Introduction
Neuronal death in the brain can cause Alzheimer's
disease (AD), which is the most common irreparable and progressive
neurodegenerative disease. In the human brain, increased oxidative
stress associated with reactive oxygen species (ROS) can cause
disorders of the central nervous system. In addition, oxidative
stress is associated with diverse neurodegenerative diseases
resulting in neurodegenerative processes (1,2). The
most important ROS, superoxide and hydroxyl radicals, can cause
oxidative stress to molecular organisms. Oxidative
stress-associated apoptotic action is connected to neuronal cell
death and critical neuronal disorders, including ischemia. A
well-known neuronal transmitter, glutamate, is unduly released
during neuroinflammation (3). The
irregular discharge of glutamate into the extracellular area
inhibits the cysteine/glutamate antiporter, which transports
cysteine into the cytoplasm while removing glutamate from the
cells. This subsequently suppresses glutathione biosynthesis and
can cause the increases in ROS (4). In addition, nuclear factor erythroid
2-related factor 2 (Nrf2)/antioxidant response element (ARE) serve
a key role against cellular oxidative stress (5). Nrf2/ARE also activates downstream
signaling through inducible antioxidant enzymes, including heme
oxygenase 1 (HO-1) and reduced nicotinamide-adenine dinucleotide
phosphate dehydrogenase [quinone] 1 (6). Among these enzymes, HO-1 is
recognized to be a protective gene against oxidative stress, which
catalyzes the metabolism of heme to yield carbon monoxide,
bilirubin/biliverdin and iron (7).
HO-1 is a stress-responsive enzyme generated by the stimulation of
heat shocks, oxidants and heavy metals. HO-1 serves vital roles in
the prevention of oxidative stress and inflammation in cells
(8). In HT22 cells, HO-1 exhibits
a protective action against glutamate-associated neurotoxicity
(9,10).
The Aceraceae plant Acer nikoense (commonly
known as Nikko maple or megusurinoki in Japanese) is indigenous to
Japan and the stem bark of A. nikoense is used in Japanese
folk medicine for the treatment of hepatic sicknesses and eye
diseases (11). Furthermore, the
stem bark of A. nikoense is ingested as a health food in
Japan (12). Various
diarylheptanoids and multiple types of phenolic compounds have been
identified from the stem bark (13), and tannin, coumarins, lignans,
triterpenes, flavonoids and sterols have been characterized in the
leaves and wood of A. nikoense (14). In addition, a number of
bioactivities in A. nikoense extracts have been reported,
including inhibition of the Na+-glucose cotransporter
(15), inhibition of nitric oxide
production (16), anti-oxidant
(17), anti-inflammatory effects
(18), hepatoprotective effects
(19) and stimulating osteoblast
differentiation (20). Acerogenin
C was isolated from A. nikoense, and previous studies have
determined the estrogenic and antiproliferative activities of
acerogenin C (21,22). However, there have been no studies
on the mechanisms underlying the antineurodegenerative actions of
acerogenin C. In the present study, the neuroprotective effects of
acerogenin C on glutamate-stimulated toxicity in HT22 mouse
hippocampal cells through Nrf2-associated HO-1 expression were
investigated.
Materials and methods
Chemicals and reagents
Acerogenin C was isolated from A. nikoense as
previously described (10). All
cell culture-associated reagents were obtained from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Lipofectamine 2000™ was
obtained from Invitrogen (Thermo Fisher Scientific, Inc.). The
anti-HO-1 (catalog no. sc-10789), Nrf-2 (catalog no. sc-722), p-Akt
(catalog no. sc-514032), Akt (catalog no. sc-5298), proliferating
cell nuclear antigen (PCNA; catalog no. sc-56) and β-actin (catalog
no. sc-1616) antibodies, and small interfering RNA (siRNA) were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The
HO-1 inducer cobalt protoporphyrin IX (CoPP) and all other chemical
reagents were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany).
HT22 cell culture
Mouse hippocampal HT22 cells were donated from
Professor Youn-Chul Kim (Wonkwang University, Iksan, Korea). The
cells (5×106 cells/dish) were cultured in Dulbecco's modified
Eagle's medium containing streptomycin (100 µg/ml), 10% fetal
bovine serum (FBS), and penicillin G (100 U/ml) at 37°C in an
atmosphere containing 5% CO2 and 95% air.
HT22 cell viability assays
HT22 cells were maintained at 2×104 cells/well and
treated with acerogenin C (1, 10, 20, 40 and 50 µM) for 48 h.
Alternatively, HT22 cells were pretreated with acerogenin C (10, 20
and 40 µM) or trolox (50 µM) for 12 h, and then treated with
glutamate (5 mM) for 12 h. In addition, HT22 cells were treated
with acerogenin C (30 µM) in the presence or absence of 50 µM tin
protoporphyrin XI (SnPP), (HO)-1 siRNA, or 10 µM LY294002, and then
exposed to glutamate (5 mM) for 12 h. All incubations were
performed at 37°C and 5% CO2. Following incubation, the
cell culture medium was removed from each well and replaced with
fresh medium. Cells were incubated with 0.5 mg/ml MTT for 1 h and
the formed formazan crystals were dissolved in 150 µl 99.7%
dimethyl sulfoxide. Optical density was measured at a wavelength of
590 nm on a microplate reader (Bio-Rad, Hercules, CA, USA).
Extraction of cytoplasmic and nuclear
cells
HT22 cells homogenized in PER-Mammalian Protein
Extraction buffer (Pierce; Thermo Fisher Scientific, Inc.)
supplemented with 1 mM phenylmethylsulfonyl fluoride and protease
inhibitor cocktail I (EMD Millipore, Billerica, MA, USA).
Cytoplasmic and nuclear fractions were separated using NE-PER
Nuclear and Cytoplasmic Extraction reagents (Pierce; Thermo Fisher
Scientific, Inc.).
Western blot analysis
HT22 cells were incubated with acerogenin C (10, 20
and 40 µM) or CoPP (20 µM) for 12 h. Alternatively, HT22 cells were
treated with 30 µM of acerogenin C for 0, 30, 60 and 120 min, and
transiently transfected with Nrf2 siRNA and then treated with 30 µM
acerogenin C for 12 h (Fig. 4B).
