Introduction
Gangliosides belong to a heterogeneous family of
lipids known as glycosphingolipids, which are ubiquitously
expressed in vertebrate cells, and are particularly abundant in the
central nervous system (1). Within
mammalian cells, gangliosides are predominantly localized on the
plasma membrane (2,3), where they form cell surface
microdomains, including caveolae, lipid rafts, and
glycolipid-enriched microdomains or cholesterol (2,4,5).
Specifically, gangliosides are established to have various
functions, including in cell proliferation, differentiation, immune
response, adhesion, migration, apoptosis, and cell-cell and
cell-substratum interactions (6).
Gangliosides are classified by the presence of one or more sialic
acid residues linked to different galactose and/or sialic acid
residues, and are classified into asialo (o)-, a-, b- and c-series
gangliosides, respectively (7).
Stem cells are widely used during research into
developmental processes and offer tremendous potential in clinical
applications for transplantation and tissue regeneration therapies
(8). As they are undifferentiated,
stem cells, specifically embryonic stem cells (ESCs), have a high
potential for proliferation (self-renewal) and the capacity to
differentiate into various distinct cell types (multipotency or
pluripotency) (9). Induced
pluripotent stem cells (iPSCs) have been generated from mouse
fibroblasts via retroviral introduction of four defined
transcription factors: POU domain, class 5, transcription factor
1 (Oct-4), SRY (sex determining region Y)-box 2
(Sox-2), c-Myc and Kruppel-like factor 4
(Klf-4) (10). Induction of
pluripotency can be also achieved in mouse spermatogonial stem
cells by self-reprogramming process (11,12).
Reprogrammed pluripotent stem cells, such as iPSCs and
germline-derived pluripotent stem cells, are indistinguishable from
ESCs in terms of morphology, self-renewal, expression of ESC
markers, and their differentiation ability (10,11,13,14).
Neural stem cells (NSCs) are known to be self-renewing, multipotent
cells that can differentiate into brain-forming cells, such as
neurons and glial cells (astrocytes and oligodendrocytes) (15). NSCs highly express nestin, Musashi
RNA-binding protein 1 (Musashi-1), Sox-2 and paired box 6
(Pax-6).
Stage-specific embryonic antigens
(SSEA)
Carbohydrate-associated molecules are known to be
involved in controlling cell surface interactions during
development. Specifically, SSEA series were originally identified
by defined carbohydrate epitopes associated with the lacto- and
globo-series glycolipids, such as SSEA-1, SSEA-3 and SSEA-4
(16,17). These SSEA series were expressed in
various tissues, cancer and cancer stem cells (18–23).
Notably, SSEA series are present in pluripotent stem cells, such as
ESCs and iPSCs. SSEA-1 (also termed CD15 and Lewis x) is present on
