Introduction
Endoplasmic reticulum (ER) stress is caused by the
accumulation of unfolded or misfolded proteins in the ER. Many
age-associated diseases have been closely related with ER stress
including Alzheimer's and idiopathic pulmonary fibrosis (IPF)
(1,2). Furthermore, a series of previous
research indicated that increased ER stress may be a key component
in the pathogenesis of various age-associated diseases (3,4). In
normal conditions, proteins involved in ER stress bind with glucose
regulated protein-78 (GRP78), also called immunoglobulin
heavy-chain-binding protein. However, under stress due to various
factors, the unfolded protein response (UPR) is activated, which
contains three important forms of signaling: protein kinase R-like
endoplasmic reticulum kinase (PERK) signaling, activating
transcription factor 6α (ATF6α) signaling and inositol-requiring
enzyme 1 (IRE-1) signaling. With accumulation of abnormally folded
or improperly assembled proteins in the ER, GRP78 dissociates with
the above three proteins, initiating signaling cascades to protect
the cell from ER stress. Among the three proteins, PERK senses
accumulation of misfolded proteins in the ER and, once activated,
undergoes autophosphorylation and dimerization and inactivates the
α-subunit of eukaryotic translational initiation factor 2 (eIF2a)
to attenuate translation of proteins. C/EBP homologous protein
(CHOP) is an important molecule involved in PERK signaling cascades
and initiates apoptosis (5).
Another protein, ATF6α during ER stress is cleaved by protease and
releases its cytoplasmic domain into the cytosol, which binds to
cis-acting ER stress response elements and activates the
transcription of ER chaperones involved in protein-folding, such as
GRP78. The third protein, IRE-1, is a transmembrane protein that
has intrinsic serine/threonine kinase and endonuclease activity,
which is activated in response to ER stress and cleaves a
26-nucleotide intron sequence from the transcription factor, X-box
binding protein (XBP)-1. Spliced XBP-1 translocates to the nucleus
and promotes the transcription of target genes, which are related
with degradation of misfolded proteins (5,6).
Emerging evidence has indicated that the ER gets
involved in the pathogenesis of idiopathic pulmonary fibrosis,
while the involvements of three signaling pathways and the process
of lung fibrosis remains unclear. Pulmonary viral infection often
triggers a hyperinflammatory response by an expansion of ER stress
(7). In the present study, the
authors observed the UPR induced by respiratory syncytial virus
infection (RSV) infection in the pathogenesis of progressive lung
fibrosis.
Materials and methods
Ethics statement
This study was approved by the Animal Care and Use
Committee of Qingdao University (Qingdao, China) and conducted in
compliance with the guidelines of the National Institutes of Health
for the care and the use of laboratory animals.
Preparation of RSV
The strain A2 of human RSV was propagated in HeLa
cells (both from the Virology Institute of Wuhan, Wuhan, China) in
minimal essential medium supplemented with 2% heat-inactivated
fetal bovine serum (both from Invitrogen; Thermo Fisher Scientific,
Waltham, MA, USA). When 75% of the cells formed syncytia, the cells
were harvested and disrupted by sonication using the same culture
medium. The suspension was clarified by centrifugation at 10,000 ×
g for 15 min at 4°C and stored in aliquots at −70°C. Sham
inoculum was prepared from uninfected HeLa cell lysate using the
same procedure. The viral titer in the inoculums was determined by
plaque assay (8) on the same day
as the mice were inoculated.
Establishment of RSV+bleomycin-induced
animal models
Ten-week-old male C57BL/6 mice (n=80; weight, 20–25
g) were purchased from the Affiliated Hospital of Qingdao
University (Qingdao, China). Mice were given free access to food
and water and were maintained with a 12-h light/dark cycle. Mice in
the RSV group were endotracheally injected 100 µl of PBS and 100 µl
of RSV (2×106 pfu/ml). Mice in the bleomycin group were
endotracheally injected with 100 µl bleomycin (1 mg/ml) in sterile
PBS and 100 µl Sham inoculum. Mice in the RSV+bleomycin group were
endotracheally injected RSV (100 µl, 2×106 pfu/ml) and
bleomycin (100 µl, 1 mg/ml) (9).
Combined sham inoculums in place of RSV, and PBS in place of
bleomycin were used as a control. At 7, 14 or 21 days following
injection, the animals were sacrificed, and analyzed. All
experimental animals used in the current study were maintained
under the protocol approved by the Institutional Animal Care and
Use Committee of Qingdao University.
Cellular classification of
bronchoalveolar lavage fluid (BALF)
BALFs were centrifuged at 1,000 × g for 5 min
at room temperature and the cell pellets were smeared onto glass
slides. Cells were air dried and stained with Wright stain, which
allows differential counting of various cells. In each sample, ≥300
cells per sample were counted under a light microscope.
