Introduction
Gliomas are the most common and most fatal tumors of
all types of brain malignancy (1,2). The
general treatment measures against gliomas include surgery,
radiotherapy and chemotherapy; however, the prognosis is poor, and
the median survival time of gliomas is only 6–14 months (3–5).
Gliomas are often resistant to antitumoral chemotherapeutic
strategies, thus limiting the efficacy of treatment (4). The pharmacological activities of
traditional Chinese medicines have attracted increasing attention
regarding their therapeutic effects against glioblastoma (6–8).
Saikosaponin D (SSd) is one of the major Saponin
components derived from the dried roots of Bupleurum
falactum, a traditional Chinese medicine plant. It has been
reported that SSd exerts anticancer activities in cervical and lung
cancer cells, and hepatocellular carcinoma cells (9–12).
SSd may potentiate tumor necrosis factor (TNF)-α-mediated cell
death via suppression of TNF-α-induced nuclear factor (NF)-κB
activation, while inducing apoptosis by enhancing the loss of
mitochondrial membrane potential (13). In addition, SSd has been
demonstrated as an inhibitor of cell survival signaling, and
subsequently attenuates the expression of B-cell lymphoma-extra
large (14). However, the
molecular mechanisms remain unknown.
Although previous studies have indicated that SSd
exerts anticancer activities in various tumor cell lines (9–12),
the effects of it in central nervous system (CNS) malignant tumors
remain unknown. In the present study, the growth potential,
proliferation inhibition and apoptosis induction effects of SSd on
human U87 glioma cells were investigated. In addition, the possible
mechanisms underlying SSd-induced growth arrest and apoptosis in
glioma cells were evaluated.
Materials and methods
Chemicals and reagents
SSd (purity, 96%) was purchased from the National
Institutes for Food and Drug Control of China (Beijing, China). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) was
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Akt (cat. no.
4691), phosphorylated (p)-Akt (cat. no. 4060), extracellular
signal-regulated kinases (ERK; cat. no. 4695), p-ERK (cat. no.
4370), c-Jun N-terminal kinases (JNK; cat. no. 9258), p-JNK (cat.
no. 4668), cleaved caspase-3 (cat. no. 9664), β-actin (cat. no.
12620) and peroxidase-conjugated anti-rabbit (cat. no. 7074)
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Chemicals for buffer preparations were
purchased from Sigma-Aldrich (Merck KGaA).
Cell line and cell culture
Human U87 glioma cells were obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China) and grown
in DMEM containing 10% fetal bovine serum (Haoyang Biological
Products Technology Co., Ltd., Tianjin, China), 100 U/ml
penicillin, 100 µg/ml streptomycin, and incubated in a humidified
atmosphere of 5% CO2 at 37°C.
Cell viability assay
Cell viability was assessed using an MTT bromide
assay. U87 cells (200 µl; 3×104 cells/ml) were seeded into 96-well
tissue culture plates. Following overnight incubation, the cells
were exposed to serial concentrations of SSd (1, 2, 3, 4, 5, 6, 7
and 8 µM) or control medium for 48 h. Subsequently, 10 µl MTT
reagents was added to each well and incubated at 37°C for 4 h,
followed by the addition of 150 µl dimethyl sulfoxide to each well.
The plates were agitated for 10 min. Then, the absorbance was read
at a wavelength of 490 nm using an iMark™ microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each experiment
was performed with six replicate wells for each condition, and the
data were obtained from three independent experiments. The
half-maximal inhibitory concentration (IC50) of SSd was
calculated using the Logit method (15).
Hoechst 33258 staining and
immunofluorescence
U87 cells in 6-well plates were treated with
different concentrations of SSd for 48 h, washed with
phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde
for 10 min. Subsequently, the cells were washed with PBS, and
stained with Hoechst 33258 for 5 min at room temperature. The
nuclear morphology was observed using a laser scanning confocal
microscope. For quantification, three different fields were
randomly selected and counted under the microscope. The apoptotic
rates were calculated as the percentage of apoptotic cells relative
to the total number of cells.
Flow cytometric evaluation of
apoptosis
Cells treated with different concentrations of SSd
were seeded into 6-well plates (1.8×105 cells/well) for 48 h and
isolated with trypsin. The cells were supplemented with 100 µl DMEM
medium and analyzed according to the manufacturer's instructions
using the Muse™ Annexin V and Dead Cell Assay kit (Muse™ Cell
Analyzer; Merck KGaA).