the surface of murine embryos at the pre-implantation stage, in
mouse germ cells and on the surface of teratocarcinoma stem cells
(24). SSEA-1 is also produced in
the thyroid, oviduct epithelium, endometrium and epididymis, and in
certain areas of the brain and kidney tubules in adults (18,25).
SSEA-1 production increases upon differentiation in human cells and
decreases during differentiation in mice.
SSEA-3 and SSEA-4 are synthesized during oogenesis
and are present in the membranes of oocytes, zygotes and early
cleavage-stage embryos in human (24,26).
They are present in undifferentiated primate ESCs, human embryonic
germ cells, teratocarcinoma stem cells and ESCs (27).
Biosynthesis of gangliosides
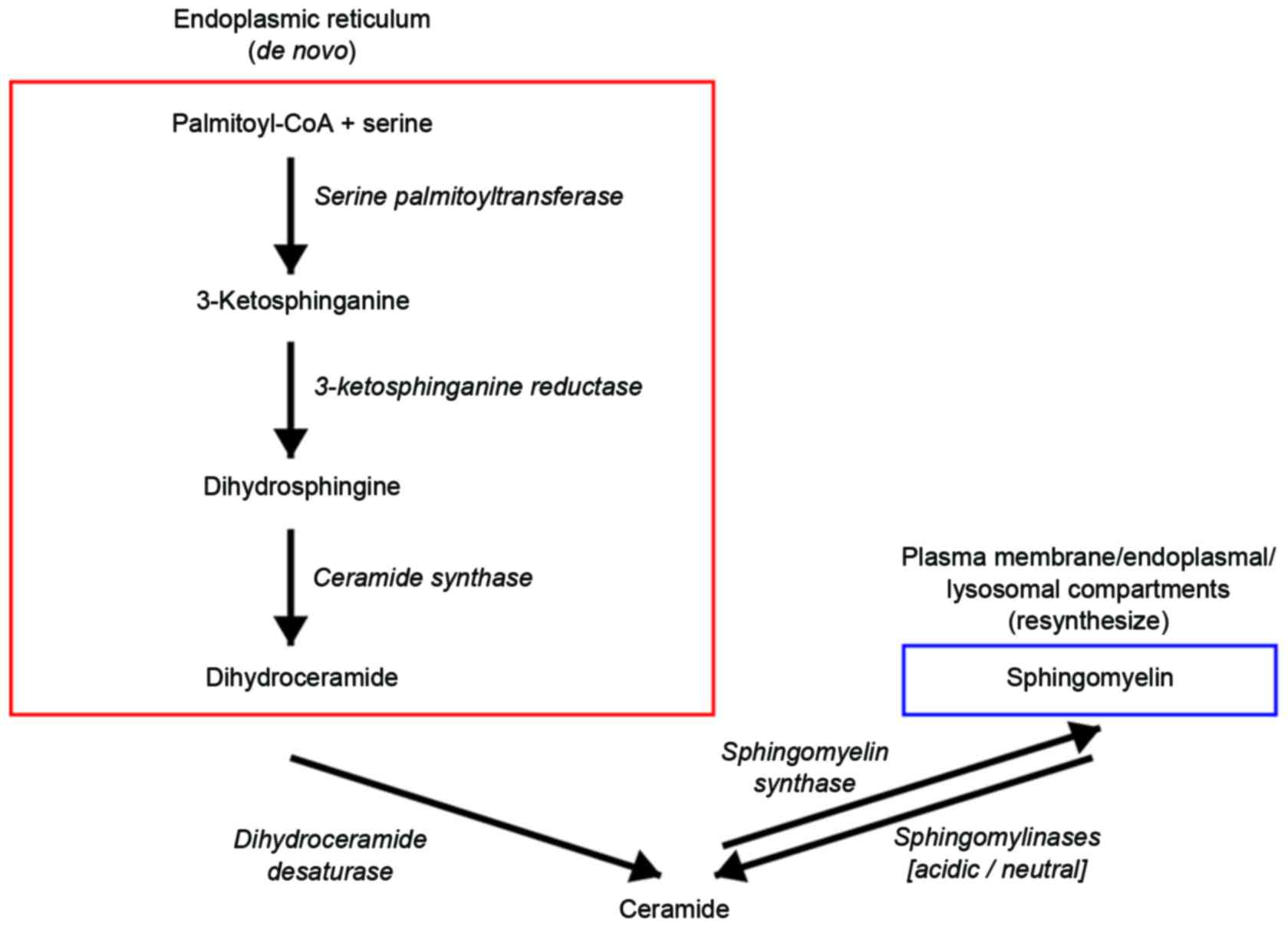
Ganglioside biosynthesis and degradation occurs
through several events: i) De novo ganglioside biosynthesis
in the endoplasmic reticulum and Golgi apparatus, followed by
vesicular sorting to the plasma membrane; ii) enzyme-assisted
chemical modifications of molecules at the plasma membrane level;
iii) internalization of gangliosides via endocytosis and recycling
to the plasma membrane; iv) direct glycosylations following sorting
from endosomes to the Golgi apparatus; v) degradation at the late
endosomal/lysosomal level with formation of fragments of sugars
(glucose, galactose, hexosamine, sialic acid) and lipids (ceramide,
sphingosine, fatty acid); vi) metabolic recycling of these
fragments for biosynthetic purposes (salvage pathways); and vii)
further degradation of fragments to waste products (Fig. 1) (28).
Ceramides, a group of higher glycosphingolipids, are
glucosylated by a glucosyl-transferase (29–31).
An uncharacterized flippase enzyme caused Glc-ceramide to flip to
the lumenal side of the cis-Golgi stack, where further
glycosylations takes place. The first glycosylation, catalyzed by
lactosyl (Lac)-ceramide synthase is galactosylation of Glc-ceramide
to Lac-ceramide (Fig. 2) (32–34).
Lac-ceramide is sialosylated to produce GM3, GD3 and GT3 molecules
via the action of three sialyltransferases (SAT) I, II and III,
each recognizing their specific acceptor substrate (35,36).
GM3, GD3 and GT3 are the starting points for the ‘a-series’,
‘b-series’ and ‘c-series’ gangliosides, respectively. In each
ganglioside series, N-acetyl-galactosaminyltransferase,
galactosyl-transferase and SAT IV, in sequence, add an
N-acetylgalactosamine, galactose and sialic acid group to the
gangliosides, respectively, to produce more complex gangliosides.
Further sialosylations can be performed by SAT V. From Lac-ceramide
a further group of glycosphingolipids (‘O-series’) can be produced
by the sequential action of N-acetyl-galactosaminyltransferase,
galactosyl-transferase and sialyl-transferase IV and V, producing
asialo-GM2 (GA2), asialo-GM1 (GA1), and gangliosides GM1b, GD1c and
GD1α (28).
