Introduction
Bone marrow mesenchymal stem cells (BMSCs) have the
potential to transdifferentiate into cardiomyocyte-like cells
(CLCs) in the heart and appear as functional phenotypes of
myocardial cells (1–3). It has been of great interest in
treatment of myocardial infarction to use stem cells to prevent
deterioration of heart failure and even to repair the damaged
myocardium. It has been reported that growth factors added to the
local microenvironment can improve the survival rate and
functioning of the transplanted cells (4,5).
Insulin-like growth factor-1 (IGF-1) is a potent
mitogen that has been proposed to play an important role in the
regulation of cell proliferation, apoptosis, and tumorigenicity
(6–8). The IGF-1 receptor (IGF-1R) and the
insulin receptor are structurally highly related glycoproteins.
Both these receptors possess two extracellular α-subunits, which
bind the ligand and two β-subunits. The receptors also span the
membrane and possess the intracellular autophosphorylation sites.
IGF-1 is involved in normal cell proliferation and has been
implicated in oncogenesis. The IGF-1 ligand, its receptor, the
IGF-1 binding proteins, and the specific proteolytic enzymes which
degrade these proteins constitute an important regulatory system
which plays a pivotal role in normal and neoplastic cell growth
(9). It has been reported that
IGF-1 promotes migration of endothelial cells and cardiac resident
progenitor cells (10–13). Li et al (4) also found that IGF-1 can simulate
transdifferentiation of BMSCs into the cardiac phenotype and
enhance the expression of GATA-4, but the mechanism is not
clear.
In the present study, BMSCs were isolated from rat
femurs and tibias and the cells were purified at passage 6 (P6).
IGF-1 and IGF-1R kinase inhibitor I-OMe AG538 were added to detect
if IGF-1 could induce BMSCs to transdifferentiate into CLCs and if
I-OMe AG538 could inhibit IGF-1-mediated receptor activation and
downstream signaling. Our study shows that I-OMe AG 538 could
inhibit IGF-1-induced CLCs in BMSCs.
Materials and methods
Isolation and culture of BMSCs
BMSCs were isolated according to the method
described by Panepucci et al (14). In brief, femurs and tibias from SD
rats (male, weighing 150±5 g) were removed. Muscle and extraosteal
tissue were trimmed under sterilized conditions. Bone marrow cells
were flushed and were transferred into culture flasks in 5%
CO2 incubator at 37°C. The culture medium contained 10%
fetal calf serum (FCS), (HyClone, Tauranga, New Zealand) and
DMEM/F12 (Gibco, Grand Island, NY, USA) containing 100 U/ml
penicillin, 100 mg/ml streptomycin, 2 mM L-glutamine
(Sigma-Aldrich, St. Louis, MO, USA). Three days later, BMSCs
adhered to the bottom of culture plates, and the hematopoietic
cells remained suspended in the medium. Fresh medium was changed
every 3 days. The sub-confluent cells in the seed cultures were
removed from the flasks by 0.25 trypsin (Sigma-Aldrich) treatment 7
days after the initial plating. They were labeled as P1 and
continued to culture until P6.
Drugs
I-OMe AG538 was purchased from Sigma-Aldrich. Stock
solution of this drug was prepared in DMSO and stored at −20°C.
Working dilutions of all drugs were prepared immediately before
use.
In vitro cytotoxicity
To study the inhibition effects of I-OMe AG538 in
standard or no-serum medium, 1,000–10,000 cells were plated into
96-well plates in DMEM/F12 plus 10% FCS. After 24 h, medium was
replaced by DMEM/F12 plus 10% FCS or without (control) various
concentrations of the compound (10 nmol/l-100 µmol/l) for ≤3 days.
MTT solution was added to the plate (5 mg/ml) 20 µl/well, then
incubated for 4 h and washed. In order to monitor at OD 490 nm, 150
µl DMSO was added to the plate for 10 min. IC50 (drug
concentration resulting in 50% inhibition of growth) values of
inhibitor was determined using the GraphPad Prism 5 Demo program,
GraphPad Software, Inc. (La Jolla, CA, USA).
Immunocytochemical staining
When BMSCs were cultured at P6, they were already
purified. To identify if these cells were BMSCs, cells cultured on
35 mm culture dish were fixed with 4% paraformaldehyde for 20 min.
After being washed 3 times with PBS for 5 min, the culture dish was
covered with 0.01% Triton X-100 (Gen-View Scientific, Inc., El
Monte, CA, USA) for 10 min then were covered with 3%
H2O2 for 10 min and blocked with normal goat
serum for 20 min at room temperature. After removal of serum, rat
monoclonal CD29 antibody (dilution, 1:200; cat. no. 121409), rat
monoclonal CD44 antibody (dilution, 1:200; cat. no. 203901) and rat
monoclonal CD45 antibody (dilution, 1:200; cat. no. 202211) were
added followed by HRP goat anti-rat IgG secondary antibody
(dilution, 1:500; cat. no. 405405) (all from BioLegend, Inc., San
Diego, CA, USA) after washing with PBS. The cells were stained
using AEC staining kit and then haematoxylin. PBS was added for the
control group.
In the second experiment, to evaluate the ability of
IGF-1 induced BMSCs to transdifferentiate into CLCs, 15 ng/ml IGF-1
group and control group containing 10% FCS were used in induction
of BMSCs. These cells were observed for morphological changes under
an inverted microscope (BX-42; Olympus, Tokyo, Japan). The
expression of troponin-T and troponin-I was detected by
immunocytochemistry.
In the third experiment, to evaluate the ability of
IGF-1 to induce cell recovery from the cytotoxic effects of I-OMe
AG538, 2×104/ml cells were plated into 35 mm culture
dish in DMEM/F12 plus 10% FCS. After 24 h, 15 ng/ml IGF-1 was added
to the medium and 300 nmol/l, replaced by DMEM/F12 plus 10% FBS or
the compound (300 nmol/l) every 6 h ≤3 days. After 72 h of
treatment, the identification of BMSCs by immunocytochemical stain
was performed as previously described. The primary antibody was rat
monoclonal troponin-T antibody (dilution, 1:500; cat. no. ab50576)
and rat polyclonal troponin-I antibody (dilution, 1:500; cat. no.
ab47003) (both from Abcam, Cambridge, UK). PBS was used in the
control group.
