Introduction
Gastroparesis is one of the common chronic
complications caused by diabetes mellitus, which primarily
manifests as bloating, nausea and vomiting, and results in low
gastric motility, delayed gastric emptying and prolonged gastric
transit time (1). Although the
apoptosis of gastric smooth muscle cells has been shown to be
important in the occurrence of diabetic gastroparesis (2,3), the
mechanisms upstream of this process remain to be elucidated. The
phosphoinositide-3-kinase (PI3K)-protein kinase B (AKT)-mammalian
target of rapamycin (mTOR) pathway is an important intracellular
signaling cascade capable of affecting a variety of cell behaviors,
including cell proliferation, growth, apoptosis and metabolism
(4,5). PI3K phosphorylates
phosphatidylinositol 4,5-bisphosphate into phosphatidylinositol
3,4,5-triphosphate to recruit and activate AKT, which subsequently
activates its downstream target molecule, mTOR. mTOR is also a
downstream target of 5′ adenosine monophosphate-activated protein
kinase (AMPK), which is widely involved in mediating cell
metabolism, and has important biological roles in regulating cell
apoptosis, physiological and pathological processes (6). AMPK phosphorylates and activates
tuberous sclerosis complex 2 (TSC-2) upstream of mTOR to promote
the formation of a TSC-1/TSC-2 complex, which is capable of
inhibiting the activity of another upstream guanosine
triphosphate-binding protein, Ras homolog enriched in brain,
ultimately reducing the activity of mTOR (7,8).
mTOR downstream targets include ribosomal S6 protein
kinase (S6K) and eukaryotic translation initiation factor 4-binding
protein 1 (4E-BP1) (9,10). The phosphorylation of mTOR has been
shown to activate the p70 form of S6K (p70S6K) and inhibit the
binding of 4E-BP1 to eukaryotic translation initiation factor 4E
(eIF4E), thereby releasing eIF4E and improving the translation of
anti-apoptotic proteins (11).
However, the correlation between cell apoptosis, and
the PI3K-AKT-mTOR and AMPK-mTOR pathways in diabetic gastroparesis
has not been reported. In the present study, a rat model of
diabetic gastroparesis was established to examine the apoptosis of
gastric smooth muscle cells, and the key proteins involved in
PI3K-AKT-mTOR and AMPK-mTOR signaling, including PI3K and
phosphorylated forms of AKT (p-AKT), AMPK (p-AMPK), TSC-2
(p-TSC-2), mTOR (p-mTOR), p70S6k (p-p70S6K) and 4E-BP1 (P-4E-BP1).
The aim of the present study was to elucidate the pathogenesis of
diabetic gastroparesis to provide a scientific theoretical basis
and experimental evidence for novel clinical treatments.
Materials and methods
Experimental animals
A total of 40 adult male Sprague-Dawley rats,
weighing 200±20 g, were provided by Yanbian University Experimental
Animal Center (Yanji, China). The rats were housed at room
temperature (18–25°C), with 50–80% relative humidity and a 12-h
light/dark cycle, and allowed free access to food and water. The
study was approved by the ethics committee of Yanbian University
College of Medicine (Yanji, China).
Preparation of the diabetic model and
experimental groups
Streptozotocin (STZ) solution (0.5%; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was prepared using citrate buffer
(pH 4.0; 0.1 mol/l). Following 1 week of adaptive feeding, the rats
were deprived of food for 12 h with access to water, and received
an intraperitoneal injection of 65 mg/kg STZ to establish the
diabetic model. The breeding conditions remained unchanged. At 7
days post-injection, tail vein blood was collected and the success
of the model was determined based on a glucose concentration
>350 mg/dL (12,13). The model rats were randomly divided
into groups of 10, including a normal control (NC) group and
diabetic model (DM) groups examined 2 weeks (DM2W), 4 weeks (DM4W)
and 6 weeks (DM6W) later.
Preparation of diabetic gastroparesis
model
The rates of gastric residual pigment were detected,
as they reflect the capacity for gastric emptying in the animal.
Following 24 h of fasting, the rats were administered with 0.4 ml
of 1 mg/ml methylene blue solution and sacrificed by cervical
dislocation 30 min later. The whole stomach was immediately
harvested, and gastric residue was collected by removing the
stomach mucosal layer and collecting the antral circular muscle
strips, which were placed in liquid nitrogen. The residues were
rinsed with saline and centrifuged at 744 × g for 15 min at 4°C,
following which the supernatant was collected and optical density
(OD) was detected at 640 nm using a spectrophotometer. The gastric
residual pigment ratio was calculated as: OD value of detection
tube/OD value of standard tube ×100%. In terms of comparing the
diabetic model groups with the NC group, P<0.05 was considered
to indicate successful diabetic gastroparesis model establishment
(14).
Preparation of gastric smooth muscle
cell suspensions
To prepare cell suspensions, gastric smooth muscle
was mechanically triturated into chyle-like shapes, and then
filtered and centrifuged at 136 × g for 3 min at 4°C. The
supernatant was discarded and the cells were cultured in Dulbecco's
modified Eagle's medium (DMEM). The filtered tissues were stirred
in 10X volume of collagenase (0.1%) in water at 37°C and sampled
every 30 min, three times. Between each step, the cells were
centrifuged at 136 × g for 3 min at 4°C, the supernatant was
discarded and the cell suspension was resuspended in DMEM. Trypan
blue staining was used to account for necrotic cells. The cells
were counted under a CKX41SF inverted microscope (Olympus
Corporation, Tokyo, Japan) and the cell density was adjusted to
1×106 cells/ml for further use.
