Introduction
Chronic prostatitis (CP) is a common urology disease
and voiding dysfunction is the primary clinical manifestation
(1), which is an important factor
that affects the quality of life of patients with CP. It has been
reported that the presence of urinary tract obstruction was
revealed in patients with CP by urodynamic examination. Urinary
tract obstruction primarily includes unstable bladder, sphincter
spasm and detrusor-external sphincter dyssynergia (2).
Non-neuronal interstitial cells of Cajal (ICCs) are
present in the gastrointestinal tract and are closely associated
with neural stromal cells (3).
Rhythmic contraction of gastrointestinal smooth muscle is regulated
by ICCs (4). In addition, ICCs are
associated with a variety of gastrointestinal motility disorders in
pathological conditions (5).
Sergeant et al (6) reported
that ICC-like cells are expressed in the bladder and have an
important role in the regulation of bladder activity. At present,
it has been demonstrated that ICC-like cells are present in various
locations in the urinary system and are morphologically and
immunologically similar to ICC stromal cells (7,8).
These cells have important roles in the motility of smooth muscle
in the urinary system, which include storage and downward
transportation of urine (9). The
structure and function of ICC-like cells is associated with urine
carriage disorders (10,11). Therefore, ICC-like cells of the
urinary system are an important area of research in
urodynamics.
The receptor c-Kit is also termed the stem cell
factor (SCF) receptor (12), and
belongs to the tyrosine kinase receptor type III family (13). It is a type of transmembrane
protein tyrosine kinase in ICC-like cells (11,14).
c-Kit has an essential role in maintaining the development, normal
morphology and function of ICC-like cells (15). However, the overexpression of c-Kit
in the bladder has been demonstrated to cause voiding dysfunction
in patients with CP (16).
Therefore, reducing the expression of c-Kit protein in ICC-like
cells may effectively improve detrusor hyperactivity in patients
with CP.
Resveratrol (17)
(trans-3,4,5-trihydroxy stilbene) is present in numerous plants and
foods, including cassia, pine trees, grapes, wine, mulberry and
peanuts. Resveratrol was originally used as a phytoalexin (18), and the extensive anti-inflammatory
effects of resveratrol have been of interest to researchers
(19). Furthermore, resveratrol
has potent effects in reversing multidrug resistance (20). It is reported that resveratrol
effectively relieves asthma by inhibiting the
phosphatidylinositol3-kinase (PI3K) signaling pathway (21). The PI3K signaling pathway is a
downstream pathway of c-Kit (22).
Therefore, resveratrol may have the potential to improve urinary
function in patients with CP. Solifenacin is currently used to
improve overactive bladders in patients with CP by inhibiting
muscarinic receptors (23),
however, it does not effectively improve prostatitis. Due to the
different targets, resveratrol and solifenacin may have potential
pharmacological synergy in patients with CP.
The present study aimed to investigate whether
resveratrol may improve overactive bladder in rats with CP and to
investigate the underlying molecular mechanisms. Furthermore, the
potential pharmacological synergy of resveratrol and solifenacin is
also investigated as a treatment for rats with CP.
Materials and methods
Chemicals
Resveratrol (>99% purity) was purchased from
Dalian Meilun Biotechnology Co., Ltd. (Dalian, China). Solifenacin
was purchased from Beijing Alliesyn Technology Co., Ltd. (Beijing,
China). Diphtheria, pertussis and tetanus (DPT) vaccine was
obtained from Wuhan Institute of Biological Products Co., Ltd.
(Wuhan, China). All other chemicals used in the present study were
of analytical grade and commercially available.
Animals
A total of 24 male Sprague-Dawley rats (age, 6
weeks; weight, 180±20 g) were purchased from the Experimental
Animal Center of Dalian Medical University (permit no. SCXK
2008-0002; Dalian, China). The rats were housed in a controlled
temperature of 22±2°C and relative humidity of 60±5%, under a 12 h
light/dark cycle with ad libitum access to water and food; rats
were fasted overnight and provided with water prior to surgery. All
animal experiments were approved by the ethics committee of Dalian
Medical University and performed in accordance with the
institutional guidelines.
Prostatic protein isolation
Rat prostatic protein was purified from male
Sprague-Dawley rats. After male Sprague-Dawley rats (weight, ~200
g) were sacrificed, prostate tissue was removed under sterile
conditions and washed with saline solution. Prostatic tissue was
subsequently placed into a physiological saline solution containing
0.5% Triton-X-100 and homogenized in an ice-water bath with a glass
homogenizer. Subsequently, the homogenized liquids were centrifuged
(10,000 × g) for 10 min at 4°C. Proteins were quantified using a
bicinchoninic acid (BCA) assay kit (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and diluted to 15 mg/ml with
PBS buffer (0.1 mol/l; pH 7.2).
Rat model of CP
To establish the CP model, rats were subcutaneously
injected with DPT vaccine (0.5 ml/kg) on day 0. Subsequently, they
were intradermally injected at multiple points with a mixture (1.0
ml) of purified rat prostatic protein and Freund's Complete
Adjuvant (1:1) on days 0, 15 and 30 (24). The rat model of CP was completed at
day 45.
Rats were randomly divided into the following four
groups (n=6 per group): Control group, normal rats received orally
administered saline for 10 days; CP group, CP model rats received
orally administered saline for 10 days; resveratrol group, CP model
rats received orally administered resveratrol (10 mg/kg) (25) for 10 days; and combination group,
CP model rats received orally administered resveratrol (10 mg/kg)
and solifenacin (0.7 mg/kg) (26)
for 10 days.
