Introduction
Presently, the primary treatments of cancer include
surgery, radiotherapy, chemotherapy and targeted therapy.
Radiotherapy is the main treatment used for cancer (1). It is widely used for the treatment of
tumors in rectal cancer, non-small cell lung cancer and breast
cancer (2–4).
For example, radiotherapy is the preferred and
primary treatment for nasopharyngeal cancer (NPC) (5). Following radiotherapy, the five-year
survival percentage for patients with NPC has increased from 50–60%
to a current percentage of ~70%. However, following radical
radiotherapy, ~20% of NPC patients still have residual tumors
(6). Local treatment failure and
distant metastasis are the key factors which prevent therapeutic
efficacy, and intrinsic or acquired radiation resistance is one of
the primary reasons causing the failure of radiotherapy and worse
treatment outcomes (7,8).
Thus, it is important to gain further insights into
the molecular mechanisms of chemotherapeutic drug resistance, and
find novel strategies involving improved therapeutic benefits for
cancer patients.
microRNAs (miRNAs) are a class of small RNAs with an
approximate length of 19–25 nucleotides, which are endogenous short
noncoding RNAs (9). miRNAs bind to
the 3′-untranslated region of target mRNAs, followed by degradation
of the mRNA or inhibition of its translation. They may also
regulate the expression of target genes at the post-transcriptional
level (10), and are associated
with regulating cell growth, proliferation, differentiation and
apoptosis (11). miRNAs are
observed in many cancers, and are involved in malignant tumor
development and progression (12).
Several studies have reported that the expressions of miRNAs are
closely associated with the radiosensitivity of tumors, and
upregulated or downregulated expression of miRNAs can increase
tumor radiosensitivity (13). To
address these possibilities, the following study characterized the
effects of miRNAs on the radiation resistance of tumor cells, in
order to provide a biochemical basis for further studies, which may
use this biomarker to predict the radiosensitivity of cancer
patients.
Materials and methods
Cells and cell culture
The CNE-2 cell line, which is suspected to be
identical to CNE-1 and cross-contaminated with HeLa (cervical
carcinoma) as well as an additional unknown cell line (14), was cultured in RPMI 1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 1% penicillin-streptomycin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and grown at 37°C in an incubator with an
atmosphere of 5% CO2.
Development of acquired radioresistant
cells
CNE-2 cells were cultured in 25 cm2
culture flasks. The cells were exposed to a series of increasing
X-ray doses using a medical electronic linear accelerator (Varian
Medical Systems, Palo Alto, CA, USA), in order to establish the
radiation resistant tumor cells (CNE-2R). The distance of the
source was 100 cm, and the radiation field was 10×10 cm at a dose
rate of 300 cGy/min. Cells were irradiated twice with 2 Gy, four
times with 4 Gy, and five times with 6 Gy with X-ray radiation over
a period of 6 months, so that the CNE-2 cells were exposed to a
total dose of 50 Gy. Following several cell passages, the radiation
resistant tumor cells were established.
Apoptosis assay
The cells were radiated with 0 and 4 Gy of
radiation. Following 12 h, many cells were detached with trypsin
without the use of EDTA. Cells were harvested in culture medium and
centrifuged for 5 min at 300 × g, and 1×105 cells were
treated with 5 µl Annexin V/PE and 10 µl 7-AAD (BD Biosciences,
Franklin Lakes, NJ, USA). The cells were gently vortexed and
incubated for 15 min at room temperature in the dark. A total of
400 µl 1X binding buffer was added into solution, the cells were
analyzed using a Gallios flow cytometer (Beckman Coulter, Inc.,
Brea, CA, USA).
Cell cycle assay
The cells were digested with trypsin, and were
centrifuged for 5 min at 300 × g. Then the cells were resuspended
twice with 2 ml PBS, fixed with 1 ml 70% ice cold ethanol, and
stored at 4°C overnight. Then 100 µg/ml RNase and 50 µg/ml PI were
added, and following incubation in the dark for 30 min, the cell
cycle was detected using a Gallios flow cytometer.
Cell viability assay
The cells (5×103) were seeded into
96-well plates. After the cells adhered to the plate, they were
irradiated with different doses of X-ray radiation using 0, 4, 8
and 12 Gy, followed by incubation for 24 h. Medium (100 µl) was
mixed with 10 µl of CCK-8 solution (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan), and a total of 110 µl of the mixed solution
was added to each well, followed by incubation of the cells for 2
h. The absorbance at 450 nm of the cells was then read using a
microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
Colony formation assay
The surviving cells were characterized using a
colony formation assay. Two hundred cells were seeded into six-well
plates. After the cells adhered, they were exposed to X-ray
radiation with single doses of 0, 4, 8 and 12 Gy, and the cells
were incubated for 2 weeks without disturbance. After 2 weeks,
there were >50 cells in each colony. The cells were fixed with
4% paraformaldehyde for 30 min and stained with crystal violet
solution for 30 min, and the colonies counted. The percentage of
cell survival was calculated as follows: The numbers of colonies at
× Gy/(the numbers of cells seeded at a × Gy colony forming
rate).
MicroRNA microarray analysis
CapitalBio Technology Co., Ltd. (Beijing, China)
conducted the present study's Agilent Human miRNA microarrays,
which were 8×60 K microarrays used for detecting miRNA expression
profiles of the CNE-2 and CNE-2R cells. There were many
differential expressions of miRNAs using the microRNA microarray.
Among these differential expressions, miR-210 had a significant
fold change and a high probe signal, so miR-210 was chosen for
further assays. Differences of miR-210 in the CNE-2 and CNE-2R
cells were measured by RT-qPCR.
Overexpression of miR-210 in CNE-2
cells and downregulation of miR-210 in CNE-2R cells
CNE-2R cells were transfected with
LV-hsa-miR-210-inhibitor, which is a lentivirus inhibiting miR-210
loaded with green fluorescent protein (GFP; GeneChem Co., Ltd.,
Shanghai, China), and CNE-2 cells were transfected with
LV-hsa-miR-210 + GFP, which is a lentivirus expressing miR-210
(GeneChem Co., Ltd.). Following infection with the virus, the
infected cells were screened by puromycin for 1 month to establish
stable downregulated and upregulated cells. Overexpression of
miR-210 in CNE-2 cells resulted in CNE-2-miR-210 cells, and
downregulation of miR-210 in CNE-2R cells resulting in
CNE-2R-miR-210-inhibitor cells.