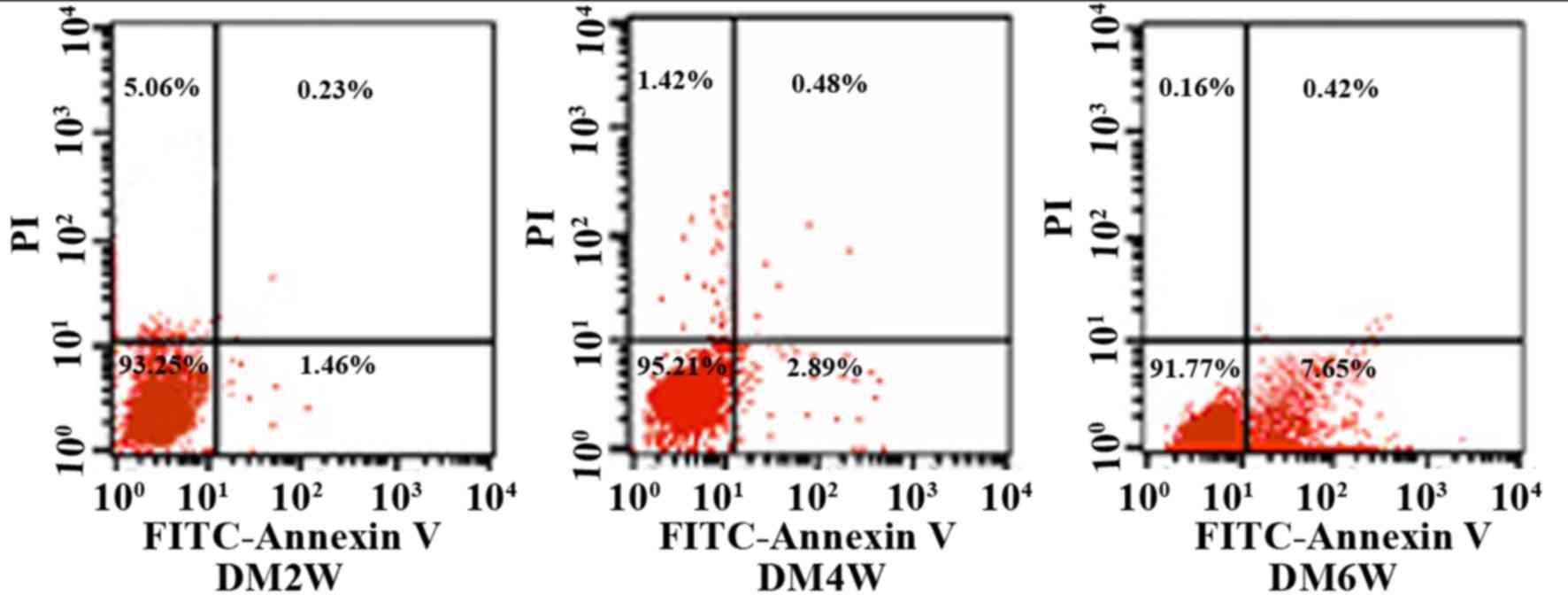
Detection of gastric smooth muscle
cell apoptotic rates using flow cytometry
An Annexin V-FITC/propidium iodide (PI) flow
cytometry kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to
detect cell apoptosis. Annexin V is regarded as a sensitive
indicator of early apoptosis as it binds to phosphatidylserine on
the cell membranes of early apoptotic cells, whereas PI stains cell
nuclei red by readily crossing the cell membranes of late apoptotic
and dead cells. Briefly, the cells were rinsed with
phosphate-buffered saline twice and centrifuged at 243 × g for 5
min at 4°C, following which 1×105 cells were collected and
suspended in 500 µl binding buffer mixed with 5 µl Annexin V-EGFP
and 5 µl PI. This mixture was incubated at room temperature in the
dark for 5–15 min. Flow cytometry was used to observe cell
apoptosis in the DM2W, DM4W and DM6W groups. The cells were divided
into four quadrants, the abscissa was FITC-Annexin V, and the
ordinate was PI. The upper right quadrant represented late
apoptotic cells, the upper left quadrant represented necrotic
cells, the lower left quadrant represented normal cells, and the
lower right quadrant represented early apoptotic cells. Apoptotic
rates were measured as the percentage of apoptotic cells to total
cells.
Western blot assay of protein
expression
The stomach muscle tissues were homogenized to
extract total protein, the protein concentration was determined
with a spectrophotometer at a wavelength of 596 nm. The protein
samples were boiled for 2 min and 40 µg of proteins was loaded and
separated via SDS-PAGE on a 10% gel. The protein was transferred
onto a polyvinylidene difluoride (PVDF) membrane using a semi-dry
transfer method. The sample was rinsed with 5% non-fat milk in
TBS-T buffer [25 mmol/l Tris, 150 mmol/l NaCl and 1% Tween 20 (pH
7.5)] and blocked on the PVDF membrane. The cells were then
cultured with antibodies (all purchased from Cell Signaling
Technology, Inc., Danvers, MA, USA) against PI3K (cat. no. 4263S,
1:1,000), p-AKT (Ser473; cat. no. 4060S; 1:500), p-mTOR (Ser2481;
cat. no. 2974S; 1:1,000), p-AMPK (Thr172; cat. no. 2531S; 1:1,000),
p-TSC-2 (Thr1462; cat. no. 3617S; 1:1,000), p-p70S6K (Thr389; cat.
no. 9234S; 1:500), P-4E-BP1 (Thr37/46; cat. no. 2855S; 1:500), and
β-actin (1:500) at 4°C overnight. The cells were washed with 0.01 M
PBS and incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:1,000; Sigma-Aldrich; Merck KGaA) at room
temperature for 1 h. A gel imaging analysis system was used to
acquire and analyze images; β-actin (cat. no. A5316-.2ML; 1:500;
Sigma-Aldrich; Merck KGaA) served as a reference against which
protein content was calculated.
Statistical analysis
SPSS 19.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistical analysis. Measurement data are expressed as
the means ± standard deviation. Differences between groups were
compared using an independent sample t-test and one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of gastric residual pigment
ratios
The results of the present study indicated the
gastric residual pigment ratio was 40.24±2.15 in the NC group and
50.65±3.31 in the DM6W group (n=10; P<0.05; Table I), suggesting that the diabetic
rats exhibited symptoms of stomach paresis at 6 weeks, with
symptoms worsening with time.
 | Table I.Comparison of gastric residual pigment
ratio and apoptotic rates. |
Table I.
Comparison of gastric residual pigment
ratio and apoptotic rates.
| Group | Gastric residual
pigment ratio (%) | Apoptotic rate
(%) |
|---|
| NC | 40.24±2.15 | – |
| DM2W | 42.82±3.19 | 1.54±0.46 |
| DM4W | 44.72±3.10 | 2.75±0.54 |
| DM6W |
50.65±3.31a |
7.48±0.36b |
Comparison of gastric smooth muscle
cell apoptotic rates
The results of the present study showed that
apoptotic gastric smooth muscle cells were apparent in each
diabetic model group, and the rates of apoptosis increased as the
disease progressed. The rates of apoptosis were 1.54±0.46 in the
DM2 W group, 2.75±0.54 in the DM4 W group, and 7.48±0.36 in the DM6
W group (n=10; P<0.05; Table I;
Fig. 1).