In addition, HT22 cells were pre-incubated with or without 10 µM
LY294002 for 1 h and then incubated in the absence or presence of
30 µM of acerogein C for 60 min (p-AKT) or 12 h (HO)-1. Pelleted
HT22 cells were obtained by centrifugation at 200 × g for 3 min at
room temperature. Following the washing of cells with PBS, they
were lysed using Tris-HCl buffer (20 mM; pH 7.4) supplemented with
a protease inhibitor mixture containing chymostatin (1 mg/ml),
aprotinin (5 mg/ml), pepstatin A (5 mg/ml) and phenylmethylsulfonyl
fluoride (0.1 mM). Equal amounts of protein were resolved using
SDS-PAGE and transferred to a Hybond-enhanced chemiluminescence
nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membrane was blocked with 5% skimmed milk at room
temperature for 1 h, and then incubated with anti-HO-1, anti-Nrf2,
anti-p-Akt, anti-Akt, anti-PCNA, or anti-β-actin antibodies (all of
which were used at a 1:1,000) at 4°C overnight. The membrane was
subsequently incubated with horseradish peroxidase-conjugated
anti-goat (catalog no. ap106p; 1:1,000), rabbit (catalog no.
ap132p; 1:1,000) and mouse (catalog no. ap160p; 1:1,000) secondary
antibodies, obtained from EMD Millipore, followed by Enhanced
Chemiluminescence (GE Healthcare, Chicago, IL, USA) detection
substrate. Secondary antibodies were diluted in 3% skimmed milk in
TBST buffer. The bands were quantified by densitometry (ImageJ
software version 1.47; National Institutes of Health, Bethesda, MD,
USA). Nuclear and cytoplasmic extracts of cells were prepared using
NE-PER reagents, as per the manufacturer's protocol (Thermo Fisher
Scientific, Inc.).
Transfection
Lipofectamine 2000™ (Invitrogen; Thermo Fisher
Scientific, Inc.) containing Opti-MEM without FBS were used to
temporarily transfect HT22 cells with 50 nM HO-1 siRNA (catalog no.
sc-35554) and Nrf2 siRNA (catalog no. sc-37030) (both from Santa
Cruz Biotechnology, Inc.) for 6 h. The cell culture medium was
replaced with fresh medium with 10% FBS.
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and quantified
spectrophotometrically at 260 nm (ND-1000; Thermo Fisher
Scientific, Inc.), Total RNA (1 µg) was reverse-transcribed into
cDNA using the High Capacity RNA kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The obtained cDNA was amplified using the
SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd., Dalian,
China) and a StepOnePlus Real-Time PCR (Applied Biosystems).
Reaction mixture contained diethyl pyrocarbonate-treated water,
primer, and SYBR-Green PCR Master Mix. The sequences of primer were
designed by PrimerQuest (Integrated DNA Technologies, Inc.,
Coralville, IA, USA). The primer sequences are: HO-1 forward,
5′-CTCTTGGCTGGCTTCCTT-3′ and reverse, 5′-GGCTCCTTCCTCCTTTCC-3′; and
GAPDH forward, 5′-ACTTTGGTATCGTGGAAGGACT-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′. The thermal cycling conditions used
were as follows: Pre-denaturation at 95°C for 10 min, denaturation
at 95°C for 15 sec and annealing at 60°C for 1 min. A total of 40
cycles were performed. The data was analyzed using StepOne software
(version 2.3; Applied Biosystems; Thermo Fisher Scientific, Inc.),
and the cycle number at the linear amplification threshold
(quantification cycle; Cq) was recorded for the endogenous control
gene and the target gene. Relative gene expression (target gene
expression normalized to the expression of the endogenous control
gene) was calculated using the comparative Cq method
(2−ΔΔCq) (23).
Statistical analysis
All data were expressed as the mean ± standard
deviation from at least three independent experiments. To compare
each experimental group, one-way analysis of variance was used
followed by the Newman-Keuls post hoc test. Statistical analysis of
all data was conducted using GraphPad Prism software (version 3.03;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of acerogenin C on cytotoxicy
induced by glutamate and ROS generation in hippocampal HT22
cells
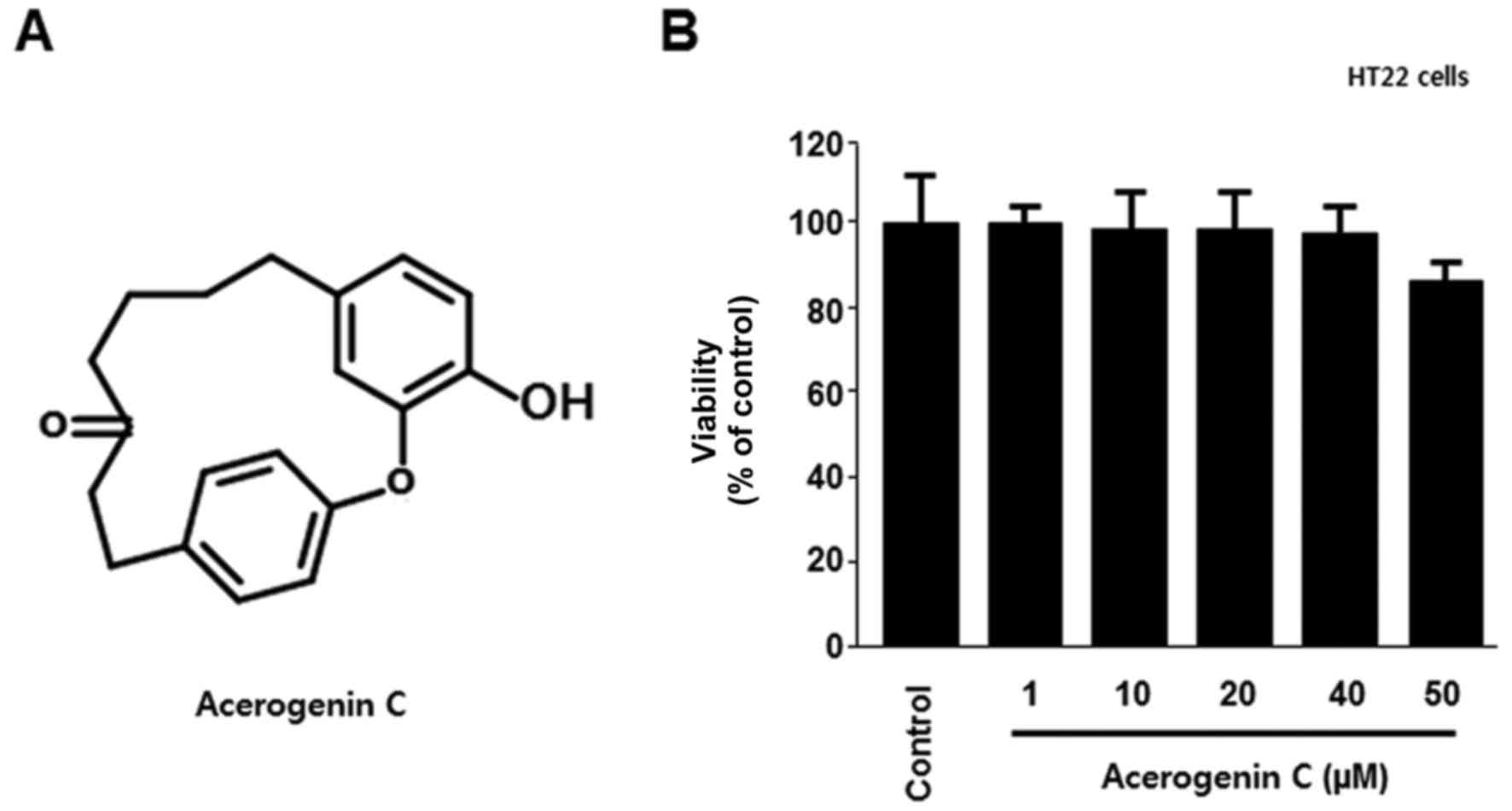
To verify the cytotoxic effects of acerogenin C
(Fig. 1A), its effect on the
viability of HT22 cells was analyzed using the MTT assay. There
were no significant effects at 1–50 µM acerogenin C; however, the
viability notably decreased at 50 µM (Fig. 1B). In addition, the neuroprotective
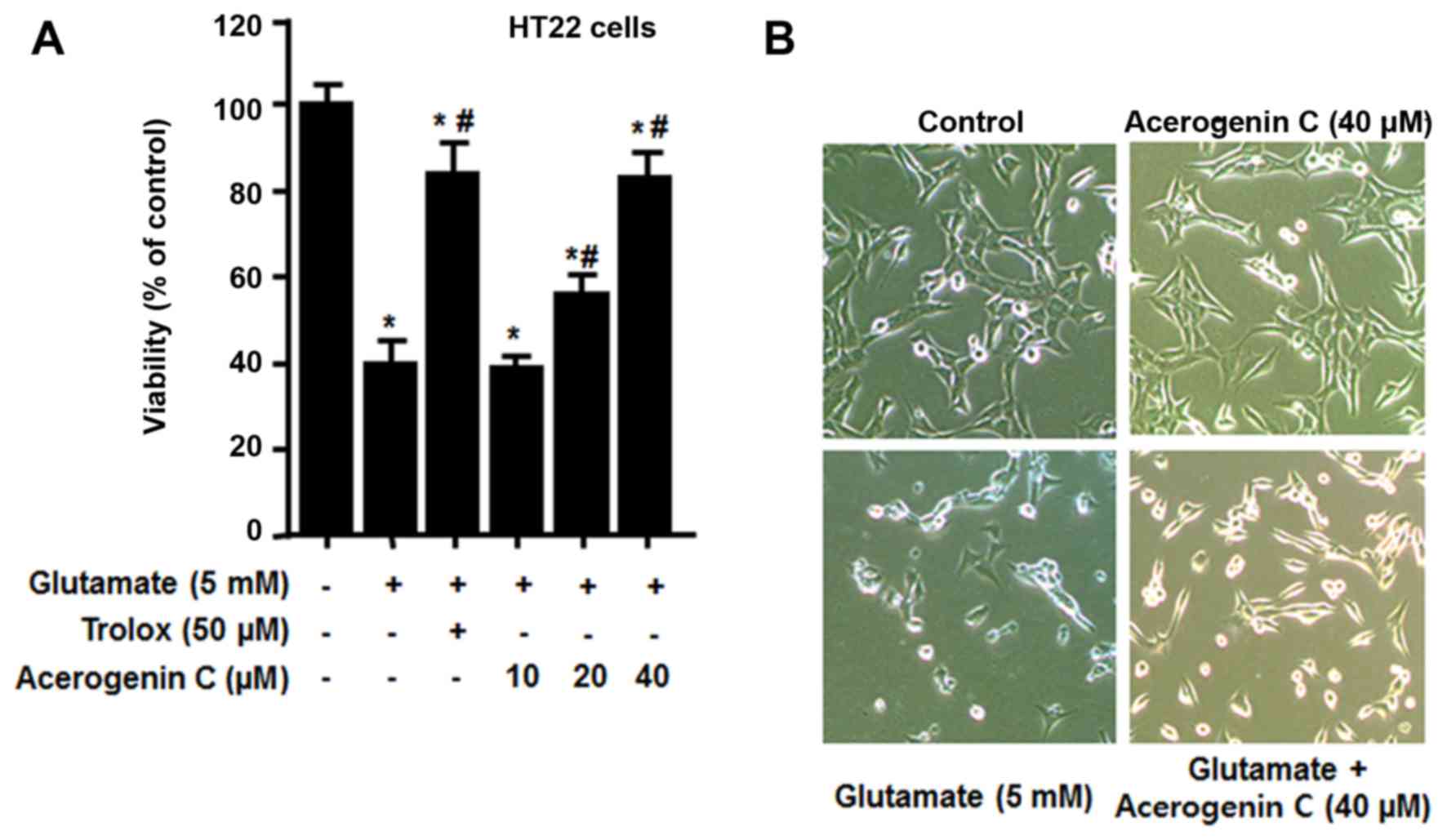
effect of acerogenin C on HT22 cells against glutamate-associated
cytotoxicity and ROS generation was investigated. To demonstrate
the neuroprotective effect of acerogenin C, HT22 cells pretreated
with different concentrations of acerogenin C (10, 20 and 40 µM)
for 3 h and 5 mM glutamate for 12 h were subject to an MTT assay.