Cytokines in BALF
Interleukin (IL)-1, -5 and -8 productions in BALF
were measured using ELISA kits for mouse IL-1β (70-EK201B2/2),
mouse IL-5 (70-EK2052) (both from Multi Sciences (Lianke) Biotech
Co., Ltd., Hangzhou, China) and mouse IL-8 (kt21138; MoShaKe
Biotech Co., Ltd., Wuhan, China) according to the manufacturer's
protocol. Briefly, a total of 100 µl BALF supernatants were added
into a 96-well plate and incubated for 1 h, followed by 100 µl
enzyme-linked antibodies for 30 min at 37°C. Following three washes
with washing buffer, the chromogenic reagent was added and
incubated for 30 min. 2 M H2SO4 was added to
terminate reaction. Absorbance was determined at 450 nm using a
microplate reader [Tecan Sunrise; Tecan (Shanghai) Trading Co.,
Ltd., Shanghai, China]. Each sample was repeated three times.
Immunohistochemistry
Mice were sacrificed with an intraperitoneal
injection of sodium pentobarbitone (100 mg/kg) and the right lung
lobes were obtained. Lobes were inflated (10% paraformaldehyde
perfusion via trachea), harvested, fixed in 4% paraformaldehyde
overnight, embedded in paraffin and cut into 5 µm sections.
Endogenous peroxidase was inhibited by soaking tissue sections in
3% H2O2. Following rinsing in PBS, sections
were incubated with goat serum (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) to block nonspecific binding of antibodies, and
were then incubated overnight at 4°C with rabbit anti-human RSV
polyclonal antibodies (orb101035; Biorbyt LLC, San Francisco, CA,
USA; dilution, 1:250). Following washing in PBS, the sections were
incubated with biotinylated goat anti rabbit IgG (Vector BA-1,000;
Vector Laboratories, Inc., Burlingame, CA, USA) at 37°C for 1 h,
and washed again. Following washing again in PBS, the signal was
detected with 3,3′-diaminobenzidine. Omission of primary or
secondary antisera was included as a method of providing a control
for each biopsy.
Immunofluorescence
Following soaking the sections in 3%
H2O2 for 15 min and 10 µl of goat serum for
30 min, sections were incubated overnight at 4°C with rabbit anti
collagen type I polyclonal antibodies (1:150; SAB4500362;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following washing
in PBS, the sections were incubated with fluorescein
isothiocyanate-labeled goat anti rabbit IgG (1:1,000; A22120;
Beyotime Institute of Biotechnology, Haimen, China) for 1 h, and
washed again. The slides were finally mounted with mounting media
(Thermo Fisher Scientific, Inc.) and examined by fluorescence
microscopy.
Western blot analysis
Lung tissues were washed by ice-cold PBS twice and
extracted in radioimmunoprecipitation assay buffer (Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China) containing 1 mM
phenylmethylsulfonyl fluoride, 5 mM β-glycerophosphate and 1%
standard protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) on
ice for 30 min. Protein concentrations were measured by Bradford
assay (Thermo Fisher Scientific, Inc.) and adjusted to a final
concentration of 10 mg/ml. Lung homogenates (50 µg) were subjected
to separation by 12% SDS-PAGE, then transferred to polyvinylidene
difluoride membranes (Beijing Dingguo Changsheng Biotechnology Co.,
Ltd.) and blocked with 5% skimmed milk. After heat-induced epitope
retrieval (Microwave on high for 15 min), the slides were incubated
with rabbit anti-mouse p-PERK (1:150; sc-13073), anti-GRP78 (1:250;
sc-13968), anti-CHOP (1:200; sc-575), anti-ATF6α (1:100; sc-22799),
anti-XBP-1 (1:150; sc-7160) (all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and rabbit anti-collagen type I polyclonal
antibodies (1:200; SAB4500362; Sigma-Aldrich; Merck KGaA) overnight
at 4°C. Blots were developed using corresponding horseradish
peroxidase-conjugated goat anti-rabbit antibodies (1:2,000; A0208;
Beyotime Institute of Biotechnology) at 37°C for 1 h, and detected
using an enhanced chemiluminescence system (Amersham ECL Plus; GE
Healthcare Life Sciences, Chalfont, UK). Band intensities were
quantified by Image Gauge (version 3.4) software with the LAS-1,000
plus system (Fujifilm Holdings Corporation, Tokyo, Japan). β-actin
served as a loading control.
Statistical analysis
The statistical significance of the data was
analyzed using the SPSS software (version 14.0; SPSS Inc., Chicago,
IL, USA). Data were expressed as means ± standard deviation.