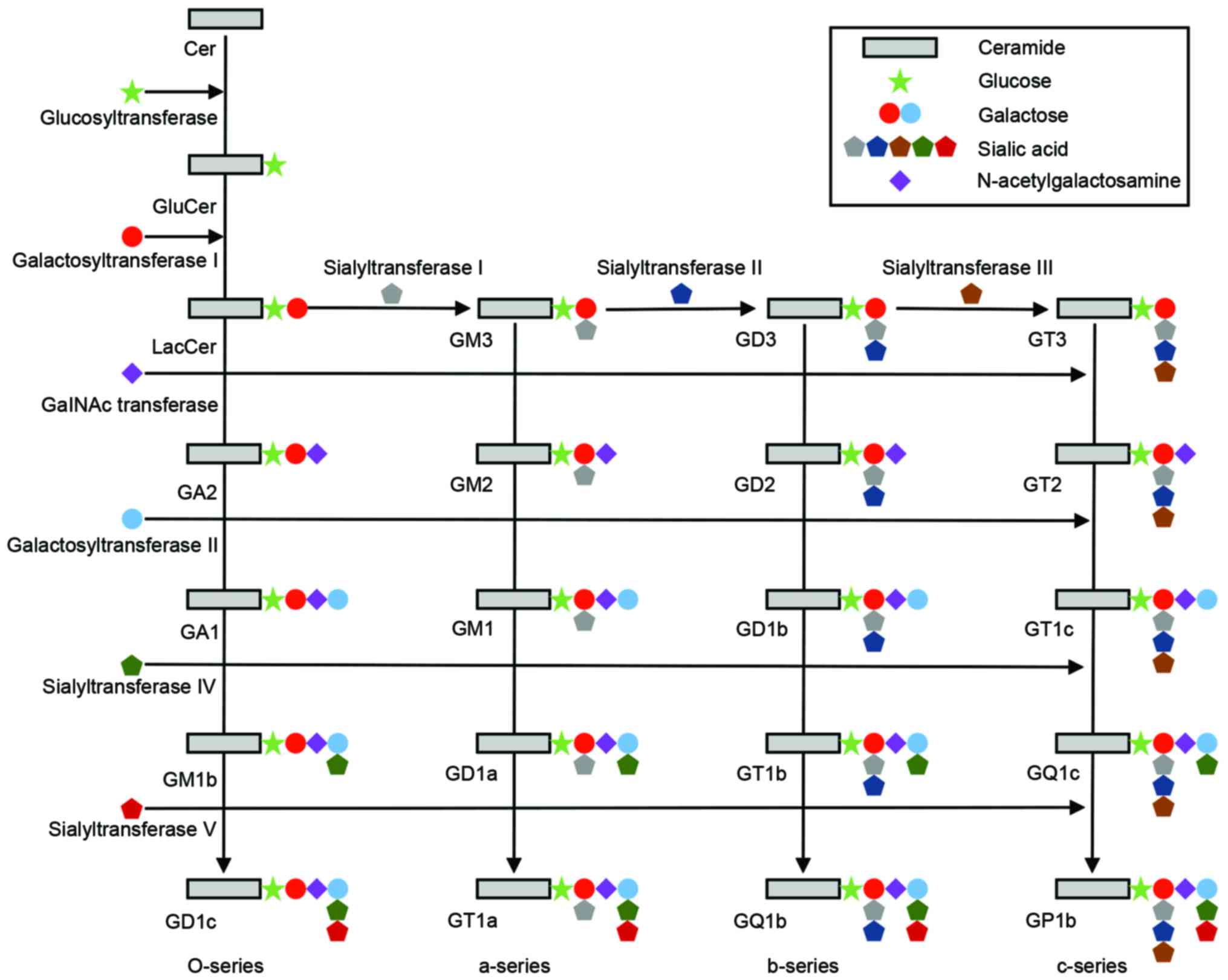 | Figure 2.Schematic diagram of the ganglioside
biosynthetic pathways. The o-series (GA2, GA1, GM1b and GD1c), the
a-series (GM3, GM2, GM1, GD1a and GT1a), the b-series (GD3, GD2,
GD1b, GT1b and GQ1b) and the c-series (GT3, GT2, GT1c, GQ1c and
GP1c), and the corresponding glucosyltransferase,
galactosyltransferases, GalNAc transferase, and sialyltransferases
are shown. Cer, ceramide. |
Expression patterns of gangliosides in mouse
pluripotent stem cells
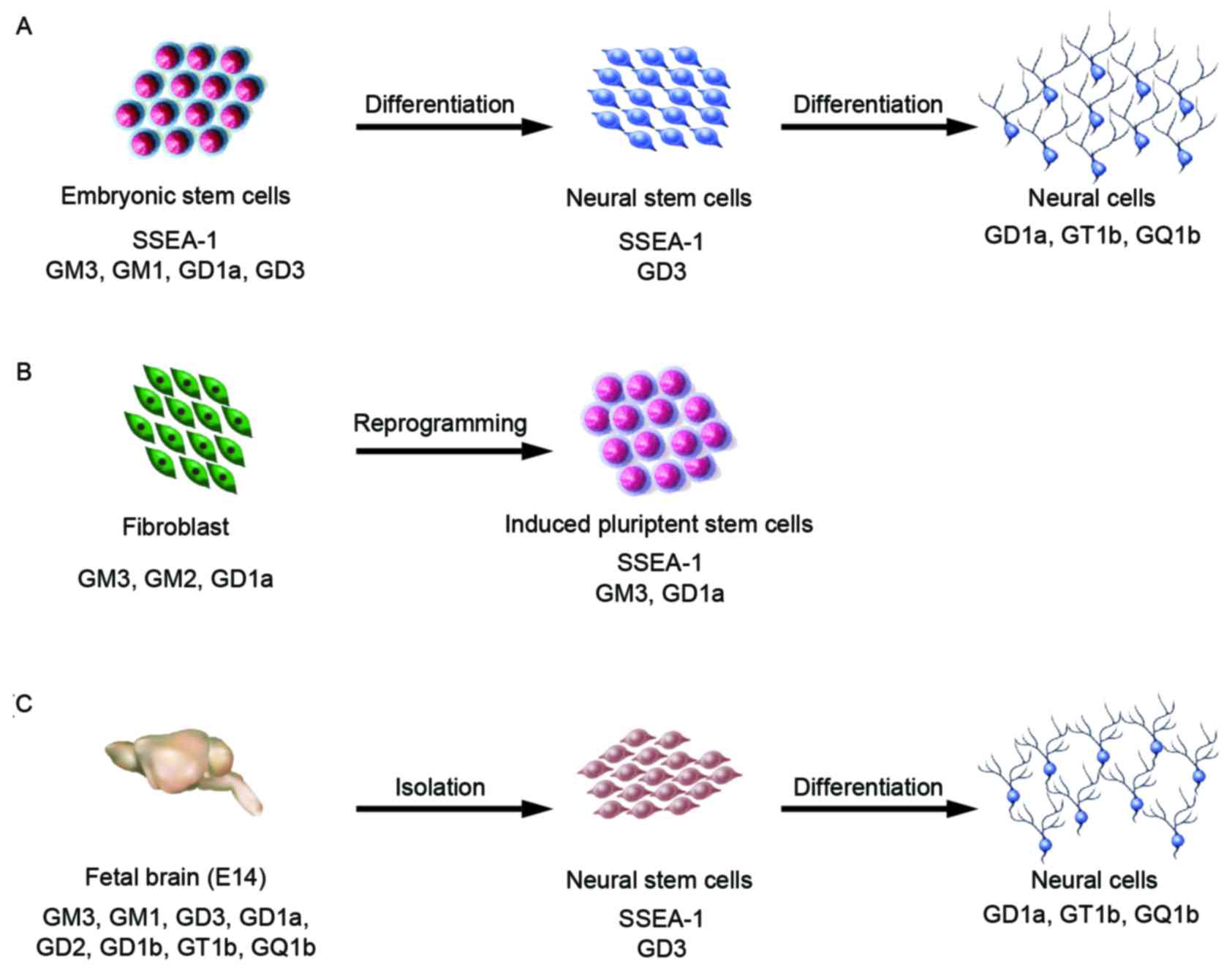
Mouse embryonic stem cells (mESCs) are derived from
the inner cell mass of blastocysts (37). Established mESCs express various
carbohydrate antigens, including glycolipids. Among those, SSEA-1
is the most well-established specific marker.
In E14 and Oct-4 promoter-EGFP (OG2) mESCs, small
amounts of a-series gangliosides, GM3, GM1 and GD1a, were detected
by high-performance thin-layer chromatography and
immunocytochemistry analysis (38,39).
Furthermore, in TC-1 mESCs, only glucosylceramide and
lactosylceramide were present (40); however, J1 mESCs contained GM3,
GM1, and GD3 (Fig. 3A) (41–43).
Furthermore, 9-O acetyl GD3 was detected in 129S6/B6-F1/DsRed.T3
mESCs (44). GM3 and GD3 are known
to be involved in cell adhesion and proliferation via
mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinases (ERK) 1/2 phosphorylation (Table I) (41,42,45,46).
Specifically, small hairpin RNA knock-down of UDP-glucose ceramide
glucosyltransferase (UGCG) to reduce glucosylceramide
synthesis was demonstrated to inhibit activation of the Ras-MAPK
pathway and cell proliferation (41).
 | Table I.Function and role of gangliosides in
mouse stem cells. |
Table I.
Function and role of gangliosides in
mouse stem cells.
| Ganglioside | Function/role |
|---|
| GM3 | Cell adhesion,
proliferation and neural differentiation, induction of neural
precursor cells, facilitates neurite formation, neural maturation,
activates ERK1/2 MAPK phosphorylation |
| GM1 | Promotes neural
differentiation, regulate neurogenesis and regeneration, protects
against apoptosis, cell proliferation, activates ERK1/2 MAPK