Western blotting
Following SSd treatment for 48 h, U87 cells were
washed with ice-cold PBS three times and resuspended in 100 µl
radioimmune precipitation buffer [100 mM NaCl and 100 mM sodium
fluoride, 20 mM Tris-HCl (pH 7.4), 2.5 mM EDTA, 1% SDS, 1% Triton
X-100 and 1% sodium deoxycholate]. The lysate was centrifuged at
12,000 × g for 20 min, and 50 µg cell lysate protein was used for
western blotting. Proteins were separated by 10% SDS-PAGE and
transferred to nitrocellulose membranes. Membranes were incubated
overnight individually with primary antibodies at a dilution of
1:1,000 in TBS containing 0.1% Tween-20 at 4°C, and the membranes
were incubated with peroxidase-conjugated anti-rabbit IgG (1:5,000)
for 2 h at room temperature. All bands were detected using the
enhanced chemiluminescence (ECL) system (Tanon Science &
Technology Co., Ltd., Shanghai, China) according to the
manufacturer's instructions (16).
Statistical analysis
The data are presented as the mean ± standard
deviations as indicated. Statistical analyses for comparison of
mean values were performed by one-way analysis of variance followed
by Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference. All data were derived from
three independent experiments.
Results
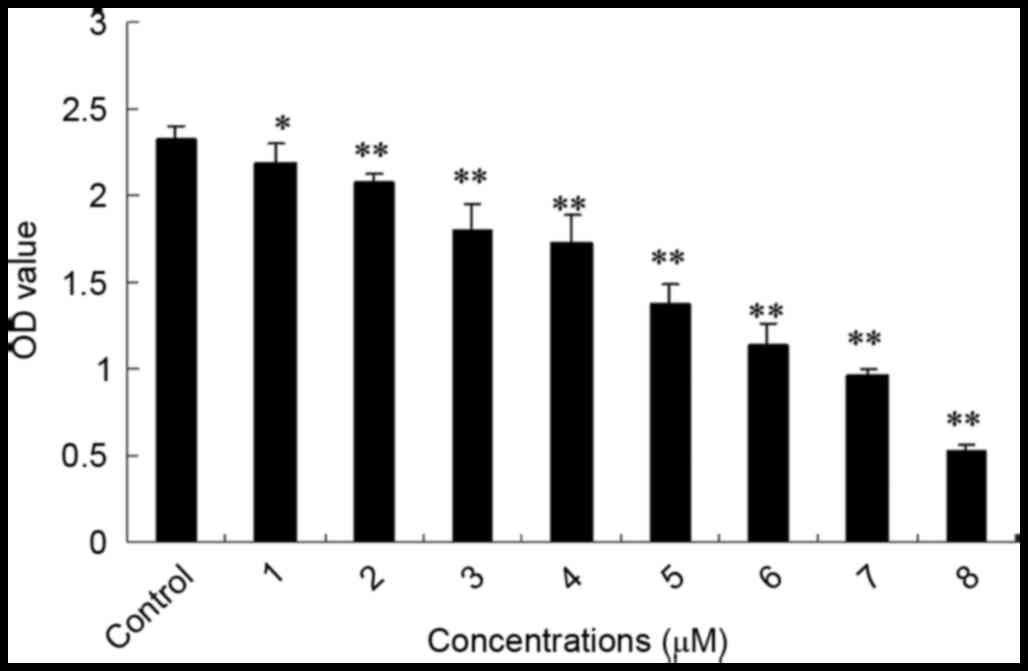
SSd treatment inhibited the
proliferation of U87 cells
The mitochondria of living cells break down MTT to
produce formazan, and the quantity of formazan corresponds with the
living cell number. To determine whether SSd inhibits U87 glioma
cell growth, the cell viability rate was assessed using the MTT
method. In the present study, obvious inhibitory effects on the
proliferation of U87 cells were observed with SSd exposure. As
shown in Fig. 1, following
treatment with 1–8 µM SSd for 48 h, the proliferation rate of U87
cells was significantly reduced in a dose-dependent manner when
compared with the control group. Additionally, the half maximal
inhibitory concentration (IC50) value of SSd was 4.79
µM. The result indicates that SSd inhibited the viability of U87
glioma cells.
