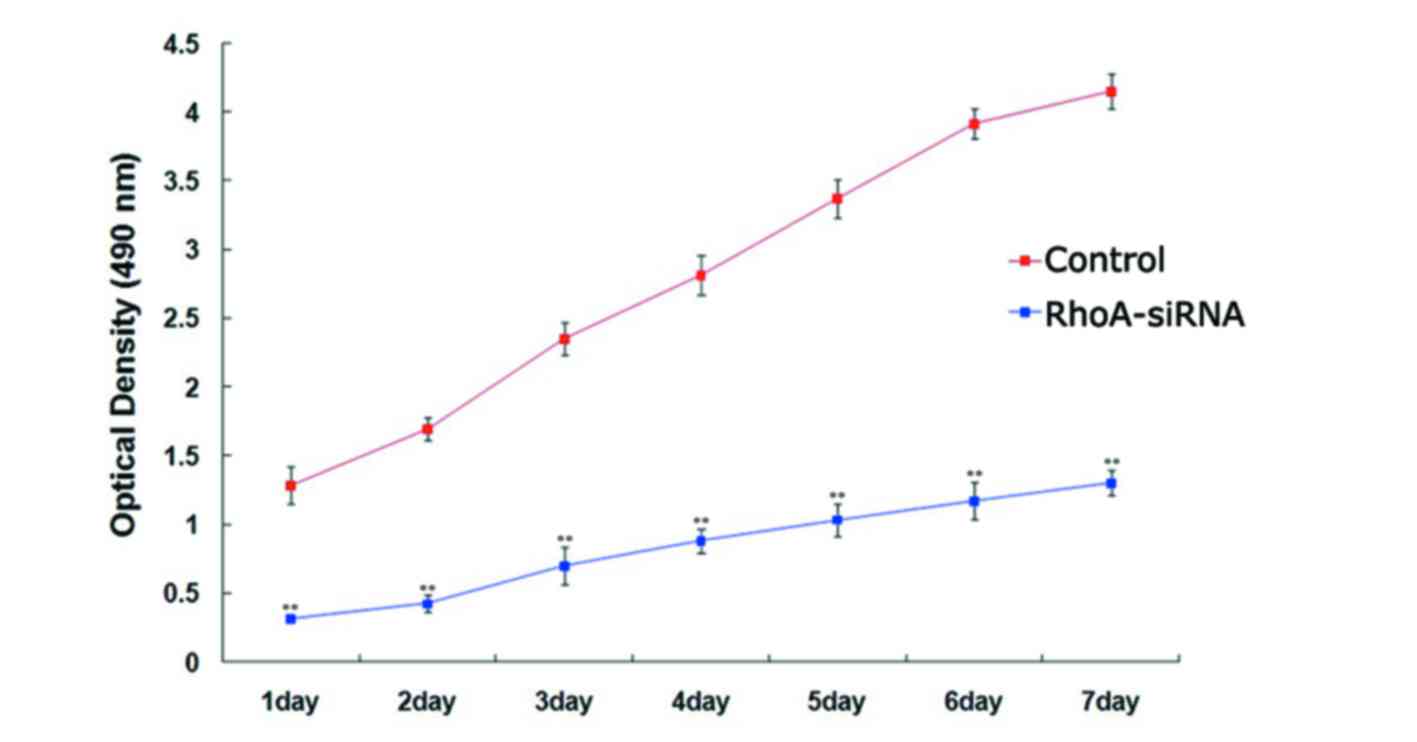
RhoA silencing decreases proliferation
of cultured SPCA1 lung cancer cells
The proliferation rate of stable RhoA
siRNA-transfected cells was significantly lower when compared with
the control cells every day for 7 days (P<0.01; Fig. 2), as determined using the MTS
assay. These results suggested that silencing of RhoA expression
significantly decreased SPCA1 cell proliferation.