RT-qPCR
Total cellular RNA was extracted with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
then the RNA samples were used for cDNA synthesis using an
All-in-One™ miRNA First-Strand cDNA Synthesis kit
(GeneCopoeia, Inc., Rockville, MD, USA). miR-210 was detected with
RT-qPCR by the All-in-One™ miRNA RT-qPCR kit
(GeneCopoeia, Inc.). U6 was used as the control for detection of
miR-210. The miR-210 RT-qPCR primer was purchased from GeneCopoeia,
Inc. All steps followed the manufacturer's protocols. The results
were calculated using the 2−ΔΔCq method (15).
Statistical analysis
SPSS software (version, 19.0; IBM SPSS, Armonk, NY,
USA) was used for all the statistical analyses. One-way analysis of
variance with Student-Newman-Keuls post hoc tests for multiple
comparisons or a Student's t-test were used for the appropriate
types of data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Radiosensitivity of the CNE-2R
cells
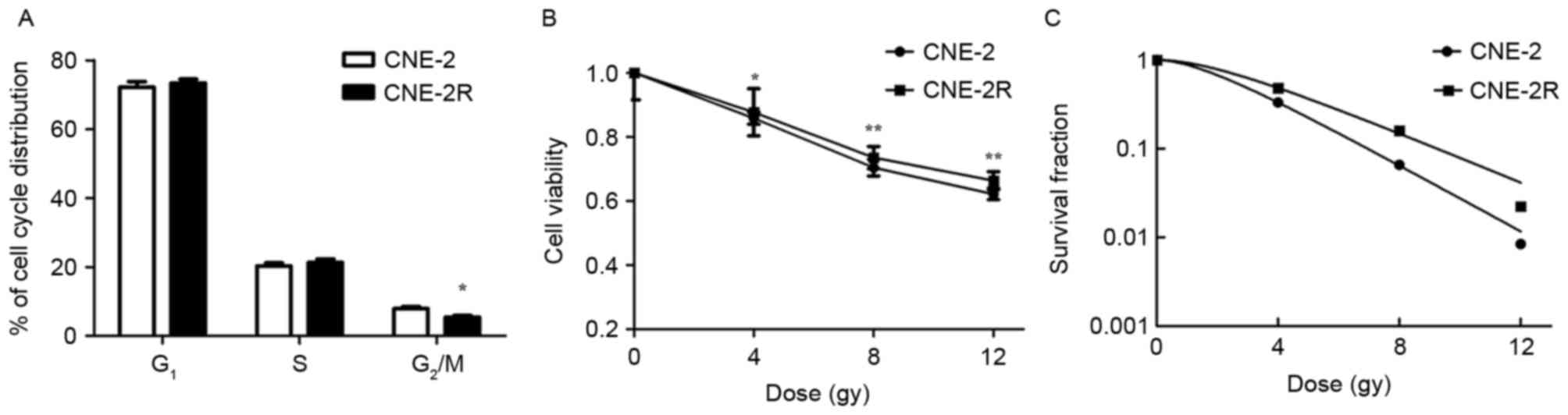
To verify whether the radioresistant tumor cells
were established, the radiosensitivity of the CNE-2R cells was
compared with the parental cells. The percentage of CNE-2 cells in
G2/M phase was significantly greater than CNE-2R cells
(Fig. 1A; P<0.05). At the same
time, determination of cell viabilities indicated that the CNE-2R
cells had lower decreases in viability than the CNE-2 cells when
irradiated with 4, 8 and 12 Gy of radiation (Fig. 1B; P<0.05). Furthermore, the
radiosensitivities of the CNE-2 and CNE-2R cells were compared
using the colony formation assay, which reported that the CNE-2R
cells had a greater percentage of radioresistant cells than the
CNE-2 cells (Fig. 1C). These
results demonstrated that the CNE-2R cells were more radioresistant
than the CNE-2 cells, and that radioresistant tumor cells were
established using the stated protocol.
Expression of miRNAs and RT-qPCR
verification between the CNE-2 cells and CNE-2R cells
Microarray analyses were used to compare the
patterns of miRNA expression between the acquired radioresistant
CNE-2R cells and their parental CNE-2 cells. There were 92 miRNAs
with expression changes greater than 2-fold in CNE-2R cells,
involving 60 miRNAs that were upregulated and 32 miRNAs that were
downregulated (Table I). The
expression of miR-210 in CNE-2R cells was 247.25-fold higher than
the expression in CNE-2 cells (P=0.0006).
 | Table I.Differential expression of miRNAs in
CNE-2 and CNE-2R cells. |
Table I.
Differential expression of miRNAs in
CNE-2 and CNE-2R cells.