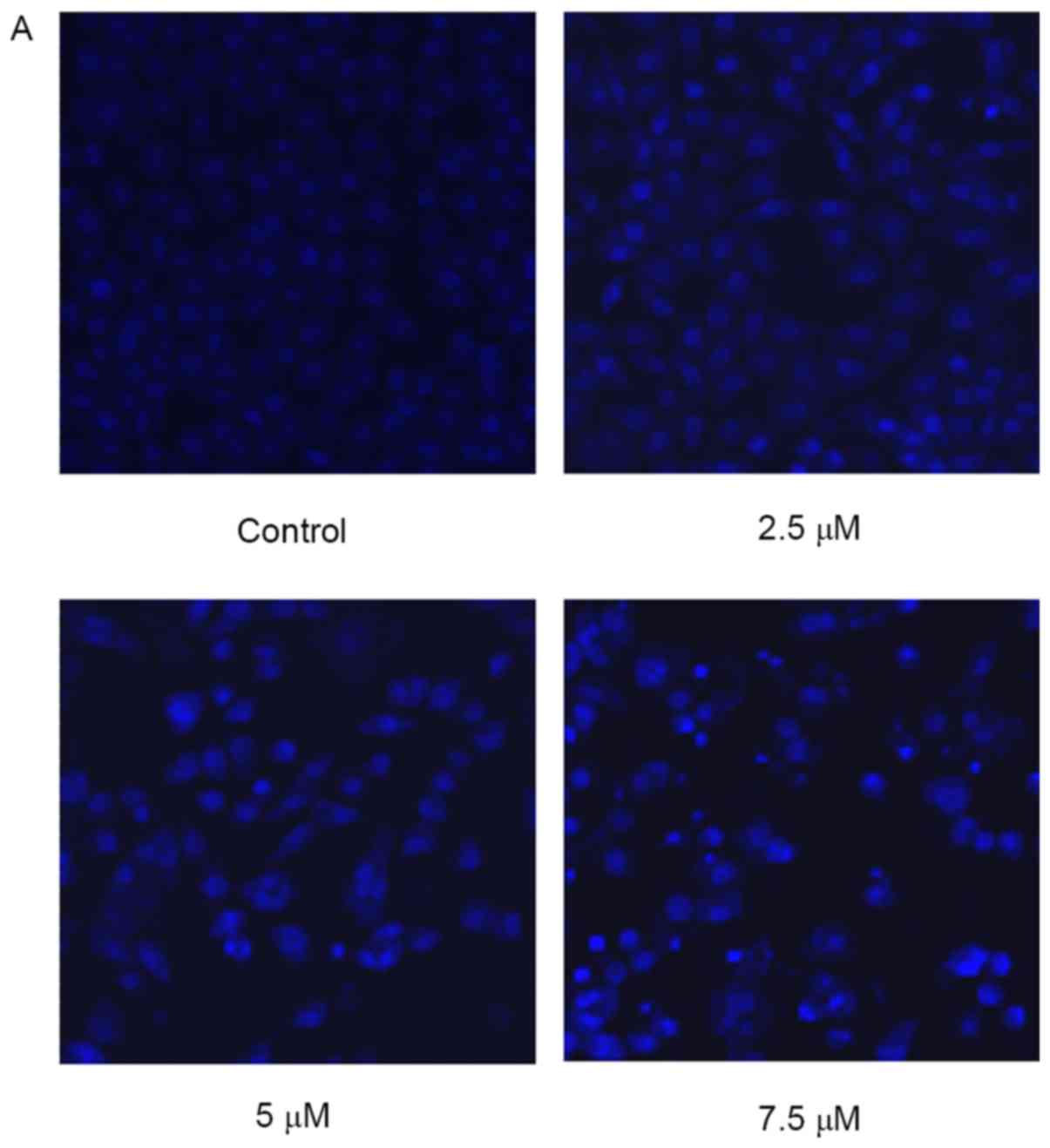
Effect of SSd treatment on changes of
cellular morphology
When detecting nuclear morphological changes, U87
cells were stained with Hoechst 33258 and observed under a laser
scanning confocal microscope. Intact nuclei in the living cells
were stained blue, and the morphology was round or oval, while the
condensed or fragmented nuclei in apoptotic cells were stained
bright blue. As illustrated in Fig.
2, the cells treated with 2.5 µM SSd began to exhibit nuclear
morphological changes when compared with the control cells, and the
nuclei of cells treated with 7.5 µM SSd were markedly brighter,
indicating a high prevalence of nuclear chromatin and
fragmentation.