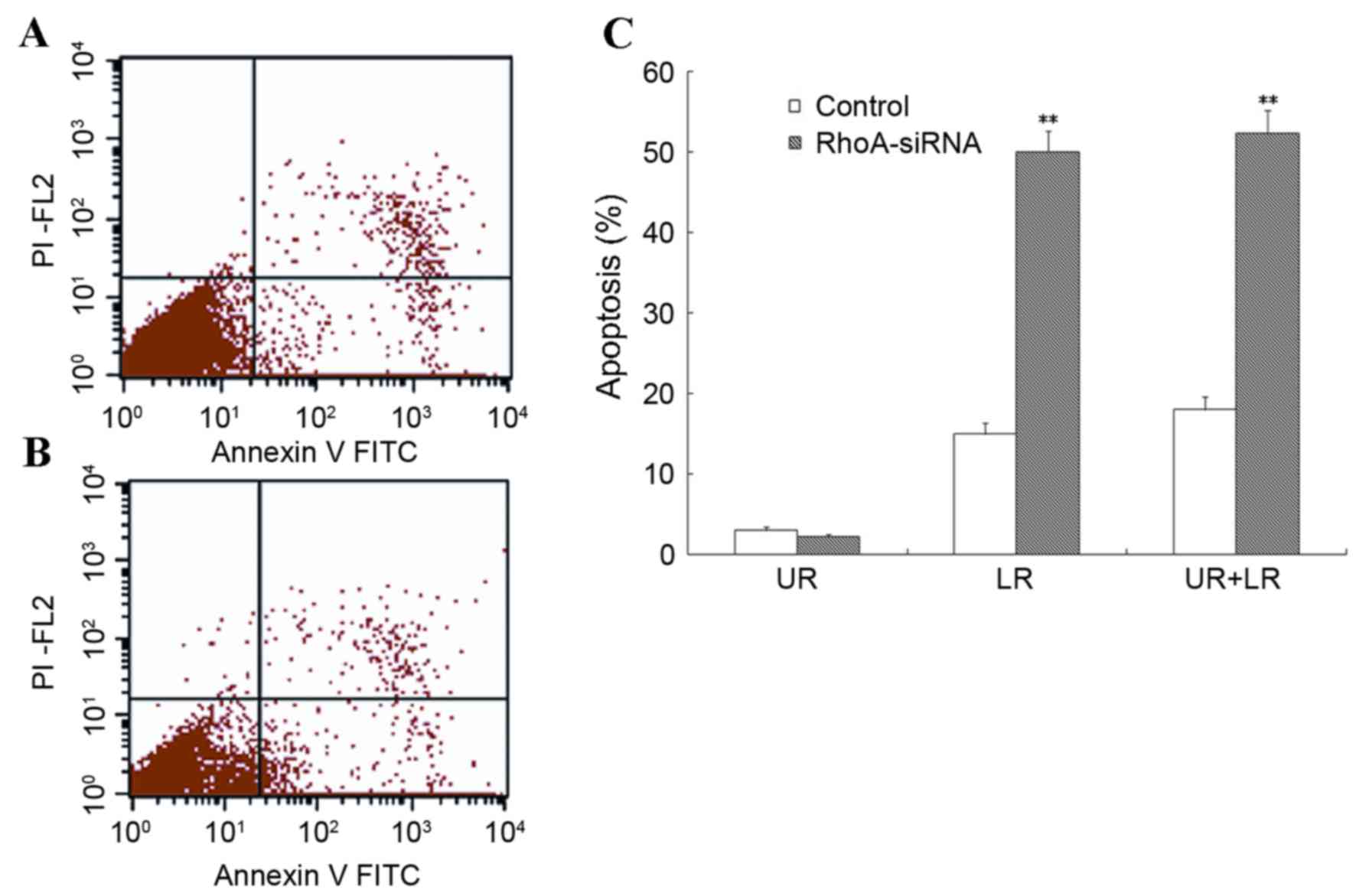
RhoA silencing increases apoptosis of
cultured SPCA1 lung cancer cells
Flow cytometry was used to assess the level of
apoptosis in RhoA siRNA-transfected SPCA1 cells. As shown in
Fig. 3, stable RhoA
siRNA-transfected cells exhibited a significant increase in the
rate of apoptosis when compared with the control cells (P<0.01).