The results demonstrated that the viability of 20 and 40 µM
acerogenin C-treated cells was significantly increased compared
with untreated cells (Fig. 2).
Trolox, well-known for its anti-oxidative effect, was used as a
positive control and provided a notable cytoprotective effect at a
concentration of 50 µM.
Effect of acerogenin C against
cytotoxicy induced by glutamate via HO-1 in hippocampal HT22
cells
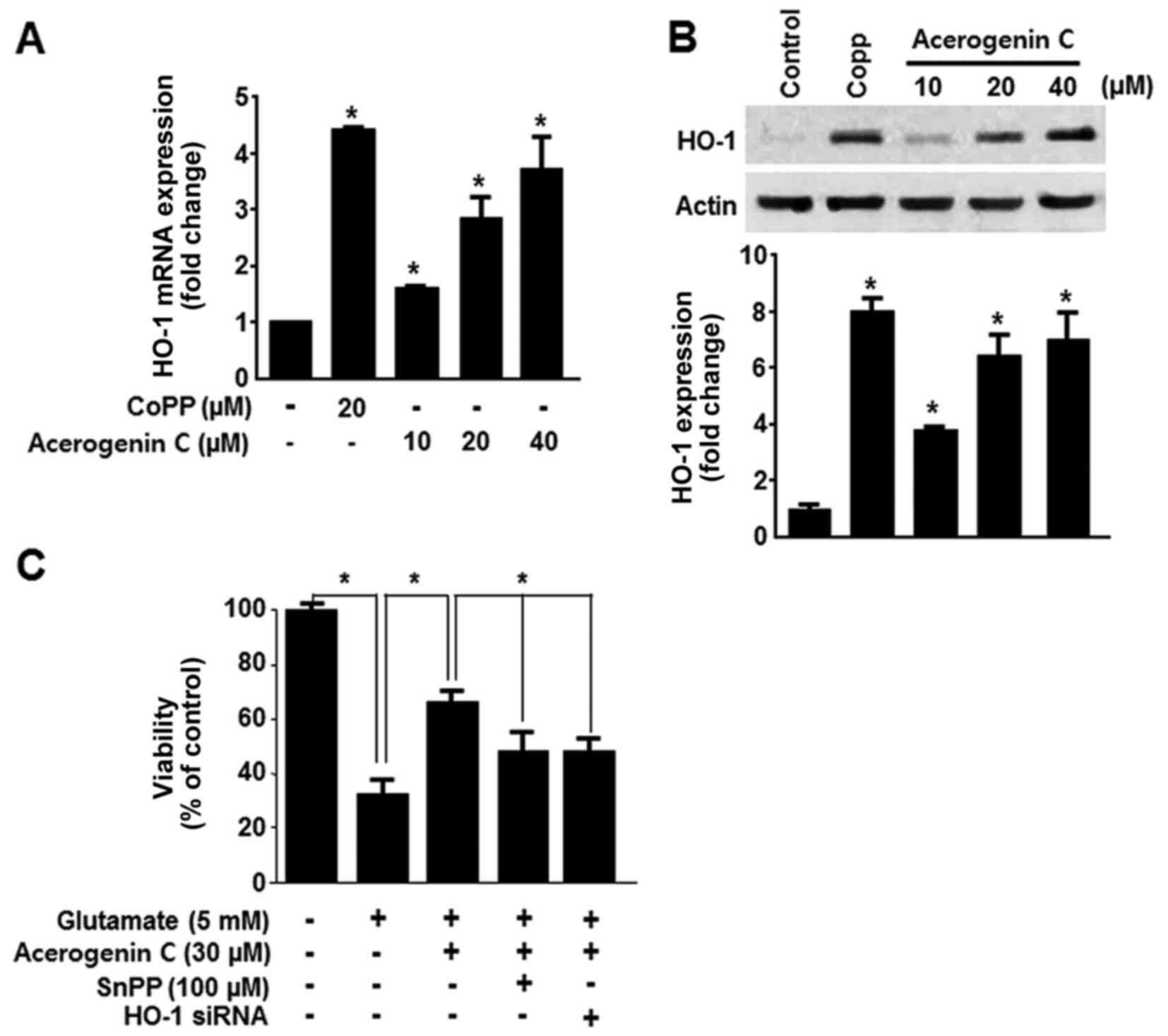
HO-1 expression serves a key cytoprotective role in
HT22 cells. It catalyzes the oxygen-dependent degradation of heme,
producing CO, free iron and bilirubin/biliverdin (7). Consequently, the HO-1 induced
protective effect in HT22 cells was investigated. HO-1 mRNA
expression was evaluated following treatment with acerogenin C.
Cobalt protoporphyrin (CoPP) was used as a positive control. CoPP
is a well-known HO-1 inducer. Treatment with cerogenin C increased
HO-1 mRNA and protein expression in HT22 cells in a dose-dependent
manner (Fig. 3A and B).
Subsequently it was demonstrated that tin protoporphyrin XI (SnPP),
a known inhibitor of HO-1 expression, and HO-1 siRNA significantly
blocked the protective effect of acerogenin C (Fig. 3C). These results suggest that HO-1
contributes to the cytoprotective effect of acerogenin C in HT22
cells.
