Introduction
Mangiferin (MGF) is a naturally occurring
polyphenolic compound commonly present in Mangifera indica
and Salacia reticulata (1,2). MGF
has a variety of pharmacological effects, including antiviral and
an antioxidant activity (3,4).
There have been a number of studies that have used MGF and S.
reticulata for the treatment of diabetes mellitus; MGF and
S. reticulata reportedly inhibit the activity of
α-glucosidase and suppress the mRNA expression of
fructose-1,6-bisphosphatase and glucose-6-phosphatase (5,6). The
effects of MGF and S. reticulata on bone and cartilage
disease have also been reported; S. reticulata leaf
ameliorated the symptoms of rheumatoid arthritis (RA) by reducing
bone tissue destruction in type II collagen antibody-induced
arthritic mice (7). Furthermore, a
previous study reported that MGF inhibits osteoclastic
differentiation of RAW 264.7 cells (8).
Osteoblasts and osteoclasts have critical roles in
bone tissue; bone metabolism is maintained by the balance of bone
formation by osteoblasts and bone resorption by osteoclasts.
Osteoblast- and osteoclast-associated bone diseases include
osteoporosis, osteopetrosis and RA (9–11).
RA is also a chronic inflammatory disease, characterized by
inflammatory cell infiltration, synovial hyperplasia, and
destruction of cartilage and bone (12–14);
this bone tissue destruction is induced and promoted by osteoclast
activation (15). In addition,
osteoblasts produce receptor activator nuclear factor-κB ligand
(RANKL) as an osteoclast differentiation factor (16).
RA treatment is currently primarily based on the
administration of anti-inflammatory drugs, immunosuppressive drugs
and antibody medicines (17–19);
calcium, steroids and bisphosphonates have also been used to treat
the deconstruction of bone (20–22).
In addition, bone resorption can be suppressed and bone formation
can be enhanced by estrogen receptors, such as estrogen receptor-α
(ERα) and ERβ (23).
It was previously reported that the leaf of S.
reticulata ameliorated the symptoms of arthritis in a mouse
model of RA, and suppressed cell proliferation and gene expression
of matrix metalloproteinase 3, RANKL, cathepsin K and c-fos mRNA in
murine synovial cells derived from mice with RA (8). This improvement in RA symptoms was
potentially associated with the MGF contained in S.
reticulata. The present study examined whether MGF directly
affects osteoblast and osteoclast proliferation and
differentiation. It was aimed to investigate whether MGF affects
cell proliferation, cell differentiation, and gene expression in
cultured osteoblasts and osteoclasts.
Materials and methods
Ethics statement
The use of experimental animals was approved, and
this study was performed in accordance with the National Institute
of Health (NIH) Guide for the Care and Use of Laboratory Animals
and the Institutional Animal Care and Use Committee of Josai
University (permit no. H22064; Saitama, Japan).
Cell and culture conditions
MC3T3-E1 cells were purchased from RIKEN Cell Bank
(RIKEN BioResource Center, Tsukuba, Japan). The cells were cultured
in α-minimal essential medium (MEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
penicillin (50 IU/ml). Cells were subcultured every 2nd day using
trypsin/EDTA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and
were maintained at 37°C in a humidified atmosphere of 5%
CO2 and 95% air.
Osteoclast-like cells (OCL) were derived from the
bone marrow of three 8-week-old ddY male mice (Tokyo Laboratory
Animals Science. Tokyo. Japan). Bone marrow macrophage (BMM) cells
were prepared immediately from the femur and tibia of ddY mice. The
BMM cells were induced by macrophage colony stimulating factor
(M-CSF) from bone marrow cells. Cell suspensions were plated at
1×104 cells/well in a 96-well plate in α-MEM containing
10% FBS, penicillin, and supplemented with M-CSF (20 ng/ml) and
RANKL (10 ng/ml) to induce osteoclast differentiation. The cells
were cultured as described previously (24).
Cell proliferation assay
MC3T3-E1 cells were plated in 96-well microplates
(BD Biosciences, Franklin Lakes, NJ, USA) at a density of 3×103
cells/well. After 24 h of incubation, MGF (1×10-3, 10–4, 10–5, 10–6
and 10–7 M) dissolved in dimethyl sulfoxide was mixed in the
culture medium and added to each well. The control group was
treated with medium only. After 2 h, the cell proliferation reagent
WST-1 (Roche Diagnostics, Indianapolis, IN, USA) was added to each
well (1/10 volume of the previously added medium), and the plates
were incubated for 30 min. Cell proliferation was then measured at
450 nm using a spectrophotometer (Wallac 1420ARVO.SX multilabel
counter; PerkinElmer, Inc., Waltham, MA, USA). All experiments were
performed in triplicate with independent samples.
