Introduction
The prevalence of obesity among children and
adolescents has greatly increased and has become a global public
health concern in recent decades (1). Obesity is classified as an excessive
accumulation of adipose tissue due to an increase in cell number
and volume. Adipose tissue stores a great deal of energy and
secretes large amounts of adipokines, including tumor necrosis
factor-α (TNF-α), interleukin-6 (IL-6), leptin and resistin
(2). These are associated with an
increased risk of several common diseases, including obesity,
insulin resistance, diabetes, hypertension, angiocardiopathy and
cancers (3). Additionally, free
fatty acids (FFAs) and glucose, as energy-source materials, are
associated with obesity and obesity-associated insulin resistance
(4,5). However, the molecular mechanisms of
the effects of these adipokines and energy-source materials on
obesity and obesity associated insulin resistance remain to be
fully elucidated.
Consequently, the present study aimed to examine the
potential molecular mechanisms of adipokines and energy-source
materials affecting obesity development and obesity-associated
insulin resistance in human mature adipocytes. MicroRNAs (miRs),
which are small non-coding RNAs that regulate gene expression at
post-transcriptional level, are involved in the regulation of
adipogenesis, obesity, insulin resistance and diabetes (6). miR-21 is one of the most researched
miRNAs with regards to cellular growth, proliferation, apoptosis
and migration (7). The use of
miR-21 as a potential molecular marker has been the focus of
numerous studies in recent years (8,9).
Previous reports have indicated that miR-21 is associated with
metabolic syndrome and is involved in human adipose tissue-derived
mesenchymal stem cell (hASC) proliferation and differentiation
(10–13). Therefore, miR-21 may be a key
regulatory factor of obesity and obesity-associated insulin
resistance.
The aim of the present study was to investigate the
effects of adipokines and energy-source materials on miR-21
expression in human mature adipocytes, and to preliminarily assess
the potential role of adipokines and energy-source materials via
observing the impact of miR-21 in obesity and obesity-associated
insulin resistance.
Materials and methods
Cell culture, differentiation and
treatment
Human preadipocytes (ScienCell Research
Laboratories, Inc., Carlsbad, CA, USA) were supplemented with 5%
fetal bovine serum, 1% preadipocyte growth supplement and 1%
penicillin/streptomycin solution (all from ScienCell Research
Laboratories, Inc.) at 37°C in 5% CO2, and maintained in
preadipocyte medium (PAM; ScienCell Research Laboratories, Inc.).
In order to induce differentiation, confluent human preadipocytes
(day 0) were cultured in serum-free PAM containing 50 nM insulin,
100 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine and 100 µM
rosiglitazone (all from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The medium was changed every 2 days for the first 4 days.
Thereafter, the medium was replaced with serum-free PAM containing
50 nM insulin. The media was replaced every 2 days, until
accumulation of lipid droplets was observed (days 14–17). When
>80% of the cells acquired the morphological and biochemical
properties of mature adipocytes, cells were prepared for the
experiments. Following overnight incubation in serum-free PAM,
human mature adipocytes were treated with 1 mM FFAs, 10 ng/ml
TNF-α, 30 ng/ml IL-6, 30 ng/ml leptin and 60 ng/ml resistin (all
Sigma-Aldrich; Merck KGaA), and 5 or 25 mM glucose for 4, 8, 24 or
48 h. Adipocytes were collected and prepared for further
investigation.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from adipocytes using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
was quantified using the One Drop spectrophotometer. Mature miRNA
quantification was performed by TaqMan miRNA analysis of miR-21.
Generation of cDNA was synthesized using the TaqMan MicroRNA
reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction mixture volume was 15 µl and
contained 200 ng total RNA, 50 nM stem-loop RT primer (ABI
Scientific, Sterling, VA, USA) RT buffer, 0.25 mM of each dNTP,
3.33 U/ml MultiScribe reverse transcriptase and 0.25 U/ml RNase
inhibitor (all Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermocycling conditions were as follows: 30 min at 16°C, 30
min at 42°C, and 5 min at 85°C. For RT-qPCR, the reaction mixture
contained the following: 1.33 µl (1:15 dilution) cDNA, 1.5 mM
forward primer (ABI Scientific), 0.2 mM TaqMan probe (ABI
Scientific), 0.7 mM reverse primer (ABI Scientific) and TaqMan
Universal PCR MasterMix (all from Applied Biosystems; Thermo Fisher
Scientific, Inc.) totaling 20 µl. The thermocycling conditions of
reactions were as follows: 10 min at 95°C and 40 cycles of 15 sec
at 95°C and 1 min at 60°C. All PCR experiments were implement using
the ABI 7500 real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The PCR results were analyzed and
expressed relative to the miRNA expression of the quantitation
cycle value (Cq). U6 small nucleolar RNA (snRU6) and miR-103 (ABI
Scientific) were used as references to obtain the relative
fold-change in expression in target samples using the comparative
Cq method.