phosphorylation |
| GD1a | Induction of early
neural differentiation |
| GD3 | Induction of early
neural differentiation, brain development, cell adhesion and
proliferation, neural maturation, facilitates neurite formation,
activates ERK1/2-MAPK phosphorylation, induction of neural
precursor cells, neural stem cell markers |
| GT1b | Necessary for
induction neural differentiation, enhances actin-rich dendrite
generation, increased in brain synapses |
| GQ1b | Neurite outgrowth
during early neural differentiation, neural differentiation through
the ERK1/2-MAPK pathway |
| SSEA-1 | Expression on pre-
and post-implantation mouse embryo and teratocarcinoma cells,
expression in thyroid tissue, expression in human renal tumors |
| SSEA-3 | Expression in human
teratocarcinoma cells, expression in colorectal cancer, significant
markers for breast cancer stem cells |
| SSEA-4 | Expression in human
teratocarcinoma cells, expression in oral cancer cell, expression
in basaloid lung cancer |
OG2 mouse embryonic fibroblast (MEF) and mESCs
produce GM3, GM1, and GD1a, but GM1 is not found in OG2 MEF-derived
iPSCs (Fig. 3B) (39). Analysis of the cell proliferation
rate in OG2 mESCs and iPSCs revealed that iPSCs have a lower
proliferation than that of mESCs. GM1 is known to affect cell
proliferation via the ERK 1/2-MAPK pathway and protects against
apoptosis in various cell types (Table
I) (39,42,47–49).
Ganglioside patterns in mouse neural stem
cells (mNSCs)
NSCs, also referred to as multipotent neural
progenitor cells, can differentiate to cells of the neural linage,
including neurons and glial cells (astrocytes and oligodendrocytes)
(50). The NSCs highly express
nestin, Musashi-1 and Sox-2; however, these marker molecules are
intracellular or nuclear proteins. Several studies demonstrated
that there are high levels of b-series gangliosides in NSCs
(51–55). GD3 is a b-series disialoganglioside
that is frequently detected in vertebrate embryos and immature
proliferative cells (56,57). Particularly, GD3 and 9-O acetyl GD3
have been biochemically detected in mNSCs (44,53,58).