Alkaline phosphatase (ALP)
activity
After 21 days of treatment with MGF, MC3T3-E1 cells
were fixed with 100% methanol. The fixed cells were incubated in
ALP staining solution for 20 min at 37°C; the ALP staining solution
consisted of 0.05 M AMP buffer at pH 9.8, 10 mM naphthol AS-BI
phosphate (Sigma-Aldrich; Merck KGaA), and 1 mM fast red violet LB
salt (Sigma-Aldrich; Merck KGaA). The ALP stained areas were then
inspected using a light microscope (Penguin 600CL; Pixera
Corporation, Tokyo, Japan). The ALP activity was quantified by
densitometric analysis using ImageJ software (version 1.3; National
Institutes of Health, Bethesda, MD, USA). All experiments were
performed in triplicate with independent samples.
Tartrate-resistant acid phosphatase
(TRAP) activity
After 6 days of culture, the OCL were fixed with
100% methanol. The fixed cells were incubated in the presence of L
(+)-tartrate buffer (0.335 mol/l, pH 4.9±0.1) with a TRAP kit
(Sigma-Aldrich; Merck KGaA) for 5 min at 37°C. Following staining,
TRAP stained areas were inspected using a light microscope (Penguin
600CL). TRAP-positive cells containing three or more nuclei were
counted as multinuclear osteoclasts. The TRAP activity was
quantified by densitometric analysis using ImageJ software. All
experiments were performed in triplicate with independent
samples.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA from MC3T3-E1, BMM and OCL were extracted
from the cultures using TRIzol reagent, and RNA was extracted
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was prepared from 1 µg total RNA using the
SuperScript First-Strand Synthesis system for RT-PCR (Invitrogen;
Thermo Fisher Scientific, Inc.). Amplification was performed in 10
µl of reaction mixture containing 1 µl cDNA using EX Taq (Takara
Bio, Inc., Otsu, Japan). The primer sequences used for each PCR are
outlined below. Initial denaturation was performed at 94°C for 30
sec, annealing temperatures ranged from 58–62°C for 30 sec, and
extension was done at 72°C for 3 min. A final extension was
performed at 72°C for 3 min. PCR cycles varied from 20–60 cycles,
due to certain genes, particularly RANK and cathepsin K, exhibiting
multiple variations and therefore requiring further cycles in order
to confirm gene expression.
Primers were as follows: Cathepsin K,
5′-CCAGTGTGGTTCCTGTTGG-3′ (forward) and 5′-TTGCCGTGGCGTTATACAT-3′
(reverse); ERα, 5′-AATTCTGACAATCGACGCCAG-3′ (forward) and
5′-GTGCTTCAACATTCTCCCTCCTC-3′ (reverse); ERβ,
5′-CAAGCTCATCTTTGCTCCAG-3′ (forward) and 5′-GCAGATGTTCCATGCCCTTG-3′
(reverse); nuclear factor of activated T-cells, cytoplasmic 1
(NFATc1), 5′-CACCAAAGTCCTGGAGATCC-3′ (forward) and
5′-GAAACGCTGGTACTGGCTTC-3′ (reverse); NF-κB,
5′-GCTTTGCAAACCTGGGAATA-3′ (forward) and 5′-TCCGCCTTCTGCTTGTAGAT-3′
(reverse); RANK, 5′-CCAGGGGACAACGGAATCAG-3′ (forward) and
5′-GGCCGGTCCGTGTACTCATC-3′ (reverse); osteoclast-associated
receptor (OSCAR), 5′-TGTTCTGGAACTGCTGGTAACG-3′ (forward) and
5′-GATGAGGTTTCCCTGGGTATAG-3′ (reverse); runt-related transcription
factor 1 (RunX1), 5′-GGTCGTTGAATCTCGCTACC-3′ (forward) and
5′-ACTTCCTCTGCTCCGTGCTA-3′ (reverse); RunX2,
5′-ACACCTACTCTCATACTGGGATGAGGAATG-3′ (forward) and
5′-ATGGTGGAGATCATCGCGGACCACCCGGCC-3′ (reverse); GAPDH,
5′-TTGACCTCAACTACATGG-3′ (forward) and 5′-CAGGGTGGTGGACCTCAT-3′
(reverse).
The PCR products were separated on 2% agarose gel
with Tris-acetate-EDTA buffer and visualized with ethidium bromide.
All gels were digitally imaged and the band intensities of these
digital images were determined using ImageJ software. Each mRNA
level was normalized against the GAPDH mRNA level. All data are
presented as the fold change in the target/GAPDH ratio. All
experiments were performed in triplicate with independent
samples.
Statistical analysis
All experiments were performed with triplicate
independent samples and were repeated at least three times, giving
qualitatively identical results. Statistical analysis was carried
out with Stat-Mate III version 3.18 (ATMS Co., Ltd., Tokyo, Japan).
Data were analyzed using the Student's t-test, with P<0.05
considered to indicate a statistically significant difference.