Statistical analyses were performed using an unpaired Student's
t-test for the comparison of data from different treatment groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RSV persistence in bleomycin-induced
pulmonary fibrosis animal models
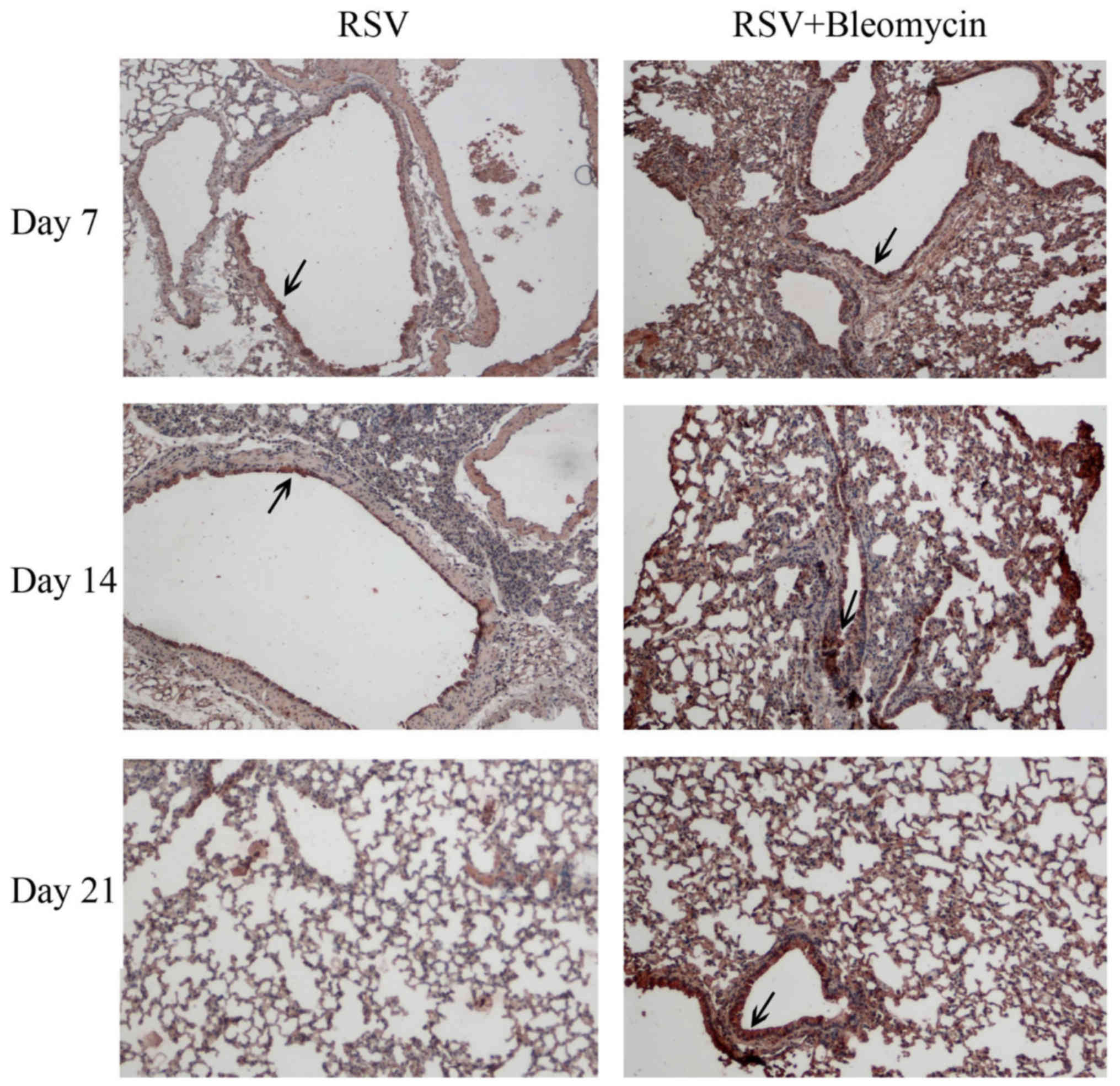
The results of immnohistochemistry indicated that
RSV (dark brown staining) was evident in the airway epithelium and
peaked at day 7 and decreased with infection time in the RSV alone
group. In RSV+bleomycin group, the RSV infection was localized
predominantly to alveoli and was persistent in the lungs from day 7
to day 21 (Fig. 1). No viruses
were observed in uninfected animals (data not shown).