Expression of key PI3K-AKT-mTOR and
AMPK0mTOR pathway proteins in rat gastric muscle
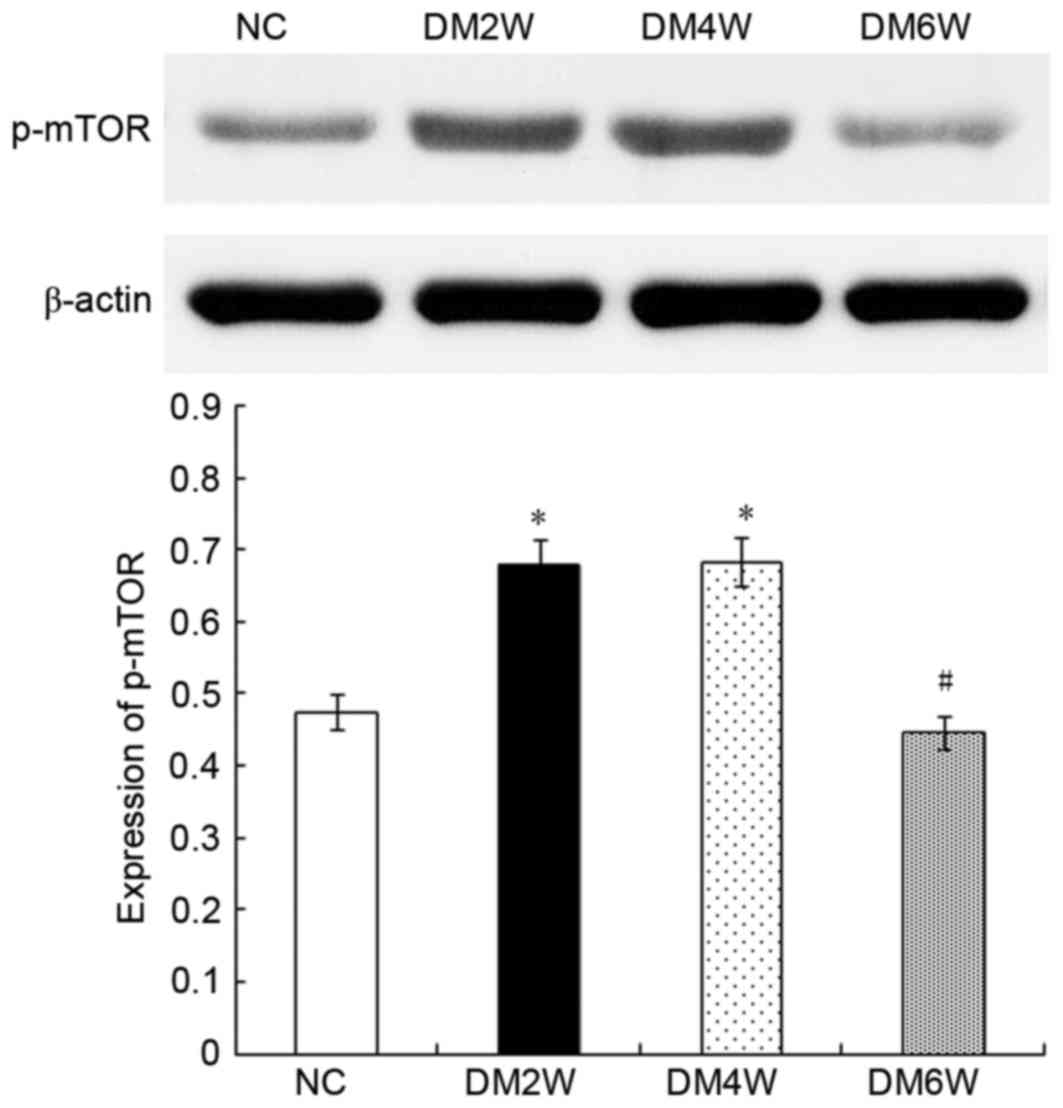
Relative protein expression of p-mTOR
The expression of p-mTOR was 0.48±0.03 in the NC
group, which was lower than the level observed in the DM2W
(0.67±0.04) and DM4W (0.68±0.03) groups (n=10; P<0.01). Compared
with the DM4W group, the DM6W group showed lower levels of p-mTOR
(0.45±0.02; n=10; P<0.01). As shown in Fig. 2, no significant difference was
observed between the NC and DM6W groups.
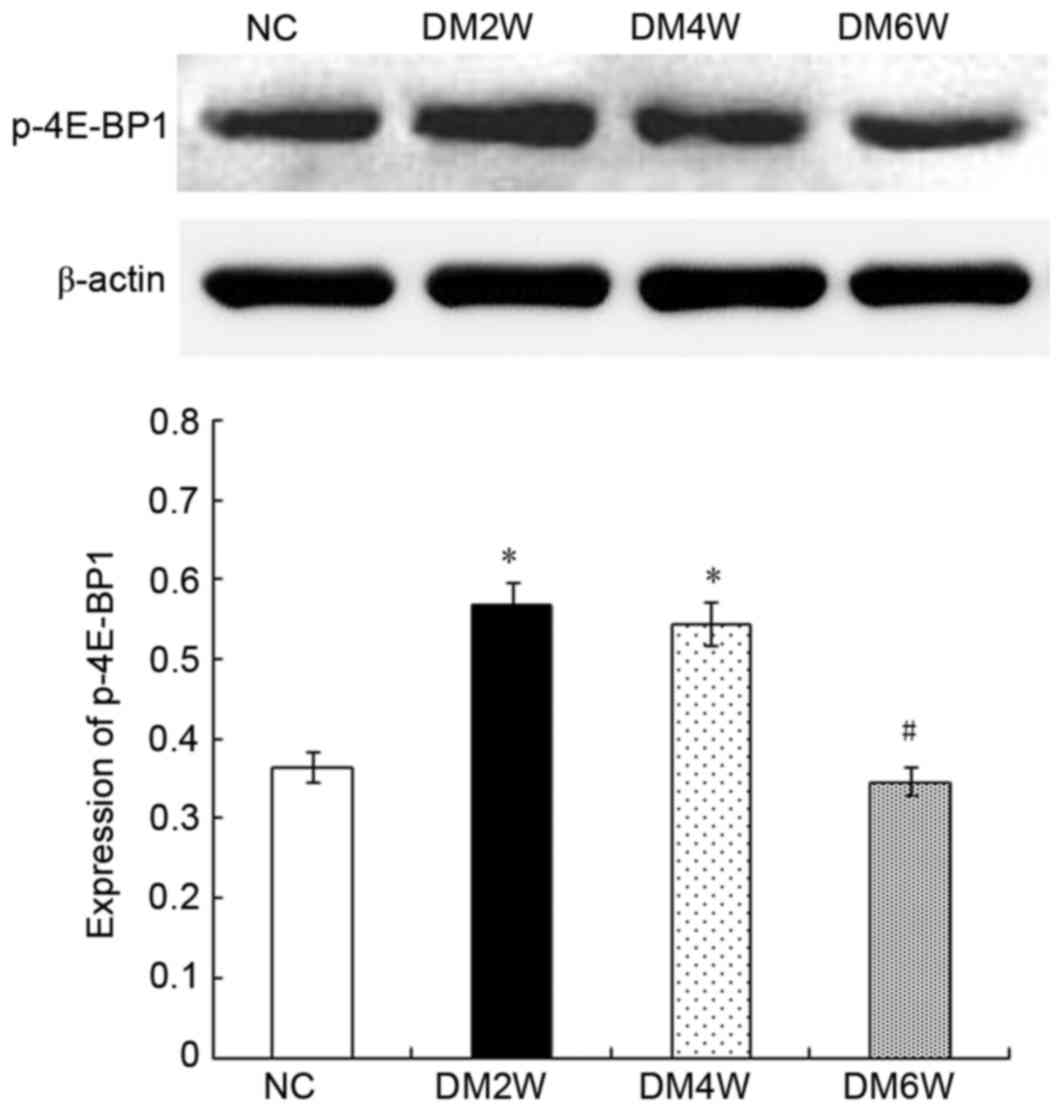
Relative protein expression of P-4E-BP1
Compared with the NC group (0.36±0.05), the
expression levels of P-4E-BP1 were higher in the DM2W (0.57±0.03)
and DM4W (0.54±0.04) groups (n=10; P<0.01). However, compared
with the DM4W group, the DM6W group exhibited a lower expression
level of P-4E-BP1 (0.35±0.02; n=10; P<0.01). No significant
difference was observed between the NC and DM6W groups, as shown in
Fig. 3.