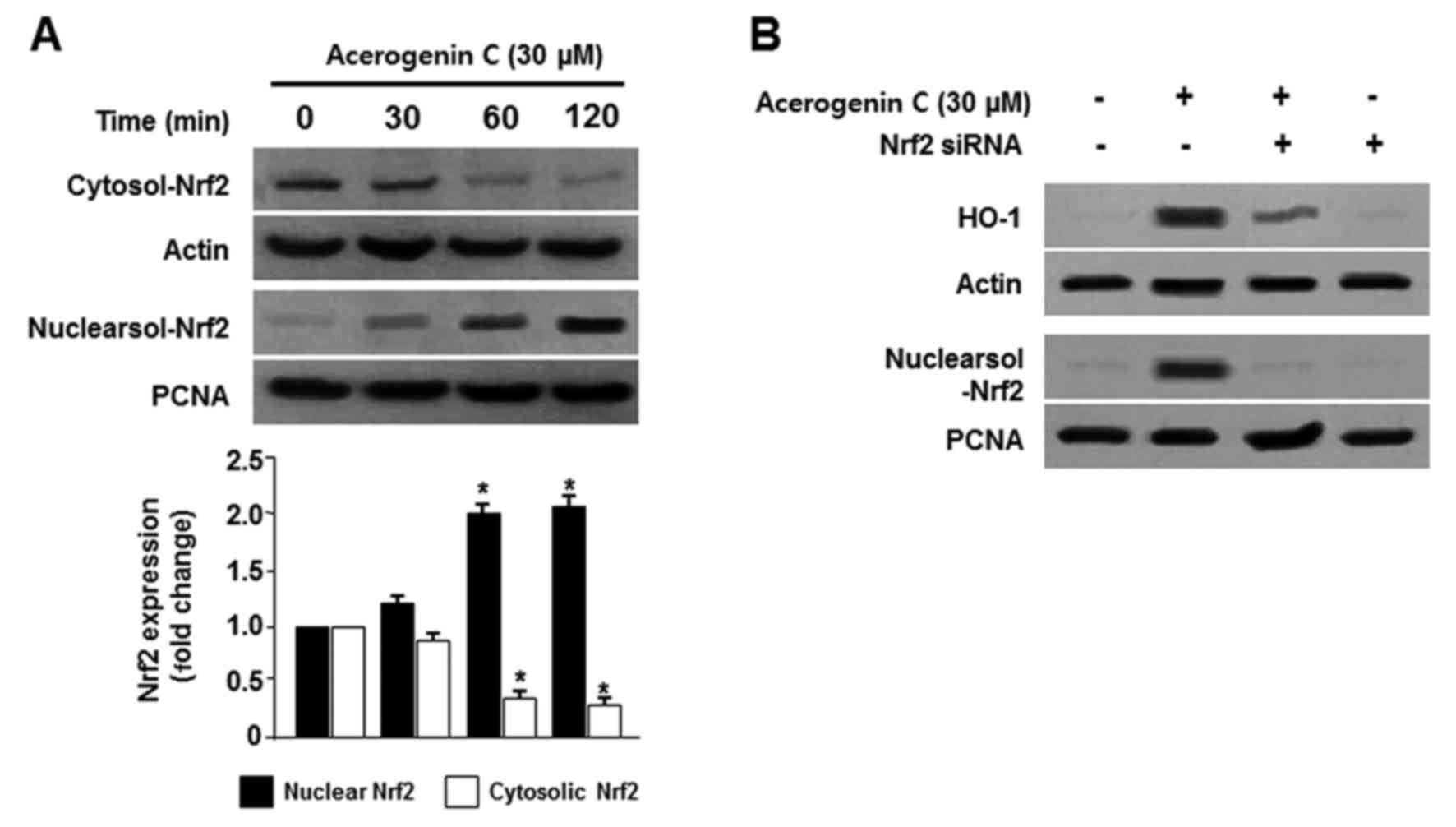
Effect of acerogenin C on the nuclear
translocation of Nrf2 in hippocamppal HT22 cells
The Nrf2/HO-1 pathway is important in the prevention
of oxidative stress-induced damage. Under normal conditions, Nrf2
is located within cell cytoplasm. During oxidative stress, Nrf2 is
phosphorylated and translocated into the nucleus, and binds to the
specific ARE sequences (5,6). Therefore, the translocation of Nrf2
to nuclei in HT22 cells was observed. Cells were treated with
acerogenin C for 0, 30, 60 and 120 min, and Nrf2 protein levels
were detected by western blotting. Cytosolic Nrf2 levels were
decreased; however, nuclear Nrf2 levels were increased following
treatment with acerogenin C (Fig.
4A). PNCA is widely used as a control in antibody validation of
nuclear protein. In addition, the role of Nrf2 in HO-1 activity was
analyzed using Nrf2 siRNA. Hippocampal HT22 cells were temporarily
transfected with Nrf2 siRNA and were treated with acerogenin C to
induce HO-1 activity. Nrf2 siRNA inhibited the nuclear
translocation of Nrf2 and transient transfections with Nrf2 siRNA
also eliminated the induction of HO-1 expression by acerogenin C
(Fig. 4B). These results
demonstrated that HO-1 induction by acerogenin C is associated with
the Nrf2 nuclear translocation pathway in HT22 cells.