Bladder pressure and volume test in
rats
Rats were anesthetized with an intraperitoneal
injection of pentobarbital (60 mg/kg) prior to surgery.
Subsequently, the rats were fixed on an operating frame and the
upper edge of the pubic symphysis skin was cut. The bladder was
exposed and placed in the incision to avoid affecting the abdominal
pressure on the detrusor pressure. Two 24-gauge tubes were inserted
into the bladder and fixed, and the bladder was irrigated by saline
(0.4 ml/min) via one 24-gauge tube. The other tube was connected to
Medlab biological signal acquisition system (Medlab GmbH,
Stutensee, Germany) via a pressure transducer. The maximum capacity
of the bladder, residual urine volume and maximum voiding pressure
were measured.
Morphological changes
Following bladder pressure and volume analysis, rats
were sacrificed. The prostate and bladder were removed and fixed
for 2 days at 25°C in 10% (v/v) neutral formalin and then embedded
in paraffin. Tissue samples were sectioned in 4 µm slices and
processed by standard histological techniques. Prostatic tissue was
stained with haematoxylin and eosin (H&E) and examined for
morphological changes. The protein expression of c-Kit, SCF, AKT
and phosphorylated-AKT (p-AKT) was investigated in bladder tissue
using western blot analysis, immunohistochemical staining and
immunofluorescence labeling.
Western blot analysis
According to the manufacturer's protocol, proteins
were extracted from rat bladders using a Total Protein Extraction
kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Protein was
quantified using a BCA assay kit (Beijing Solarbio Science &
Technology Co., Ltd.), with bovine serum albumin (BSA) as the
standard. Proteins (20 µg) were resuspended in electrophoresis
sample buffer containing 5% β-mercaptoethanol and separated by
electrophoresis on a pre-cast 10% SDS-polyacrylamide gel (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), followed by electrotransfer
to a polyvinylidene fluoride membrane (EMD Millipore, Billerica,
MA, USA). Membranes were blocked using 5% non-fat milk in TBS with
0.1% Tween-20 (TBST) for 2 h at 37°C. β-actin served as a loading
control. Membranes were incubated overnight at 4°C with a 1:1,000
dilution of polyclonal antibodies for c-Kit (sc-168), SCF
(sc-9132), AKT (sc-8312) and p-AKT (sc-16646-R; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and with a 1:1,500 dilution
of monoclonal antibody for β-actin (bsm-33036M; BIOSS, Beijing,
China). Following washing with TBST, the blots were incubated with
a 1:1,000 dilution of goat anti-rabbit IgG-HRP (sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h at 37°C. Following extensive washing
with TBST, protein bands were visualized by enhanced
chemiluminescence (ECL) using ECL plus reagents from Beyotime
Institute of Biotechnology according to the manufacturer's
protocol. Emitted light was documented with a BioSpectrum®-410
multispectral imaging system with a Chemi HR 410 camera (UVP, Inc.,
Upland, CA, USA). Protein bands were visualized and photographed
under transmitted ultraviolet light. Blots were semi-quantified by
densitometric analysis using the Image Lab software version 4.0
(Bio-Rad Laboratories, Inc.). Experiments were repeated 6
times.
Immunohistochemical staining
Histological sections of rat bladder (4 µm) were
mounted on poly-L-lysine-coated slides. Slides were deparaffinized
in xylene and rehydrated in graded alcohols. Sections were
pretreated with citrate buffer (0.01 mol/l citric acid; pH 6.0) for
20 min at 95°C. Subsequently, at room temperature, sections were
immersed in PBS containing 3% H2O2 for 10
min. Following exposure to 10% normal goat serum (ZSGB-BIO,
Beijing, China) in PBS for 30 min at room temperature, the tissue
sections were incubated at 4°C overnight with rabbit polyclonal
anti-c-Kit (1:100; sc-168; Santa Cruz Biotechnology, Inc.).
Sections were rinsed with PBS, incubated with biotinylated goat
anti-rabbit IgG kit (SP-9001; OriGene Technologies, Inc., Beijing,
China) for 20 min at room temperature and treated with
3,30-diaminobenzidine chromogen for 5 min at room temperature.
Finally, sections were counterstained with hematoxylin for 6 min
and imaged under a light microscope. A total of 6 sections and 10
randomly selected fields/section were imaged per animal.
Immunofluorescence labeling
After rinsing with PBS, rat bladder sections (4 µm)
were permeabilized with 0.1% Triton X-100 in PBS for 10 min and
blocked with 2% BSA in PBS for 30 min at 37°C. The specimen slides
were incubated with primary anti-c-Kit antibody (1:100; sc-168;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. The specimens
were subsequently washed three times with PBS and incubated with a
cyanine 3-conjugated AffiniPure goat anti-rabbit IgG (H+L) (1:100;
sa00009-4; ProteinTech Group, Inc., Chicago, IL, USA) at 37°C for 1
h. Following additional washes, the immunofluorescent images were
captured by 80i Nikon microscope (Nikon Corporation, Tokyo, Japan).