| No. | miRNA | Fold
changea (log2) | P-value |
|---|
| 1 | miR-105-5p | 28.179 | 0.0032 |
| 2 | miR-1228-3p | 98.961 | 0.0172 |
| 3 | miR-1228 | 97.514 | 0.0042 |
| 4 | miR-130a-3p | 2.396 | 0.0901 |
| 5 | miR-149-5p | 44.556 | 0.0503 |
| 6 | miR-181d | 43.655 | 0.0082 |
| 7 | miR-182-5p | 35.525 | 0.0260 |
| 8 | miR-192-5p | 107.700 | 0.0081 |
| 9 | miR-194-5p | 40.462 | 0.0206 |
| 10 | miR-197-3p | 2.151 | 0.0095 |
| 11 | miR-210 | 247.250 | 0.0006 |
| 12 | miR-212-3p | 51.685 | 0.0225 |
| 13 | miR-296-5p | 40.781 | 0.0304 |
| 14 | miR-301b | 19.520 | 0.0087 |
| 15 | miR-3132 | 112.260 | 0.0059 |
| 16 | miR-3162-3p | 207.030 | 0.0203 |
| 17 | miR-338-3p | 4.461 | 0.0048 |
| 18 | miR-340-5p | 39.523 | 0.0076 |
| 19 | miR-342-3p | 2.702 | 0.0095 |
| 20 | miR-34a-3p | 48.242 | 0.0207 |
| 21 | miR-34b-3p | 41.055 | 0.0190 |
| 22 | miR-3591-3p | 142.980 | 0.0303 |
| 23 | miR-361-3p | 95.404 | 0.0040 |
| 24 | miR-3653 | 64.164 | 0.0073 |
| 25 | miR-3663-3p | 157.820 | 0.0206 |
| 26 | miR-3940-5p | 52.816 | 0.0104 |
| 27 | miR-4254 | 141.580 | 0.0065 |
| 28 | miR-4433-5p | 106.840 | 0.0081 |
| 29 | miR-4449 | 61.067 | 0.0009 |
| 30 | miR-4478 | 40.268 | 0.0054 |
| 31 | miR-4484 | 65.965 | 0.0070 |
| 32 | miR-451b | 211.190 | 0.0009 |
| 33 | miR-452-5p | 153.160 | 0.0013 |
| 34 | miR-454-3p | 65.327 | 0.0305 |
| 35 | miR-4634 | 81.783 | 0.0097 |
| 36 | miR-4649-3p | 69.214 | 0.0063 |
| 37 | miR-4665-3p | 85.470 | 0.0130 |
| 38 | miR-4701-5p | 142.820 | 0.0066 |
| 39 | miR-4730 | 31.110 | 0.0411 |
| 40 | miR-4787-5p | 64.610 | 0.0225 |
| 41 | miR-484 | 81.568 | 0.0020 |
| 42 | miR-5010-3p | 119.350 | 0.0145 |
| 43 | miR-503-5p | 67.102 | 0.0176 |
| 44 | miR-5100 | 2.127 | 0.0105 |
| 45 | miR-517c-3p | 62.576 | 0.0067 |
| 46 | miR-521 | 58.890 | 0.0112 |
| 47 | miR-522-3p | 108.351 | 0.0054 |
| 48 | miR-532-3p | 65.069 | 0.0092 |
| 49 | miR-572 | 56.282 | 0.0132 |
| 50 | miR-629-3p | 2.002 | 0.0051 |
| 51 | miR-629-5p | 53.444 | 0.0066 |
| 52 | miR-6510-5p | 95.523 | 0.0110 |
| 53 | miR-6512-5p | 2.148 | 0.0098 |
| 54 | miR-6514-3p | 118.251 | 0.0109 |
| 55 | miR-6515-3p | 90.446 | 0.0230 |
| 56 | miR-664b-3p | 64.357 | 0.0080 |
| 57 | miR-6716-3p | 32.462 | 0.0102 |
| 58 | miR-766-3p | 50.140 | 0.0077 |