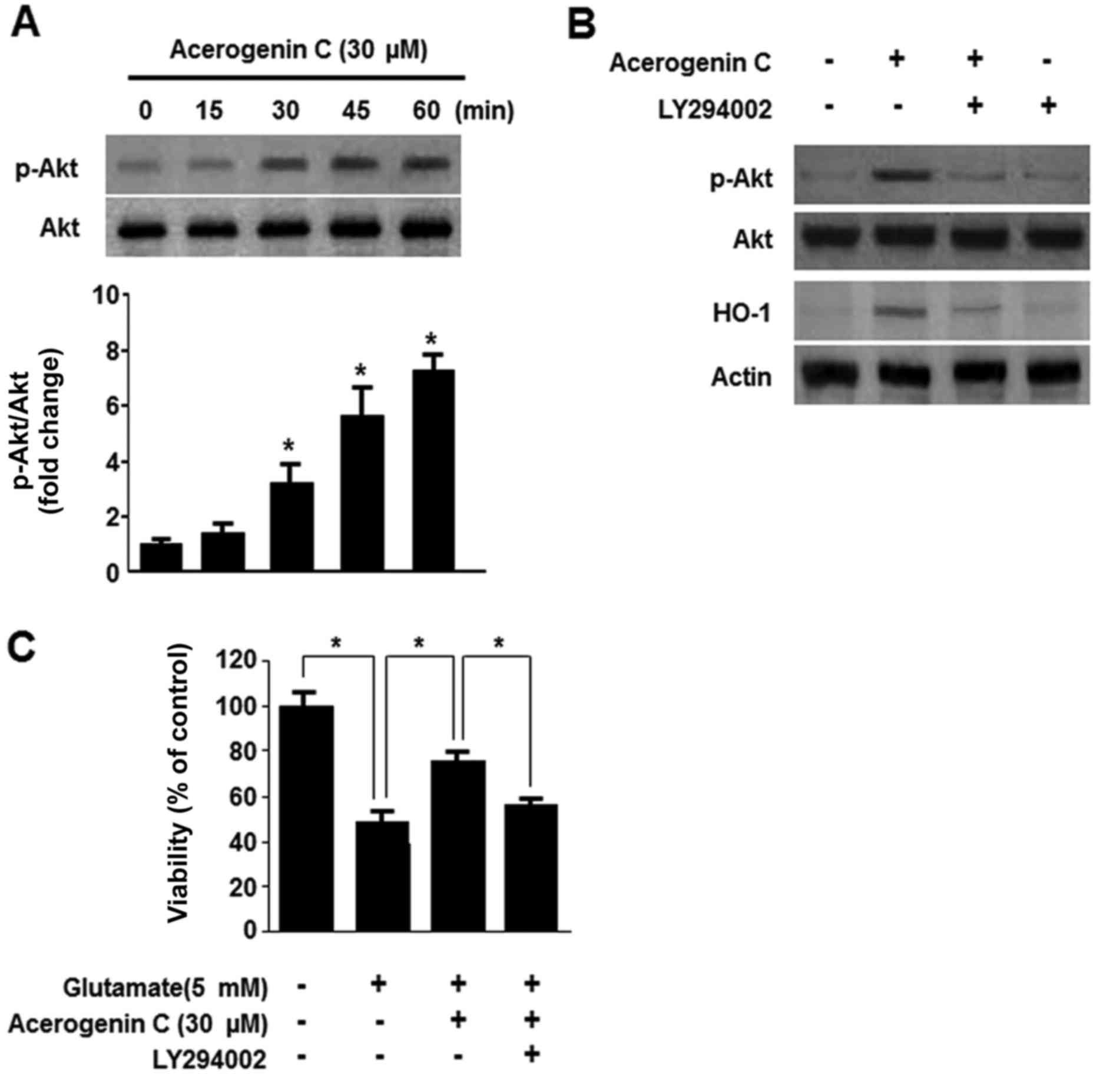
Effect of acerogenin C on the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway in
the induction of HO-1 expression
Multiple studies demonstrated that the PI3K/Akt
pathway is associated with the expression of HO-1 (24). Therefore, it was investigated
whether acerogenin C induces expression of HO-1 via the PI3K/Akt
signaling pathway. Following treatment with 40 µM acerogenin C, the
levels of Akt were significantly increased (Fig. 5A). In addition, treatment with
LY294002, a known inhibitor of PI3K, abolishes HO-1 expression and
cytoprotection by acerogenin C (Fig.
5B and C). Therefore, acerogenin C-induced HO-1 expression is
associated with the PI3K/Akt signaling pathway in HT22 cells.
Discussion
Since the beginning of medicine, humans have
depended on chemical compounds taken from animals, plants and
micro-organisms to cure diseases. These are referred to ‘natural
products’. Natural products have been the most useful sources of
lead compounds in the development of drugs. The worth of these
‘natural products’ can be measured using three criteria: Their
frequency of use in treatment; the number of chemicals of wide
structural variety they contain; and the number of diseases they
treat or prevent (25).
Neurodegeneration is typically caused when the neuron loses its
ability to function properly and its structures, and includes
neuron damage. These neurodegenerative processes can be the cause
of a number of neurodegenerative diseases, including Parkinson's
disease, AD and Huntington's disease. In particular, brain tissue
is susceptible to oxidative stress as well as inflammation which
may occur through physiological or pathological processes (26).
In previous studies, research was focused on the
identification of different bioactive natural compounds, which
regulate HO-1 specifically, and their molecular impression on
neurodegenerative diseases (27,28).
The hippocampus has a significant role in the formation of memory
and spatial processing, as well as learning and pattern separation.
An immortalized mouse hippocampal cell line, HT22, which resembles
neuronal cells; however, lacks functional ionotropic glutamate
receptors (29). It is well known
that high concentrations of glutamate do not activate glutamate
receptors in these cells. HT22 cells are thus relevant to the
independent study of oxidative glutamate toxicity (30). In the present study, it was
established that acerogenin C exhibits neuroprotective action
against glutamate-induced cytotoxicity in HT22 cells. Acerogenin C
repressed the generation of ROS induced by glutamate and
glutamate-induced cell death in HT22 cells. Furthermore, the
induction of HO-1 is involved in the cytoprotective effect of
acerogenin C against oxidative stress induced by glutamate in HT22
cells (31). Therefore, it was
investigated whether acerogenin C stimulated HO-1 expression in a
dose-dependent manner and suppressed the generation of ROS induced
by glutamate. As a result, the cytoprotective effect of acerogenin
C may be arbitrated by the induction of HO-1 expression.
The neuroprotective effect of acerogeninC in HT22
cells against cytotoxicity induced by glutamate was investigated.
The results demonstrated that with acerogenin C (10–40 µM) for 12 h
suppressed glutamate-induced cell death in a dose-dependent manner.
In a previous study, expression of HO-1 appeared to serve important
roles in the protection of neuronal cells (7). Therefore, in the present study it was
determined whether acerogenin C-induced HO-1 expression. In HT22
cells, acerogenin C increased HO-1 mRNA expression. To corroborate
the cytoprotective effect associated with acerogenin C-mediated
HO-1 expression, it was investigated whether the effect of
acerogenin C-mediated HO-1 expression was reversed by pretreatment
with an inhibitor (SnPP) of HO-1. Furthermore, it is well-known
that Nrf2 initiates antioxidant protein expression, including HO-1.
It was demonstrated that treatment with acerogenin C induces Nrf2
translocation to the nuclei in HT22 cells and an associated
decrease in Nrf2. Nrf2 siRNA completely inhibited nuclear
translocation. Furthermore, temporary transfection with Nrf2 siRNA
inhibited induction of HO-1 expression by acerogenin C in HT22
cells. These results demonstrated that the upregulation of HO-1
caused by treatment with acerogenin C is associated with the Nrf2
nuclear translocation pathway in HT22 cells. In addition, it was
investigated whether upstream signaling pathways, including
PI3K/Akt, were involved in the regulation of HO-1 protein.