A total of 6 sections and 10 randomly selected fields/section were
imaged per animal.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. Statistically significant differences
were compared using one-way analysis of variance followed by post
hoc Tukey or Dunnett's tests for multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Assessment of CP
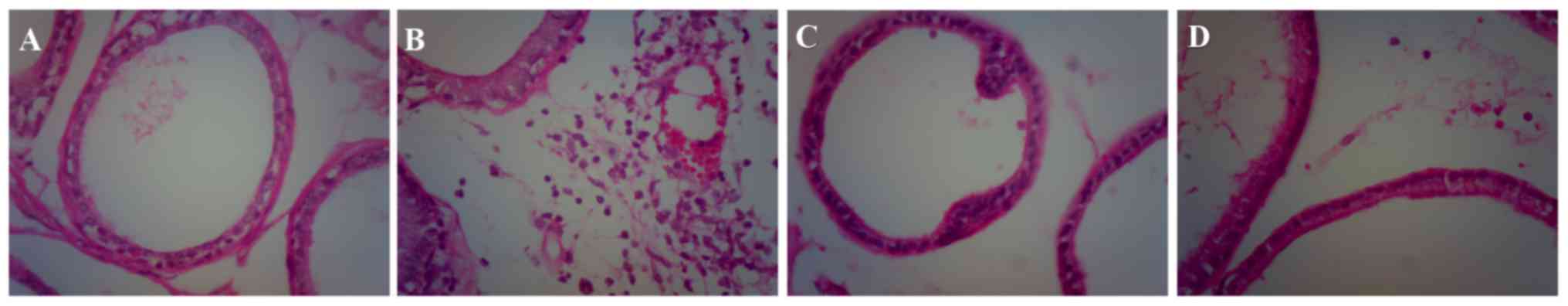
Histological examination of H&E-stained sections
was performed to determine the extent of CP. H&E-stained
prostate from the CP group demonstrated that the prostate tissues
were infiltrated by a large number of inflammatory cells (Fig. 1), which indicates that the rat
model of CP was established successfully (27).
Resveratrol improves overactive
bladder in rats with CP
The results of the bladder pressure and volume
analysis demonstrate that the maximum capacity of the bladder,
residual urine volume and maximum voiding pressure in the rats of
control group were 0.57 ml, 0.17 ml and 29.62 cm H2O,
respectively (Table I). The
maximum capacity of the bladder, residual urine volume and maximum
voiding pressure in the rats of CP group increased by 0.71, 2.06
and 0.27 fold, respectively, compared with the control group
(Table I). These results also
indicate that the model of CP rats was successfully established.
Following treatment with resveratrol in CP rats, the maximum
capacity of the bladder, residual urine volume and maximum voiding
pressure in the rats of resveratrol group was reduced by 25.77,
44.23 and 13.32%, respectively, compared with the CP group
(Table I), which indicates that
resveratrol may effectively improve overactive bladder in rats with
CP and, as the reduction observed with combination treatment was
larger, pharmacological synergy of resveratrol and solifenacin may
improve overactive bladder.
 | Table I.Resveratrol improves overactive
bladder in rats with CP. |
Table I.
Resveratrol improves overactive
bladder in rats with CP.
| Group | Maximum capacity
(ml) | Maximum voiding
pressure (cmH2O) | Residual urine volume
(ml) |
|---|
| Control | 0.57±0.09 | 27.62±2.98 | 0.17±0.11 |
| CP |
0.97±0.12a |
35.21±0.89a |
0.52±0.17a |
| Resveratrol |
0.72±0.03b |
30.51±1.14b |
0.29±0.02b |
| Combination |
0.60±0.08c |
28.78±1.03c |
0.23±0.16c |
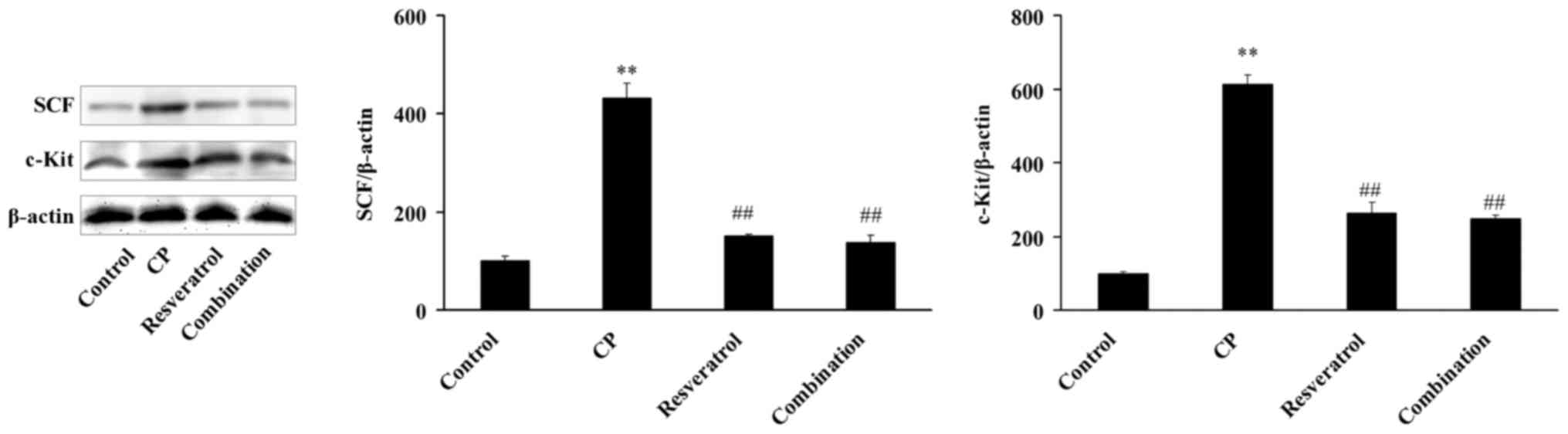
Resveratrol downregulates the protein
expression of SCF and c-Kit
The results of western blot analysis demonstrate
that the protein expression of SCF and c-Kit in the bladder of rats
in the CP group was 4.32 and 6.13 times higher than in the control
group (Fig. 2), respectively.