These results suggested that RhoA silencing was associated with a
marked increase in the level of apoptosis in SPCA1 cells.
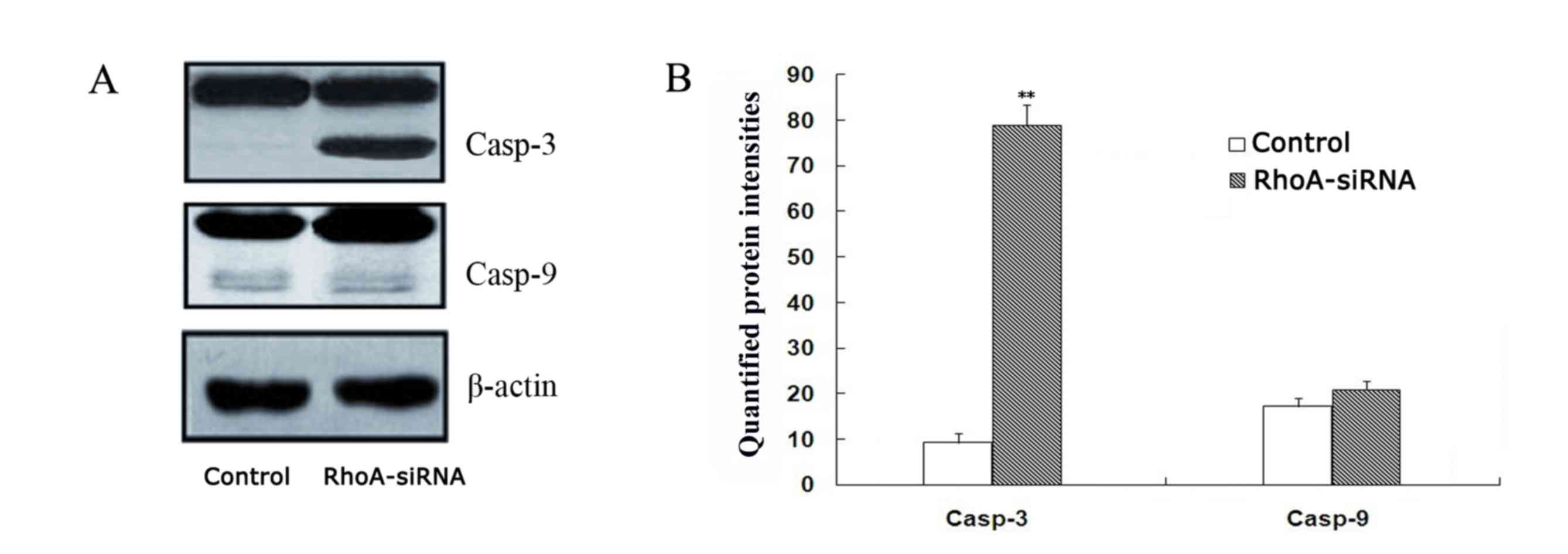
RhoA silencing induces activation of
caspase-3 in cultured SPCA1 lung cancer cells
As shown in Fig. 4,
a significant increase in caspase-3 expression in stable RhoA
siRNA-transfected cells was observed when compared with the control
cells, as determined by western blotting (P<0.01). By contrast,
caspase-9 activation remained unaffected between the two groups
(Fig. 4). These results suggest
that RhoA silencing may activate caspase-3.