Therefore, it was hypothesized that the mechanism by which
acerogenin C defends against anti-oxidative injury is mediated by
Nrf2.
Nrf2 exhibits neuroprotective effects and serves a
key role in phase II detoxification. Nrf2 a cap‘n’collar
transcription factor that regulates the production of multiple
anti-oxidative enzymes (32,33).
Nrf2 exhibits protective action in different organs and tissues,
including the brain, heart and liver (34–36).
Nrf2 activation attenuates ROS and inhibits glutamate- and
H2O2-induced neurotoxicity to protect
neuronal cells (6,37). Nrf2 is significantly required for
inducible protein expression, including HO-1 expression (38). In the present study, it was
demonstrated that acerogenin C initiates Nrf2 translocation into
the nucleus and suggested that Nrf2 performs a vital function in
the induction of HO-1 by acerogenin C. PI3K/Akt signaling is
associated with the regulation of HO-1 via the activation of the
Nrf2 signaling pathway (39,40).
Stimulation of the PI3K/Akt signaling pathway may be associated
with acerogenin C-induced HO-1 expression.
In conclusion, it was demonstrated that
glutamate-induced oxidative stress was decreased by acerogenin C.
Acerogenin C also exhibits inhibitory effects on ROS production in
HT22 cells. Pretreatment with acerogenin C and LY294002 led to
decreased HO-1 expression in HT22 cells. Therefore, acerogenin C
induced HO-1 expression via the Nrf2 and PI3K/Akt signaling
pathways in HT22 cells. In conclusion, the present study indicates
that acerogenin C may be a potential candidate in the treatment of
different neurodegenerative diseases.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
no. 2015R1C1A1A02036465).
References
|
1
|
Jazwa A and Cuadrado A: Targeting heme
oxygenase-1 for neuroprotection and neuroinflammation in
neurodegenerative diseases. Curr Drug Targets. 11:1517–1531. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mosley RL, Benner EJ, Kadiu I, Thomas M,
Boska MD, Hasan K, Laurie C and Gendelman HE: Neuroinflammation,
Oxidative Stress and the Pathogenesis of Parkinson's Disease. Clin
Neurosci Res. 6:261–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caceres LG, Aon Bertolino L, Saraceno GE,
Zubilete MA Zorrilla, Uran SL, Capani F and Guelman LR:
Hippocampal-related memory deficits and histological damage induced
by neonatal ionizing radiation exposure. Role of oxidative status.
Brain Res. 1312:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conrad M and Sato H: The oxidative
stress-inducible cystine/glutamate antiporter, system × (c) (−):
Cystine supplier and beyond. Amino Acids. 42:231–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim JL, Wilhelmus MM, de Vries HE,
Drukarch B, Hoozemans JJ and van Horssen J: Antioxidative defense
mechanisms controlled by Nrf2: State-of-the-art and clinical
perspectives in neurodegenerative diseases. Arch Toxicol.
88:1773–1786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ouyang Y, Chen Z, Tan M, Liu A, Chen M,
Liu J, Pi R and Fang J: Carvedilol, a third-generation β-blocker
prevents oxidative stress-induced neuronal death and activates
Nrf2/ARE pathway in HT22 cells. Biochem Biophys Res Commun.
441:917–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haines DD, Lekli I, Teissier P, Bak I and
Tosaki A: Role of haeme oxygenase-1 in resolution of oxidative
stress-related pathologies: Focus on cardiovascular, lung,
neurological and kidney disorders. Acta Physiol (Oxf). 204:487–501.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryter SW, Otterbein LE, Morse D and Choi
AM: Heme oxygenase/carbon monoxide signaling pathways: Regulation
and functional significance. Mol Cell Biochem 234–235. 249–263.
2002. View Article : Google Scholar
|
|
9
|
Kim DC, Cho KH, Ko W, Yoon CS, Sohn JH,
Yim JH, Kim YC and Oh H: Anti-inflammatory and cytoprotective
effects of TMC-256C1 from marine-derived Fungus Aspergillus sp.
SF-6354 via up-regulation of heme oxygenase-1 in murine hippocampal
and microglial cell lines. Int J Mol Sci. 17:5292016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee DS, Cha BY, Woo JT, Kim YC and Jang
JH: Acerogenin A from Acer nikoense Maxim prevents oxidative
stress-induced neuronal cell death through Nrf2-mediated heme
oxygenase-1 expression in mouse hippocampal HT22 cell line.
Molecules. 20:12545–12557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagai M, Kubo M, Fujita M, Inoue T and
Matsuo M: Studies on the constituents of Aceraceae plants. II.
Structure of aceroside I, a glucoside of a novel cyclic
diarylheptanoid from Acer nikoense Maxim. Chem Pharm Bull.
26:2805–2810. 1978. View Article : Google Scholar
|
|
12
|
Morikawa T, Tao J, Ueda K, Matsuda H and
Yoshikawa M: Medicinal foodstuffs. XXXI. Structures of new aromatic
constituents and inhibitors of degranulation in RBL-2H3 cells from
a Japanese folk medicine, the stem bark of Acer nikoense. Chem
Pharm Bull (Tokyo). 51:62–67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue T, Ishidate Y, Fujita M, Kubo M,
Fukushima M and Nagai M: Studies on the constituents of Aceraceae
plants. I. Constituents in the leaves and the stem bark of Acer
nikoense Maxim. Yakugaku Zasshi. 98:41–46. 1978.(In Japanese).
|
|
14
|
Furukawa N, Nagumo S, Inoue T and Nagai M:
Studies on the constituents of aceraceae plants VII.
Coumarinolignans from the wood of Acer nikoense. Shoyakugaku
Zasshi. 42:163–165. 1988.
|
|
15
|
Morita H, Deguchi J, Motegi Y, Sato S,
Aoyama C, Takeo J, Shiro M and Hirasawa Y: Cyclic diarylheptanoids
as Na+-glucose cotransporter (SGLT) inhibitors from Acer nikoense.