Results
Effects of mangiferin on cell
proliferation, cell differentiation and gene expression regulation
of MC3T3-E1 cells
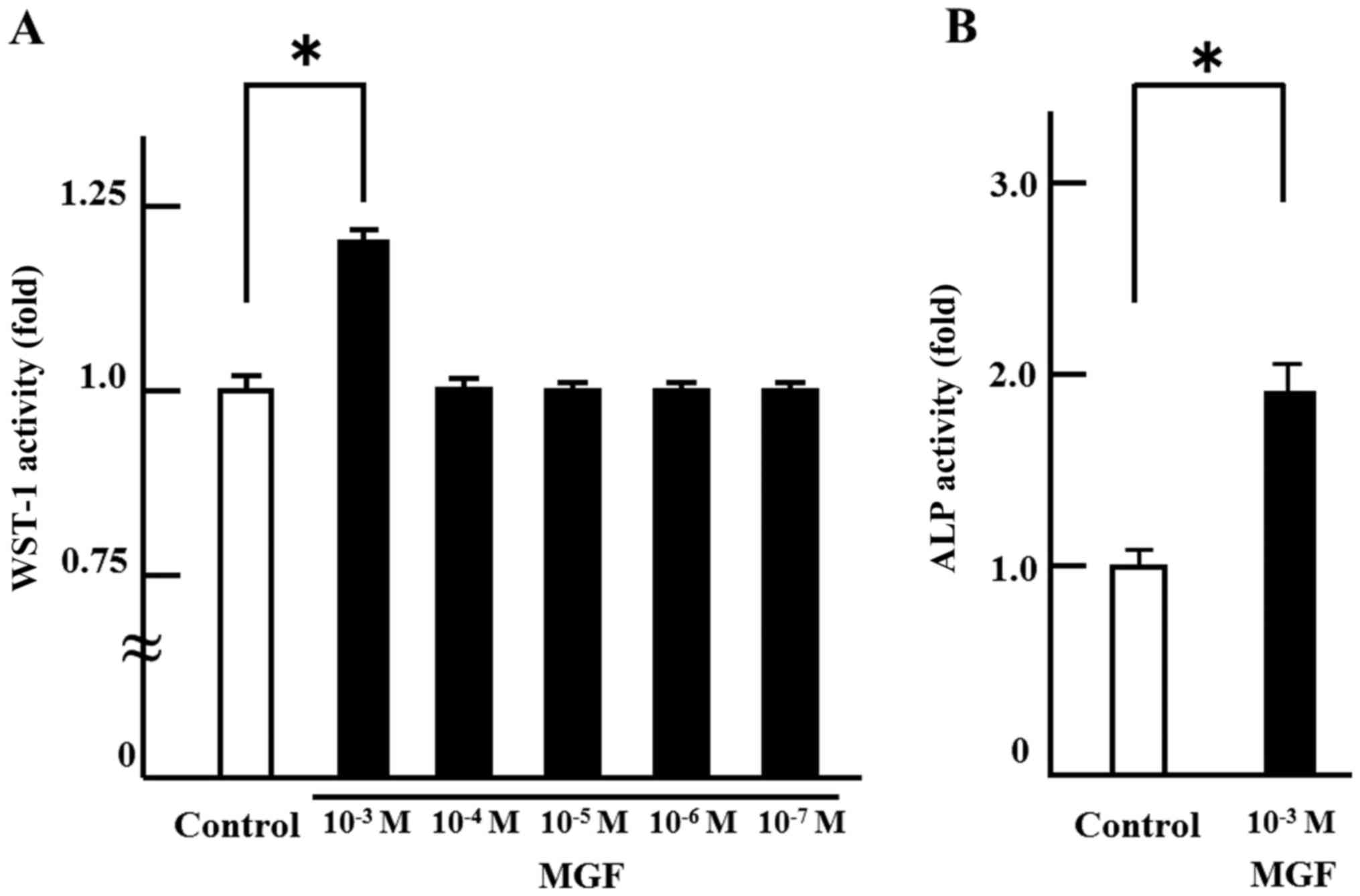
In order to investigate the effect on cell
proliferation, MGF was added at concentrations ranging from 10-3 M
to 10-7 M. There was no change in cell proliferation with MGF at
concentrations of 10-4 M to 10-7 M compared with the control. Only
10-3 M MGF resulted in an increase in WST-1 activity; 10-3 M MGF
induced >20% cell proliferation of MC 3T3-E1 cells compared with
controls (Fig. 1A). Thus, 10-3 M