GD3 is present in the subventricular zone of the lateral ventricle
where NSCs are localized (59,60).
Furthermore, GD3 expression in mouse neuroepithelial cells is
enriched in NSCs, radial glia, embryonic-, postnatal-, and
adult-NSCs (Fig. 3C) (53,58,61).
Furthermore, NSCs differentiated from mESCs also express GD3
(unpublished data) (Fig. 3A).
The GD3 concentration is known to be high in
embryonic brains, which predominantly consist of undifferentiated
neural progenitor cells; however, the concentration in the brain
rapidly decreases after birth (62). It has been demonstrated that NSCs
derived from GD3-synthase knockout mice could not be maintained in
in vitro culture (55);
thus, it is speculated that GD3 has a major role in the maintenance
of NSCs (51,54,63).
It has been reported that when the GD3 level was reduced by an
inhibitor of glucosylceramide synthesis, basic fibroblast growth
factor-induced proliferation was repressed in primary mNSCs.
Additionally, GD3 interacts with epidermal growth factor receptor
(EGFR) (55,64). These finding imply that GD3 may
induce early neural precursor cell differentiation and neurite
formation (Table I) (41,43).
Expression patterns of gangliosides in
differentiated neural cells
The pattern of ganglioside synthesis changes
dramatically during nervous system development (65–67).
Thus, gangliosides, including sulfatide (for the myelin sheath in
the peripheral and central nerve system), galactosylceramide (for
oligodendrocytes) and A2B5 (c-series gangliosides; for neural stem
cells, oligodendrocytes, and astrocytes) are considered to be
useful as differentiation markers of specific neural lineages
(68). It was reported previously
that the GD3 level is high in the brain and is involved in
embryonic brain development; however, its concentration rapidly
decreases during neural development, whereas other gangliosides,
including GD1a, GT1b, and GQ1b, increase during aging and neural
development (62). In addition, it
was demonstrated that correlative changes of ganglioside
composition accompany normal development in vitro and in
vivo. Furthermore, b-series gangliosides, including GT1b and
GQ1b, are present in mouse neuroepithelial cells (58,69).
Our previous studies demonstrated that GD3, GT1b,
and GQ1b are present in cells during retinoic acid-induced neural
differentiation of mESCs and embryonic carcinoma cells (Fig. 3A) (42,43).
A number of approaches have been reported to determine the role of
gangliosides during neural differentiation (70,71).
Overproduction of gangliosides can facilitate neurite formation,
which is part of the neural maturation process (43). By contrast, knock-down of
Ugcg was reported to result in a decrease in the neural
differentiation rate of mESCs and human dental pulp-derived
mesenchymal stem cells (41,72).
Therefore, these gangliosides have important regulatory roles in
neural differentiation in vitro (41–43,73).
GD3 is involved in the early neural differentiation
and maturation process (63,74),
while GD1a induces early neural differentiation (43). By contrast with GD3, GT1b is
necessary for the induction of neural differentiation and
drastically enhances actin-rich dendrite generation (42,75).
Furthermore, it GT1b syntheses is increased in brain synapses
(74). GQ1b promotes neurite
outgrowth during early neural differentiation via the ERK 1/2-MAPK
pathway (Table I) (43,73,76).
Conclusion
Gangliosides are located on the plasma membrane and
have roles in various functions of mouse stem cells. As described
above, specific gangliosides are detected in mESCs, mouse iPSCs,
mNSCs and differentiated neural cells. These gangliosides regulate
cell proliferation and differentiation via the MAPK-ERK 1/2
pathway. Furthermore, gangliosides have been demonstrated to be
useful marker molecules for detecting or sorting mouse stem cells
and differentiated neural cells. Nevertheless, the functional roles
of gangliosides during cellular differentiation and proliferation
require further investigation. Identification of the gangliosides
present in stem cells should be performed to thoroughly
characterize marker gangliosides, and contribute to progression in
basic stem cell research and clinical applications.
Acknowledgements
This paper was supported by Wonkwang University
(Iksan, Korea) in 2015.
Glossary
Abbreviations
Abbreviations:
|
ESCs
|
embryonic stem cells
|
|
iPSCs
|
induced pluripotent stem cells
|
|
MEF
|
mouse embryonic fibroblast
|
|
NSCs
|
neural stem cells
|
References
|
1
|
Schengrund CL: The role(s) of gangliosides
in neural differentiation and repair: A perspective. Brain Res
Bull. 24:131–141. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hakomori S: Structure, organization, and
function of glycosphingolipids in membrane. Curr Opin Hematol.
10:16–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu RK, Nakatani Y and Yanagisawa M: The
role of glycosphingolipid metabolism in the developing brain. J
Lipid Res. 50 Suppl:S440–S445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson RG: The caveolae membrane system.