However, following treatment with resveratrol, the protein
expression of SCF and c-Kit in the rat bladders was significantly
reduced compared with the CP group (Fig. 2). However, no significant
differences in the protein expression of SCF and c-Kit were
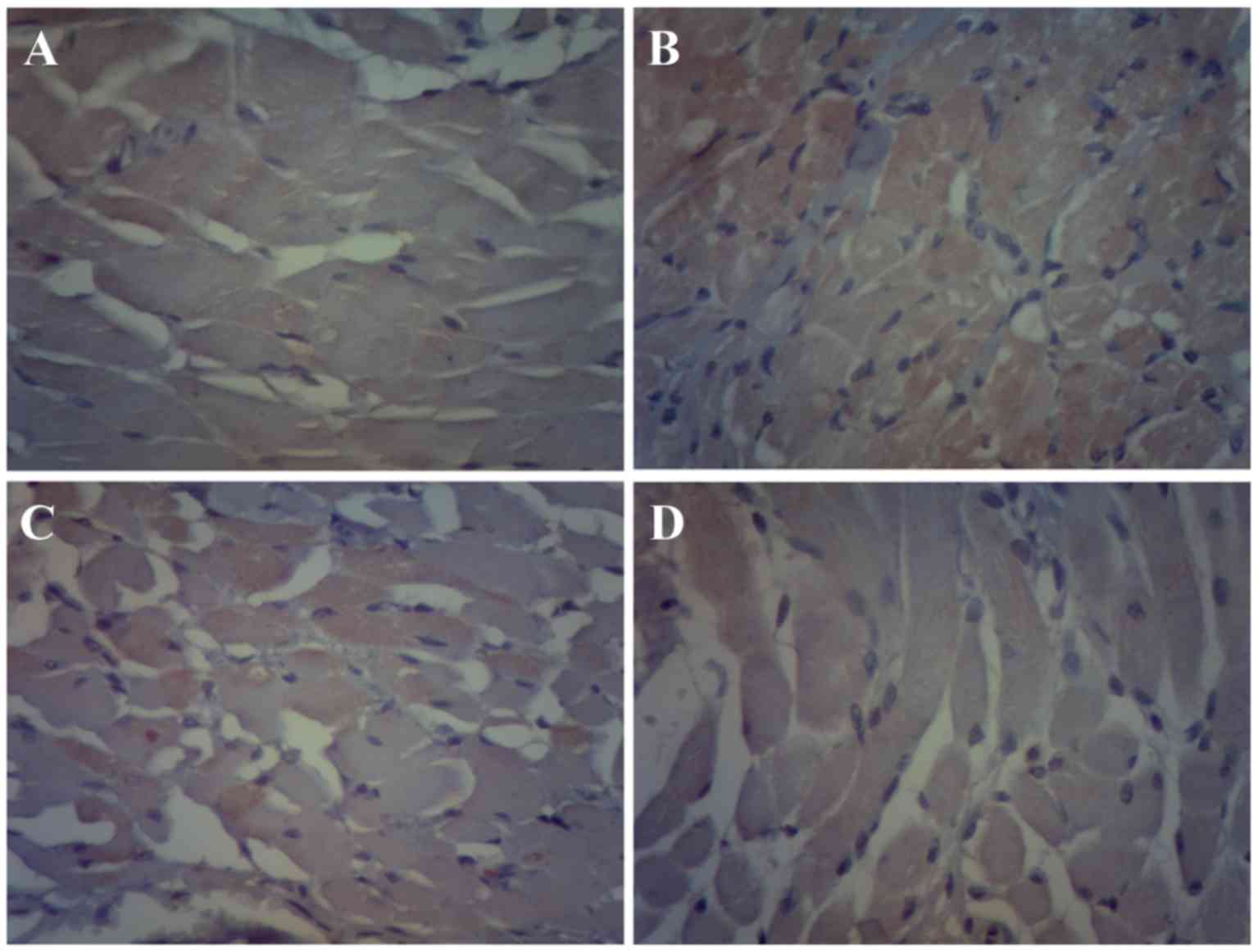
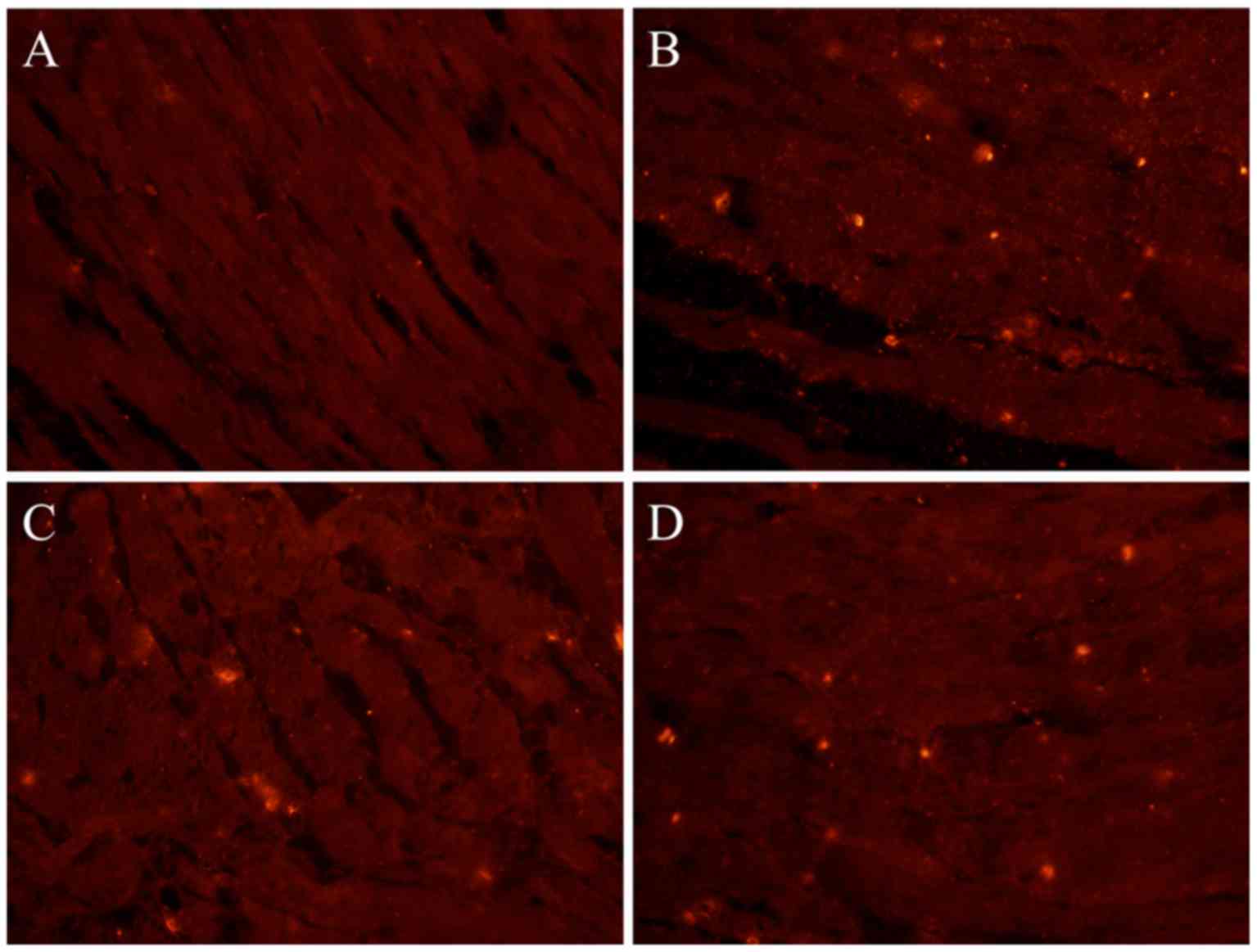
observed between resveratrol and combination groups (Fig. 2). Immunohistochemistry and
immunofluorescence results were consistent with western blot
analysis results. The protein expression of c-Kit in the bladder
tissue of the CP group was significantly increased compared with
the control group (Figs. 3 and
4). However, following treatment
with resveratrol or resveratrol + solifenacin, the protein
expression of c-Kit in the bladder tissue was reduced compared with
the CP group (Figs. 3 and 4), with no differences observed between
resveratrol and combination groups.
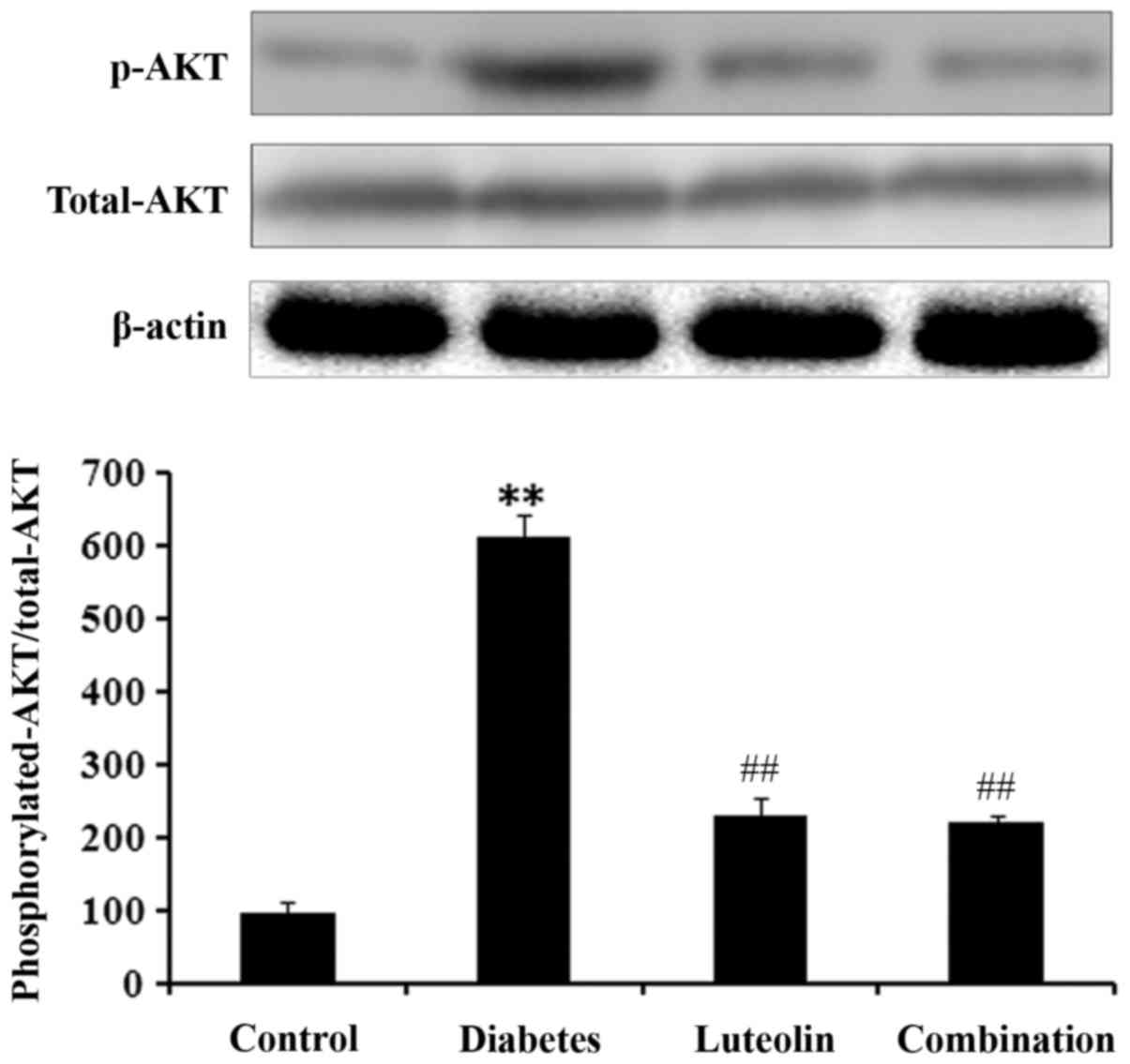
Resveratrol inhibits PI3K/AKT pathway
activity
The PI3K/AKT pathway is a downstream signaling
pathway of SCF/c-Kit, therefore, the present study investigated the
pathway activity of PI3K/AKT. The level of p-AKT in the CP group
was 6.31 times higher than in the control group (Fig. 5). However, following treatment with
resveratrol or resveratrol + solifenacin, p-AKT levels were
significantly reduced compared with the CP group (Fig. 5). The results indicate that
resveratrol may suppress the activity of the PI3K/AKT signaling
pathway in rats with CP.
Discussion
The c-Kit protein was first discovered in 1987, and
the human c-Kit gene is located at 4q11-12 and encodes a c-Kit