Western blotting
Constitutive activation of IGF-1R was evaluated on a
panel of BMSCs. To evaluate the ability of IGF-1 to induce cell
recovery from the cytotoxic effects of I-OMe AG538 toward IGF-1R
kinase and signaling, starved BMSCs were treated with doses of 300
nmol/l (corresponding to IC50 value) I-OMe AG538 for 3 h
followed by stimulation with IGF-1 (Peprotech, Inc., Rocky Hill,
NJ, USA) for 5 to 30 min. In a second experiment, I-OMe AG538
inhibitory effects were followed on IGF-1R-related signaling
pathways by exposing BMSCs to 300 nmol/l compound for 1 to 48 h in
standard medium. To determine phosphorylation status of Erk1/2 and
Akt, two downstream mediators of mitogen-activated protein kinase
(MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways, cell
lysates were prepared with a buffer containing 50 mmol/l MOPS, 320
mmol/l Sucrose, 100 mmol/l Kcl, 0.5 mmol/l MgCl2, 1
mmol/l DTT, 50 mmol/l NaF, 20 mmol/l NaPPi, 20 mmol/l
β-glycerophosphate, 1 mmol/l Na3VO4, 1 mmol/l
EDTA, and protease inhibitors (0.5 mmol/l phenylmethylsulfonyl
fluoride, 0.5 mmol/l leupeptin).
In the third experiment, to evaluate the ability of
IGF-1 to induce BMSCs to transdifferentiate into CLCs which had
been added with 300 nmol/l I-OMe AG538, we collected BMSCs which
were induced by IGF-1 for 3 days. The experiment followed as above.
Protein concentration was determined by BCA protein assay (Thermo
Labsystems, Santa Rosa, CA, USA) and equivalent amounts of total
cell lysate (50 µg) were separated by 4 or 10% SDS-PAGE under
denaturing conditions and transferred onto nitrocellulose membrane.
Membranes were incubated with 5% bovine serum albumin confining
liquid overnight, then with primary antibodies. Rabbit monoclonal
phospho-IGF-1R antibody (dilution, 1:1,000; cat. no. 3918S); rabbit
monoclonal IGF-1R antibody (dilution: 1:1,000; cat. no. 14534S);
rabbit monoclonal phospho-Akt antibody (dilution, 1:1,000; cat. no.
12178S); rabbit monoclonal Akt antibody (dilution, 1:1,000; cat.
no. 11848) and rabbit monoclonal Erk1/2 antibody (dilution,
1:1,000; cat. no. 4348S) were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Then the goat anti-rabbit
secondary antibody (dilution, 1:2,000; cat. no. ab6721; Abcam,
Cambridge, MA, USA) was added. In all experiments, final
concentration of DMSO in the medium was <0.001%, and in the
present study, it had no effect on cell growth inhibition. This
method was described by Scotlandi et al (15).
Results
Morphological changes of BMSCs in
vitro
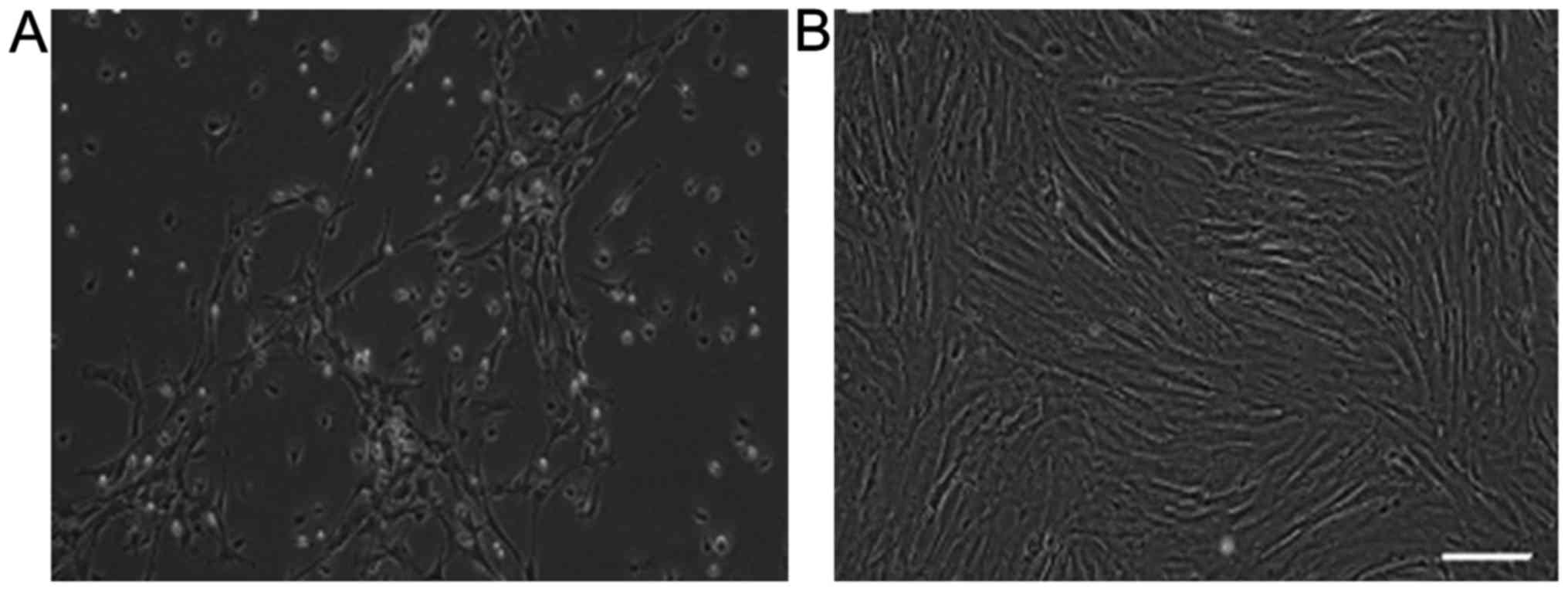
BMSCs were successfully expanded and used for
subsequent experiments. At first, single cells of spindle
morphology were observed 3–4 days after the initial seeding. The
morphologic appearance of the cell population was fairly
homogeneous (Fig. 1A). About 1
week later, most cells appeared as fibroblasts with spindle or
shuttle shape. The colonies continuously grew, reaching 80–90%
confluence in 2 weeks. However, there were some blood cells in the
view. When they were labeled at P6, cells were already purified and
there were no blood cells in the view and the confluence was
whirlpool-like (Fig. 1B).