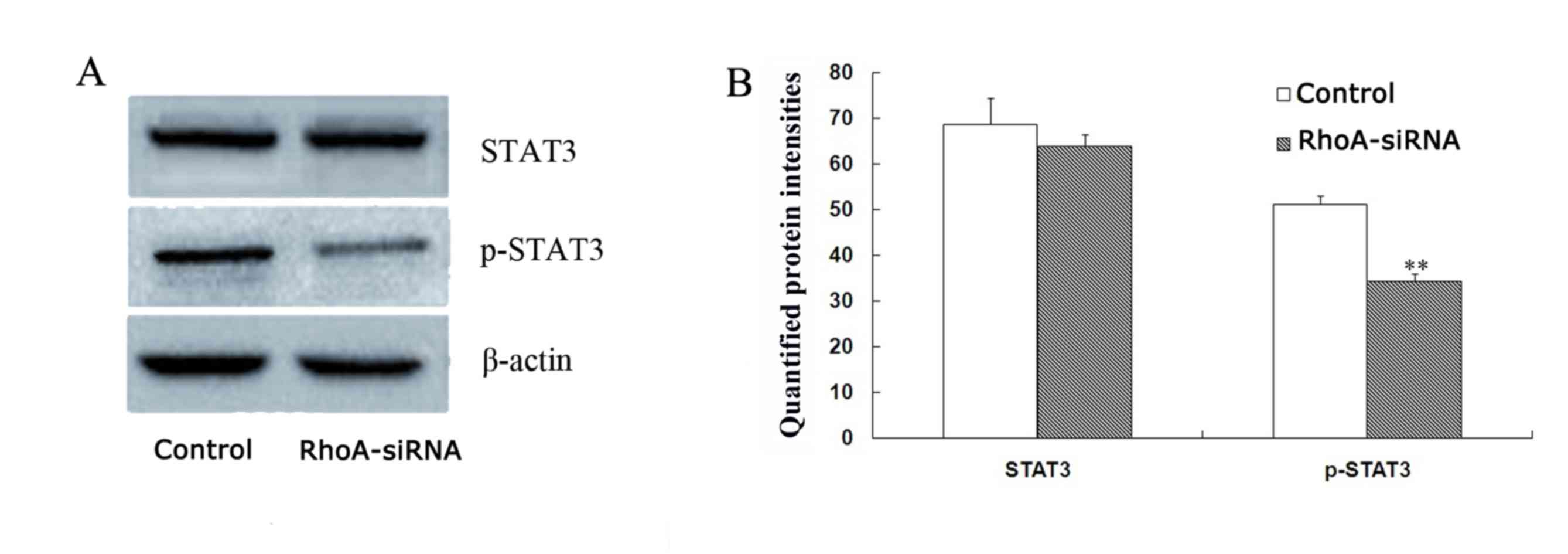
RhoA silencing downregulates STAT3
phosphorylation in cultured SPCA1 lung cancer cells
The level of phopho-STAT3 were decreased by 33.1% in
stable RhoA siRNA-transfected cells when compared with control
cells (P<0.01; Fig. 5) as
determined by western blotting analysis. By contrast, no
significant difference in STAT3 protein expression levels between
the groups was observed (Fig. 5).
Therefore, RhoA silencing may decrease the expression levels of
phospho-STAT3 in SPCA1 cells.
Discussion
The deregulation of cell proliferation and apoptosis
pathways is a prerequisite for the development of lung cancer
(17). At present, current cancer
therapies consist of agents that target signaling pathways involved
in cell proliferation, which result in cell death, for example,
gemcitabine hydrochloride (18).
However, further investigation into the mechanisms involved in
cancer development is required in order to develop novel and
improved anti-cancer therapies.
Activation of the Rho/Rho kinase pathway is
important for cell migration, cell-to-cell adhesion, invasion and
mitosis (19,20), and may additionally be predominant
in cancer growth and invasion (21). In non-small cell lung cancers,
inhibition of the Rho/Rho-kinase pathway impedes tumour migration
and invasion (10,11). The aim of the present study was to
assess the consequences of RhoA silencing in lung cancer cells at
various stages of carcinogenesis, including proliferation and
apoptosis.