| 59 | miR-940 | 55.310 | 0.0220 |
| 60 | miR-95 | 2.049 | 0.0035 |
| 61 | miR-1224-5p | 2.115 | 0.0089 |
| 62 | miR-125a-3p | 3.413 | 0.0113 |
| 63 | miR-1268a | 3.235 | 0.0248 |
| 64 | miR-1268b | 2.005 | 0.0016 |
| 65 | miR-1275 | 2.876 | 0.0105 |
| 66 | miR-1287 | 2.052 | 0.0077 |
| 67 | miR-1290 | 4.105 | 0.0080 |
| 68 | miR-135a-3p | 40.335 | 0.0211 |
| 69 | miR-188-5p | 2.468 | 0.0370 |
| 70 | miR-21-3p | 2.590 | 0.0087 |
| 71 | miR-22-5p | 2.110 | 0.0112 |
| 72 | miR-221-3p | 2.293 | 0.0099 |
| 73 | miR-221-5p | 2.174 | 0.0115 |
| 74 | miR-3135b | 3.459 | 0.0063 |
| 75 | miR-3648 | 2.135 | 0.0061 |
| 76 | miR-3656 | 3.438 | 0.0124 |
| 77 | miR-3679-5p | 2.105 | 0.0035 |
| 78 | miR-4298 | 86.930 | 0.0039 |
| 79 | miR-4455 | 7.343 | 0.0091 |
| 80 | miR-4459 | 2.076 | 0.0022 |
| 81 | miR-4466 | 2.209 | 0.0231 |
| 82 | miR-4485 | 2.076 | 0.0103 |
| 83 | miR-4487 | 2.446 | 0.0007 |
| 84 | miR-4532 | 3.176 | 0.0176 |
| 85 | miR-4672 | 2.512 | 0.0233 |
| 86 | miR-483-5p | 91.404 | 0.0145 |
| 87 | miR-5001-5p | 2.079 | 0.0069 |
| 88 | miR-574-5p | 3.520 | 0.0201 |
| 89 | miR-5787 | 6.642 | 0.0088 |
| 90 | miR-6087 | 3.059 | 0.0121 |
| 91 | miR-642a-3p | 2.042 | 0.0084 |
| 92 | miR-663a | 156.200 | 0.0092 |
Downregulation of miR-210 in CNE-2R
cells and overexpression of miR-210 in CNE-2 cells
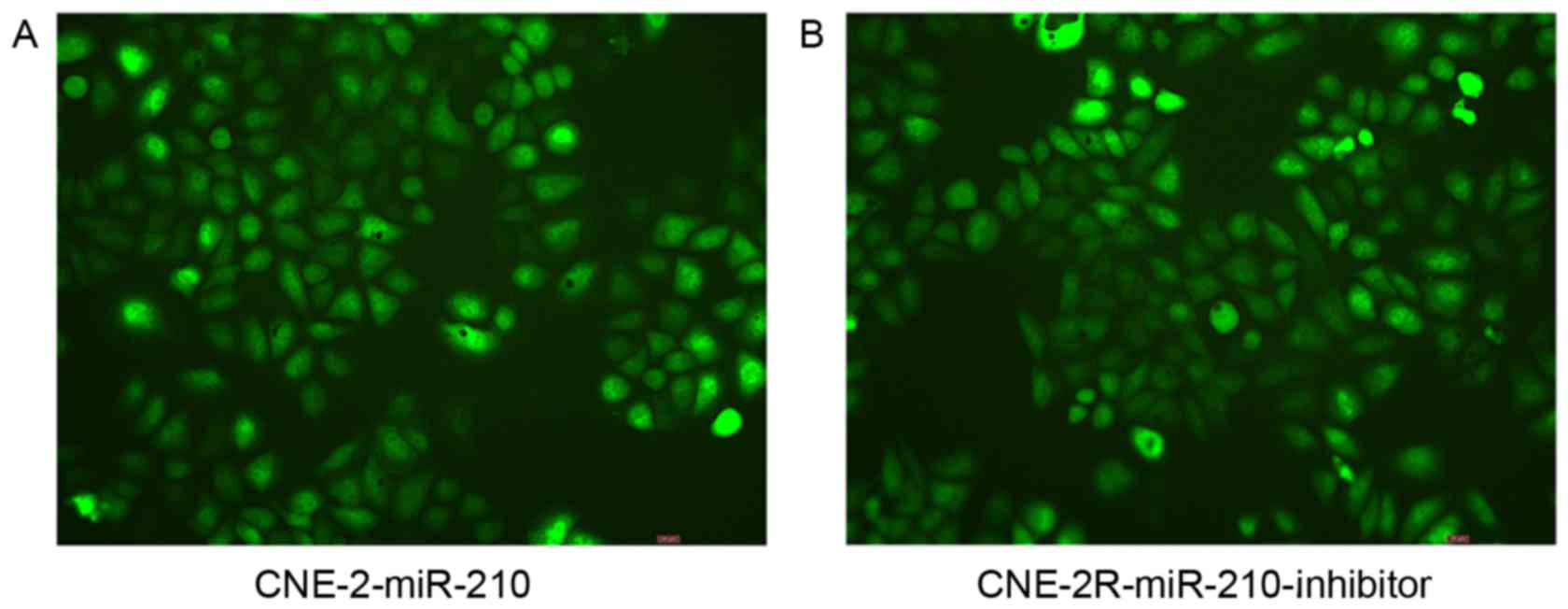
In order to determine the relationship between
miR-210 and radioresistance, the authors transfected LV-hsa-miR-210
into CNE-2 cells (Fig. 2A), and
transfected the LV-hsa-miR-210-inhibitor into CNE-2R cells
(Fig. 2B). Following infection for
72 h, 95% of the CNE-2R cells and 95% of the CNE-2 cells expressed
green fluorescent protein, as assessed with a fluorescence
microscope. Next, the expressions of miR-210 in
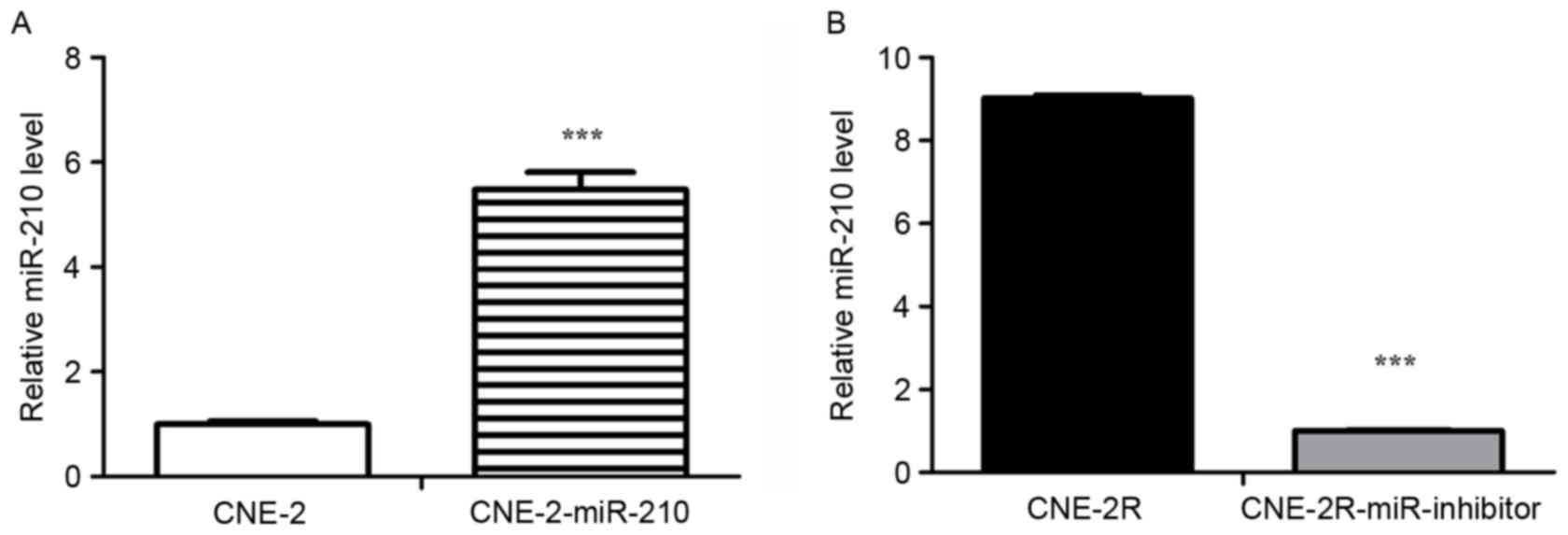
CNE-2R-miR-210-inhibitor and CNE-2-miR-210 cells were measured by
RT-qPCR. The expression of miR-210 in CNE-2-miR-210 cells was
significantly greater than CNE-2 cells (Fig. 3A; P<0.05), and the expression of
miR-210 was significantly lower in CNE-2R-miR-210-inhibitor cells
compared to CNE-2R cells (Fig. 3B;
P<0.05). Consistent with these expected results, downregulation
of miR-210 expression in CNE-2R cells and overexpression in CNE-2
cells was identified.