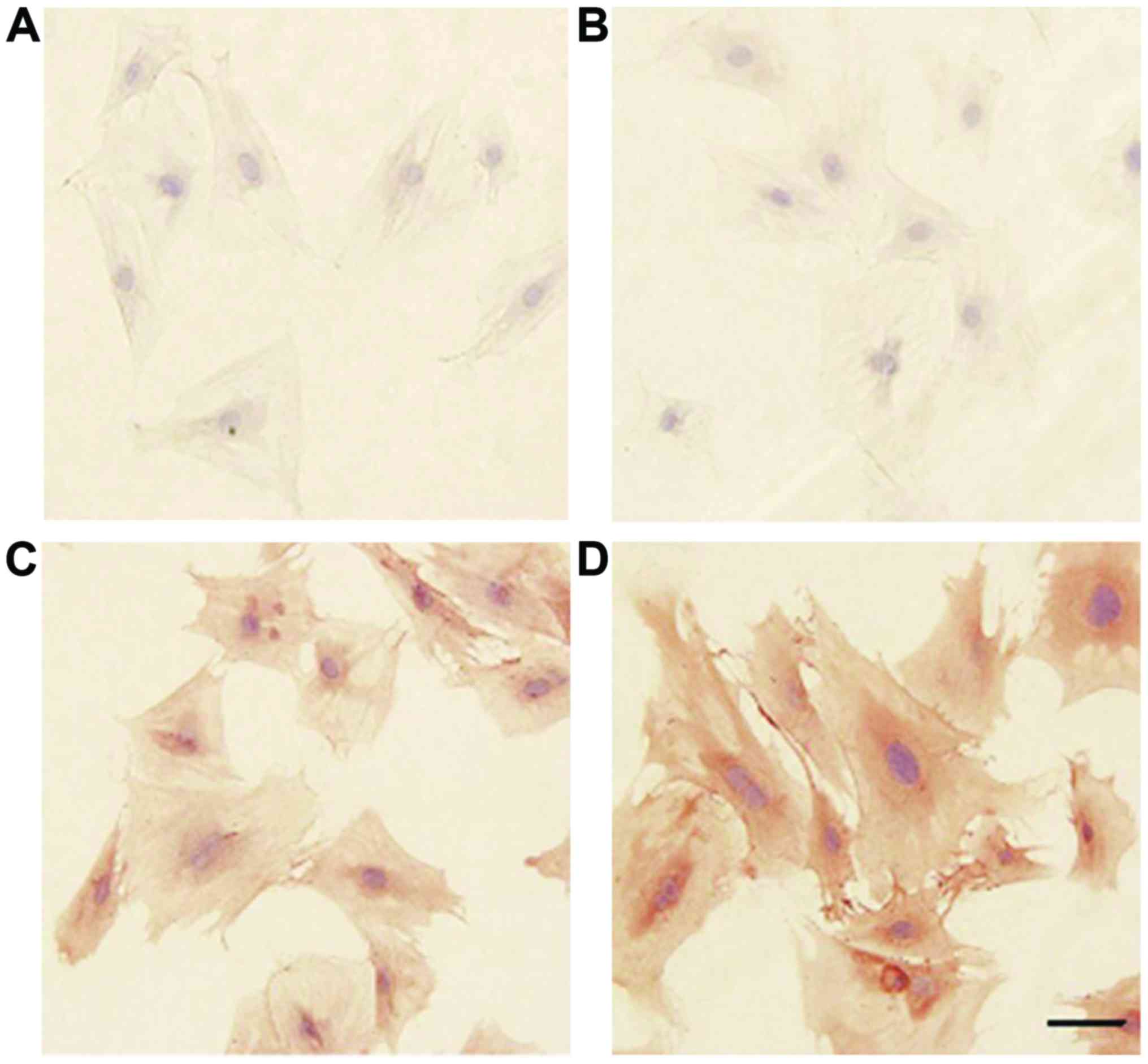
The identification of BMSCs
All P6 BMSCs express CD29 (Fig. 2C) and CD44 (Fig. 2D), but there was no staining for
CD45 (Fig. 2B). This demonstrated
that these cells were BMSCs but haemopoietic stem cells. We
confirmed that the method to isolate BMSCs was feasible and highly
purified cells could be obtained with this method.
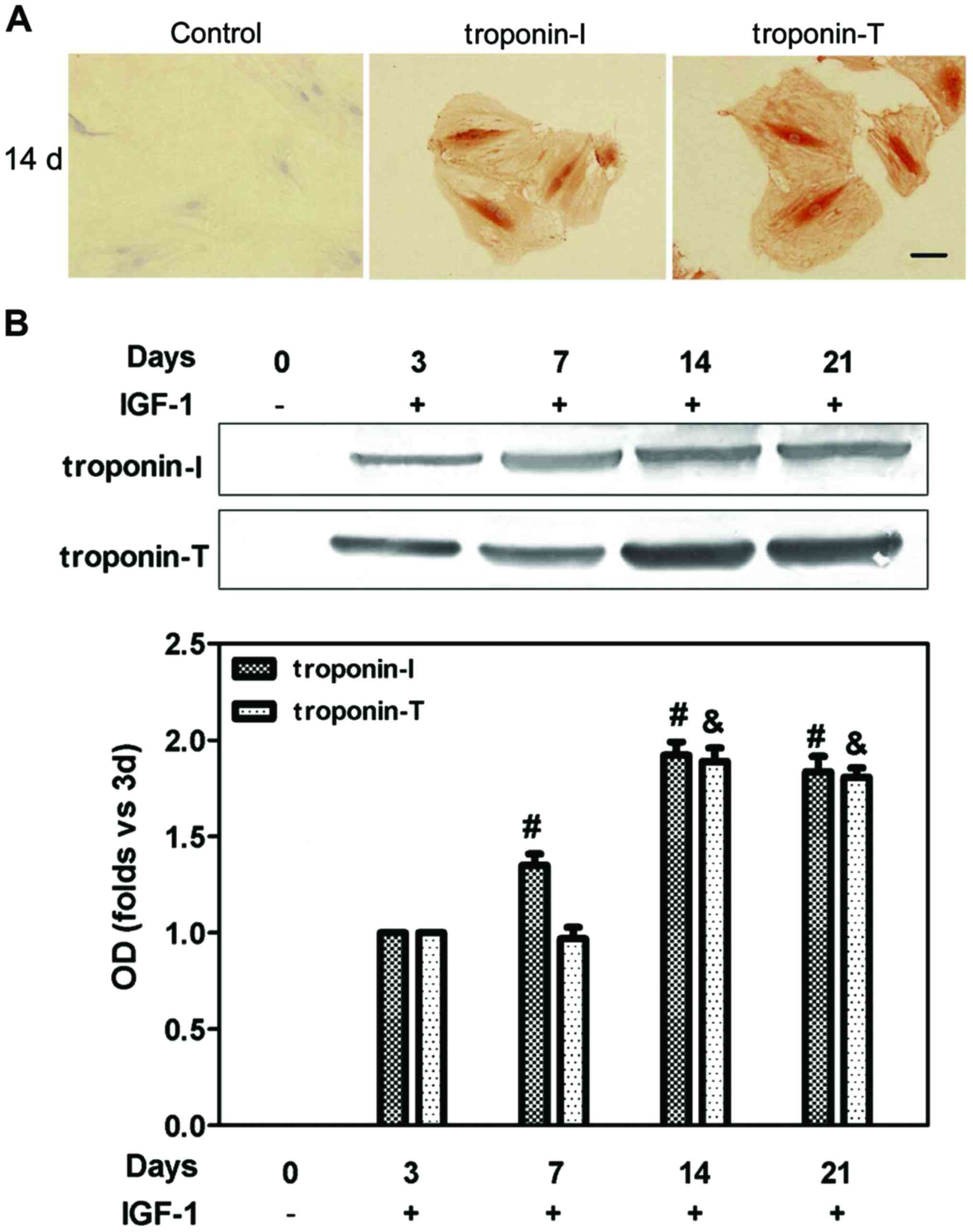