protein that is a transmembrane glycoprotein of receptor tyrosine
kinases class III (28). The c-Kit
receptor is widely distributed on the surface of hematopoietic stem
cells, myeloid progenitor cells, dendritic cells, mast cells,
lymphocyte precursors, melanocytes and germ cells. In the bladder,
c-Kit is primarily expressed in ICC-like cells, thus, the protein
expression level of c-Kit is an indicator of the number of ICC-like
cells (28). ICC-like cells,
detrusor smooth muscle cells and nerves interact with each other in
the bladder (29,30). The ICC-like cell is closely
associated with nerve signal transduction from the epithelium to
smooth muscle, and the structural relationships contribute to
triggering the detrusor contraction (31).
The survival, proliferation and differentiation of
cells are closely associated with the activation of SCF/c-Kit
signaling pathway (32–34). SCF is a c-Kit ligand and cytokine.
It is considered important for c-Kit activation, survival and the
function of ICCs (15). However,
the excessive activation of SCF/c-Kit signaling pathway may cause
abnormal proliferation of ICC-like cells in the bladder, therefore
potentially causing urinary dysfunction. Therefore, the present
study investigated whether the abnormal proliferation of ICC-like
cells is one of the causes of voiding dysfunction in rats with
CP.
A rat model of CP was established by immunological
methods. After the model established, changes in rat prostate
morphology was investigated by H&E staining. The results
indicate that the prostate tissues of rats with CP were infiltrated
by a large number of inflammatory cells. Subsequently, bladder