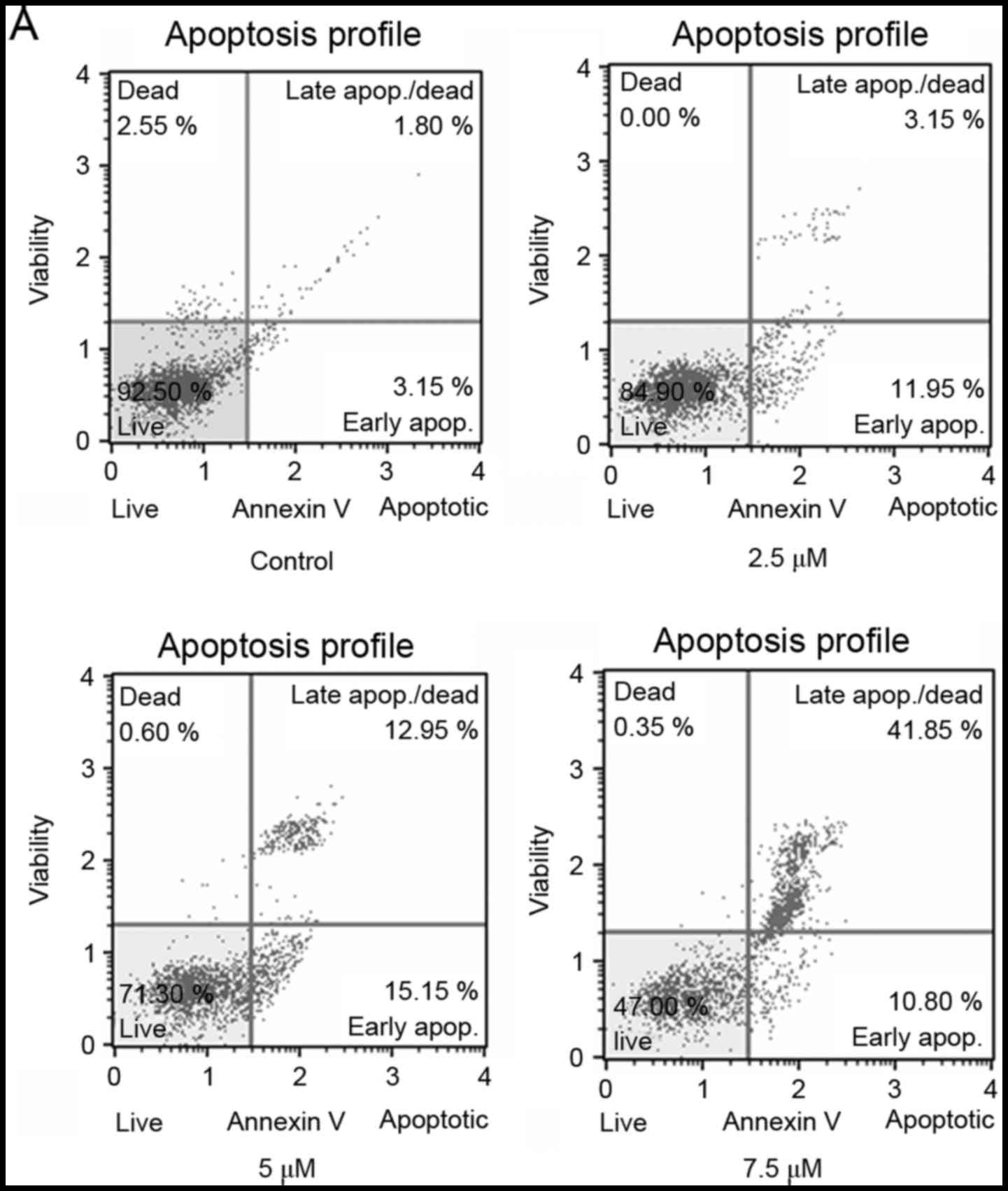
SSd treatment induced apoptosis in U87
cells
To further evaluate whether the inhibition of cell
proliferation in U87 cells was associated with the induction of
apoptosis, Annexin V staining was used to assess the rate of
apoptotic cells following SSd treatment. As shown in Fig. 3, treatment with SSd significantly
increased the proportion of Annexin V-positive cells in a
dose-dependent manner. The results indicate that SSd treatment
inhibited the viability of U87 cells by inducing apoptosis.