Annu Rev Biochem. 67:199–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simons K and Toomre D: Lipid rafts and
signal transduction. Nat Rev Mol Cell Biol. 1:31–39. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hakomori S, Yamamura S and Handa AK:
Signal transduction through glyco(sphingo)lipids. Introduction and
recent studies on glyco(sphingo)lipid-enriched microdomains. Ann N
Y Acad Sci. 845:1–10. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu RK, Tsai YT, Ariga T and Yanagisawa M:
Structures, biosynthesis, and functions of gangliosides-an
overview. J Oleo Sci. 60:537–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fortier LA: Stem cells: Classifications,
controversies, and clinical applications. Vet Surg. 34:415–423.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith AG: Embryo-derived stem cells: Of
mice and men. Annu Rev Cell Dev Biol. 17:435–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko K, Tapia N, Wu G, Kim JB, Bravo MJ,
Sasse P, Glaser T, Ruau D, Han DW, Greber B, et al: Induction of
pluripotency in adult unipotent germline stem cells. Cell Stem
Cell. 5:87–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ko K, Araúzo-Bravo MJ, Kim J, Stehling M
and Schöler HR: Conversion of adult mouse unipotent germline stem
cells into pluripotent stem cells. Nature Protoc. 5:921–928. 2010.
View Article : Google Scholar
|
|
13
|
Kim JB, Zaehres H, Wu G, Gentile L, Ko K,
Sebastiano V, Araúzo-Bravo MJ, Ruau D, Han DW, Zenke M and Schöler
HR: Pluripotent stem cells induced from adult neural stem cells by
reprogramming with two factors. Nature. 454:646–650. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JB, Sebastiano V, Wu G, Araúzo-Bravo
MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et
al: Oct4-induced pluripotency in adult neural stem cells. Cell.
136:411–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Molyneaux BJ, Arlotta P, Menezes JR and
Macklis JD: Neuronal subtype specification in the cerebral cortex.
Nat Rev Neurosci. 8:427–437. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shamblott MJ, Axelman J, Wang S, Bugg EM,
Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR and Gearhart
JD: Derivation of pluripotent stem cells from cultured human
primordial germ cells. Proc Natl Acad Sci USA. 95:13726–13731.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solter D and Knowles BB: Monoclonal
antibody defining a stage-specific mouse embryonic antigen
(SSEA-1). Proc Natl Acad Sci USA. 75:5565–5569. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu J, Hardin H, Zhang R, Sundling K,
Buehler D and Lloyd RV: Stage-specific embryonic antigen-1 (SSEA-1)
expression in thyroid tissues. Endocr Pathol. 27:271–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liebert M, Jaffe R, Taylor RJ, Ballou BT,
Solter D and Hakala TR: Detection of SSEA-1 on human renal tumors.
Cancer. 59:1404–1408. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki Y, Haraguchi N, Takahashi H, Uemura
M, Nishimura J, Hata T, Takemasa I, Mizushima T, Ishii H, Doki Y,
et al: SSEA-3 as a novel amplifying cancer cell surface marker in
colorectal cancers. Int J Oncol. 42:161–167. 2013.PubMed/NCBI
|
|
21
|
Cheung SK, Chuang PK, Huang HW,
Hwang-Verslues WW, Cho CH, Yang WB, Shen CN, Hsiao M, Hsu TL, Chang
CF and Wong CH: Stage-specific embryonic antigen-3 (SSEA-3) and
β3GalT5 are cancer specific and significant markers for breast
cancer stem cells. Proc Natl Acad Sci USA. 113:960–965. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noto Z, Yoshida T, Okabe M, Koike C, Fathy
M, Tsuno H, Tomihara K, Arai N, Noguchi M and Nikaido T: CD44 and
SSEA-4 positive cells in an oral cancer cell line HSC-4 possess
cancer stem-like cell characteristics. Oral Oncol. 49:787–795.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gottschling S, Jensen K, Warth A, Herth
FJ, Thomas M, Schnabel PA and Herpel E: Stage-specific embryonic
antigen-4 is expressed in basaloid lung cancer and associated with
poor prognosis. Eur Respir J. 41:656–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Knowles BB, Aden DP and Solter D:
Monoclonal antibody detecting a stage-specific embryonic antigen
(SSEA-1) on preimplantation mouse embryos and teratocarcinoma
cells. Curr Top Microbiol Immunol. 81:51–53. 1978.PubMed/NCBI
|
|
25
|
Fox N, Damjanov I, Martinez-Hernandez A,
Knowles BB and Solter D: Immunohistochemical localization of the
early embryonic antigen (SSEA-1) in postimplantation mouse embryos
and fetal and adult tissues. Dev Biol. 83:391–398. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fox N, Shevinsky L, Knowles BB, Solter D
and Dawjanov I: Distribution of murine stage-specific embryonic
antigens in the kidneys of three rodent species. Exp Cell Res.
140:331–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kannagi R, Cochran NA, Ishigami F,
Hakomori S, Andrews PW, Knowles BB and Solter D: Stage-specific
embryonic antigens (SSEA-3 and −4) are epitopes of a unique
globo-series ganglioside isolated from human teratocarcinoma cells.
EMBO J. 2:2355–2361. 1983.PubMed/NCBI
|
|
28
|
Tettamanti G:
Ganglioside/glycosphingolipid turnover: New concepts. Glycoconj J.
20:301–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basu S, Kaufman B and Roseman S: Enzymatic
synthesis of glucocerebroside by a glucosyltransferase from
embryonic chicken brain. J Biol Chem. 248:1388–1394.
1973.PubMed/NCBI
|
|
30
|
Ichikawa S, Sakiyama H, Suzuki G, Hidari
KI and Hirabayashi Y: Expression cloning of a cDNA for human
ceramide glucosyltransferase that catalyzes the first glycosylation
step of glycosphingolipid synthesis. Proc Natl Acad Sci USA.