MGF was selected for use in the subsequent experiments. In order to
investigate the effect of MGF on cell differentiation, ALP staining
was performed. MGF induced an ~2-fold increase in the ALP-stained
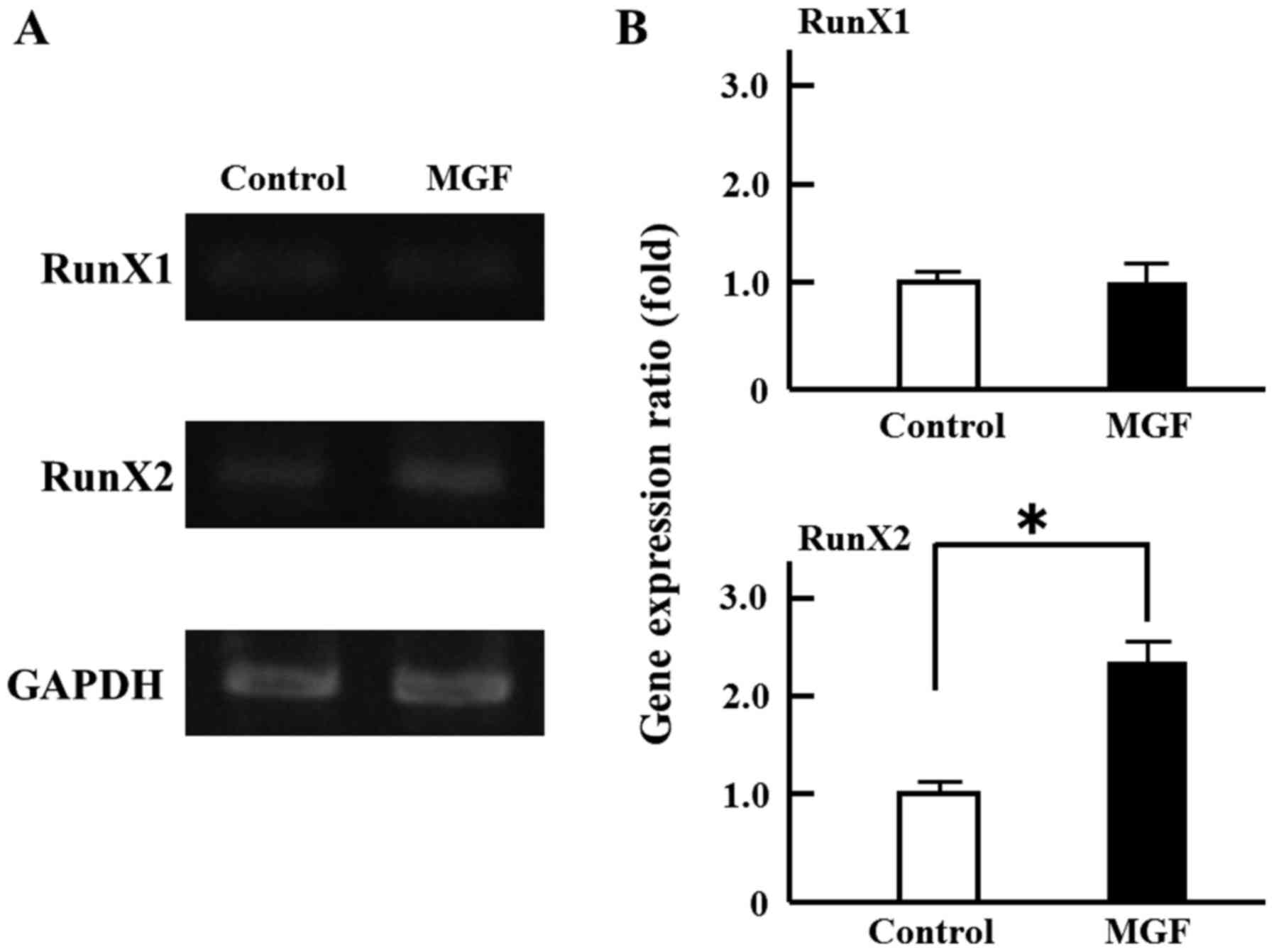
area of MC 3T3-E1 cells compared with controls (Fig. 1B). Regarding the effect of MGF on
the expression of genes involved in the differentiation of
osteoblasts, MGF induced >2-fold increase in RunX2 mRNA
expression in MC3T3-E1 cells; however, MGF did not affect the level
of RunX1 mRNA expression (Fig. 2A and
B).