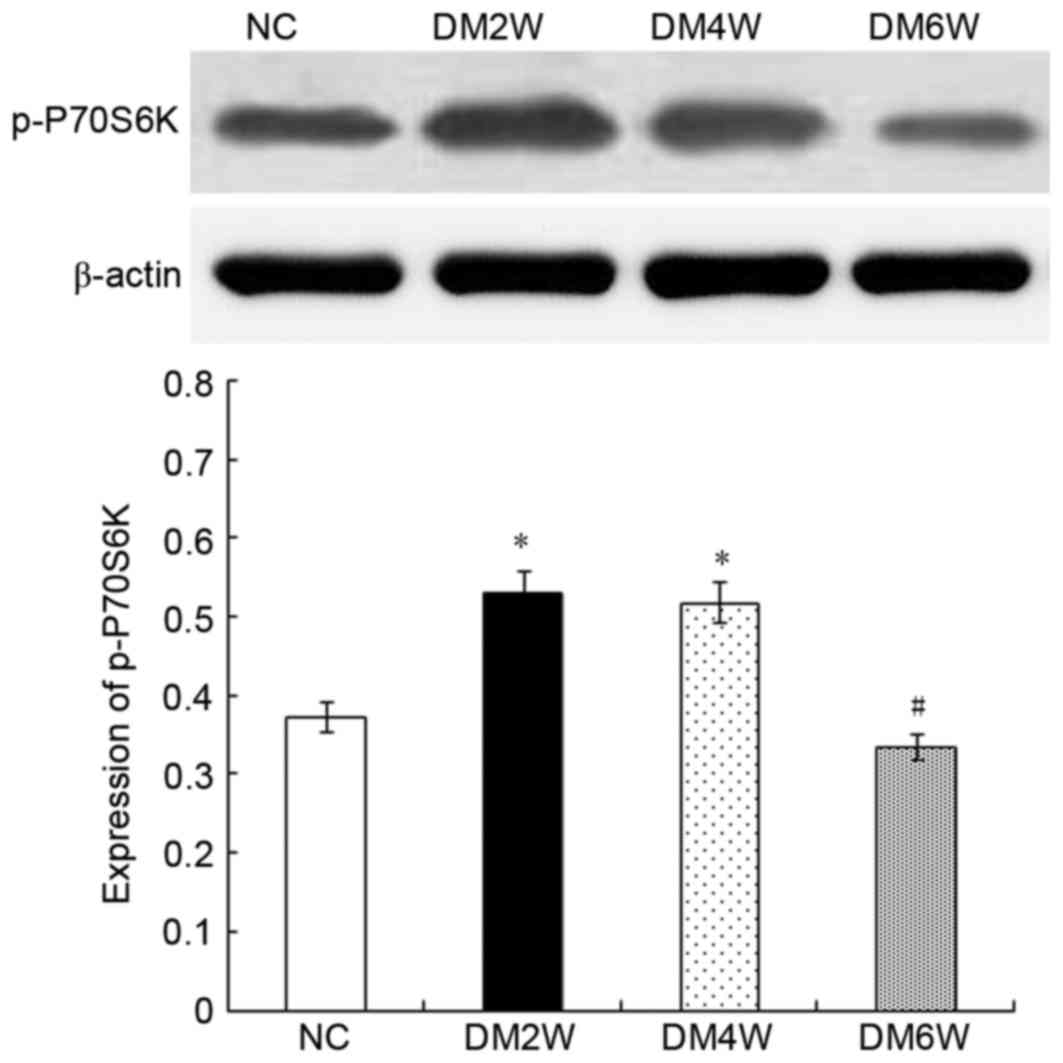
Relative protein expression of p-P70S6K
Compared with the NC group (0.37±0.04), the
expression levels of p-P70S6K were higher in the DM2W (0.53±0.02)
and DM4W (0.52±0.02) groups (n=10; P<0.01). The DM6W group
(0.34±0.02) exhibited a decreased expression level of p-P70S6K
compared with the DM4W group (n=10; P<0.01). As shown in
Fig. 4, no significant difference
was observed between the NC and DM6W groups.
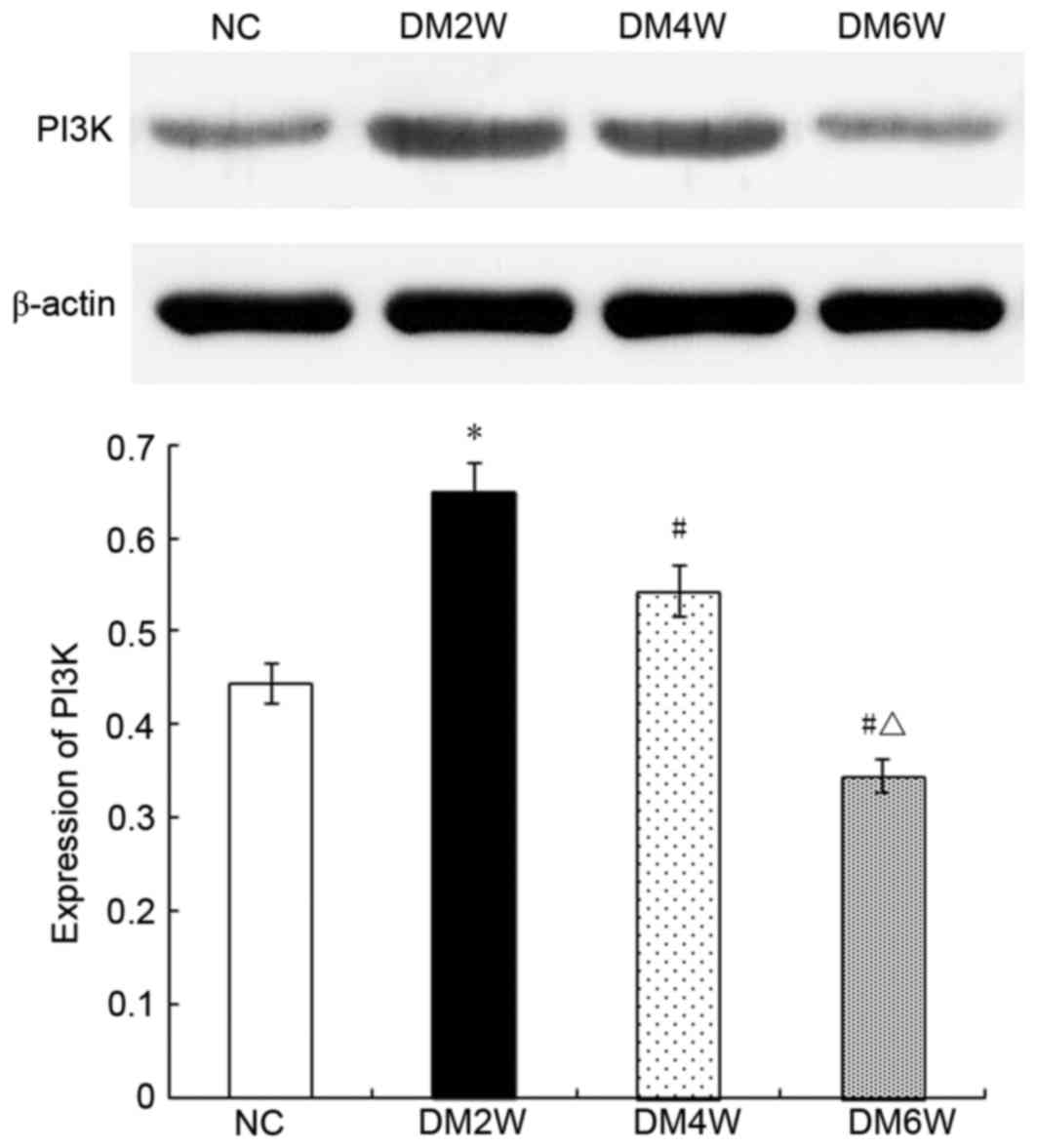
Relative protein expression of PI3K
Compared with the NC group (0.44±0.03), the
expression levels of PI3K were higher in the DM2W (0.65±0.03) and
DM4W (0.54±0.06) groups (n=10; P<0.01), whereas the DM6W group
exhibited lower expression (0.35±0.05; n=10; P<0.01). The
expression levels were also lower in the DM4W and DM6W groups
(n=10; P<0.01), as shown in Fig.