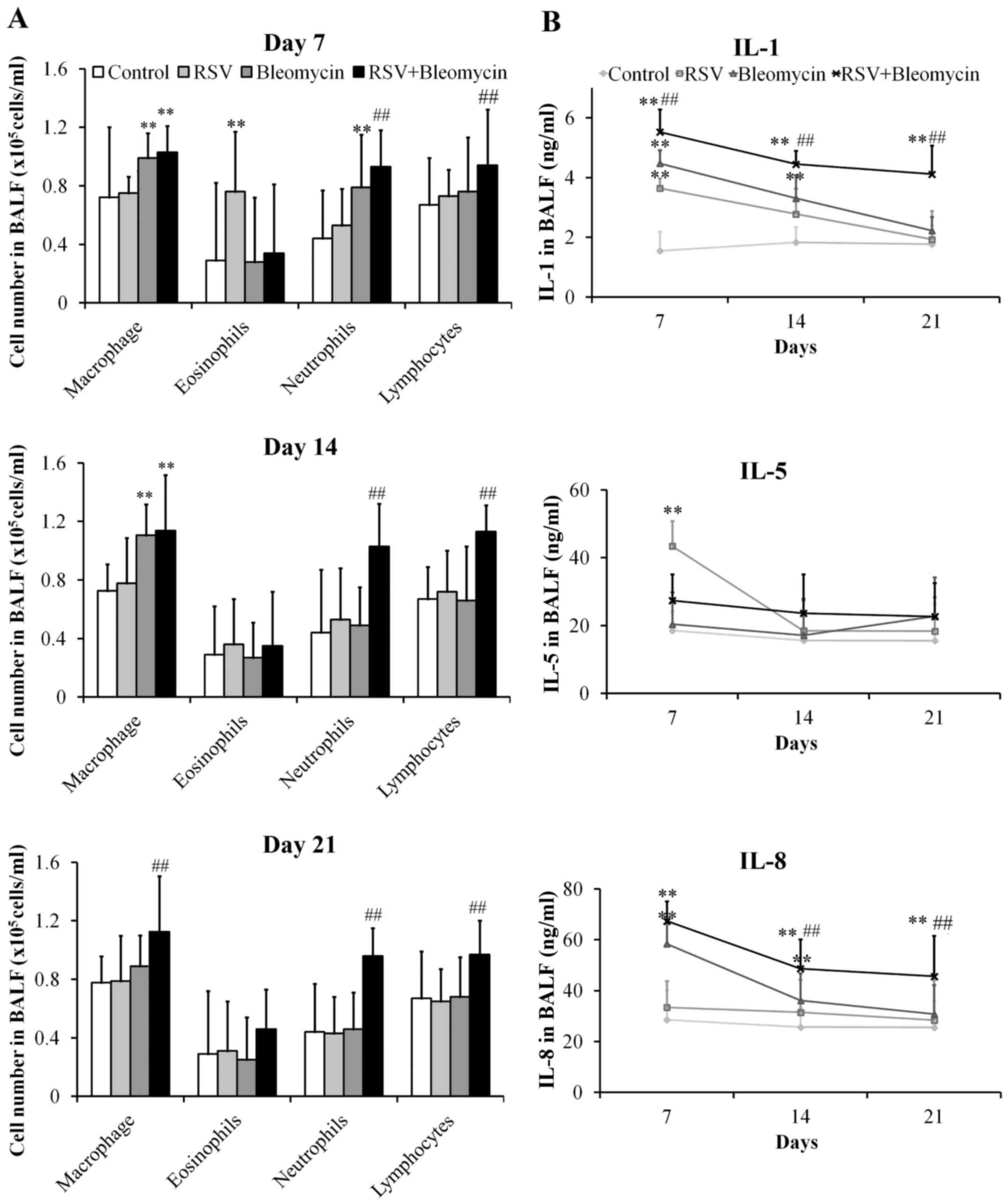
RSV promoted inflammation of
bleomycin-induced pulmonary fibrosis animal models
The lungs of mice infected with RSV presented
significant increases in the numbers of eosinophils at day 7
(P<0.01), when compared with the control group, before returning
to normal levels. In addition, the bleomycin group demonstrated
obvious increases in macrophages and neutrophils at day 7 (both
P<0.01), only macrophages at day 14 (P<0.01), when compared
with the control group before returning to normal levels of all red
blood cell types at day 21. However, the RSV+bleomycin group
exhibited obvious increases in macrophages, neutrophils and
lymphocytes at day 7, 14 and 21 (P<0.01) when compared with
bleomycin group (Fig. 2A). In
pursuit of the effects of RSV on the process of bleomycin-induced
pulmonary fibrosis, the authors investigated the production of the
pro-inflammatory cytokines IL-1, -5 and -8 in BALF. As demonstrated
in Fig. 2B, RSV promoted the
production of IL-1 and -5 at day 7 (both P<0.01), when compared
with the control group, before returning to normal levels.
Bleomycin induced release of IL-1 and -8 at day 7 and day 14 (all
P<0.01), when compared with the control group, before returning
to normal levels at day 21. However, RSV+bleomycin group presented
obvious increases in IL-1 and -8 at days 7, 14 and 21 (all
P<0.01), when compared with control or the bleomycin group.