Effects of mangiferin on cell
differentiation and gene expression regulation in osteoclast
lineage cells
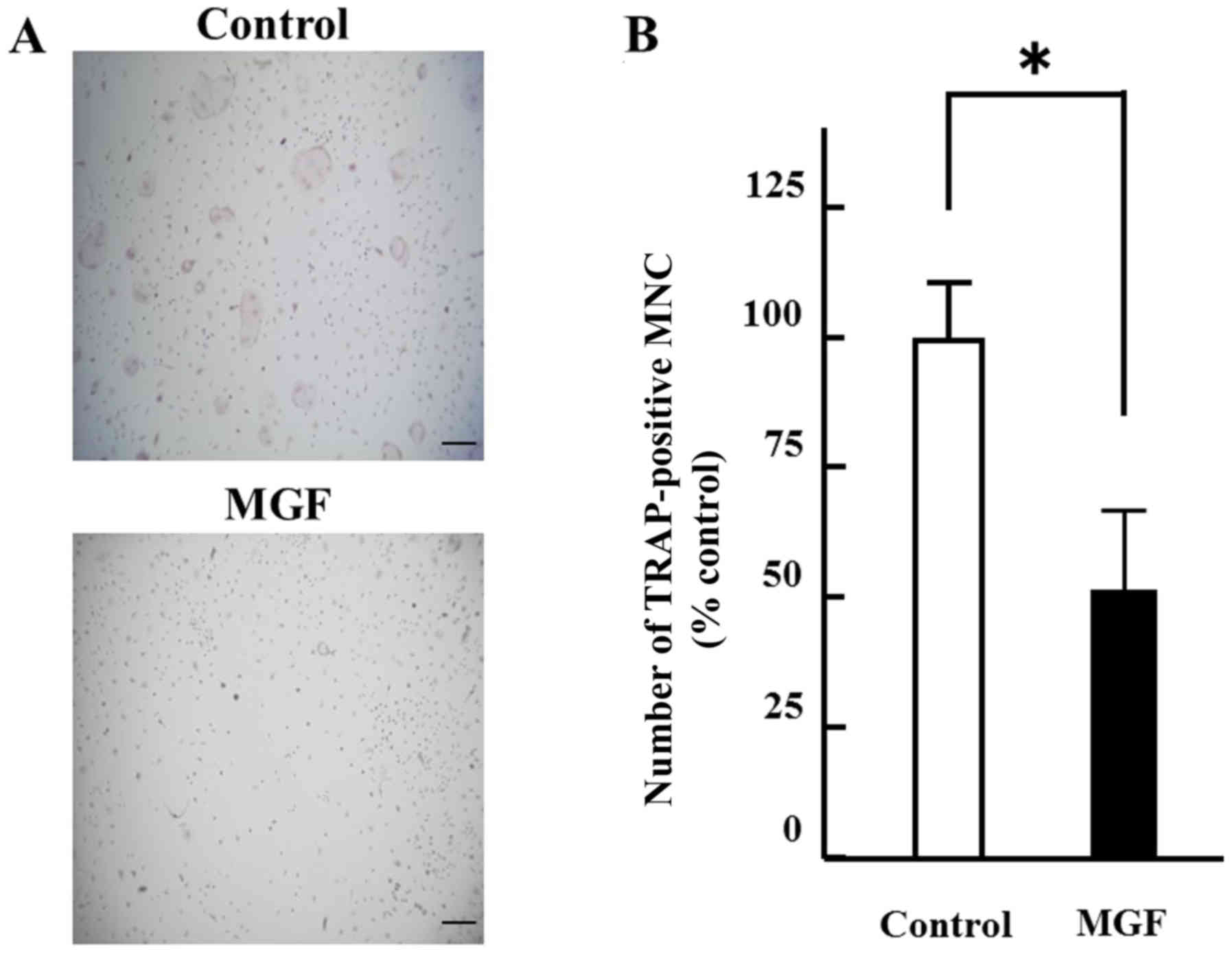
The effect of 10-3 M MGF on osteoclast
differentiation was determined. The number of TRAP-positive
multinuclear cells were counted. MGF reduced the number of
TRAP-positive multinuclear cells by ~50% compared with the vehicle
control (Fig. 3A and B). Regarding
the effect of MGF on the expression of genes involved in the
differentiation of osteoclasts, the cathepsin K and OSCAR mRNA
expression in BMM cells was ~1/4 of that in OCLs; RANK was two-fold
higher and ERα was 4-fold higher in BMM cells compared with mature
OCL cells (Fig. 4A and 4B). In
addition, MGF treatment increased the ERβ mRNA expression level in
BMM by ~5-fold compared with untreated BMM. However, MGF did not
affect the expression levels of cathepsin K, c-fos, ERα, NF-κB,
NFATc1, OSCAR or RANK mRNA in BMM.