Correlation of miR-210 expression
levels with cell cycle and apoptosis in tumor cells
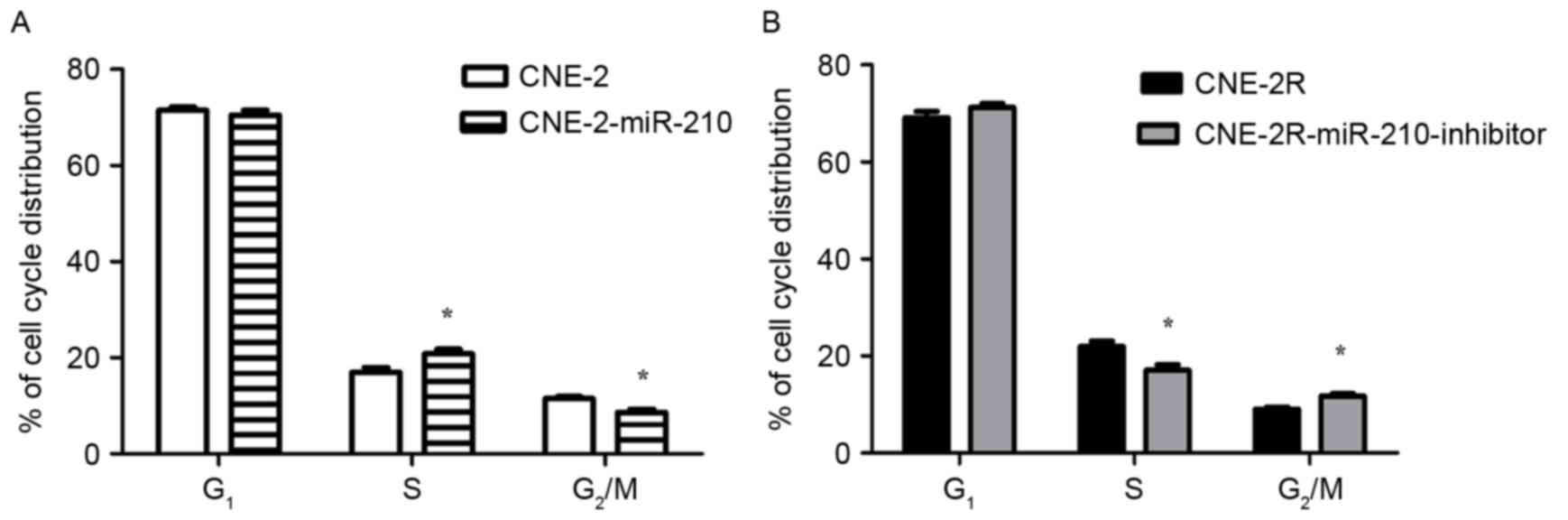
Low expression of miR-210 in CNE-2 cells, there was
a significant decrease in the percentage of cells in S phase, and a
significant increase in the percentage of cells in G2/M
phase, when compared with CNE-2-miR-210 cells (Fig. 4A; P<0.05). In addition to the
low expression of miR-210 in CNE-2R-miR-210-inhibitor cells leading
to a significant decrease in the percentage of cells in S phase,
there was a significant increase in the percentage of
CNE-2R-miR-210-inhibitor cells in G2/M phase when
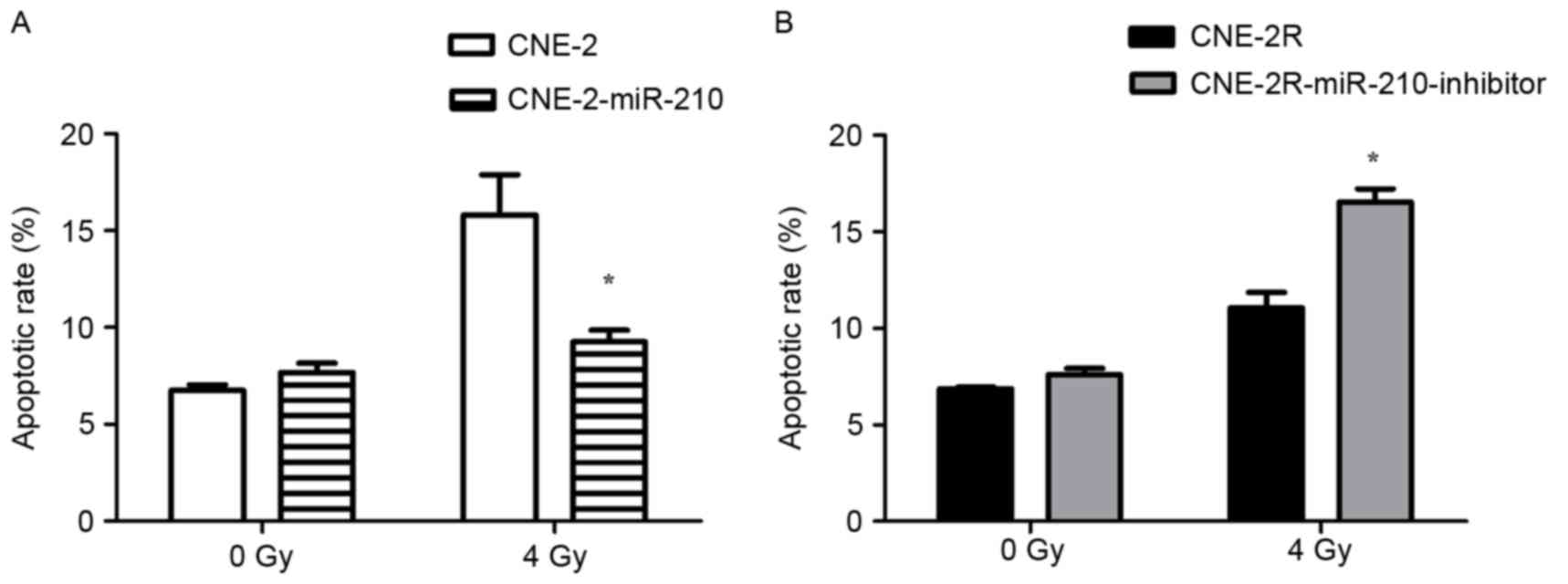
compared with the control CNE-2R cells (Fig. 4B, P<0.05). The authors exposed
cells to 0 or 4 Gy of X-ray radiation, and apoptosis was measured
by flow cytometry. The results of flow cytometry indicated that the
apoptosis percentage of CNE-2-miR-210 cells was significantly lower
than CNE-2 cells (Fig. 5A;
P<0.05), and the apoptosis percentage of
CNE-2R-miR-210-inhibitor cells was significantly greater than
CNE-2R cells (Fig. 5B; P<0.05),
following irradiation by 4 Gy. However, there were no significant
differences in the percentages of apoptosis of these cells in the
absence of irradiation (Fig. 5A and
B).