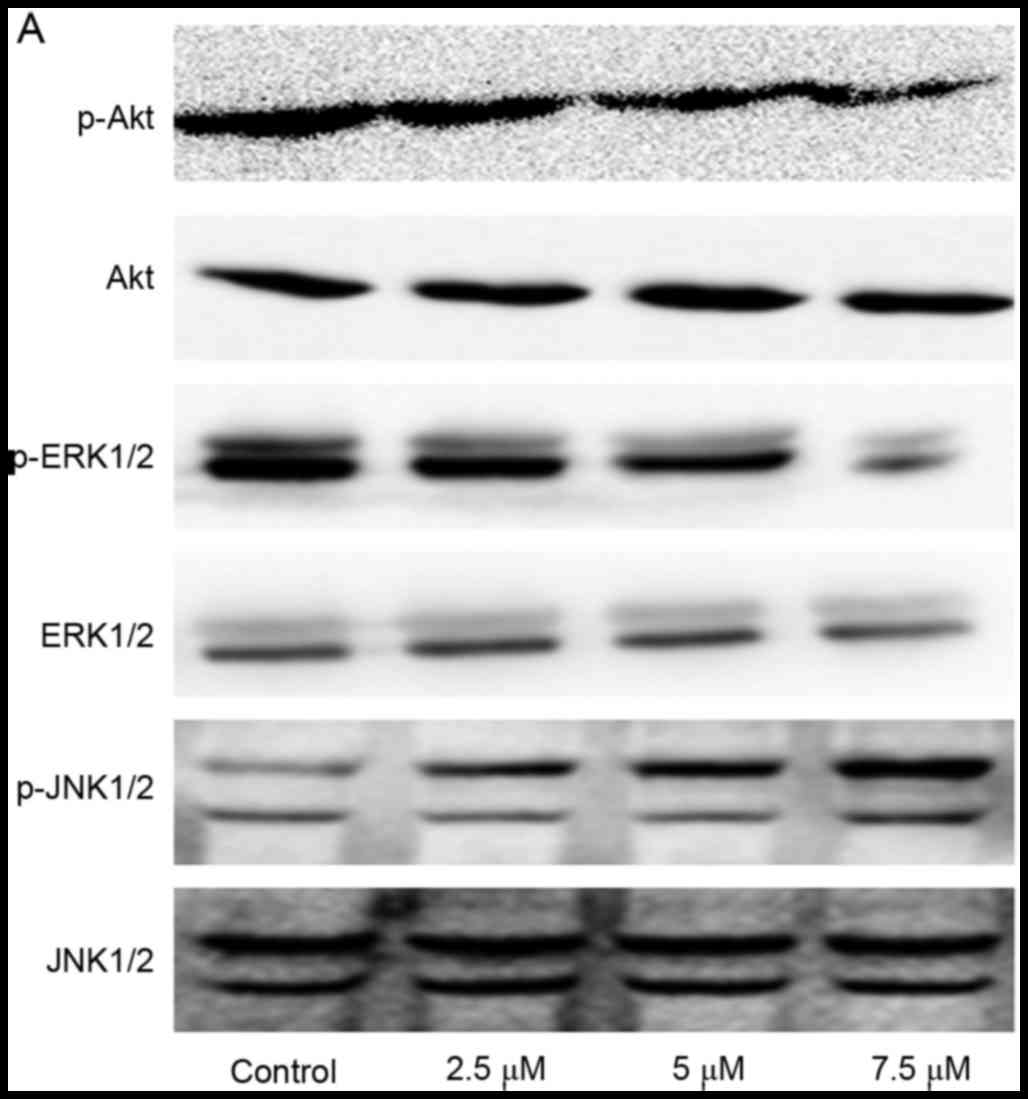
Effect of SSd treatment on
phosphatidylinositol 3-kinases (PI3K)/Akt, ERK and JNK signaling
pathways in U87 cells
To further elucidate the possible mechanisms
underlying the effects of SSd treatment on U87 cells, the
p-Akt/Akt, p-ERK/ERK, p-JNK/JNK protein expression levels with
western blot analysis. The data demonstrated that SSd exposure
induced a significant decrease of p-Akt and p-ERK relative protein
expression levels, and increased p-JNK protein expression levels
(Fig. 4; P<0.05). These results
indicated that treatment with SSd potentially downregulated the
PI3K/Akt and ERK signaling pathways, increased JNK activation and
further enhanced apoptosis in U87 cells.