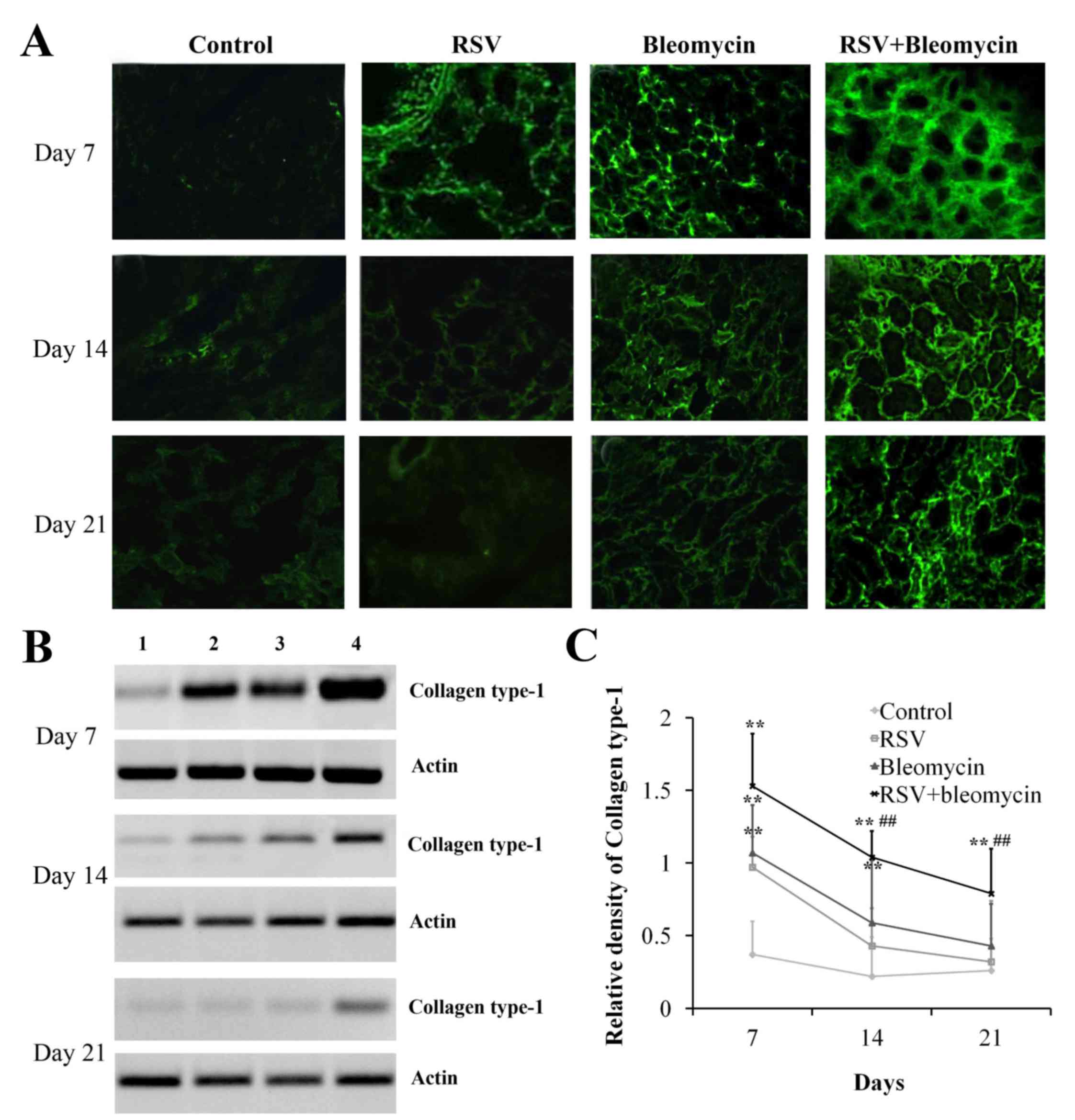
RSV promoted collagen synthesis of
bleomycin-induced pulmonary fibrosis animal models
Excessive production and deposition of extracellular
matrix components is crucial for the development of pulmonary
fibrosis. As demonstrated in Fig.
3, immunofluorescence (Fig.
3A) and western blot analyses (Fig. 3B and C) identified that RSV or
bleomycin alone promoted the accumulation of collagen type I in the
lungs at day 7, when compared with the control group (Fig. 3C; P<0.01); these effects
decreased with time. However, RSV+bleomycin promoted more collagen
type I over the time course compared with bleomycin alone.
RSV promoted expression of p-PERK,
ATF6α and GRP78 in bleomycin-induced pulmonary fibrosis animal
models
Using western blot analysis, GRP78, p-PERK, CHOP,
XBP-1 and ATF6α were detectable at low levels in control lung
tissues at days 7, 14 and 21 (Fig.
4). Mice treated with RSV demonstrated significantly elevated
levels of p-PERK, ATF6α and GRP78 at day 7 compared with those of
controls and returned to normal levels at days 14 and 21 (Fig. 4; all P<0.01). Mice treated with
bleomycin demonstrated significantly elevated levels of CHOP,
XBP-1, GRP78 and ATF6α at day 7, CHOP, XBP-1 and GRP-78 at day 14,
when compared with those of controls, before returning to normal
levels at day 21 (Fig. 4; all
P<0.01). Mice treated with combined RSV+bleomycin demonstrated
significantly elevated levels of p-PERK, XBP-1, ATF6α and GRP78
from day 7 to day 21, when compared to the control group (Fig. 4; all P<0.01).