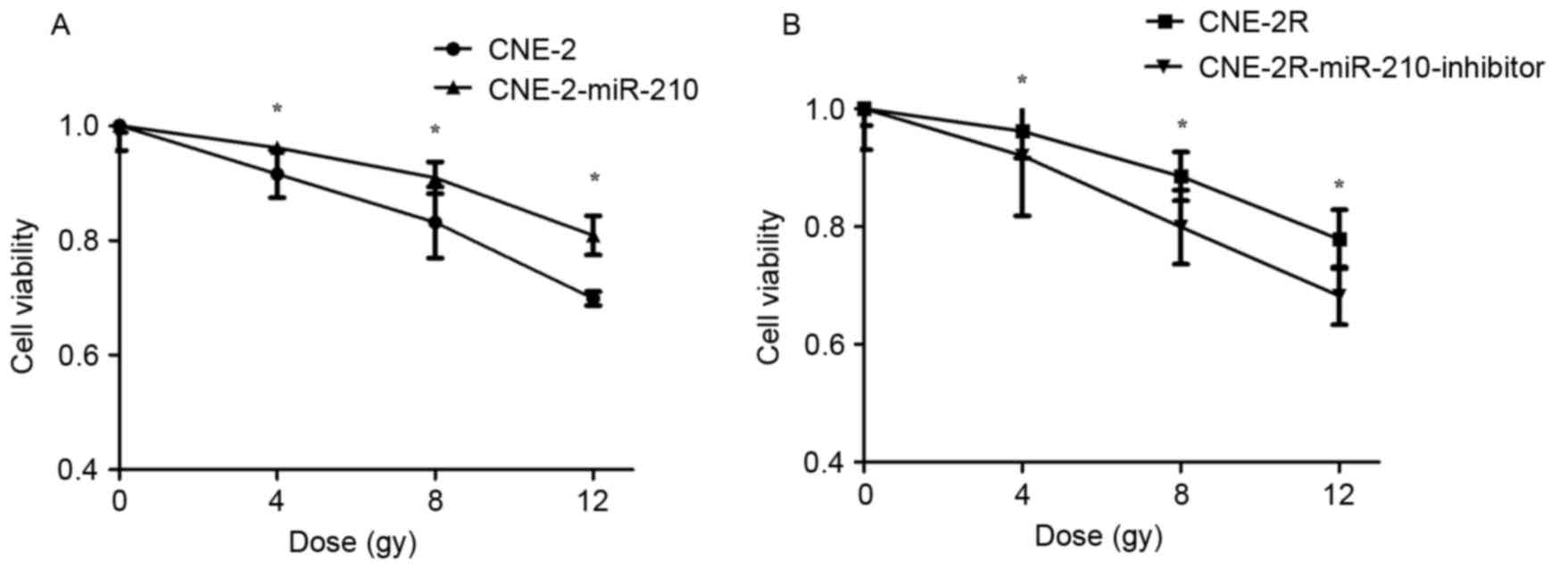
miR-210 negatively regulates cell
viability following irradiation
To determine whether miR-210 negatively regulates
cell viability, a cell viability assay was used to determine the
effect of miR-210 on cell viability with different doses of X-ray
radiation of 0, 4, 8 and 12 Gy. The cell viability of CNE-2 cells
was lower than CNE-2-miR-210 cells when they were radiated with 4,
8 and 12 Gy (Fig. 6A; P<0.05).
The cell viability of CNE-2R cells was higher than
CNE-2R-miR-210-inhibitor cells following being irradiated by 4, 8
and 12 Gy (Fig. 6B; P<0.05),
demonstrating that suppression of miR-210 expression increased the
viability of tumor cells.
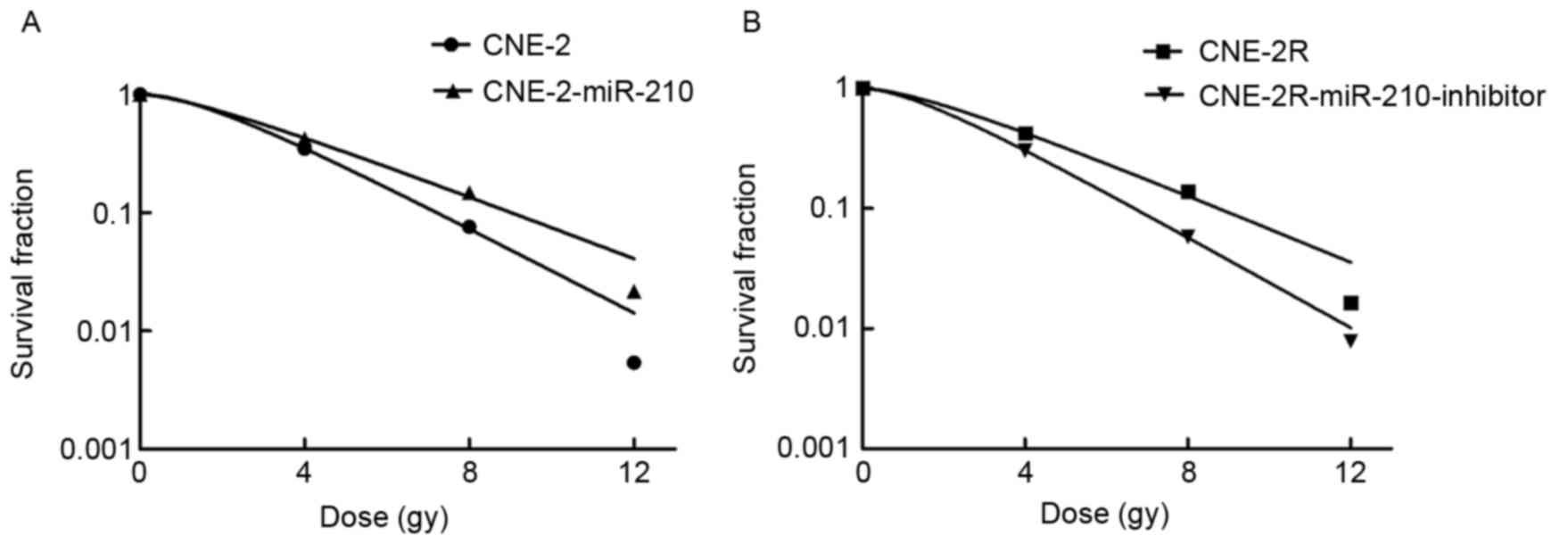
miR-210 promotes the radioresistance
of tumor cells
In order to identify and confirm that miR-210
promotes radioresistance of tumor cells, the authors irradiated
CNE-2, CNE-2R, CNE-2-miR-210 and CNE-2R-miR-210-inhibitor cells
with different doses of X-ray radiation of 0, 4, 8 and 12 Gy, and
determined the cell survival using a colony formation assay
(Fig. 7). As expected, the CNE-2
cells were more radiosensitive than CNE-2-miR-210 cells following
X-ray treatment, and CNE-2R cells were more radioresistant than the
CNE-2R-miR-210-inhibitor cells, confirming that miR-210 can promote
radioresistance of tumor cells.