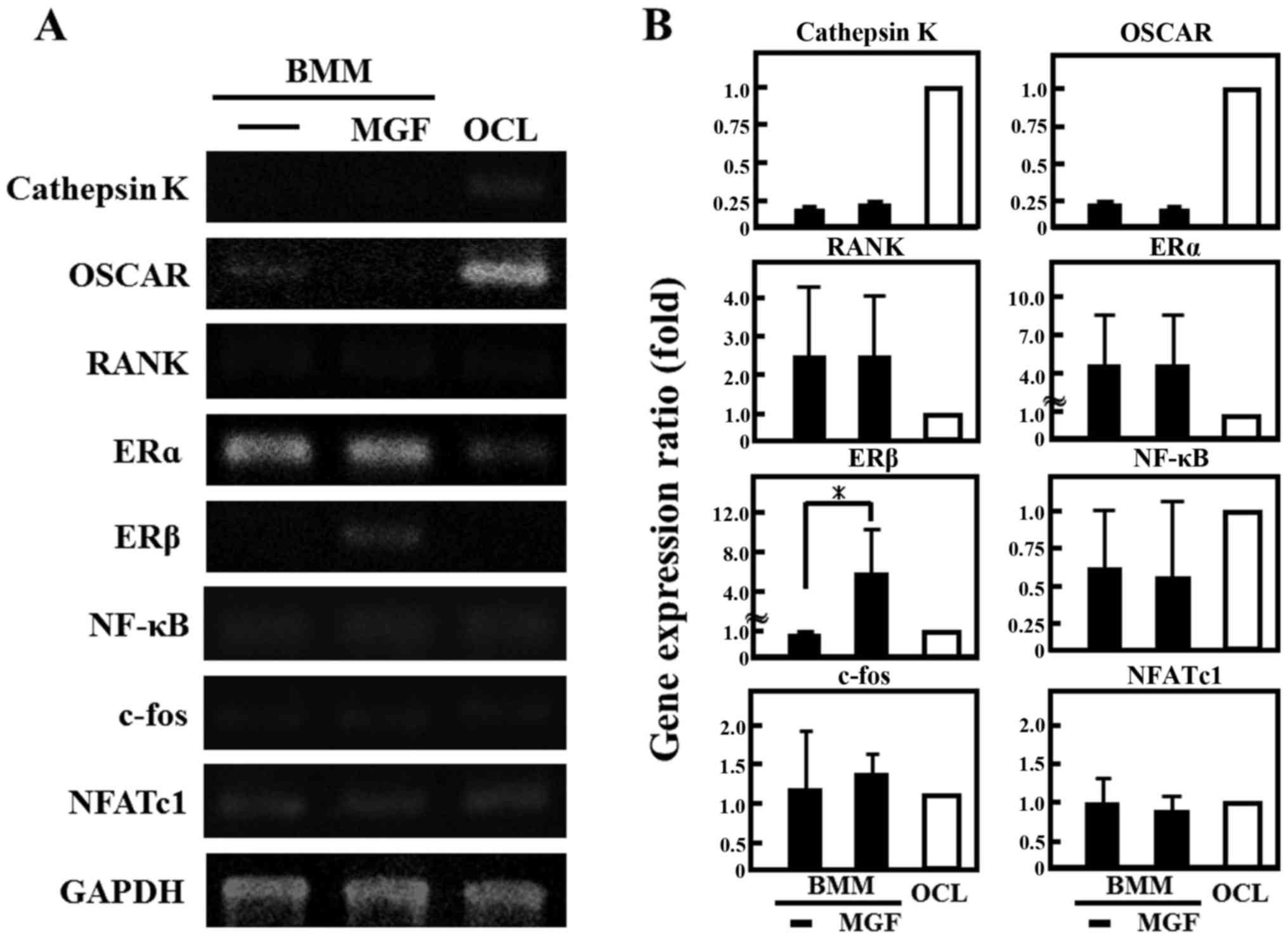 | Figure 4.Effect of MGF on gene expression of
osteoclast-associated genes. (A) The mRNA expression of cathepsin
K, OSCAR, RANK, ERα, ERβ, NF-κB, c-fos, and NFATc1 were analyzed by
reverse transcription-polymerase chain reaction. Total RNA
extracted from control OCL in the presence of macrophage colony
stimulating factor (20 ng/ml) and RANK ligand (10 ng/ml) for 3 days
and BMM cells treated with or without MGF (10−3 M) for 3
h. (B) The results are expressed as the mean ± standard deviation
of three independent experiments (n=3). *P<0.05. BMM, bone
marrow macrophages; MGF, mangiferin; OCL, osteoclast-like cells;
OSCAR, osteoclast-associated receptor; RANK, receptor activator
nuclear factor-κB; ER, estrogen receptor; NF-κB, nuclear factor-κB;
NFATc1, nuclear factor of activated T-cells, cytoplasmic 1. |
Discussion
The present study aimed to investigate whether MGF
affects cell proliferation, cell differentiation and gene
expression in cultured osteoblasts and osteoclasts. For example,
α-mangostin, a compound similar to MGF, has been reported to affect
cell proliferation in human lung adenocarcinoma cells and human
breast adenocarcinoma cells (25,26).
The present study indicated that MGF may promote cell proliferation
and cell differentiation of osteoblasts. Furthermore, MGF may
induce osteoblast differentiation from preosteoblasts to mature
osteoblasts via RunX2. It is established that RunX1 regulates the
differentiation of hematopoietic stem cells, and that RunX2
regulates the differentiation of osteoblasts (27,28).
Additionally, Runx2 and ALP are validated osteoblastic
differentiation markers (29–31).