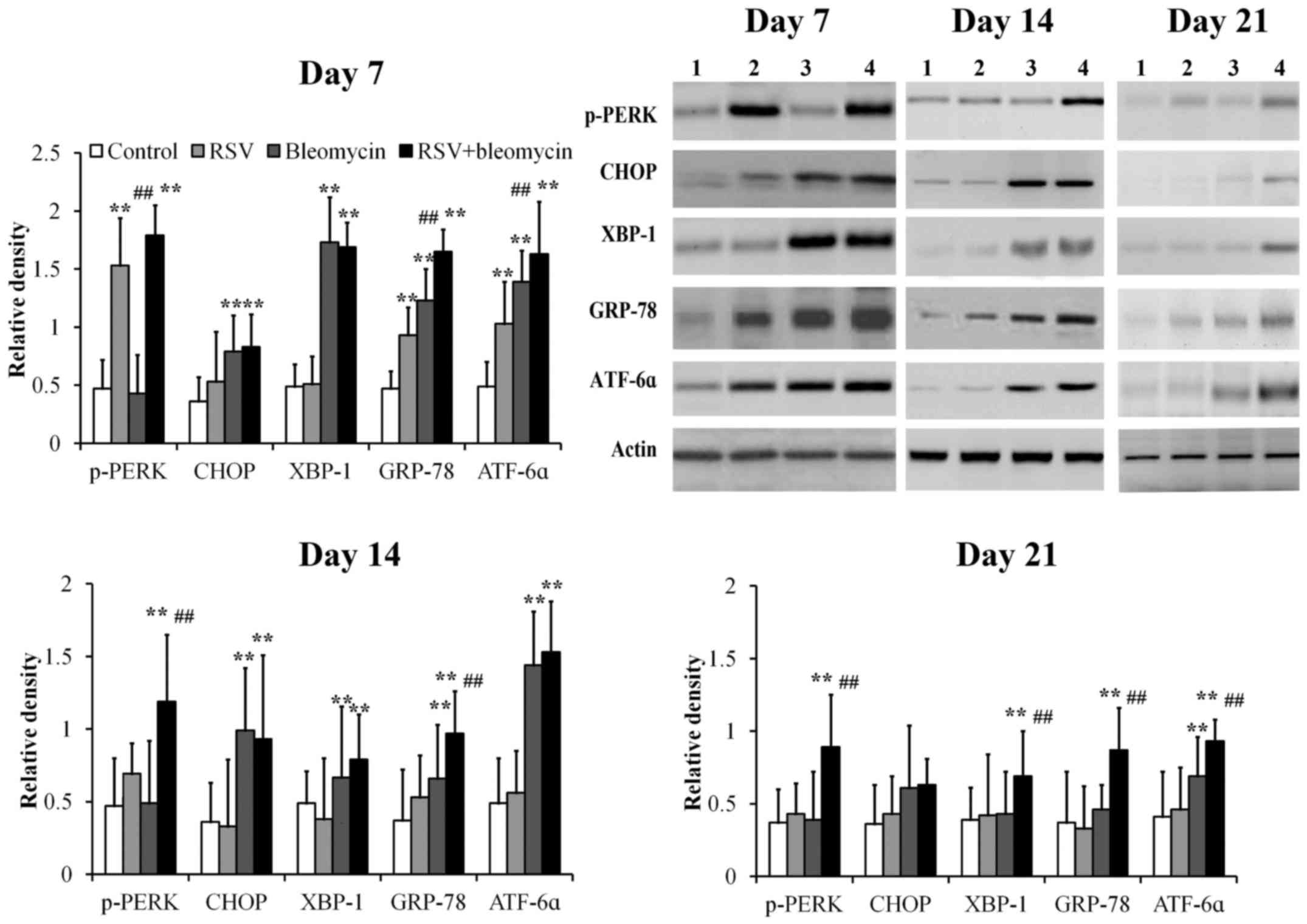 | Figure 4.The expressions of ER stress-related
proteins in lung tissues were assayed at days 7, 14 and 21 by
western blot analysis. The relative density of all tested ER stress
related proteins was weak in controls from day 7 to day 21. p-PERK,
ATF6α and GRP78 were upregulated at day 7 in RSV-infected lung
tissues, when compared with the control group. CHOP, XBP-1, GRP78
and ATF6α proteins were upregulated at day 7 and CHOP and GRP-78
were upregulated at day 14 in bleomycin-treated lung tissues,
compared to the control group. However, in RSV-infected
bleomycin-induced animal models, p-PERK, XBP-1, ATF6α and GRP78
were upregulated from day 7 to day 21. **P<0.01 vs. control;
##P<0.01 vs. bleomycin. ER, endoplasmic reticulum;
RSV, respiratory syncytial virus infection, CHOP, C/EBP homologous
protein; XBP-1, X-box binding protein; GRP-78, 78 kDa
glucose-regulated protein; ATF6α, activating transcription factor
6α; PERK, protein kinase R-like endoplasmic reticulum kinase. |
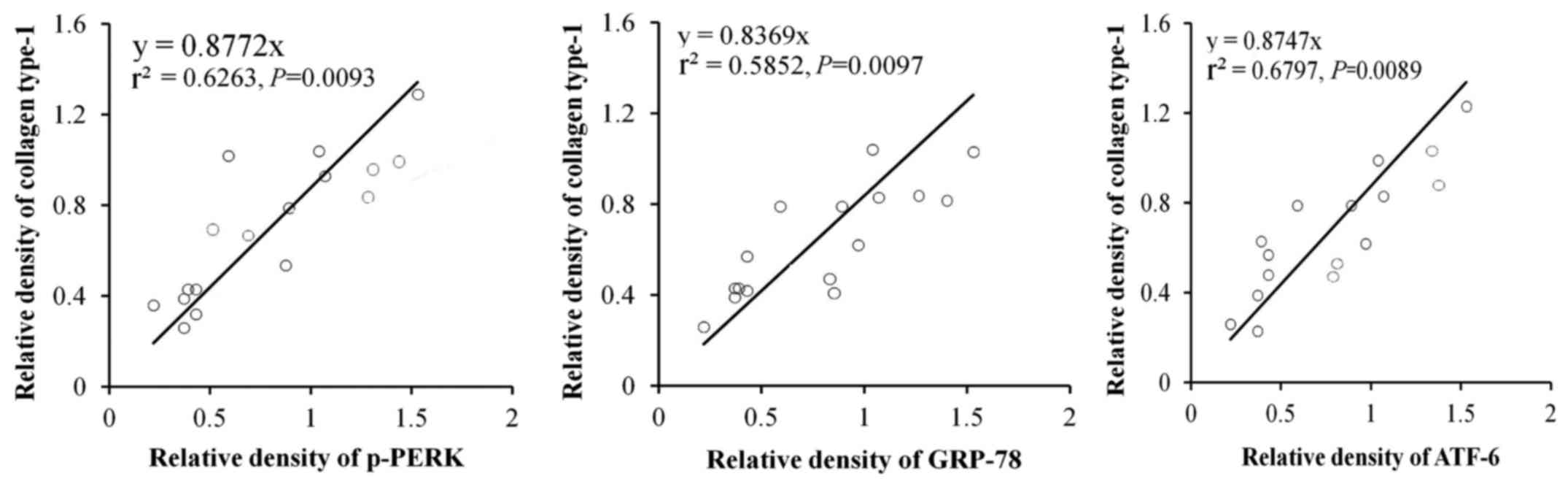
In order to further identify the effects of ER
stress-related proteins induced by RSV in the lung fibrosis, the
correlations of p-PERK, ATF6α and GRP78 with accumulation of
collagen type-1 were examined. The results indicated that p-PERK,
(P=0.0093), ATF6α (P=0.0097) and GRP78 (P=0.0089) were closely
related with accumulation of collagen type-1 (Fig. 5).
Discussion
The involvement of ER stress in the pathogenesis of
pulmonary fibrosis has been researched in previous years (10,11).
Lung microbiota is crucially important to maintain the normal
function of the lungs, and respiratory infection is a very common
factor that may induce ER stress of lung diseases (12,13).
To study the effects of viral infection on the onset and
progression of lung fibrosis, a bleomycin-induced pulmonary
fibrosis animal model was established, as it is a mature model for
studying the process of lung fibrosis by observing inflammation and
collagen deposition (14). Within
the animal models, persistent RSV immunoreactivity was observed
until day 21 in the lung tissues, following acute infection (day 7)
following the combined administration of RSV and bleomycin.
Many inflammatory cells, inflammatory media and