pressure and volume tests were performed in rats. The results
demonstrated that the maximum capacity of the bladder, residual
urine volume and maximum voiding pressure of the rats in the CP
group were significantly increased compared with the control group.
These results also indicate that the rat model of CP was
established successfully. However, after the rats were administered
resveratrol, the maximum capacity of the bladder, residual urine
volume and maximum voiding pressure of the rats in resveratrol
group were significantly reduced compared with the CP group and
were further reduced with combination treatment. The results
indicate that resveratrol may improve overactive bladder in rats
with CP, and pharmacological synergy between resveratrol and
solifenacin was observed to improve overactive bladder.
To investigate the mechanism by which resveratrol
improves overactive bladder in CP rats, the protein expression of
SCF and c-Kit in bladder of rats was examined by western blot
analysis and immunohistochemical staining. The results of western
blot analysis, immunohistochemical staining and immunofluorescence
labeling demonstrate that the protein expression of c-Kit in
bladders from the CP group were significantly increased compared
with the control group. However, following treatment with
resveratrol, the protein expression of c-Kit was reduced compare
with the CP group. In addition, the results of western blot
analysis demonstrated that SCF protein expression changes are
similar to c-Kit. Therefore, we hypothesize that resveratrol may
reduce the expression of c-Kit by downregulating SCF expression,
which improves overactive bladder in rats with CP.