93:4638–4643. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paul P, Kamisaka Y, Marks DL and Pagano
RE: Purification and characterization of UDP-glucose: Ceramide
glucosyltransferase from rat liver Golgi membranes. J Biol Chem.
271:2287–2293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Basu S, Kaufman B and Roseman S: Enzymatic
synthesis of ceramide-glucose and ceramide-lactose by
glycosyltransferases from embryonic chicken brain. J Biol Chem.
243:5802–5804. 1968.PubMed/NCBI
|
|
33
|
Nomura T, Takizawa M, Aoki J, Arai H,
Inoue K, Wakisaka E, Yoshizuka N, Imokawa G, Dohmae N, Takio K, et
al: Purification, cDNA cloning, and expression of UDP-Gal:
Glucosylceramide beta-1,4-galactosyltransferase from rat brain. J
Biol Chem. 273:13570–13577. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sundaram KS and Lev M: Purification and
activation of brain sulfotransferase. J Biol Chem. 267:24041–24044.
1992.PubMed/NCBI
|
|
35
|
Huwiler A, Kolter T, Pfeilschifter J and
Sandhoff K: Physiology and pathophysiology of sphingolipid
metabolism and signaling. Biochim Biophys Acta. 1485:63–99. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kolter T, Proia RL and Sandhoff K:
Combinatorial ganglioside biosynthesis. J Biol Chem.
277:25859–25862. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martin GR: Isolation of a pluripotent cell
line from early mouse embryos cultured in medium conditioned by
teratocarcinoma stem cells. Proc Natl Acad Sci USA. 78:7634–7638.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kimber SJ, Brown DG, Pahlsson P and
Nilsson B: Carbohydrate antigen expression in murine embryonic stem
cells and embryos. II. Sialylated antigens and glycolipid analysis.
Histochem J. 25:628–641. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ryu JS, Chang KT, Lee JT, Lim MU, Min HK,
Na YJ, Lee SB, Moussavou G, Kim SU, Kim JS, et al: Ganglioside GM1
influences the proliferation rate of mouse induced pluripotent stem
cells. BMB Rep. 45:713–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamashita T, Wada R, Sasaki T, Deng C,
Bierfreund U, Sandhoff K and Proia RL: A vital role for
glycosphingolipid synthesis during development and differentiation.
Proc Natl Acad Sci USA. 96:9142–9147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jung JU, Ko K, Lee DH, Ko K, Chang KT and
Choo YK: The roles of glycosphingolipids in the proliferation and
neural differentiation of mouse embryonic stem cells. Exp Mol Med.
41:935–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwak DH, Yu K, Kim SM, Lee DH, Kim SM,
Jung JU, Seo JW, Kim N, Lee S, Jung KY, et al: Dynamic changes of
gangliosides expression during the differentiation of embryonic and
mesenchymal stem cells into neural cells. Exp Mol Med. 38:668–676.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee DH, Koo DB, Ko K, Ko K, Kim SM, Jung
JU, Ryu JS, Jin JW, Yang HJ, Do SI, et al: Effects of daunorubicin
on ganglioside expression and neuronal differentiation of mouse
embryonic stem cells. Biochem Biophys Res Commun. 362:313–318.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Azevedo-Pereira RL, Morrot A, Machado GS,
Paredes BD, Dde C Rodrigues, de Carvalho AC and Mendez-Otero R:
Expression of ganglioside 9-O acetyl GD3 in undifferentiated
embryonic stem cells. Cell Biol Int. 39:121–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheresh DA, Pierschbacher MD, Herzig MA
and Mujoo K: Disialogangliosides GD2 and GD3 are involved in the
attachment of human melanoma and neuroblastoma cells to
extracellular matrix proteins. J Cell Biol. 102:688–696. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kwak DH, Rho YI, Kwon OD, Ahan SH, Song
JH, Choo YK, Kim SJ, Choi BK and Jung KY: Decreases of ganglioside
GM3 in streptozotocin-induced diabetic glomeruli of rats. Life Sci.
72:1997–2006. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Duan JG, Xiang T, Chen H and Liu M: Role
of extrinsic ganglioside GM1 in proliferation and differentiation
of neural stem cells. Sichuan Da Xue Xue Bao Yi Xue Ban.
38:260–263. 2007.(In Chinese). PubMed/NCBI
|
|
48
|
Gouni-Berthold I, Seul C, Ko Y, Hescheler
J and Sachinidis A: Gangliosides GM1 and GM2 induce vascular smooth
muscle cell proliferation via extracellular signal-regulated kinase
1/2 pathway. Hypertension. 38:1030–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nishio M, Tajima O and Furukawa K, Urano T
and Furukawa K: Over-expression of GM1 enhances cell proliferation
with epidermal growth factor without affecting the receptor
localization in the microdomain in PC12 cells. Int J Oncol.