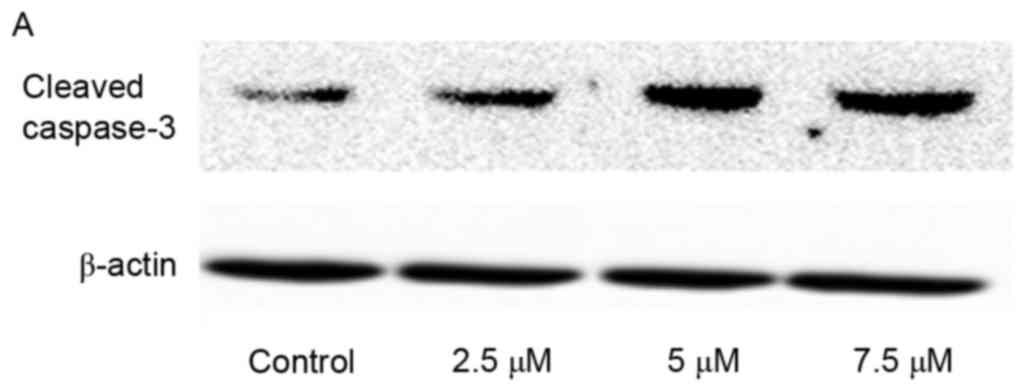
Activation of caspase-3 induced by SSd
exposure
Caspases are critical mediators during the process
of apoptosis. In all of apoptosis-associated caspases, caspase-3 is
a key effector or executioner for programmed cell death (17). Thus, to determine whether caspase-3
was involved in SSd-induced cytotoxicity, the expression levels of
cleaved caspase-3, an indicator of its activity, were examined. In
the present study, SSd treatment significantly increased the
expression level of cleaved caspase-3 (Fig. 5), indicating that SSd-induced
apoptosis in U87 cells may be associated with the promoted
activation of caspase-3.
Discussion
As a traditional Chinese medicine, B. falcatum
L is commonly administered for the treatment of hepatopathy,
inflammation and viral infection in Asia. Triterpene saponins are
the major pharmaceutical ingredients in B. falcatum L, which
are divided into saikosaponin-a, -b, -c and -d according to the
different structures. SSd is one of the most active ingredients of
triterpene saponins. During the past decades, studies have focused
on SSd, as it demonstrates numerous beneficial properties,
including anti-inflammatory, antioxidant and antitumor effects
(12,18,19).
In the present study, it was demonstrated that SSd treatment
inhibited the proliferation of human malignant glioma U87 cells,
indicating that SSd may exert potential beneficial effects in the
treatment of malignant gliomas.
Apoptosis is recognized as the most important form
of cell death in multicellular organisms, and occurs in
physiological and pathological conditions. Certain types of
cytotoxic stresses, such as hypoxia, UV, infared irradiation and
chemotherapeutic drugs, initiate apoptosis to remove target cells
(20,21). Biochemical alterations of apoptosis
include phosphatidylserine externalization, chromosomal DNA
cleavage, and activation of a family of proteases (22). Apoptosis is important for the
inhibition of cancer development (23,24).
At present, drug-induced apoptosis is considered to be the primary
strategy for curing tumors (25).
Previous studies have confirmed that SSd exposure inhibits
proliferation and promotes apoptosis in certain tumor cells
(26–28). However, whether SSd induces
apoptosis in glioma cells has yet to be elucidated. In the present
study, the addition of SSd led to increased apoptosis in human U87
glioma cells, as measured by Hoechst 33258 staining and Annexin V
staining assays, implying that SSd treatment exerts significant
cytotoxic effects by increasing apoptosis in glioma cells.
In the current study, it was also found that SSd
inhibited the PI3K/Akt signaling pathway in a dose-dependent
manner, indicating that depression of the PI3K/AKT signaling
pathway may be associated with SSd-mediated apoptosis and
proliferation inhibition. Akt, also known as protein kinase B, is a
primary downstream effector of the PI3K signal transduction
pathway, with crucial functions in regulating cell proliferation
and survival (29,30). Furthermore, it has been reported
that the Akt signaling pathway is particularly important in
preventing apoptosis (31,32) and inhibiting the activation of Akt
may induce apoptosis (33). ERK
and JNK are members of the mitogen-activated protein kinase (MAPK)
family, and have been shown to be key in cell proliferation,
differentiation, development and programmed cell death (34). Numerous studies have indicated that
abnormalities in MAPK signaling pathways were involved in the
pathological processes of various types of cancer (35–37).
In the current study, the aim was to investigate the effects of SSd
exposure on the protein expression levels of ERK and JNK. The
results demonstrated that SSd treatment significantly inhibited the
activation of ERK and stimulated the phosphorylation of JNK.