Bioorg Med Chem Lett. 20:1070–1074. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deguchi J, Motegi Y, Nakata A, Hosoya T
and Morita H: Cyclic diarylheptanoids as inhibitors of NO
production from Acer nikoense. J Nat Med. 67:234–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akazawa H, Akihisa T, Taguchi Y, Banno N,
Yoneima R and Yasukawa K: Melanogenesis inhibitory and free radical
scavenging activities of diarylheptanoids and other phenolic
compounds from the bark of Acer nikoense. Biol Pharm Bull.
29:1970–1972. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue T: Constituents of Acer nikoense and
Myrica rubra. On diarylheptanoids. Yakugaku Zasshi. 113:181–197.
1993.(In Japanese). View Article : Google Scholar
|
|
19
|
Shinoda M, Ohta S, Kumasaka M, Fujita M,
Nagai M and Inoue T: Protective effect of the bark of Acer nikoense
on hepatic injury induced by carbon tetrachloride in rats.
Shoyakugaku Zasshi. 40:177–181. 1986.
|
|
20
|
Kihara T, Ichikawa S, Yonezawa T, Lee JW,
Akihisa T, Woo JT, Michi Y, Amagasa T and Yamaguchi A: Acerogenin
A, a natural compound isolated from Acer nikoense Maxim, stimulates
osteoblast differentiation through bone morphogenetic protein
action. Biochem Biophys Res Commun. 406:211–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cha BY, Wen LS, Kotaro W, Takayuki Y,
Toshiaki T, Kiyotake K, Yuichi I, Shigeru N, Kazuo N and Woo JT:
Antiproliferative activity of acerogenin C, a
macrocyclicdiarylheptanoid, on PDGF-induced human aortic smooth
muscle cells proliferation. Sci Res Publ. 6:47–55. 2015.
|
|
22
|
Kim JS, Kim HJ, Jung CL, Nam DH, Lim JS,
Han MY and Hong YS: Estrogenic activity of acerogenin C isolated
from Acer nikoense Maxim. FASEB J. 25:771.102011.
|
|
23
|
Litvak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)). Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin D, Rojo AI, Salinas M, Diaz R,
Gallardo G, Alam J, De Galarreta CM and Cuadrado A: Regulation of
heme oxygenase-1 expression through the phosphatidylinositol
3-kinase/Akt pathway and the Nrf2 transcription factor in response
to the antioxidant phytochemical carnosol. J Biol Chem.
279:8919–8929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koehn FE and Carter GT: Rediscovering
natural products as a source of new drugs. Discov Med. 5:159–164.
2005.PubMed/NCBI
|
|
26
|
Hald A and Lotharius J: Oxidative stress
and inflammation in Parkinson's disease: Is there a causal link?
Exp Neurol. 193:279–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee DS, Jeong GS, Li B, Park H and Kim YC:
Anti-inflammatory effects of sulfuretin from Rhus verniciflua
Stokes via the induction of heme oxygenase-1 expression in murine
macrophages. Int Immunopharmacol. 10:850–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee DS and Jeong GS: Arylbenzofuran
isolated from Dalbergia odorifera suppresses
lipopolysaccharide-induced mouse BV2 microglial cell activation,
which protects mouse hippocampal HT22 cells death from
neuroinflammation-mediated toxicity. Eur J Pharmacol. 728:1–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao G, Li R, Chen X, Zhang W, Du S and
Yuan Y: Sodium valproate prevents radiation-induced injury in
hippocampal neurons via activation of the Nrf2/HO-1 pathway.
Neuroscience. 331:40–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang B, Liu H, Yue L, Li X, Zhao L, Yang
X, Wang X, Yang Y and Qu Y: Neuroprotective effects of
pterostilbene against oxidative stress injury: Involvement of
nuclear factor erythroid 2-related factor 2 pathway. Brain Res.
1643:70–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li B, Jeong GS, Kang DG, Lee HS and Kim
YC: Cytoprotective effects of lindenenyl acetate isolated from
Lindera strychnifolia on mouse hippocampal HT22 cells. Eur J
Pharmacol. 614:58–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee DS, Ko W, Kim DC, Kim YC and Jeong GS:
Cudarflavone B provides neuroprotection against glutamate-induced
mouse hippocampal HT22 cell damage through the Nrf2 and PI3K/Akt
signaling pathways. Molecules. 19:10818–10831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang B, Liu H, Yue L, Li X, Zhao L, Yang
X, Wang X, Yang Y and Qu Y: Neuroprotective effects of
pterostilbene against oxidative stress injury: Involvement of
nuclear factor erythroid 2-related factor 2 pathway. Brain Res.
1643:70–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sandberg M, Patil J, D'Angelo B, Weber SG
and Mallard C: NRF2-regulation in brain health and disease:
Implication of cerebral inflammation. Neuropharmacology.
79:298–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mann GE: Nrf2-mediated redox signalling in
vascular health and disease. Free Radic Biol Med. 75 Suppl
1:S12014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krajka-Kuźniak V, Paluszczak J and
Baer-Dubowska W: Xanthohumol induces phase II enzymes via Nrf2 in
human hepatocytes in vitro. Toxicol In Vitro. 27:149–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saw CL, Guo Y, Yang AY, Paredes-Gonzalez
X, Ramirez C, Pung D and Kong AN: The berry constituents quercetin,
kaempferol, and pterostilbene synergistically attenuate reactive
oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food
Chem Toxicol. 72:303–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim
HR, Jeon SB, Jeon WK, Chae HJ and Chung HT: Hydrogen sulfide
inhibits nitric oxide production and nuclear factor-kappaB via heme
oxygenase-1 expression in RAW264.7 macrophages stimulated with
lipopolysaccharide. Free Radic Biol Med. 41:106–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alam J, Stewart D, Touchard C, Boinapally
S, Choi AM and Cook JL: Nrf2, a Cap'n'Collar transcription factor,
regulates induction of the heme oxygenase-1 gene. J Biol Chem.
274:26071–26078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elbirt KK, Whitmarsh AJ, Davis RJ and
Bonkovsky HL: Mechanism of sodium arsenite-mediated induction of
heme oxygenase-1 in hepatoma cells. Role of mitogen-activated
protein kinases. J Biol Chem. 273:8922–8931. 1998. View Article : Google Scholar : PubMed/NCBI
|