Various downstream signaling pathways of SCF/c-Kit
are activated when SCF specifically binds with c-Kit and forms a
homodimer, including PI3K (22),
Ras/Erk phospholipase C-γ and JAK/STAT signaling pathways (35). Phosphorylation of AKT is an
important process in PI3K signal transduction. Therefore, the
present study examined the levels of p-AKT by western blot
analysis. p-AKT levels in the CP group were significantly higher
compared with the control group. However, following treatment with
resveratrol, p-AKT levels were significantly reduced compared with
the CP group. The results indicate that resveratrol may suppress
PI3K/AKT pathway activity, and that resveratrol may improve
overactive bladder in rats with CP by downregulating the expression
of c-Kit.
Traditional medicines have been associated with
therapeutic efficacy and reduced side effects in numerous diseases.
Currently, the separation, extraction and investigation of the
pharmacological activity of monomer components of traditional
medicines are increasingly performed in studies. Furthermore, the
combination of traditional medicine and chemical drugs has emerged
as a novel research direction. Solifenacin is a selective
antagonist of muscarinic acetylcholine M3 receptors. It is used in
the treatment of overactive bladder, which includes urgency,
frequency and urge incontinence. Therefore, the present study
investigated whether there is potential pharmacological synergy
between resveratrol and solifenacin in the treatment of rats with
CP. The results of bladder pressure and volume tests indicated that
the combination of resveratrol and solifenacin strengthened the
improvement in overactive bladder. However, no differences in the
protein expression of SCF, c-Kit and p-AKT were observed between
resveratrol and combination groups by western blot analysis,
immunohistochemical staining and immunofluorescence labeling. The
results indicate that resveratrol and solifenacin may act on
different targets to exhibit pharmacological synergy in the
treatment of CP.
In conclusion, resveratrol may improve overactive
bladder by downregulating the expression of SCF, c-Kit and p-AKT in
the bladder of rats with CP. Furthermore, resveratrol and
solifenacin may exhibit pharmacological synergy in the treatment of
CP in rats.
Acknowledgements
The present study was conducted with the technical
support of the Second Affiliated Hospital of Dalian Medical
University (Dalian, China).
References
|
1
|
Cho DS, Choi JB, Kim YS, Joo KJ, Kim SH,
Kim JC and Kim HW: Heart rate variability in assessment of
autonomic dysfunction in patients with chronic prostatitis/chronic
pelvic pain syndrome. Urology. 78:1369–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao LM, Shi BY and Liang CQ: Ambulatory
urodynamic monitoring of external urethral sphincter behavior in
chronic prostatitis patients. Asian J Androl. 1:215–217.
1999.PubMed/NCBI
|
|
3
|
Wei J, Li N, Xia X, Chen X, Peng F, Besner
GE and Feng J: Effects of lipopolysaccharide-induced inflammation
on the interstitial cells of Cajal. Cell Tissue Res. 356:29–37.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin DH, Lee MJ, Jiao HY, Choi S, Kim MW,
Park CG, Na J, Kim SW, Park IK, So I and Jun JY: Regulatory roles
of endogenous mitogen-activated protein kinases and tyrosine
kinases in the pacemaker activity of colonic interstitial cells of
cajal. Pharmacology. 96:16–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Kong D, He Y, Wang X, Gao L, Li J,
Yan M, Liu D, Wang Y, Zhang L and Jin X: The impact of inflammatory
cells in malignant ascites on small intestinal ICCs' morphology and
function. J Cell Mol Med. 19:2118–2127. 2015.PubMed/NCBI
|
|
6
|
Sergeant GP, Hollywood MA, McCloskey KD,
Thornbury KD and McHale NG: Specialised pacemaking cells in the
rabbit urethra. J Physiol. 526:359–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faussone-Pellegrini MS, Serni S and Carini
M: Distribution of ICC and motor response characteristics in
urinary bladders reconstructed from human ileum. Am J Physiol.
273:G147–G157. 1997.PubMed/NCBI
|
|
8
|
Shafik A, El Sibai O and Ahmed I: The
identification of specialized pacemaking cells in the anal
sphincters. Int J Colorectal Dis. 21:453–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thornbury KD, Hollywood MA, McHale NG and
Sergeant GP: Cajal beyond the gut: Interstitial cells in the
urinary system-towards general regulatory mechanisms of smooth
muscle contractility? Acta Gastroenterol Belg. 74:536–542.
2011.PubMed/NCBI
|
|
10
|
Hashitani H and Lang RJ: Functions of
ICC-like cells in the urinary tract and male genital organs. J Cell
Mol Med. 14:1199–1211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lang RJ and Klemm MF: Interstitial cell of
Cajal-like cells in the upper urinary tract. J Cell Mol Med.
9:543–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lennartsson J and Rönnstrand L: Stem cell
factor receptor/c-Kit: From basic science to clinical implications.