RNAi is considered to be a precise method of gene
silencing, which can involve the use of siRNA (22). This method was used to construct an
RhoA siRNA expression vector in the present study, which was
subsequently transfected into SPCA1 lung cancer cells. A stable
RhoA siRNA-transfected SPCA1 cell line was successfully established
and it was verified that RhoA siRNA reduced RhoA expression in
SPCA1 lung cancer cells. It was demonstrated that knockdown of RhoA
resulted in inhibition of lung cancer cell viability. Using flow
cytometry analysis, it was subsequently demonstrated that a
knockdown of RhoA significantly induced apoptosis in SPCA1
cells.
Western blot analysis was then performed in order to
examine the activation of caspases as a possible mechanism
underlying the increased levels of apoptosis observed in SPCA1
cells in response to RhoA knockdown. A significant increase in
caspase-3, but not caspase-9 expression, was observed in RhoA
siRNA-transfected SPCA1 cells. This was consistent with the results
obtained from FACS analysis. Therefore, RhoA knockdown induced
strong activation of caspase-3 in SPCA1 cells, which suggests that
caspase-dependent apoptosis may have contributed to the RhoA
knockdown-mediated, anti-proliferative effects observed in SPCA1
cells.
Cellular transformation in response to constitutive
activation of STAT3 is frequently observed in numerous human cancer
cells (23). In the present study,
western blotting was used to determine whether the observed RhoA
siRNA-mediated inhibition of SPCA1 cell proliferation was the
result of STAT3 inactivation, by determining the phosphorylation
status of STAT3. RhoA knockdown resulted in a significant decrease
in phospho-STAT3 (Tyr-705) levels, however, no effect on total
STAT3 protein levels was observed. The results suggested that RhoA
knockdown induced cell growth inhibition and apoptotic cell death
in SPCA1 cells, which may be associated with STAT3
inactivation.
In conclusion, the results of the present study
suggest that RhoA may be important for lung cancer cell
proliferation and apoptosis. Alterations in caspase-3 and
phospho-STAT3 levels and activation may be the underlying molecular
mechanisms associated with the effect of RhoA on SPCA1 cell
proliferation and apoptosis. These results provide an improved
understanding of the cellular pathways involved in human lung
cancer, and may facilitate the development of novel therapies to
treat patients with this disease.
Acknowledgements
The present study was supported by The Specialized
Research Fund for the Doctoral Program of Higher Education of China
(grant no. 20122104120015).
References
|
1
|
Lee C, Raffaghello L and Longo VD:
Starvation, detoxification, and multidrug resistance in cancer
therapy. Drug Resist Updat. 15:114–122. 2012. View Article : Google Scholar :
|
|
2
|
Street CA and Bryan BA: Rho kinase
proteins-pleiotropic modulators of cell survival and apoptosis.
Anticancer Res. 31:3645–3657. 2011.
|
|
3
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and nonmuscle myosin II: Modulated by
G proteins, kinases, and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003. View Article : Google Scholar
|
|
4
|
Zicha D, Dobbie IM, Holt MR, Monypenny J,
Soong DY, Gray C and Dunn GA: Rapid actin transport during cell
protrusion. Science. 300:142–145. 2003. View Article : Google Scholar
|
|
5
|
Brown M, Roulson JA, Hart CA, Tawadros T
and Clarke NW: Arachidonic acid induction of Rho-mediated
transendothelial migration in prostate cancer. Br J Cancer.
110:2099–2108. 2014. View Article : Google Scholar :
|
|
6
|
Ridley AJ: Rho proteins and cancer. Breast
Cancer Res Treat. 84:13–19. 2004. View Article : Google Scholar
|
|
7
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
8
|
Burbelo P, Wellstein A and Pestell RG:
Altered Rho GTPase signaling pathways in breast cancer cells.