cytokines are involved in lung inflammation. Among then, IL-5 is
considered as the key chemokine for the recruitment and activation
of eosinophils and IL-8 for neutrophils (15). In the present study, the authors
demonstrated that, when co-administered, bleomycin and RSV promoted
the counts of various leukocytes and release of IL-1 and -8, when
compared with pulmonary fibrosis induced by bleomycin alone.
The production and deposition of collagen and other
extracellular matrix components is a characteristic feature of
pulmonary fibrosis (16). Using
RSV or bleomycin alone was self-limiting to induce collagen
deposition in the lungs. However, beyond promoting inflammation
following acute bleomycin administration, RSV was also able to
promote tissue fibrosis at earlier time points, lasting for a
longer period of time.
The activation of ER stress and UPR were first
observed in patients with familial interstitial pneumonia (FIP)
with a mutation in surfactant protein C (SFTPC) (17). The presence of UPR was demonstrated
in type II alveolar epithelial cells, which specifically express
SFTPC. In a subsequent study, it was demonstrated that prominent ER
stress in alveolar epithelial cells was also observed in patients
with FIP without SFTPC mutation, as well as in patients with
sporadic IPF (18). Potential
causes of ER stress in the lungs may include exposure to inhaled
particles or smoke or infections (17,19).
It was indicated in the present study that infection with RSV leads
to the development of ER stress in the lungs and the subsequent
exaggerated lung fibrosis in bleomycin-induced pulmonary fibrosis,
whereas RSV alone only developed mild reversible infections. This
suggested that activation of ER stress serves a role in the
severity of the fibrotic process. Of the three key transducers of
the UPR, the roles of PERK, ATF6α and IRE1-XBP1 signaling are all
involved in exaggerated lung fibrosis (20).
In summary, the current study demonstrated that RSV
exaggerated the fibrotic process induced by bleomycin. Further
studies investigating the mechanism of RSV administration on the
course of fibrotic lung diseases would be of great benefit to the
field. Based on these results, the authors suggest that ER stress
induced by RSV infection are involved in pulmonary fibrosis.