5.
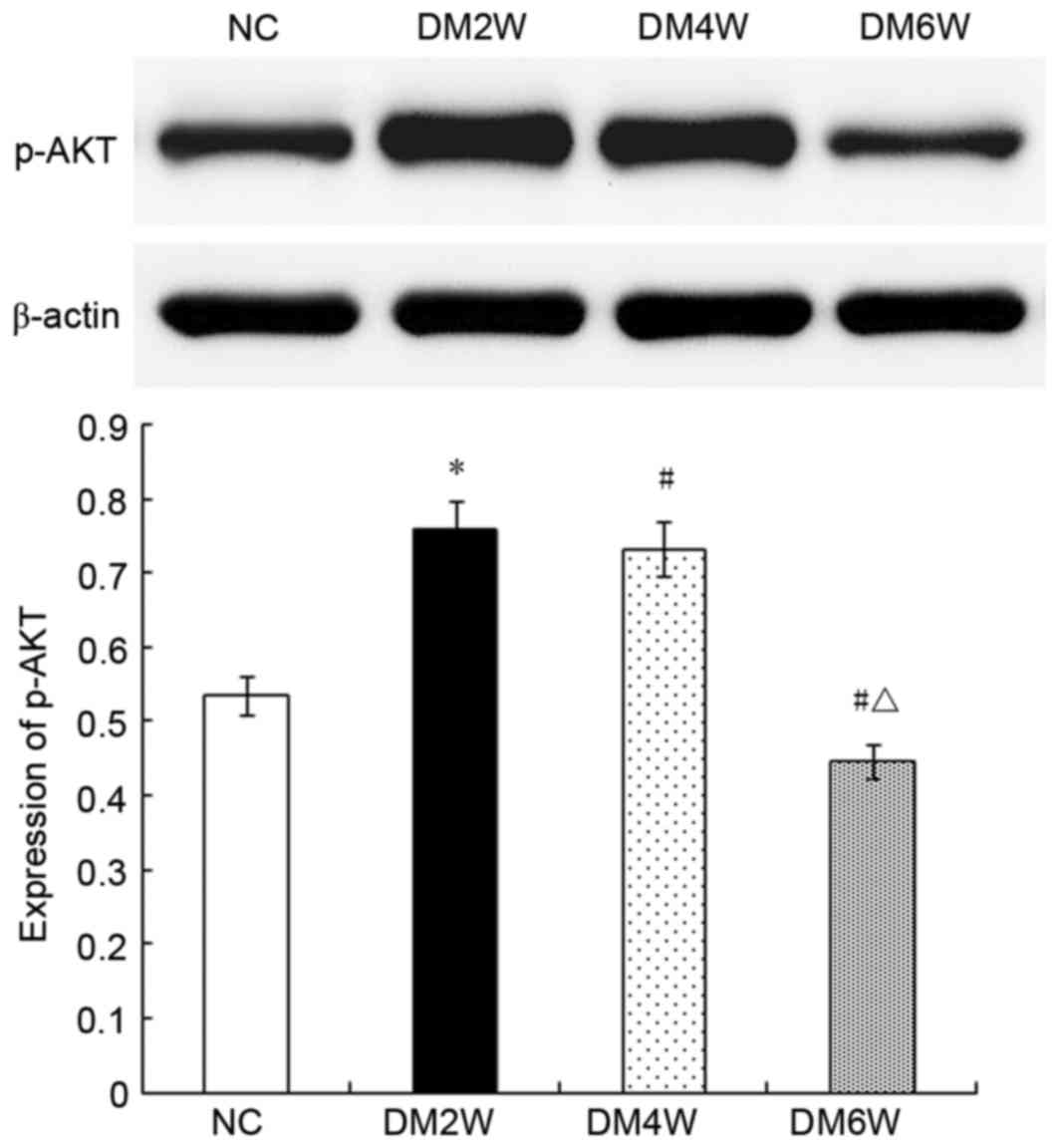
Relative protein expression of p-AKT
The expression levels of p-AKT were higher in the
DM2W (0.75±0.02) and DM4W (0.73±0.03) groups, compared with that in
the NC group (0.53±0.02; n=10; P<0.01). By contrast, the DM6W
group exhibited lower expression (0.44±0.02; n=10; P<0.01). As
shown in Fig. 6, the expression
levels were significantly lower in the DM4W and DM6W groups,
compared with that in the DM2W group (n=10; P<0.01).