There are three apoptotic pathways found in mammals:
The extrinsic pathway (death receptor-mediated pathway), the
intrinsic pathway (mitochondrial-mediated pathway) and the granzyme
B pathway. The key regulatory factors in these three pathways are
the caspases (cysteinyl aspartate-specific proteinases), which are
activated following cell damage and are responsible for regulating
cell apoptosis (38). Accordingly,
in recent years, much attention has been paid to developing
anticancer therapeutic strategies that modulate the activation of
caspases to inhibit tumor progression. Caspase-3 is a major
mediator during the execution period of cell apoptosis. When
activated by upstream signaling molecules, such as caspase-8,
caspase-9 or caspase-10, caspase-3 would be either partially or
totally responsible for the proteolysis of many downstream key
proteins associated with apoptosis (39). In order to elucidate the molecular
mechanisms responsible for SSd-induced apoptosis in U87 cells, the
activity of caspase-3 was analyzed in the present study. The
findings indicated that the addition of SSd to U87 cells promoted
caspase-3 activity, demonstrating the role of capase-3 activation
in SSd-induced apoptosis.
In conclusion, the present study demonstrated that
SSd treatment in human glioma cells inhibits cell proliferation,
downregulates phosphorylation of Akt and ERK, upregulates JNK and
caspase-3 activities and eventually causes cell apoptosis.
Collectively, these observations provide further understanding of
the pharmacological activity of SSd. However, future work is
required to elucidate the underlying mechanisms of SSd-induced
tumor cell apoptosis.
Acknowledgements
The present study was supported by the Department of
Education of Jilin Province (grant no. 2015407) and Project of
Science & Technology Development of Jilin Province (grant no.
201205079).
References
|
1
|
Crocetti E, Trama A, Stiller C, Caldarella
A, Soffietti R, Jaal J, Weber DC, Ricardi U, Slowinski J and
Brandes A; RARECARE working group, : Epidemiology of glial and
non-glial brain tumours in Europe. Eur J Cancer. 48:1532–1542.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ricard D, Idbaih A, Ducray F, Lahutte M,
Hoang-Xuan K and Delattre JY: Primary brain tumours in adults.
Lancet. 379:1984–1996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de-Almeida-Sassi F, Lunardi-Brunetto A,
Schwartsmann G, Roesler R and Abujamra AL: Glioma revisited: From
neurogenesis and cancer stem cells to the epigenetic regulation of
theniche. J Oncol. 2012:5378612012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lima FR, Kahn SA, Soletti RC, Biasoli D,
Alves T, da Fonseca AC, Garcia C, Romão L, Brito J, Holanda-Afonso
R, et al: Glioblastoma: Therapeutic challenges, what lies ahead.
Biochim Biophys Acta. 1826:338–349. 2012.PubMed/NCBI
|
|
5
|
Shahar T, Nossek E, Steinberg DM, Rozovski
U, Blumenthal DT, Bokstein F, Sitt R, Freedman S, Corn BW, Kanner
AA and Ram Z: The impact of enrollment in clinical trials on
survival of patients with glioblastoma. J Clin Neurosci.
19:1530–1534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shao J, Zheng D, Jiang Z, Xu H, Hu Y, Li X
and Lu X: Curcumin delivery by methoxy polyethylene
glycol-poly(caprolactone) nanoparticles inhibits the growth of C6
glioma cells. Acta Biochim Biophys Sin (Shanghai). 43:267–274.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YB, Hu Y, Li Z, Wang P, Xue YX, Yao
YL, Yu B and Liu YH: Artemether combined with shRNA interference of
vascular cell adhesion molecule-1 significantly inhibited the
malignant biological behavior of human glioma cells. PLoS One.
8:e608342013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang FY, Hu Y, Que ZY, Wang P, Liu YH,
Wang ZH and Xue YX: Shikonin inhibits the migration and invasion of
human glioblastoma cells by targeting phosphorylated β-catenin and
phosphorylated PI3K/Akt: A potential mechanism for the anti-glioma
efficacy of a traditional chinese herbal medicine. Int J Mol Sci.
16:23823–23848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tundis R, Bonesi M, Deguin B, Loizzo MR
and Menichini F, Conforti F, Tillequin F and Menichini F: Cytotoxic
activity and inhibitory effect on nitric oxide production of
triterpene saponins from the roots of Physospermum verticillatum
(Waldst & Kit) (Apiaceae). Bioorg Med Chem. 17:4542–4547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q, Zheng XL, Yang L, Shi F, Gao LB,
Zhong YJ, Sun H, He F, Lin Y and Wang X: Reactive oxygen
species-mediated apoptosis contributes to chemosensitization effect
of saikosaponins on cisplatin-induced cytotoxicity in cancer cells.