References
|
1
|
De Felice FG and Lourenco MV: Brain
metabolic stress and neuroinflammation at the basis of cognitive
impairment in Alzheimer'sdisease. Front Aging Neurosci. 7:942015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pluquet O, Pourtier A and Abbadie C: The
unfolded protein response and cellular senescence. A review in the
theme: Cellular mechanisms of endoplasmic reticulum stress
signaling in health and disease. Am J Physiol Cell Physiol.
308:C415–C425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reinhardt S, Schuck F, Grösgen S,
Riemenschneider M, Hartmann T, Postina R, Grimm M and Endres K:
Unfolded protein response signaling by transcription factor XBP-1
regulates ADAM10 and is affected in Alzheimer's disease. FASEB J.
28:978–997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanjore H, Blackwell TS and Lawson WE:
Emerging evidence for endoplasmic reticulum stress in the
pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung
Cell Mol Physiol. 302:L721–L729. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bettigole SE and Glimcher LH: Endoplasmic
reticulum stress in immunity. Annu Rev Immunol. 33:107–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mera K, Kawahara K, Tada K, Kawai K,
Hashiguchi T, Maruyama I and Kanekura T: ER signaling is activated
to protect human HaCaT keratinocytes from ER stress induced by
environmental doses of UVB. Biochem Biophys Res Commun.
397:350–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reed M, Morris SH, Owczarczyk AB and
Lukacs NW: Deficiency of autophagy protein Map1-LC3b mediates
IL-17-dependent lung pathology during respiratory viral infection
via ER stress-associated IL-1. Mucosal Immunol. 8:1118–1130. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung MB, Sampayo-Escobar V, Green R,
Moore ML, Mohapatra S and Mohapatra SS: Respiratory syncytial
virus-infected mesenchymal stem cells regulate immunity via
interferon beta and indoleamine-2,3-dioxygenase. PLoS One.
11:e01637092016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan Y, Yang T, Liu S, Liu H, Xiang Y, Qu
F, Li H and Qin X: Infection with respiratory syncytial virus
alters peptidergic innervation in the lower airways of guinea-pigs.
Exp Physiol. 93:1284–1291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mo XT, Zhou WC, Cui WH, Li DL, Li LC, Xu
L, Zhao P and Gao J: Inositol-requiring protein 1-X-box-binding
protein 1 pathway promotes epithelial-mesenchymal transition via
mediating snail expression in pulmonary fibrosis. Int J Biochem
Cell Biol. 65:230–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffman SM, Tully JE, Nolin JD, Lahue KG,
Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME
and Anathy V: Endoplasmic reticulum stress mediates house dust
mite-induced airway epithelial apoptosis and fibrosis. Respir Res.
14:1412013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Wu G, Qin X, Ma Q, Zhou Y, Liu S
and Tan Y: Expression of nodal on bronchial epithelial cells
influenced by lung microbes through DNA methylation modulates the
differentiation of T-helper cells. Cell Physiol Biochem.
37:2012–2022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan YR, Peng D, Chen CM and Qin XQ:
Nonstructural protein-1 of respiratory syncytial virus regulates
HOX gene expression through interacting with histone. Mol Biol Rep.
40:675–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashley SL, Jegal Y, Moore TA, van Dyk LF,
Laouar Y and Moore BB: γ-Herpes virus-68, but not Pseudomonas
aeruginosa or influenza A (H1N1), exacerbates established murine
lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 307:L219–L230.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang JS, Tan YR, Xiang Y, Luo ZQ and Qin
XQ: Regulatory peptides modulate adhesion of polymorphonuclear
leukocytes to bronchial epithelial cells through regulation of
interleukins, ICAM-1 and NF-kappaB/IkappaB. Acta Biochim Biophys
Sin (Shanghai). 38:119–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tilbury K, Hocker J, Wen BL, Sandbo N,
Singh V and Campagnola PJ: Second harmonic generation microscopy
analysis of extracellular matrix changes in human idiopathic
pulmonary fibrosis. J Biomed Opt. 19:0860142014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lawson WE, Crossno PF, Polosukhin VV,
Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB,
et al: Endoplasmic reticulum stress in alveolar epithelial cells is
prominent in IPF: Association with altered surfactant protein
processing and herpesvirus infection. Am J Physiol Lung Cell Mol
Physiol. 294:L1119–L1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lawson WE, Cheng DS, Degryse AL, Tanjore
H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB,
et al: Endoplasmic reticulum stress enhances fibrotic remodeling in
the lungs. Proc Natl Acad Sci USA. 108:10562–10567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao H, Yang J, Shan L and Jorgensen ED:
Measuring the impact of cigarette smoke on the UPR. Methods
Enzymol. 489:147–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nanua S, Sajjan U, Keshavjee S and
Hershenson MB: Absence of typical unfolded protein response in
primary cultured cystic fibrosis airway epithelial cells. Biochem
Biophys Res Commun. 343:135–143. 2006. View Article : Google Scholar : PubMed/NCBI
|