Breast Cancer Res Treat. 84:43–48. 2004. View Article : Google Scholar
|
|
9
|
Morgan-Fisher M, Wewer UM and Yoneda A:
Regulation of ROCK Activity in cancer. J Histochem Cytochem.
61:185–198. 2013. View Article : Google Scholar :
|
|
10
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar :
|
|
11
|
Yang X, Liu Y, Zong Z and Tian D: The Rho
kinase inhibitor fasudil inhibits the migratory behaviour of 95-D
lung carcinoma cells. Biomed Pharmacother. 64:58–62. 2010.
View Article : Google Scholar
|
|
12
|
Yang X, Di J, Zhang Y, Zhang S, Lu J, Liu
J and Shi W: The Rho-kinase inhibitor inhibits proliferation and
metastasis of small cell lung cancer. Biomed Pharmacother.
66:221–227. 2012. View Article : Google Scholar
|
|
13
|
Bass BL: Double-stranded RNA as a template
for gene silencing. Cell. 101:235–238. 2000. View Article : Google Scholar
|
|
14
|
Yang X, Zheng F, Zhang S and Lu J: Loss of
RhoA expression prevents proliferation and metastasis of SPCA1 lung
cancer cells in vitro. Biomed Pharmacother. 69:361–366. 2015.
View Article : Google Scholar
|
|
15
|
Shin JY, Kim JO, Lee SK, Chae HS and Kang
JH: LY294002 may overcome 5-FU resistance via down-regulation of
activated p-AKT in Epstein-Barr virus-positive gastric cancer
cells. BMC Cancer. 10:4252010. View Article : Google Scholar :
|
|
16
|
Chen T, Xu Y, Guo H, Liu Y, Hu P, Yang X,
Li X, Ge S, Velu SE, Nadkarni DH, et al: Experimental therapy of
ovarian cancer with synthetic makaluvamine analog: In vitro and in
vivo anticancer activity and molecular mechanisms of action. PLoS
One. 6:e207292011. View Article : Google Scholar :
|
|
17
|
Morgensztern D, Campo MJ, Dahlberg SE,
Doebele RC, Garon E, Gerber DE, Goldberg SB, Hammerman PS, Heist
RS, Hensing T, et al: Molecularly targeted therapies in non-small
cell lung cancer annual update 2014. J Thorac Oncol. 10(1 Supp 1):
S1–S63. 2015. View Article : Google Scholar :
|
|
18
|
Pooja D, Panyaram S, Kulhari H, Reddy B,
Rachamalla SS and Sistla R: Natural polysaccharide functionalized
gold nanoparticles as biocompatible drug delivery carrier. Int J
Biol Macromol. 80:48–56. 2015. View Article : Google Scholar
|
|
19
|
Ying H, Biroc SL, Li WW, Alicke B, Xuan
JA, Pagila R, Ohashi Y, Okada T, Kamata Y and Dinter H: The Rho
kinase inhibitor fasudil inhibits tumor progression in human and
rat tumor models. Mol Cancer Ther. 5:2158–2164. 2006. View Article : Google Scholar
|
|
20
|
Rath N and Olson MF: Rho-associated
kinases in tumorigenesis: Re-considering ROCK inhibition for cancer
therapy. EMBO Rep. 13:900–908. 2012. View Article : Google Scholar :
|
|
21
|
Nakajima M, Hayashi K, Katayama K, Amano
Y, Egi Y, Uehata M, Goto N and Kondo T: Wf-536 prevents tumor
metastasis by inhibiting both tumor motility and angiogenic
actions. Eur J Pharmacol. 459:113–120. 2003. View Article : Google Scholar
|
|
22
|
Wagner A, Röhrs V, Kedzierski R, Fechner H
and Kurreck J: A novel method for the quantification of
adeno-associated virus vectors for RNA interference applications
using quantitative polymerase chain reaction and purified genomic
adeno-associated virus DNA as a standard. Hum Gene Ther Methods.
24:355–363. 2013. View Article : Google Scholar
|
|
23
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar
|