Target gene analysis
It was predicted that miRNAs exerted their functions
via regulating the expression of the target genes miRbase
(http://mirbase.org/index.shtml),
miRecords (http://mirecords.biolead.org), Tarbase (http://microrna.gr/tarbase) and TargetScan (http://www.targetscan.org). There were many potential
target genes, such as HIF-1α, CTR1A, ADAMTS5 and CAMTA1. In future
studies, the authors will use the luciferase reporter to identify
target genes, and western blot analyses to detect differences in
the translation products of these target genes. Furthermore, it is
planned to characterize the mechanism of target gene
radiosensitivity.
Discussion
Radiotherapy has emerged as the most important
treatment for tumor, because it inhibits tumor growth and prolongs
patient survival (16,17). Radiotherapy, which primarily
involves the induction of DNA damage, is the most common
therapeutic method for many types of cancers (18). Numerous studies have reported that
miRNAs are involved in the control of DNA damage or its repair
mechanisms (19,20). In addition, miRNAs are involved in
various malignant cell behaviors, including radioresistance
(21–24). miR-205, miR-7, miR-100 and miR-101
have been reported to be associated with tumor radioresistance
(25–28). For example, miR-218 is often absent
in cervical cancer, which sensitizes human cervical cancer cells to
radiotherapy by promoting apoptosis (29). miR-148b increases the
radiosensitivity of non-Hodgkin lymphoma (NHL) cells via enhancing
radiation-induced apoptosis, which reported that miR-148b serves a
significant role in the response of NHL to radiation (30). miR-504 is downregulated following
radiotherapy of NPC, which can be regarded as a new radioresistant
biomarker to monitor the tumor response to radiation treatment
(31). Taken together, these
studies have indicated that miRNAs are crucial in mediating the
radioresistance to cancer. However, there have been few reports of
the possible role of miR-210 in radiation resistant tumor cells.
Therefore, the objective of the present study was to investigate
the possible correlation between miR-210 and the radiosensitivity
of tumor cells.
The results demonstrated that miR-210 induced cell
cycle arrest, and cells that had high expressions of miR-210 in the
S phase were significantly decreased while the percentage of cells
in G2/M phase increased. There are significant
differences in the radiosensitivity of cells in the cell cycle. The
radiosensitivity of the G2/M phase is high, and the S
phase is the most radioresistant (32). In addition, increased expression of
miR-210 suppressed cell apoptosis. Similar to the trends observed
for cell viability, the higher the expression level of miR-210, the
higher the cell viability after irradiation. The colony formation
assay confirmed that tumor cells with high levels of miR-210 were
less sensitive to radiation. Based on these results, the expression
of miR-210 is inversely correlated with the radiosensitivity of
tumor cells.
However, the radiation resistance mechanism of
miR-210 remains unclear (33). It
has been reported that vascular endothelial growth factor (VEGF) is
one of the targets of miRNA-210 (34). VEGF can contribute to
tumorigenesis, by promoting angiogenesis and enhancing the blood
supply (35). Moreover, miR-210
has been reported to be the most highly induced miRNA in hypoxic
cells (36), and hypoxia reduces
the radiosensitivity of tumor cells both in vitro and in
vivo (37). Previous studies
have reported that some miRNAs can regulate the DNA damage response
to radiation and participate in DNA repair pathways (38,39),
but the mechanisms involved in radioresistance, angiogenesis,
apoptosis, cell cycle control and DNA damage/repair are still
unknown. Further studies are needed to identify the binding sites
of miR-210 and characterize signaling pathways of miR-210.
Taken together, the presented findings provide
insight into improving tumor radiosensitivity, and strongly suggest
that miR-210 expression is negatively associated with the
radiosensitivity of tumor cells. In addition, the results
demonstrated that miR-210 may be used to predict the therapeutic
effects of radiotherapy in cancer patients, suggesting that miR-210
could be a prognostic biomarker for cancer patients with
radiotherapy, and may provide novel insights into the
identification of therapeutic targets.
Acknowledgments
The current work was supported by Natural Science
Foundation Project of Chongqing (grant no. cstc2012jjA10138), and
the Oncology of National Clinical Specialist Project (grant no.
[2013] 544).
References
|
1
|
Tamura M, Ito H and Matsui H: Radiotherapy
for cancer using X-ray fluorescence emitted from iodine. Sci Rep.
7:436672017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Serbanescu GL, Gruia MI, Bara M and Anghel
RM: The evaluation of the oxidative stress for patients receiving
neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J
Med Life. 10:992017.PubMed/NCBI
|
|
3
|
Yoon SM, Shaikh T and Hallman M:
Therapeutic management options for stage III non-small cell lung
cancer. World J Clin Oncol. 8:1–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Son SH, Choi KH and Kim SW: Dosimetric
comparison of simultaneous integrated boost with whole-breast
irradiation for early breast cancer. PLoS One. 12:e01735522017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang N, Liang SB, Deng YM, Lu RL, Chen
HY, Zhao H, Lv ZQ, Liang SQ, Yang L, Liu DS and Chen Y: Primary
tumor regression speed after radiotherapy and its prognostic
significance in nasopharyngeal carcinoma: A retrospective study.
BMC cancer. 14:1362014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen ZT, Liang ZG and Zhu XD: A Review:
Proteomics in Nasopharyngeal Carcinoma. Int J Mol Sci.
16:15497–15530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen MY, Jiang R, Guo L, Zou X, Liu Q, Sun
R, Qiu F, Xia ZJ, Huang HQ, Zhang L, et al: Locoregional
radiotherapy in patients with distant metastases of nasopharyngeal
carcinoma at diagnosis. Chin J Cancer. 32:604–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutajulu SH, Kurnianda J, Tan IB and
Middeldorp JM: Therapeutic implications of Epstein-Barr virus
infection for the treatment of nasopharyngeal carcinoma. Ther Clin
Risk Manag. 10:721–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedersen CC, Refsgaard JC, Østergaard O,
Jensen LJ, Heegaard NH, Borregaard N and Cowland JB: Impact of
microRNA-130a on the neutrophil proteome. BMC immunol. 16:702015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Dong G, Wang B, Gao W and Yang Q:
miR-543 promotes gastric cancer cell proliferation by targeting
SIRT1. Biochem Biophys Res Commun. 469:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee D, Sun S, Zhang XQ, Zhang PD, Ho AS,
Kiang KM, Fung CF, Lui WM and Leung GK: MicroRNA-210 and
endoplasmic reticulum chaperones in the regulation of
chemoresistance in glioblastoma. J Cancer. 6:227–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Han W, Ni TT, Lu L, Huang M, Zhang
Y, Cao H, Zhang HQ, Luo W and Li H: Knockdown of microRNA-1323
restores sensitivity to radiation by suppression of PRKDC activity
in radiation-resistant lung cancer cells. Oncol Rep. 33:2821–2828.