The ability of IGF-1 to induce BMSCs
to transdifferentiate into CLCs
After BMSCs were exposed to 15 ng/ml of IGF-1 for 21
days, the morphology of BMSCs did not change. The expression of
troponin-T and troponin-I were significantly higher in the 14-day
group than control group (Fig. 3)
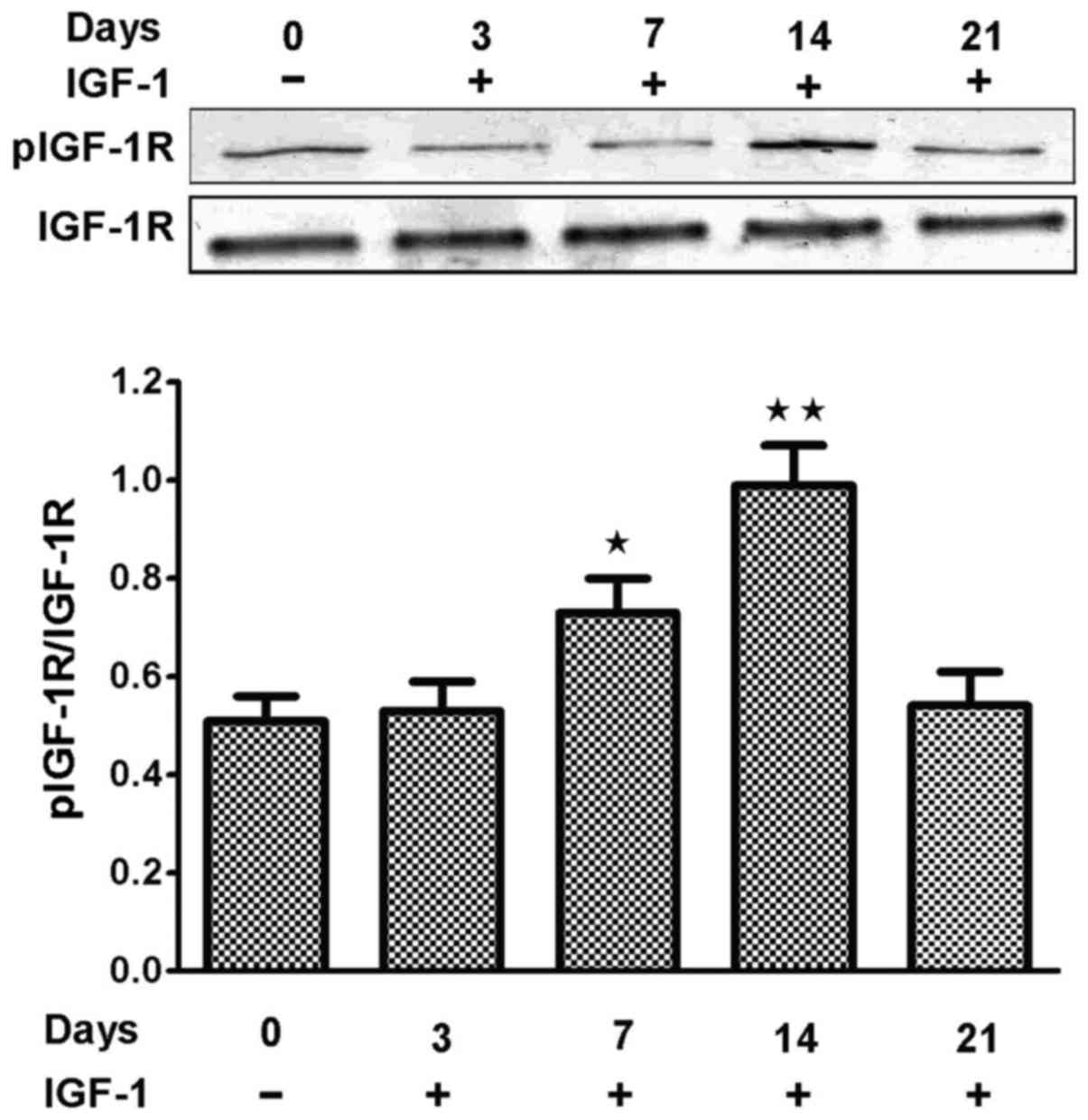
and the levels of pIGF-1R were higher in the 14-day group than
other groups (Fig. 4).