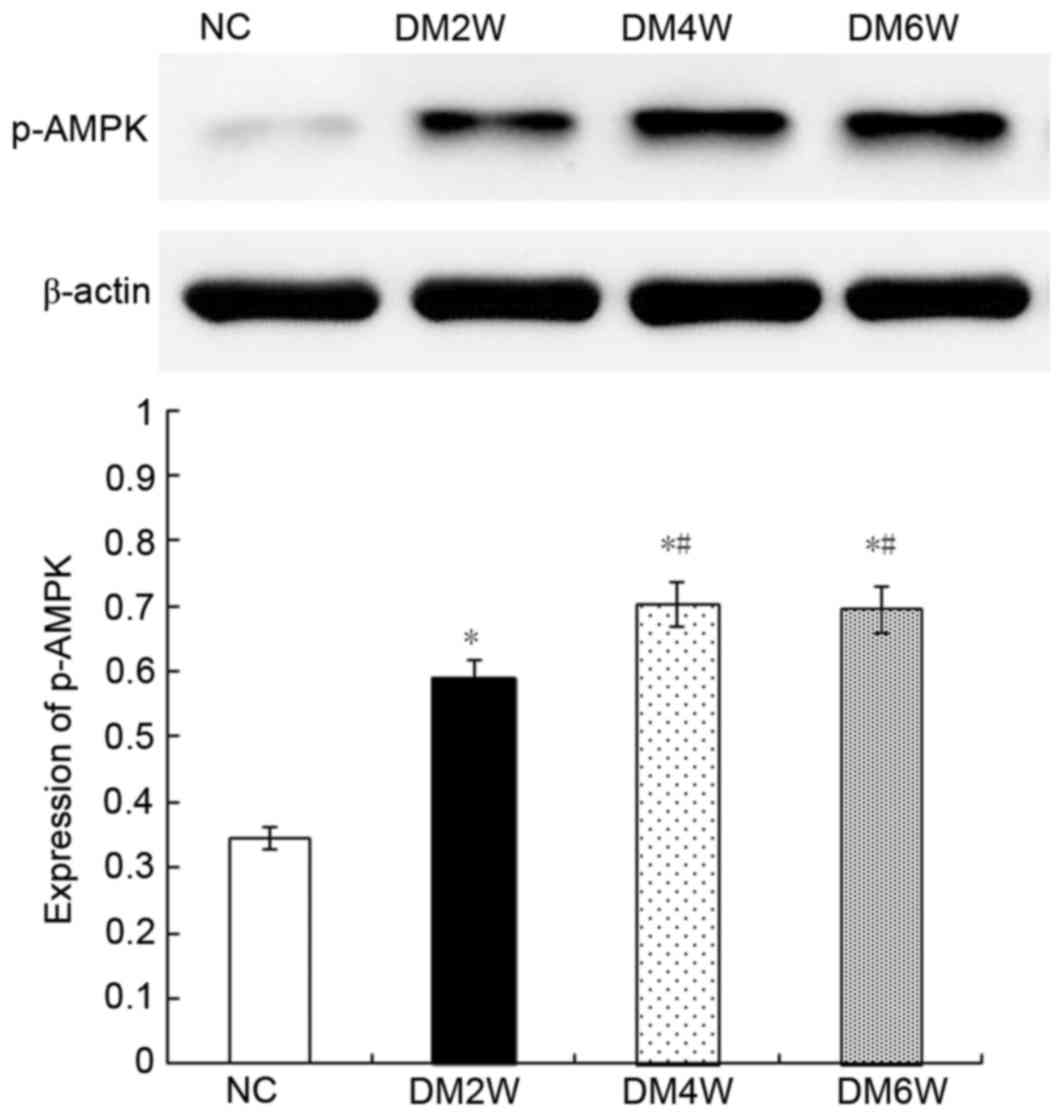
Relative protein expression of p-AMPK
Compared with the NC group (0.34±0.04), the
expression levels of p-AMPK were higher in the DM2W (0.58±0.05),
DM4W (0.70±0.05) and DM6W (0.69±0.05) groups (n=10; P<0.01). The
expression levels were increased in the DM4W and DM6W groups,
compared with that in the DM2W group (n=10; P<0.01); however, no
significant difference was observed between the DM4W and DM6W
groups, as shown in Fig. 7.
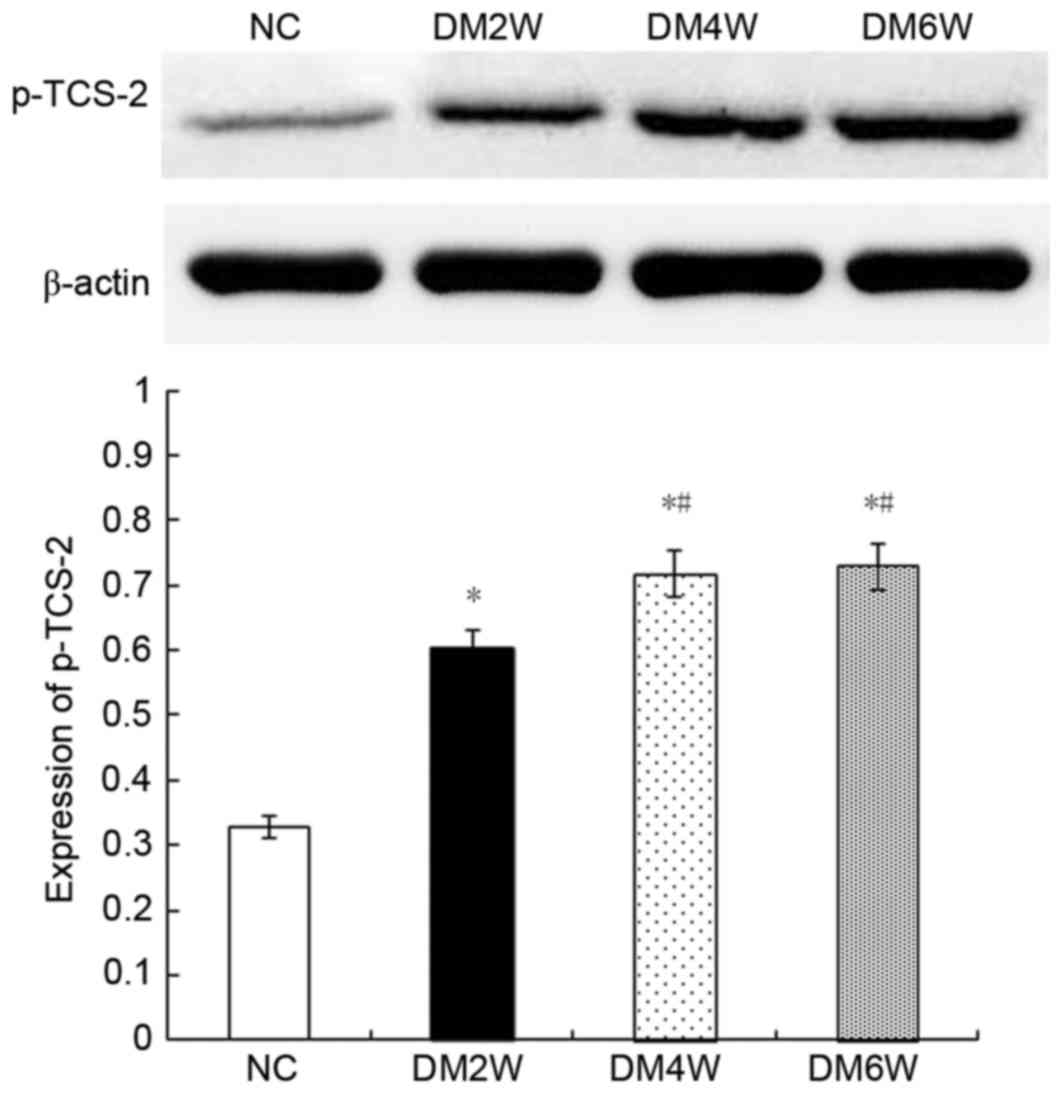
Relative protein expression of p-TCS-2
Compared with the NC group (0.32±0.02), the
expression levels of p-TCS-2 were higher in the DM2W (0.60±0.03),
DM4W (0.72±0.02) and DM6W (0.73±0.04) groups (n=10; P<0.01).