J Exp Clin Cancer Res. 29:1592010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang BF, Lin S, Bai MH, Song LQ, Min WL,
Wang M, Yang P, Ma HB and Wang XJ: Effects of SSd combined with
radiation on inhibiting SMMC-7721 hepatoma cell growth. Med Sci
Monit. 20:1340–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong VK, Li T, Law BY, Ma ED, Yip NC,
Michelangeli F, Law CK, Zhang MM, Lam KY, Chan PL and Liu L:
Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell
death in apoptosis-defective cells. Cell Death Dis. 4:e7202013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong VK, Zhang MM, Zhou H, Lam KY, Chan
PL, Law CK, Yue PY and Liu L: Saikosaponin-d enhances the
anticancer potency of TNF-α via overcoming its undesirable response
of activating NF-kappa B signalling in cancer cells. Evid Based
Complement Alternat Med. 2013:7452952013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu YL, Kuo PL, Chiang LC and Lin CC:
Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in
induction of apoptosis and cell cycle arrest by saikosaponin d in
human hepatoma cell lines. Cancer Lett. 213:213–221. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Acharya AS, Acharya NK and Dash AP:
Software for estimating LD50 and LD90 by logit analysis. Comput
Methods Programs Biomed. 34:255–256. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nesbitt SA and Horton MA: A nonradioactive
biochemical characterization of membrane proteins using enhanced
chemiluminescence. Anal Biochem. 206:267–272. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin X, Wu S, Wang Q, Shi Y, Liu G, Zhi J
and Wang F: Saikosaponin-D reduces H2O2-induced PC12 cell apoptosis
by removing ROS and blocking MAPK-dependent oxidative damage. Cell
Mol Neurobiol. 36:1365–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang P, Ren J, Tang J, Zhang D, Li B and
Li Y: Estrogen-like activities of saikosaponin-d in vitro: A pilot
study. Eur J Pharmacol. 626:159–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kin KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Townson JL, Naumov GN and Chambers AF: The
role of apoptosis in tumor progression and metastasis. Curr Mol
Med. 3:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saddoughi SA, Song P and Ogretmen B: Roles
of bioactive sphingolipids in cancer biology and therapeutics.
Subcell Biochem. 49:413–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He S, Lu G, Hou H, Zhao Z, Zhu Z, Lu X,
Chen J and Wang Z: Saikosaponin-d suppresses the expression of
cyclooxygenase-2 through the phospho-signal transducer and
activator of transcription 3/hypoxia-inducible factor-1α pathway in
hepatocellular carcinoma cells. Mol Med Rep. 10:2556–2562.
2014.PubMed/NCBI
|
|
27
|
Jang MJ, Kim YS, Bae EY, Oh TS, Choi HJ,
Lee JH, Oh HM and Lee SW: Saikosaponin D isolated from Bupleurum
falcatum inhibits selectin-mediated cell adhesion. Molecules.
19:20340–20349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu RY and Li JP: Saikosaponin-d inhibits
proliferation of human undifferentiated thyroid carcinoma cells
through induction of apoptosis and cell cycle arrest. Eur Rev Med
Pharmacol Sci. 18:2435–2443. 2014.PubMed/NCBI
|
|
29
|
Davis WJ, Lehmann PZ and Li W: Nuclear
PI3K signaling in cell growth and tumorigenesis. Front Cell Dev
Biol. 3:242015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wymann MP, Zvelebil M and Laffargue M:
Phosphoinositide 3-kinase signalling-which way to target? Trends
Pharmacol Sci. 24:366–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
OuYang F, Wang G, Guo W, Zhang Y, Xiang W
and Zhao M: AKT signalling and mitochondrial pathways are involved
in mushroom polysaccharide-induced apoptosis and G1 or S phase
arrest in human hepatoma cells. Food Chem. 138:2130–2139. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warfel NA and Kraft AS: PIM kinase (and
Akt) biology and signaling in tumors. Pharmacol Ther. 151:41–49.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakagawa A, Sullivan KD and Xue D:
Caspase-activated phosphoinositide binding by CNT-1 promotes
apoptosis by inhibiting the AKT pathway. Nat Struct Mol Biol.
21:1082–1090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: Conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
35
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gkouveris I, Nikitakis N, Karanikou M,
Rassidakis G and Skavounou A: JNK1/2 expression and modulation of
STAT3 signaling in oral cancer. Oncol Lett. 12:699–706.
2016.PubMed/NCBI
|
|
37
|
Wang Z, Wang W, Xu S, Wang S, Tu Y, Xiong
Y, Mei J and Wang C: The role of MAPK signaling pathway in the
Her-2-positive meningiomas. Oncol Rep. 36:685–695. 2016.PubMed/NCBI
|
|
38
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mazumder S, Plesca D and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 414:13–21.
2008.PubMed/NCBI
|