I-OMe AG538 selectively inhibits
IGF-1-mediated growth and signaling
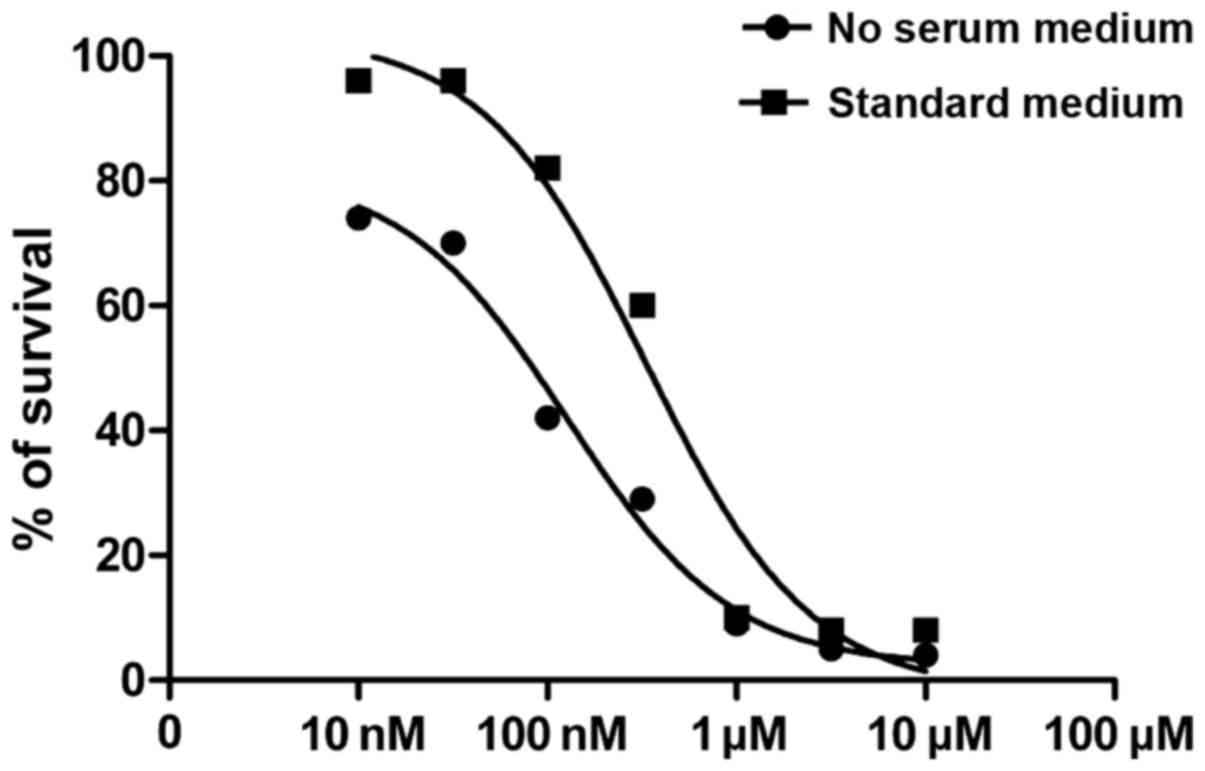
Fig. 5 demonstrates
that similar inhibitory effects were obtained in BMSCs with various
concentrations using I-OMe AG538. Growth inhibitory activity of the
compound was maintained for ≥72 h after its removal (50% of growth
inhibition with the dose of 331.7±2.3 nmol/l; P<0.05) (Fig. 5). To confirm the inhibitory
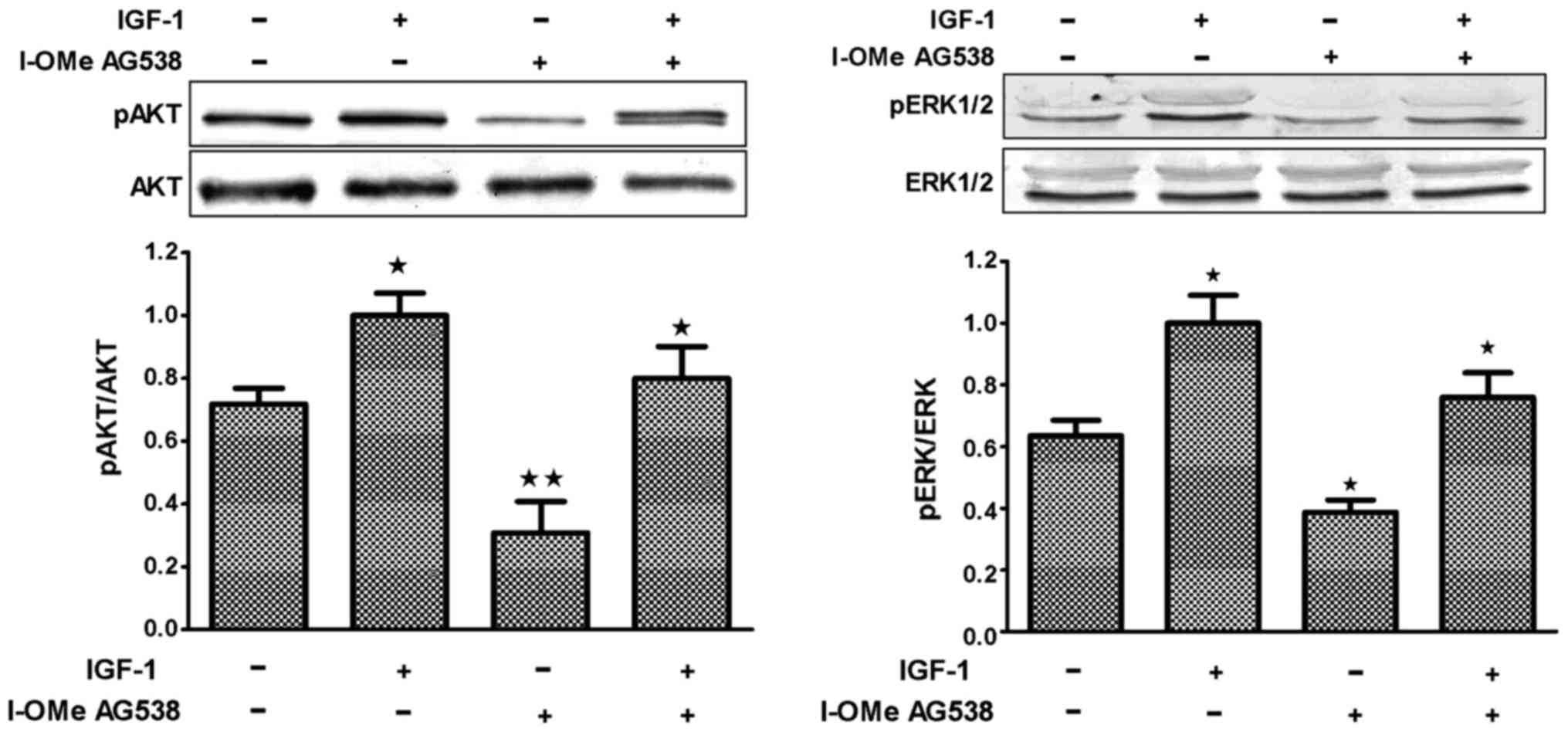
activity of I-OMe AG538 toward IGF-1R kinase and signaling, starved
BMSCs were treated with doses of 300 nmol/l for 3 h followed by
stimulation with IGF-1 for 5 to 30 min. Fig. 6 shows that both IGF-1R
autophosphorylation and the two major IGF-1R-related intracellular
signaling pathways, MAPK and PI3K pathways, were inhibited by I-OMe
AG538.