26:191–199. 2005.PubMed/NCBI
|
|
50
|
Lee SW, Lee HJ, Hwang HS and Ko K, Han DW
and Ko K: Optimization of Matrigel-based culture for expansion of
neural stem cells. Anim Cells Syst. 19:175–180. 2015. View Article : Google Scholar
|
|
51
|
Itokazu Y, Kato-Negishi M, Nakatani Y,
Ariga T and Yu RK: Effects of amyloid β-peptides and gangliosides
on mouse neural stem cells. Neurochem Res. 38:2019–2027. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Klassen H, Schwartz MR, Bailey AH and
Young MJ: Surface markers expressed by multipotent human and mouse
neural progenitor cells include tetraspanins and non-protein
epitopes. Neurosci Lett. 312:180–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakatani Y, Yanagisawa M, Suzuki Y and Yu
RK: Characterization of GD3 ganglioside as a novel biomarker of
mouse neural stem cells. Glycobiology. 20:78–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang J, Cheng A, Wakade C and Yu RK:
Ganglioside GD3 is required for neurogenesis and long-term
maintenance of neural stem cells in the postnatal mouse brain. J
Neurosci. 34:13790–13800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang J and Yu RK: Interaction of
ganglioside GD3 with an EGF receptor sustains the self-renewal
ability of mouse neural stem cells in vitro. Proc Natl Acad Sci
USA. 110:19137–19142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Irvine RA and Seyfried TN: Phylogenetic
conservation of ganglioside GD3 expression during early vertebrate
ontogeny. Comp Biochem Physiol B Biochem Mol Biol. 109:603–612.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Seyfried TN and Yu RK: Ganglioside GD3:
Structure, cellular distribution, and possible function. Mol Cell
Biochem. 68:3–10. 1985.PubMed/NCBI
|
|
58
|
Yanagisawa M, Nakamura K and Taga T: Roles
of lipid rafts in integrin-dependent adhesion and gp130 signalling
pathway in mouse embryonic neural precursor cells. Genes Cells.
9:801–809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Doetsch F, Caillé I, Lim DA,
Garcia-Verdugo JM and Alvarez-Buylla A: Subventricular zone
astrocytes are neural stem cells in the adult mammalian brain.
Cell. 97:703–716. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Goldman JE, Hirano M, Yu RK and Seyfried
TN: GD3 ganglioside is a glycolipid characteristic of immature
neuroectodermal cells. J Neuroimmunol. 7:179–192. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cammer W and Zhang H: Ganglioside GD3 in
radial glia and astrocytes in situ in brains of young and adult
mice. J Neurosci Res. 46:18–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ngamukote S, Yanagisawa M, Ariga T, Ando S
and Yu RK: Developmental changes of glycosphingolipids and
expression of glycogenes in mouse brains. J Neurochem.
103:2327–2341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu Y, Li R and Ladisch S: Exogenous
ganglioside GD1a enhances epidermal growth factor receptor binding
and dimerization. J Biol Chem. 279:36481–36489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yanagisawa M, Nakamura K and Taga T:
Glycosphingolipid synthesis inhibitor represses cytokine-induced
activation of the Ras-MAPK pathway in embryonic neural precursor
cells. J Biochem. 138:285–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bouvier JD and Seyfried TN: Ganglioside
composition of normal and mutant mouse embryos. J Neurochem.
52:460–466. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu RK: Development regulation of
ganglioside metabolism. Prog Brain Res. 101:31–44. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yu RK, Macala LJ, Taki T, Weinfield HM and
Yu FS: Developmental changes in ganglioside composition and
synthesis in embryonic rat brain. J Neurochem. 50:1825–1829. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yanagisawa M and Yu RK: The expression and
functions of glycoconjugates in neural stem cells. Glycobiology.
17:57R–74R. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yanagisawa M, Taga T, Nakamura K, Ariga T
and Yu RK: Characterization of glycoconjugate antigens in mouse
embryonic neural precursor cells. J Neurochem. 95:1311–1320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moussavou G, Kwak DH, Lim MU, Kim JS, Kim
SU, Chang KT and Choo YK: Role of gangliosides in the
differentiation of human mesenchymal-derived stem cells into
osteoblasts and neuronal cells. BMB Rep. 46:527–532. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lee SH, Kwak DH, Ryu JS, Lim MU, Kim JS,
Chang KT and Choo YK: Differential expression pattern of
gangliosides during the differentiation of human dental
pulp-derived mesenchymal stem cells into dopaminergic neural-like
cells. Anim Cells Syst. 18:210–216. 2014. View Article : Google Scholar
|
|
72
|
Ryu JS, Ko K, Lee JW, Park SB, Byun SJ,
Jeong EJ, Ko K and Choo YK: Gangliosides are involved in neural
differentiation of human dental pulp-derived stem cells. Biochem
Biophys Res Commun. 387:266–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kwak DH, Jin JW, Ryu JS, Ko K, Lee SD, Lee
JW, Kim JS, Jung KY, Ko K, Ma JY, et al: Regulatory roles of
ganglioside GQ1b in neuronal cell differentiation of mouse
embryonic stem cells. BMB Rep. 44:799–804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Vinson M, Strijbos PJ, Rowles A, Facci L,
Moore SE, Simmons DL and Walsh FS: Myelin-associated glycoprotein
interacts with ganglioside GT1b. A mechanism for neurite outgrowth
inhibition. J Biol Chem. 276:20280–20285. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Osanai T, Kotani M, Yuen CT, Kato H, Sanai
Y and Takeda S: Immunohistochemical and biochemical analyses of
GD3, GT1b, and GQ1b gangliosides during neural differentiation of
P19 EC cells. FEBS Lett. 537:73–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tsuji S, Arita M and Nagai Y: GQ1b, a
bioactive ganglioside that exhibits novel nerve growth factor
(NGF)-like activities in the two neuroblastoma cell lines. J
Biochem. 94:303–306. 1983. View Article : Google Scholar : PubMed/NCBI
|