The results of the present study indicated that MGF may inhibit
M-CSF- and RANKL-induced osteoclast formation and differentiation
from BMM to TRAP-positive multinuclear cells as osteoclasts. These
results indicate that MGF suppressed the differentiation of
osteoclasts from BMM and promoted the expression of ERβ mRNA in
BMM. These data suggest that MGF ay suppress osteoclastogenesis via
the ERβ signal. In conventional investigation of osteoclasts, the
effect on TRAP-positive cells indicates the induction of
differentiation of osteoclasts (32,33).
In addition, it is established that ER suppresses
osteoclastogenesis (34,35).
In conclusion, MGF promoted cell proliferation and
induced cell differentiation in preosteoblast MC3T3-E1 cells via
RunX2; thus, MGF may potentially promote osteoblastic bone
formation. MGF suppressed cell differentiation to mature OCL, and
promoted the mRNA expression of ERβ in BMM; thus, MGF may
potentially inhibit osteoclastic bone resorption. MGF may adjust
the balance between osteoblast and osteoclast function, and could
be useful in improving bone diseases. Further study is warranted
into the use of MGF as a treatment for bone diseases such as
osteoporosis, osteopetrosis and RA.
References
|
1
|
Makare N, Bodhankar S and Rangari V:
Immunomodulatory activity of alcoholic extract of Mangifera indica
L. In mice. J Ethnopharmacol. 78:133–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshikawa M, Ninomiya K, Shimoda H,
Nishida N and Matsuda H: Hepatoprotective and antioxidative
properties of Salacia reticulata: Preventive effects of phenolic
constituents on CCl4-induced liver injury in mice. Biol Pharm Bull.
25:72–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guha S, Ghosal S and Chattopadhyay U:
Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a
naturally occurring glucosylxanthone. Chemotherapy. 42:443–451.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sánchez GM, Re L, Giuliani A, Núñez-Sellés
AJ, Davison GP and León-Fernández OS: Protective effects of
Mangifera indica L. Extract, mangiferin and selected antioxidants
against TPA-induced biomolecules oxidation and peritoneal
macrophage activation in mice. Pharmacol Res. 42:565–573. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng J, Yang XW and Wang RF: Bio-assay
guided isolation and identification of α-glucosidase inhibitors
from the leaves of Aquilaria sinensis. Phytochemistry. 72:242–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Im R, Mano H, Matsuura T, Nakatani S,
Shimizu J and Wada M: Mechanisms of blood glucose-lowering effect
of aqueous extract from stems of Kothala himbutu (Salacia
reticulata) in the mouse. J Ethnopharmacol. 121:234–240. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sekiguchi Y, Mano H, Nakatani S, Shimizu J
and Wada M: Effects of the Sri Lankan medicinal plant, Salacia
reticulata, in rheumatoid arthritis. Genes Nutr. 5:89–96. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ang E, Liu Q, Qi M, Liu HG, Yang X, Chen
H, Zheng MH and Xu J: Mangiferin attenuates osteoclastogenesis,
bone resorption, and RANKL-induced activation of NF-κB and ERK. J
Cell Biochem. 112:89–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao B, Takami M, Yamada A, Wang X, Koga
T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, et al: Interferon
regulatory factor-8 regulates bone metabolism by suppressing
osteoclastogenesis. Nat Med. 15:1066–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soysa NS, Alles N, Weih D, Lovas A, Mian
AH, Shimokawa H, Yasuda H, Weih F, Jimi E, Ohya K and Aoki K: The
pivotal role of the alternative NF-kappaB pathway in maintenance of
basal bone homeostasis and osteoclastogenesis. J Bone Miner Res.
25:809–818. 2010.PubMed/NCBI
|
|
11
|
Childress P, Philip BK, Robling AG,
Bruzzaniti A, Kacena MA, Bivi N, Plotkin LI, Heller A and Bidwell
JP: Nmp4/CIZ suppresses the response of bone to anabolic
parathyroid hormone by regulating both osteoblasts and osteoclasts.
Calcif Tissue Int. 89:74–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funk JL, Cordaro LA, Wei H, Benjamin JB
and Yocum DE: Synovium as a source of increased amino-terminal
parathyroid hormone-related protein expression in rheumatoid
arthritis. A possible role for locally produced parathyroid
hormone-related protein in the pathogenesis of rheumatoid
arthritis. J Clin Invest. 101:1362–1371. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parekh RB, Dwek RA, Sutton BJ, Fernandes
DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T,
Matsuta K, et al: Association of rheumatoid arthritis and primary
osteoarthritis with changes in the glycosylation pattern of total
serum IgG. Nature. 316:452–457. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinitz M, Izak G, Cohen S, Ehrenfeld M
and Flechner I: Continuous production of monoclonal rheumatoid
factor by EBV-transformed lymphocytes. Nature. 287:443–445. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ainola M, Li TF, Mandelin J, Hukkanen M,
Choi SJ, Salo J and Konttinen YT: Involvement of a disintegrin and
a metalloproteinase 8 (ADAM8) in osteoclastogenesis and
pathological bone destruction. Ann Rheum Dis. 68:427–434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rudge S, Hailwood S, Horne A, Lucas J, Wu
F and Cundy T: Effects of once-weekly oral alendronate on bone in
children on glucocorticoid treatment. Rheumatology (Oxford).