I-OMe AG538 blocks the differentiation
into CLCs of BMSCs induced by IGF-1
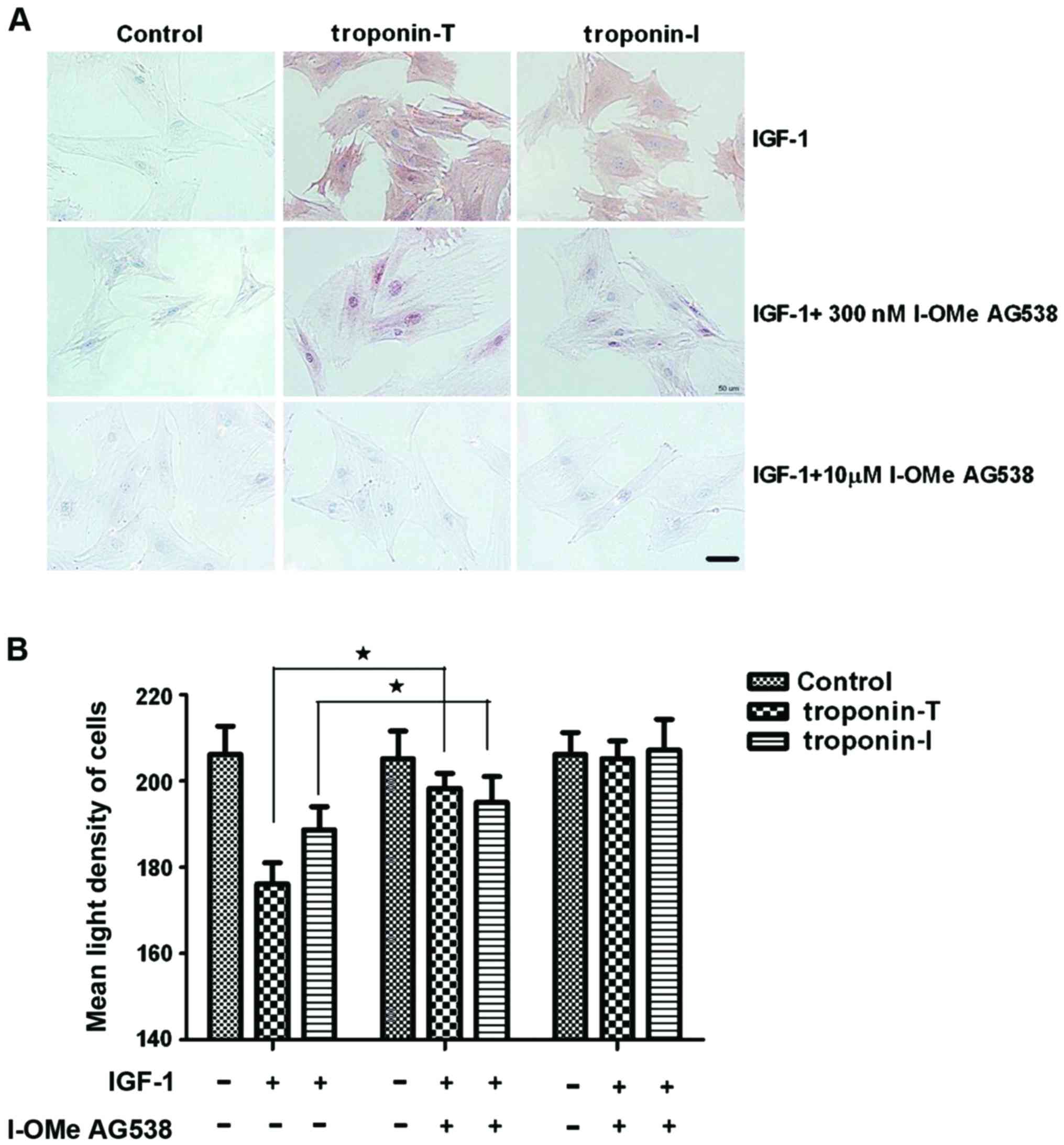
To confirm the inhibitory activity of I-OMe AG538
and whether it blocks the differentiation of BMSCs into CLCs
induced by IGF-1, BMSCs were treated with 15 ng/ml IGF-1 and 300
nmol/l or 10 µmol/l I-OMe AG538. Fig.
7 shows that the expression of troponin-T and troponin-I were
inhibited by 300 nmol/l I-OMe AG538.
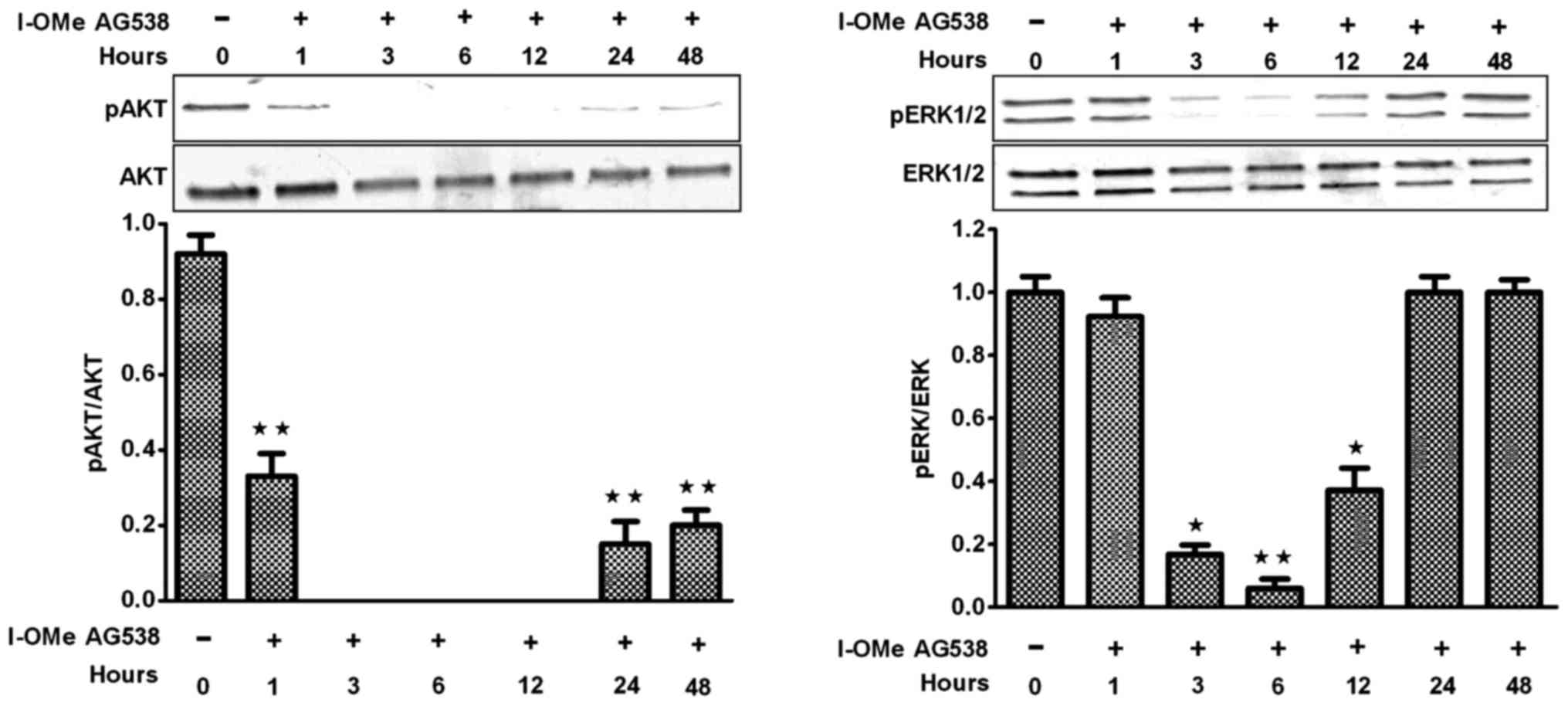
In vitro activity of I-OMe AG538 on
BMSCs
A time course evaluation of inhibitory effects of
300 nmol/l I-OMe AG538 on MAPK and PI3K signaling pathways in
standard medium, however, revealed transient effects on MAPK and
PI3K pathway, particularly for the dose of 300 nmol/l (Fig. 8).