Compared with the DM2W group, the expression was increased in the
DM4W group (n=10; P<0.01). No significant difference was
observed between the DM4W and DM6W groups, as shown in Fig. 8.
Discussion
Diabetic gastroparesis is a complex
pathophysiological process (15).
Investigations on the pathogenesis of diabetic gastroparesis
predominantly focus on high blood sugar-induced neuropathy, stomach
hormone secretion disorder, stress, and certain microvascular
diseases (16,17), whereas few report on apoptosis and
its regulatory pathways. Apoptosis can be induced and controlled by
a variety of cytokines (18). As
each tissue or cell can react differently to different factors,
each cytokine can exhibit different biological effects (19,20).
Apoptosis is triggered by anti- and pro-apoptotic factors,
including the PI3K-AKT-mTOR pathway, which inhibits cell apoptosis.
Under normal circumstances, growth factors, including insulin,
insulin-like growth factor and epidermal growth factor, induce
PI3K-AKT-mTOR activation via their respective receptors (21). By contrast, the AMPK-mTOR pathway
has a pro-apoptotic role. Under conditions of stress, including
ischemia, hypoxia or nutrient deficiency, the AMPK-mTOR pathway is
activated to relieve stress and maintain normal body metabolism
(22).
Hyperglycemia, a characteristic clinical
manifestation of diabetes mellitus, forms the basis of the majority
of diabetes complications. It enables cellular oxidative stress and
metabolic disorders, resulting in hypoxia, decreased amino acid
levels, low adenosine triphosphate and/or high adenosine
monophosphate. Previous studies have found that these changes may
alter the biological effects of mTOR through the AMPK or PI3K-AKT
pathway to regulate apoptosis (23,24).
To investigate the significance of apoptosis in diabetic
gastroparesis, in addition to changes in PI3K-AKT-mTOR and
AMPK-mTOR signaling, the present study established a diabetic rat
model to determine gastric residual pigment ratios and the timing
of the occurrence of diabetic gastroparesis. It was found that
diabetic gastroparesis was present in diabetic rats at 6 weeks,
with the diabetic rats beginning to show symptoms of gastroparesis
at 6 weeks. This differs from the findings of a previous study
(25), which may be associated
with differences between individual animals and feeding conditions.
Following establishment of the diabetic gastroparesis model, it was
found that the apoptotic rates of the gastric smooth muscle cells
during diabetic gastroparesis gradually increased, with
significance at 6 weeks. As apoptosis is a form of programmed cell
death, increased apoptotic rates directly result in the reduction
of normal functional cells, thereby prolonging gastric emptying.
This evidence indicated that increased apoptosis may be an
important cause of diabetic gastroparesis, consistent with the
findings of previous studies (3).
mTOR is a protein factor regulated by the
PI3K-AKT-mTOR pathway and the AMPK-mTOR pathway. Although the
primary activity of mTOR is the phosphorylation of mTOR, its
regulation during apoptosis is achieved by the phosphorylation of
downstream 4E-BP1 and p70S6K (11). Therefore, the expression levels of
p-mTOR, P-4E-BP1 and p-p70S6K reflect the effect of mTOR on
apoptosis. In the present study, the results of the western blot
analysis revealed increased expression levels of p-mTOR, P-4E-BP1
and p-p70S6K when diabetic gastroparesis began to occur, and
decreased expression following its establishment, indicating the
involvement of mTOR and its downstream factors in the initial
occurrence of diabetic gastroparesis. The initial increases in mTOR
activity and downstream factors were associated with the inhibition
of cell apoptosis. Of note, the rates of cell apoptosis increased
continuously during diabetic gastroparesis, indicating that the
anti-apoptotic role of mTOR was not dominant.
PI3K-AKT and AMPK are positive and negative
regulatory factors of the activity of mTOR, respectively. PI3K,
p-AKT, p-AMPK and p-TCS-2 are functional proteins involved in these
two pathways, therefore, their expression level directly affects
the activity of the pathways. Western blot analysis was performed
to investigate the regulation of mTOR activity by the PI3K-AKT and
AMPK pathways during diabetic gastroparesis, which revealed similar
changes in the expression of PI3K and p-AKT, which initially
increased and then decreased. p-AMPK and p-TCS-2 also exhibited
similar expression patterns, which initially increased and were
then maintained. Collectively, these results indicated that the
PI3K-AKT and AMPK pathways were involved in the occurrence of
diabetic gastroparesis, and the mechanism may be associated with
the mTOR-mediated regulation of apoptosis.
During the early stage of diabetic gastroparesis,
the expression of proteins in the PI3K-AKT pathway increased, which
promoted the phosphorylation of mTOR and inhibited anti-apoptotic
activity. A potential reason for this is that, in response to
initial stimulation with high glucose, gastric smooth muscle cells
compensate by increasing upstream growth factors in the PI3K-AKT
pathway by autocrine or paracrine mechanisms to maintain cellular
function, thus activating the PI3K-AKT pathway. When diabetic
gastroparesis results from continuous stimulation by hyperglycemia,
the expression of PI3K-AKT upstream growth factors was decreased,
therefore, the activity of PI3K-AKT was decreased. The changes in
AMPK differed from those of PI3K-AKT, as the expression of
functional proteins continuously increased over the duration of the
diabetic gastroparesis process, indicating activation of its
pro-apoptotic role. This is consistent with the observed increases
in apoptotic rates when diabetic gastroparesis occurred. Of note,
the activity of mTOR downstream of AMPK initially increased and
then was inhibited, suggesting that AMPK was inferior to PI3K-AKT
in regulating the activity of mTOR. During the early stage of
diabetic gastroparesis, the PI3K-AKT-mediated activation of mTOR
increased, which weakened the AMPK-induced inhibition of mTOR
activity. With prolonged duration, the activity of PI3K-AKT
decreased and that of AMPK increased. Therefore, when diabetic
gastroparesis was established, a decrease in the activity of mTOR
occurred.
In conclusion, the present study confirmed that cell
apoptosis was important in the occurrence of diabetic
gastroparesis. During this process, the PI3K-AKT-mTOR and AMPK-mTOR
pathways were activated, but were unable to regulate apoptosis.
Further investigations aim to focus on how the activated
PI3K-AKT-mTOR and AMPK-mTOR pathways are involved in establishing
diabetic gastroparesis.
Acknowledgements
This study was financially supported by grants from
the National Natural Science Foundation of China (grant nos.
81360070 and 81560142).