44:813–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hansen IB, Ellingsen T, Hornung N, Poulsen
JH, Lottenburger T and Stengaard-Pedersen K: Plasma level of
CXC-chemokine CXCL12 is increased in rheumatoid arthritis and is
independent of disease activity and methotrexate treatment. J
Rheumatol. 33:1754–1759. 2006.PubMed/NCBI
|
|
19
|
Puéchal X, Miceli-Richard C, Mejjad O,
Lafforgue P, Marcelli C, Solau-Gervais E, Steinfeld S, Villoutreix
C, Trèves R, Mariette X, et al: Anti-tumour necrosis factor
treatment in patients with refractory systemic vasculitis
associated with rheumatoid arthritis. Ann Rheum Dis. 67:880–884.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lories R: The balance of tissue repair and
remodeling in chronic arthritis. Nat Rev Rheumatol. 7:700–707.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luppen CA, Blake CA, Ammirati KM, Stevens
ML, Seeherman HJ, Wozney JM and Bouxsein ML: Recombinant human bone
morphogenetic protein-2 enhances osteotomy healing in
glucocorticoid-treated rabbits. J Bone Miner Res. 17:301–310. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Mashiba T and Burr DB:
Bisphosphonate treatment suppresses not only stochastic remodeling
but also the targeted repair of microdamage. Calcif Tissue Int.
69:281–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gruber CJ, Tschugguel W, Schneeberger C
and Huber JC: Production and actions of estrogens. N Engl J Med.
346:340–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mano H, Hakeda Y and Kumegawa M: Estrogen
directly down-regulates the bone-resorbing activity of mature
osteoclasts through nuclear estrogen receptor alpha.
Cytotechnology. 35:17–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shih YW, Chien ST, Chen PS, Lee JH, Wu SH
and Yin LT: Alpha-mangostin suppresses phorbol 12-myristate
13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3
integrin/FAK/ERK and NF-kappaB signaling pathway in human lung
adenocarcinoma A549 cells. Cell Biochem Biophys. 58:31–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YB, Ko KC, Shi MD, Liao YC, Chiang TA,
Wu PF, Shih YX and Shih YW: Alpha-mangostin, a novel dietary
xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions
through the ERK signaling pathway in MCF-7 human breast
adenocarcinoma cells. J Food Sci. 75:H13–H23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichikawa M, Goyama S, Asai T, Kawazu M,
Nakagawa M, Takeshita M, Chiba S, Ogawa S and Kurokawa M:
AML1/Runx1 negatively regulates quiescent hematopoietic stem cells
in adult hematopoiesis. J Immunol. 180:4402–4408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu JC, Lengner CJ, Gaur T, Lou Y, Hussain
S, Jones MD, Borodic B, Colby JL, Steinman HA, van Wijnen AJ, et
al: Runx2 protein expression utilizes the Runx2 P1 promoter to
establish osteoprogenitor cell number for normal bone formation. J
Biol Chem. 286:30057–30070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD,
Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS and Koh JT:
Metformin induces osteoblast differentiation via orphan nuclear
receptor SHP-mediated transactivation of Runx2. Bone. 48:885–893.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laflamme C, Curt S and Rouabhia M:
Epidermal growth factor and bone morphogenetic proteins upregulate
osteoblast proliferation and osteoblastic markers and inhibit bone
nodule formation. Arch Oral Biol. 55:689–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim TY, Wang W, Shi Z, Poh CK and Neoh KG:
Human bone marrow-derived mesenchymal stem cells and osteoblast
differentiation on titanium with surface-grafted chitosan and
immobilized bone morphogenetic protein-2. J Mater Sci Mater Med.
20:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Teramachi J, Morimoto H, Baba R, Doi Y,
Hirashima K and Haneji T: Double stranded RNA-dependent protein
kinase is involved in osteoclast differentiation of RAW264.7 cells
in vitro. Exp Cell Res. 316:3254–3262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Windahl SH, Norgård M, Kuiper GG,
Gustafsson JA and Andersson G: Cellular distribution of estrogen
receptor beta in neonatal rat bone. Bone. 26:117–121. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hiyama S, Sugiyama T, Kusuhara S and
Uchida T: Evidence for estrogen receptor expression during
medullary bone formation and resorption in estrogen-treated male
Japanese quails (Coturnix coturnix japonica). J Vet Sci.
13:223–227. 2012. View Article : Google Scholar : PubMed/NCBI
|