Discussion
The traditional belief has been that the cardiac
muscle is a terminally differentiated tissue and can only be
replaced by scar tissue after a myocardial infarction. To maintain
cardiac pumping following an infarction, remodeling of the
degenerative left ventricle occurs, causing a decrease in cardiac
function and leading to heart failure (4). BMSCs have been considered to be one
of the potential cell sources for cellular cardiomyoplasty due to
their multipotency and immunomodulatory properties (2,16,17).
Although there is great enthusiasm for repairing the heart by using
cell therapy, simple cell implantation cannot reinstate cell
transdifferentiation or reverse apoptosis. Furthermore,
experimental data indicate that only a small number of cells can be
implanted in the host tissue with normal function. Therefore,
attempts at using various approaches to enhance the effectiveness
of cell therapy have become a challenge (16). However, utilizing growth factors in
a local tissue environment to increase the survival of transplanted
cells may be practical. IGF-1 has been considered as a potent
growth hormone capable of inducing cell proliferation, limiting
apoptotic cell death, and attenuating maladaptive extracellular
matrix remodeling in the failing heart (17,18).
IGF-1 can promote angiogenesis in the infarcted myocardium, reduce
the degree of myocardial necrosis, maintain the myocardial
structure, stimulate proliferation of cardiac fibroblast and
inhibit matrix degradation. Thus, IGF-1 can prevent ventricular
dilatation and reduce load capacity of the heart (19–21).
IGF-1 plays an important role in proliferation and differentiation
of stem cells. It has been reported that IGF-1 promotes migration
of endothelial cells and cardiac resident progenitor cells
(10–12). A recent report also found that
IGF-1 increases BMSCs migratory responses in vitro via CXCR4
chemokine receptor signaling and provided substantial evidence that
PI3-kinase/Akt is the dominant signaling pathway underlying IGF-1
enhanced BMSCs migration (22).
Kofidis et al (23) found
that insulin-like growth factor promotes engraftment,
differentiation, and functional improvement after transfer of
embryonic stem cells for myocardial restoration.
IGF-1R is the most important individual component of
the IGF axis that includes the ligands IGF-1 and IGF-2, six
high-affinity IGF-binding proteins, several proteases, and three
receptors (24). In general, the
activation of IGF/IGF-1R signaling promotes proliferative and
survival mechanisms that can be usefully exploited by many cells
(25).
In the present study, in order to determine whether
15 ng/ml IGF-1 could induce BMSCs to transdifferentiate into CLCs
and have a heart cell phenotype, cells were treated with antibodies
against proteins expressed in cardiac cells, including troponin-T
and troponin-I. After BMSCs were exposed to 15 ng/ml IGF-1 for 21
days, the morphology of BMSCs did not change. Immunocytochemistry
analysis and western blotting all showed that the expression of
troponin-T and troponin-I were significantly higher in the 14 day
group than control group and the level of pIGF-1R was also higher
in 14 day group than the control group. These results suggested
that IGF-1 could induce BMSCs to transdifferentiate into CLCs and
have a heart cell phenotype.
IGF-1R kinase inhibitor I-OMe AG538 were added to
detect if I-OMe AG538 could inhibit IGF-1-mediated receptor
activation and downstream signaling. Our data showed that I-OMe
AG538 was found to fulfill the key features expected from an IGF-1R
inhibitor. It selectively inhibits IGF-1-mediated growth and signal
transduction in BMSCs. Of note, the inhibitory effects of I-OMe
AG538 were not completely reverted in the presence of 15 ng/ml
IGF-1. BMSCs are sensitive to I-OMe AG538 and therefore, when a
time course analysis of the effects of I-OMe AG538 on MAPK and PI3K
signaling was done, we observed a transient inhibitory effect on
Erk1/2 and Akt phosphorylation, which is in keeping with the
inhibitory effects on cell growth.
In conclusion, BMSCs can transdifferentiate into
CLCs and obtain the myocardial cell phenotype in the presence of
IGF-1 (13). In addition, the
induction effects of IGF-1 can be blocked by the inhibitor of
IGF-1R kinase and be time- dependent. However, as we know, PI3K/Akt
is the dominant signaling pathway underlying IGF-1 enhanced cell
survival and migration (22,26),
and the Ras/Raf/MEK/ERK MAPK pathway regulates the expression of a
large number of proteins involved in the control of cell
proliferation, differentiation, and apoptosis (27,28).
Therefore, if the effect that IGF-1 induces BMSCs to
transdifferentiate into CLCs is through the MAPK/ERK pathway, then
that needs to be further studied.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant no. 30572073); the Life Health
Science and Technology Projects funded by the Jiangsu Special Funds
(grant no. BL2012019); the Jiangsu Key Talents of Medical Science
(grant no. RC2007024); the Xuzhou Science and Technology
Development Project (grant no. XF10C029); and the Xuzhou Science
and Technology Development Project (grant no. XM12B062).