Glossary
Abbreviations
Abbreviations:
|
4E-BP1
|
eukaryotic translation initiation
factor 4-binding protein 1
|
|
AMP
|
adenosine monophosphate
|
|
AMPK
|
5′ adenosine monophosphate-activated
protein kinase
|
|
AKT
|
protein kinase B
|
|
ATP
|
adenosine triphosphate
|
|
DM2W
|
diabetic model at 2 weeks
|
|
DM4W
|
diabetic model at 4 weeks
|
|
DM6W
|
diabetic model at 6 weeks
|
|
DM8W
|
diabetic model at 8 weeks
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
eIF4E
|
eukaryotic translation initiation
factor 4E
|
|
mTOR
|
mammalian target of rapamycin
|
|
OD
|
optical density
|
|
NC
|
normal control
|
|
P-4E-BP1
|
phosphorylated eukaryotic translation
initiation factor 4-binding protein 1
|
|
p-AKT
|
phosphorylated protein kinase B
|
|
p-AMPK
|
phosphorylated 5′-adenosine
monophosphate-activated protein kinase
|
|
p-TSC-2
|
phosphorylated tuberous sclerosis
complex 2
|
|
p-mTOR
|
phosphorylated mammalian target of
rapamycin
|
|
p-p70S6K
|
phosphorylated p70 ribosomal S6
kinase
|
|
p70S6K
|
p70 ribosomal S6 kinase
|
|
PI
|
propidium iodide
|
|
PI3K
|
phosphoinositide-3-kinase
|
|
PVDF
|
polyvinylidene difluoride
|
|
STZ
|
streptozotocin
|
|
TSC-2
|
tuberous sclerosis complex 2
|
References
|
1
|
Zhao J, Frøkjaer JB, Drewes AM and
Ejskjaer N: Upper gastrointestinal sensory-motor dysfunction in
diabetes mellitus. World J Gastroenterol. 12:2846–2857. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin QH, Shen HX, Wang H, Shou QY and Liu
Q: Curcumin improves expression of SCF/c-kit through attenuating
oxidative stress and NF-κB activation in gastric tissues of
diabetic gastroparesis rats. Diabetol Metab Syndr. 5:122013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen X, Fu XS, Li CP and Zhao HX: ER
stress and ER stress-induced apoptosis are activated in gastric
SMCs in diabetic rats. World J Gastroenterol. 20:8260–8267. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu D, Qian J, Li W, Feng Q, Pan S and
Zhang S: β-hydroxyisovaleryl-shikonin induces human cervical cancer
cell apoptosis via PI3K/AKT/mTOR signaling. Oncol Lett.
10:3434–3442. 2015.PubMed/NCBI
|
|
5
|
Cui H, Wu S, Shang Y, Li Z, Chen M, Li F
and Wang C: Pleurotus nebrodensis polysaccharide(PN50G) evokes A549
cell apoptosis by the ROS/AMPK/PI3K/AKT/mTOR pathway to suppress
tumor growth. Food Funct. 7:1616–1627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo S, Yao Q, Ke Z, Chen H, Wu J and Liu
C: Resveratrol attenuates high glucose-induced oxidative stress and
cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol.
412:85–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han G, Gong H, Wang Y, Guo S and Liu K:
AMPK/mTOR-mediated inhibition of survivin partly contributes to
metformin-induced apoptosis in human gastric cancer cell. Cancer
Biol Ther. 16:77–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Liang X, Chang H, Shu F, Wu Y,
Zhang T, Fu Y, Zhang Q, Zhu JD and Mi M: Ampelopsin-induced
autophagy protects breast cancer cells from apoptosis through
Akt-mTOR pathway via endoplasmic reticulum stress. Cancer Sci.
105:1279–1287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wani ZA, Guru SK, Rao AV, Sharma S,
Mahajan G, Behl A, Kumar A, Sharma PR, Kamal A, Bhushan S and
Mondhe DM: A novel quinazolinone chalcone derivative induces
mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR
signaling pathway in human colon cancer HCT-116 cells. Food Chem
Toxicol. 87:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Cheng D, Zhou S, Zhu B, Hu T and
Yang Q: Overexpression of X-Box binding protein 1 (XBP1) correlates
to poor prognosis and up-regulation of PI3K/mTOR in human
osteosarcoma. Int J Mol Sci. 16:28635–28646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zi D, Zhou ZW, Yang YJ, Huang L, Zhou ZL,
He SM, He ZX and Zhou SF: Danusertib induces apoptosis, cell cycle
arrest, and autophagy but inhibits epithelial to mesenchymal
transition involving PI3K/Akt/mTOR Signaling Pathway in Human
Ovarian Cancer Cells. Int J Mol Sci. 16:27228–27251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu DY, Liu L, Cai YL, Li XL, Qiu ZX, Jin Z
and Xu WX: Natriuretic peptide-dependent cGMP signal pathway
potentiated the relaxation of gastric smooth muscle in
streptozotocin-induced diabetic rats. Dig Dis Sci. 55:589–595.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai YL, Xu DY, Li XL, Qiu ZX, Jin Z and Xu
WX: C-type natriuretic-peptide-potentiated relaxation response of
gastric smooth muscle in streptozotocin-induced diabetic rats.
World J Gastroenterol. 15:2125–2131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asano T, Aida S, Suemasu S and Mizushima
T: Anethole restores delayed gastric emptying and impaired gastric
accommodation in rodents. Biochem Biophys Res Commun. 472:125–130.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tack J, Carbone F and Rotondo A:
Gastroparesis. Curr Opin Gastroenterol. 31:499–505. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singla R, Homko C, Schey R and Parkman HP:
Diabetes-related autoantibodies in diabetic gastroparesis. Dig Dis
Sci. 60:1733–1737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
King RJ, Harrison L, Gilbey SG,
Santhakumar A, Wyatt J, Jones R and Bodansky HJ: Diabetic
hepatosclerosis: Another diabetes microvascular complication?
Diabet Med. 33:e5–e7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Childs BG, Baker DJ, Kirkland JL, Campisi
J and van Deursen JM: Senescence and apoptosis: Dueling or
complementary cell fates? EMBO Rep. 15:1139–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sankari SL, Babu NA, Rajesh E and Kasthuri
M: Apoptosis in immune-mediated diseases. J Pharm Bioallied Sci. 7
Suppl 1:S200–S202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vanhaesebroeck B, Stephens L and Hawkins
P: PI3K signalling: The path to discovery and understanding. Nat
Rev Mol Cell Biol. 13:195–203. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin Y, Bai Y, Ni H, Qiang L, Ye L, Shan Y
and Zhou M: Ac tivation of autophagy through calcium-dependent
AMPK/mTOR and PKCθ pathway causes activation of rat hepatic
stellate cells under hypoxic stress. FEBS Lett. 590:672–682. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar S, Guru SK, Pathania AS, Manda S,
Kumar A, Bharate SB, Vishwakarma RA, Malik F and Bhushan S:
Fascaplysin induces caspase mediated crosstalk between apoptosis
and autophagy through the inhibition of PI3K/AKT/mTOR signaling
cascade in human leukemia HL-60 cells. J Cell Biochem. 116:985–997.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma MQ, Thapalia BA and Lin XH: A 6 hour
therapeutic window, optimal for interventions targeting AMPK
synergism and apoptosis antagonism, for cardioprotection against
myocardial ischemic injury: An experimental study on rats. Am J
Cardiovasc Dis. 5:63–71. 2015.PubMed/NCBI
|
|
25
|
Jin QH, Shen HX, Wang H, Shou QY and Liu
Q: Curcumin improves expression of SCF/c-kit through attenuating
oxidative stress and NF-κB activation in gastric tissues of
diabetic gastroparesis rats. Diabetol Metab Syndr. 5:122013.
View Article : Google Scholar : PubMed/NCBI
|