2015.PubMed/NCBI
|
|
14
|
Chan SY, Choy KW, Tsao SW, Tao Q, Tang T,
Chung GT and Lo KW: Authentication of nasopharyngeal carcinoma
tumor lines. Int J Cancer. 122:2169–2171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi RC, Meng AF, Zhou WL, Yu XY, Huang XE,
Ji AJ and Chen L: Effects of home nursing intervention on the
quality of life of patients with nasopharyngeal carcinoma after
radiotherapy and chemotherapy. Asian Pac J Cancer Prev.
16:7117–7121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo P, Lan J, Ge J, Nie Q, Guo L, Qiu Y
and Mao Q: MiR-26a enhances the radiosensitivity of glioblastoma
multiforme cells through targeting of ataxia-telangiectasia
mutated. Exp Cell Res. 320:200–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Czochor JR and Glazer PM: microRNAs in
cancer cell response to ionizing radiation. Antioxid Redox Signal.
21:293–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Metheetrairut C and Slack FJ: MicroRNAs in
the ionizing radiation response and in radiotherapy. Curr Opin
Genet Dev. 23:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su H, Jin X, Zhang X, Xue S, Deng X, Shen
L, Fang Y and Xie C: Identification of microRNAs involved in the
radioresistance of esophageal cancer cells. Cell Biol Int.
38:318–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Zhang J, Zhang L, Zhu Z, Fan J,
Chen L, Zhuang L, Luo J, Chen H, Liu L, et al: MicroRNA 23b
regulates autophagy associated with radioresistance of pancreatic
cancer cells. Gastroenterology. 145:1133–1143.e12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F,
Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z and Chun-Sheng
K: MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell
proliferation and radioresistance by targeting PTEN. BMC cancer.
10:3672010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, Wang Y, Dong R, Huang X, Ding S and
Qiu H: Circulating microRNA-218 was reduced in cervical cancer and
correlated with tumor invasion. J Cancer Res Clin Oncol.
138:671–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: MiR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee KM, Choi EJ and Kim IA: microRNA-7
increases radiosensitivity of human cancer cells with activated
EGFR-associated signaling. Radiother Oncol. 101:171–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi W, Alajez NM, Bastianutto C, Hui AB,
Mocanu JD, Ito E, Busson P, Lo KW, Ng R, Waldron J, et al:
Significance of Plk1 regulation by miR-100 in human nasopharyngeal
cancer. Int J Cancer. 126:2036–2048. 2010.PubMed/NCBI
|
|
28
|
Yan D, Ng WL, Zhang X, Wang P, Zhang Z, Mo
YY, Mao H, Hao C, Olson JJ, Curran WJ and Wang Y: Targeting
DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS
One. 5:e113972010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan W, Xiaoyun H, Haifeng Q, Jing L,
Weixu H, Ruofan D, Jinjin Y and Zongji S: MicroRNA-218 enhances the
radiosensitivity of human cervical cancer via promoting radiation
induced apoptosis. Int J Med Sci. 11:691–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Y, Liu GL, Liu SH, Wang CX, Xu YL, Ying
Y and Mao P: MicroRNA-148b enhances the radiosensitivity of
non-Hodgkin's Lymphoma cells by promoting radiation-induced
apoptosis. J Radiat Res. 53:516–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao L, Tang M, Hu Z, Yan B, Pi W, Li Z,
Zhang J, Zhang L, Jiang W, Li G, et al: miR-504 mediated
down-regulation of nuclear respiratory factor 1 leads to
radio-resistance in nasopharyngeal carcinoma. Oncotarget.
6:15995–16018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmad TA Tengku, Jaafar F, Jubri Z, Rahim
K Abdul, Rajab NF and Makpol S: Gelam honey attenuated
radiation-induced cell death in human diploid fibroblasts by
promoting cell cycle progression and inhibiting apoptosis. BMC
Complement Altern Med. 14:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
In Lee S, Ji MR, Jang YJ, Jeon MH, Kim JS,
Park JK, Jeon IS and Byun SJ: Characterization and miRNA-mediated
posttranscriptional regulation of vitelline membrane outer layer
protein I in the adult chicken oviduct. In Vitro Cell Dev Biol
Anim. 51:222–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu SC, Chuang SM, Hsu CJ, Tsai CH, Wang
SW and Tang CH: CTGF increases vascular endothelial growth
factor-dependent angiogenesis in human synovial fibroblasts by
increasing miR-210 expression. Cell Death Dis. 5:e14852014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Osugi J, Kimura Y, Owada Y, Inoue T,
Watanabe Y, Yamaura T, Fukuhara M, Muto S, Okabe N, Matsumura Y, et
al: Prognostic impact of hypoxia-inducible miRNA-210 in patients
with lung adenocarcinoma. J Oncol. 2015:3167452015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harriss-Phillips WM, Bezak E and Yeoh E:
Monte Carlo radiotherapy simulations of accelerated repopulation
and reoxygenation for hypoxic head and neck cancer. Br J Radiol.
84:903–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gasparini P, Lovat F, Fassan M, Casadei L,
Cascione L, Jacob NK, Carasi S, Palmieri D, Costinean S, Shapiro
CL, et al: Protective role of miR-155 in breast cancer through
RAD51 targeting impairs homologous recombination after irradiation.
Proc Natl Acad Sci USA. 111:4536–4541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu YJ, Lin YF, Chen YF, Luo EC, Sher YP,
Tsai MH, Chuang EY and Lai LC: MicroRNA-449a enhances
radiosensitivity in CL1-0 lung adenocarcinoma cells. PLoS One.
8:e623832013. View Article : Google Scholar : PubMed/NCBI
|