References
|
1
|
Liechty KW, MacKenzie TC, Shaaban AF, Radu
A, Moseley AM, Deans R, Marshak DR and Flake AW: Human mesenchymal
stem cells engraft and demonstrate site-specific differentiation
after in utero transplantation in sheep. Nat Med. 6:1282–1286.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orlic D, Kajstura J, Chimenti S, Limana F,
Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A and Anversa
P: Mobilized bone marrow cells repair the infarcted heart,
improving function and survival. Proc Natl Acad Sci USA.
98:10344–10349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kudo M, Wang Y, Wani MA, Xu M, Ayub A and
Ashraf M: Implantation of bone marrow stem cells reduces the
infarction and fibrosis in ischemic mouse heart. J Mol Cell
Cardiol. 35:1113–1119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Z, Gu TX and Zhang YH: Hepatocyte
growth factor combined with insulin like growth factor-1 improves
expression of GATA-4 in mesenchymal stem cells cocultured with
cardiomyocytes. Chin Med J (Engl). 121:336–340. 2008.PubMed/NCBI
|
|
5
|
Rangappa S, Entwistle JW, Wechsler AS and
Kresh JY: Cardiomyocyte-mediated contact programs human mesenchymal
stem cells to express cardiogenic phenotype. J Thorac Cardiovasc
Surg. 126:124–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haylor J, Hickling H, El Eter E, Moir A,
Oldroyd S, Hardisty C and El Nahas AM: JB3, an IGF-I receptor
antagonist, inhibits early renal growth in diabetic and
uninephrectomized rats. J Am Soc Nephrol. 11:2027–2035.
2000.PubMed/NCBI
|
|
7
|
Hu C, Wu Y, Wan Y, Wang Q and Song J:
Introduction of hIGF-1 gene into bone marrow stromal cells and its
effects on the cell's biological behaviors. Cell Transplant.
17:1067–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schnabel LV, Lynch ME, van der Meulen MC,
Yeager AE, Kornatowski MA and Nixon AJ: Mesenchymal stem cells and
insulin-like growth factor-I gene-enhanced mesenchymal stem cells
improve structural aspects of healing in equine flexor digitorum
superficialis tendons. J Orthop Res. 27:1392–1398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blum G, Gazit A and Levitzki A: Substrate
competitive inhibitors of IGF-1 receptor kinase. Biochemistry.
39:15705–15712. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Urbich C, Aicher A, Heeschen C, Dernbach
E, Hofmann WK, Zeiher AM and Dimmeler S: Soluble factors released
by endothelial progenitor cells promote migration of endothelial
cells and cardiac resident progenitor cells. J Mol Cell Cardiol.
39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu M, Uemura R, Dai Y, Wang Y, Pasha Z and
Ashraf M: In vitro and in vivo effects of bone marrow stem cells on
cardiac structure and function. J Mol Cell Cardiol. 42:441–448.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su EJ, Cioffi CL, Stefansson S, Mittereder
N, Garay M, Hreniuk D and Liau G: Gene therapy vector-mediated
expression of insulin-like growth factors protects cardiomyocytes
from apoptosis and enhances neovascularization. Am J Physiol Heart
Circ Physiol. 284:H1429–H1440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo J, Lin G, Bao C, Hu Z, Chu H and Hu M:
Insulin-like growth factor 1 improves the efficacy of mesenchymal
stem cells transplantation in a rat model of myocardial infarction.
J Biomed Sci. 15:89–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panepucci RA, Siufi JL, Silva WA Jr,
Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT and Zago
MA: Comparison of gene expression of umbilical cord vein and bone
marrow-derived mesenchymal stem cells. Stem Cells. 22:1263–1278.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scotlandi K, Manara MC, Nicoletti G,
Lollini PL, Lukas S, Benini S, Croci S, Perdichizzi S, Zambelli D,
Serra M, et al: Antitumor activity of the insulin-like growth
factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal
tumors. Cancer Res. 65:3868–3876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shake JG, Gruber PJ, Baumgartner WA,
Senechal G, Meyers J, Redmond JM, Pittenger MF and Martin BJ:
Mesenchymal stem cell implantation in a swine myocardial infarct
model: Engraftment and functional effects. Ann Thorac Surg.
73:1919–1925; discussion 1926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li G, Borger MA, Williams WG, Weisel RD,
Mickle DA, Wigle ED and Li RK: Regional overexpression of
insulin-like growth factor-I and transforming growth factor-beta1
in the myocardium of patients with hypertrophic obstructive
cardiomyopathy. J Thorac Cardiovasc Surg. 123:89–95. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Welch S, Plank D, Witt S, Glascock B,
Schaefer E, Chimenti S, Andreoli AM, Limana F, Leri A, Kajstura J,
et al: Cardiac-specific IGF-1 expression attenuates dilated
cardiomyopathy in tropomodulin-overexpressing transgenic mice. Circ
Res. 90:641–648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bayes-Genis A, Conover CA and Schwartz RS:
The insulin-like growth factor axis: A review of atherosclerosis
and restenosis. Circ Res. 86:125–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beamer WG, Donahue LR and Rosen CJ:
Insulin-like growth factor I and bone: From mouse to man. Growth
Horm IGF Res. 10:S103–S105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Madry H, Zurakowski D and Trippel SB:
Overexpression of human insulin-like growth factor-I promotes new
tissue formation in an ex vivo model of articular chondrocyte
transplantation. Gene Ther. 8:1443–1449. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Yu X, Lin S, Li X, Zhang S and Song
YH: Insulin-like growth factor 1 enhances the migratory capacity of
mesenchymal stem cells. Biochem Biophys Res Commun. 356:780–784.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kofidis T, de Bruin JL, Yamane T, Balsam
LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL and Robbins RC:
Insulin-like growth factor promotes engraftment, differentiation,
and functional improvement after transfer of embryonic stem cells
for myocardial restoration. Stem Cells. 22:1239–1245. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Larsson O, Girnita A and Girnita L: Role
of insulin-like growth factor 1 receptor signalling in cancer. Br J
Cancer. 92:2097–2101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pollak MN, Schernhammer ES and Hankinson
SE: Insulin-like growth factors and neoplasia. Nat Rev Cancer.
4:505–518. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
McMahon LA, Prendergast PJ and Campbell
VA: A comparison of the involvement of p38, ERK1/2 and PI3K in
growth factor-induced chondrogenic differentiation of mesenchymal
stem cells. Biochem Biophys Res Commun. 368:990–995. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peyssonnaux C and Eychène A: The
Raf/MEK/ERK pathway: New concepts of activation. Biol Cell.
93:53–62. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong KK: Recent developments in
anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent
Patents Anticancer Drug Discov. 4:28–35. 2009. View Article : Google Scholar
|