Physiol Rev. 92:1619–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abu-Duhier FM, Goodeve AC, Care RS, Gari
M, Wilson GA, Peake IR and Reilly JT: Mutational analysis of class
III receptor tyrosine kinases (C-KIT, C-FMS, FLT3) in idiopathic
myelofibrosis. Br J Haematol. 120:464–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Biers SM, Reynard JM, Doore T and Brading
AF: The functional effects of a c-kit tyrosine inhibitor on
guinea-pig and human detrusor. BJU Int. 97:612–616. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roskoski R Jr: Structure and regulation of
Kit protein-tyrosine kinase-the stem cell factor receptor. Biochem
Biophys Res Commun. 338:1307–1315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuksel O Haki, Urkmez A and Verit A: The
role of Cajal cells in chronic prostatitis. Arch Ital UrolAndrol.
88:133–135. 2016. View Article : Google Scholar
|
|
17
|
Torres P, Poveda A, Jimenez-Barbero J,
Ballesteros A and Plou FJ: Regioselective lipase-catalyzed
synthesis of 3-o-acyl derivatives of resveratrol and study of their
antioxidant properties. J Agric Food Chem. 58:807–813. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma S, Anjaneyulu M, Kulkarni SK and
Chopra K: Resveratrol, a polyphenolic phytoalexin, attenuates
diabetic nephropathy in rats. Pharmacology. 76:69–75. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Csiszar A: Anti-inflammatory effects of
resveratrol: Possible role in prevention of age-related
cardiovascular disease. Ann N Y Acad Sci. 1215:117–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang F, Wu XN, Chen J, Wang WX and Lu ZF:
Resveratrol reverses multidrug resistance in human breast cancer
doxorubicin-resistant cells. Exp Ther Med. 7:1611–1616.
2014.PubMed/NCBI
|
|
21
|
Poolman TM, Ng LL, Farmer PB and Manson
MM: Inhibition of the respiratory burst by resveratrol in human
monocytes: Correlation with inhibition of PI3K signaling. Free
Radic Biol Med. 39:118–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vajravelu BN, Hong KU, Al-Maqtari T, Cao
P, Keith MC, Wysoczynski M, Zhao J, JB IV Moore and Bolli R: C-Kit
promotes growth and migration of human cardiac progenitor cells via
the PI3K-AKT and MEK-ERK pathways. PloS One. 10:e01407982015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SH, Byun SS, Lee SJ, Kim KH and Lee
JY: Effects of initial combined tamsulosin and solifenacin therapy
for overactive bladder and bladder outlet obstruction secondary to
benign prostatic hyperplasia: A prospective, randomized,
multicenter study. Int Urol Nephrol. 46:523–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guohong S, Qiumei Z, Baozhen P, Lijuan H,
Julaiti S, Aisikeer T, Xuan G, Lina Y, Reyihan W, Wentao Z and
Qingyang P: Effects of different Chinese herbal prescriptions on
cytokines in au- toimmune prostatitis rats. J Tradit Chin Med.
35:211–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng PW, Ho WY, Su YT, Lu PJ, Chen BZ,
Cheng WH, Lu WH, Sun GC, Yeh TC, Hsiao M and Tseng CJ: Resveratrol
decreases fructose-induced oxidative stress, mediated by NADPH
oxidase via an AMPK-dependent mechanism. Br J Pharmacol.
171:2739–2750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki M, Ohtake A, Yoshino T, Yuyama H,
Hayashi A, Ukai M, Okutsu H, Noguchi Y, Sato S and Sasamata M:
Effects of solifenacin succinate (YM905) on detrusor overactivity
in conscious cerebral infarcted rats. Eur J Pharmacol. 512:61–66.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Yuan L, Chen J, Xiong C and Ruan
J: Multitargeted protective effect of Abacopteris penangiana
against carrageenan-induced chronic prostatitis in rats. J
Ethnopharmacol. 151:343–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rivera RS, Nagatsuka H, Gunduz M, Cengiz
B, Gunduz E, Siar CH, Tsujigiwa H, Tamamura R, Han KN and Nagai N:
C-kit protein expression correlated with activating mutations in
KIT gene in oral mucosal melanoma. Virchows Arch. 452:27–32. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Metzger R, Schuster T, Till H, Franke FE
and Dietz HG: Cajal-like cells in the upper urinary tract:
Comparative study in various species. Pediatr Surg Int. 21:169–174.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnston L, Woolsey S, Cunningham RM,
O'Kane H, Duggan B, Keane P and McCloskey KD: Morphological
expression of KIT positive interstitial cells of Cajal in human
bladder. J Urol. 184:370–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCloskey KD: Interstitial cells in the
urinary bladder-localization and function. Neurourol Urodyn.
29:82–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bashamboo A, Taylor AH, Samuel K, Panthier
JJ, Whetton AD and Forrester LM: The survival of differentiating
embryonic stem cells is dependent on the SCF-KIT pathway. J Cell
Sci. 119:3039–3046. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fraser L, Taylor AH and Forrester LM:
SCF/KIT inhibition has a cumulative but reversible effect on the
self-renewal of embryonic stem cells and on the survival of
differentiating cells. Cell Reprogram. 15:259–268. 2013.PubMed/NCBI
|
|
34
|
Lorincz A, Redelman D, Horvath VJ,
Bardsley MR, Chen H and Ordog T: Progenitors of interstitial cells
of cajal in the postnatal murine stomach. Gastroenterology.
134:1083–1093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taylor ML and Metcalfe DD: Kit signal
transduction. Hematol Oncol Clin North Am. 14:517–535. 2000.
View Article : Google Scholar : PubMed/NCBI
|