Statistical analysis
Data are presented as the mean ± standard error.
Statistical analysis was performed using one-way analysis of
variance followed by the Student-Newman-Keuls post hoc test, using
the statistical software package SPSS (version 13.0; SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
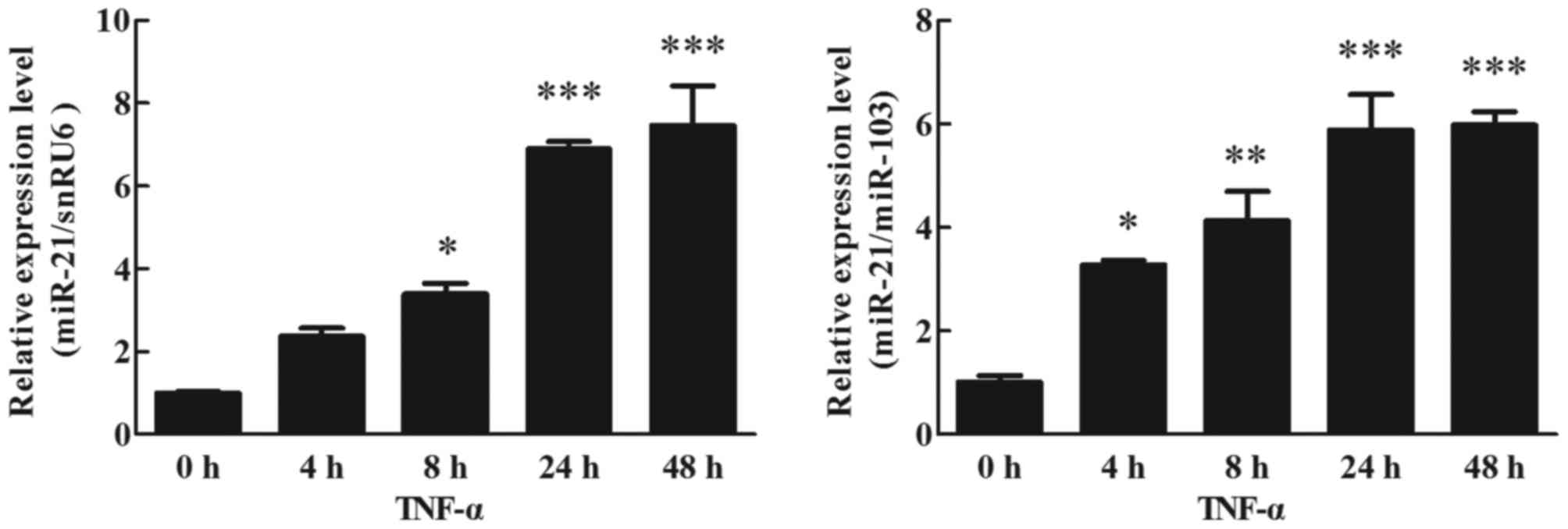
Effects of TNF-α on miR-21 expression
in human adipocytes
Differentiation of human preadipocytes was induced
and adipocyte cultures were prepared for experiments as described
in the Materials and methods section. Mature adipocytes were
cultured with TNF-α (10 ng/ml) for 48 h. Using RT-qPCR (TaqMan
probe method) to analyze the effects of miR-21 expression in
different periods (0, 4, 8, 24 and 48 h), except for the expression
of miR-21 normalized to snRU6 at 4 h, it was significantly
upregulated at 4 (P<0.05), 8 (P<0.01), 24 (P<0.001) and 48
h (P<0.001) following TNF-α stimulation, when compared with the
treated cells at 0 h (Fig. 1).
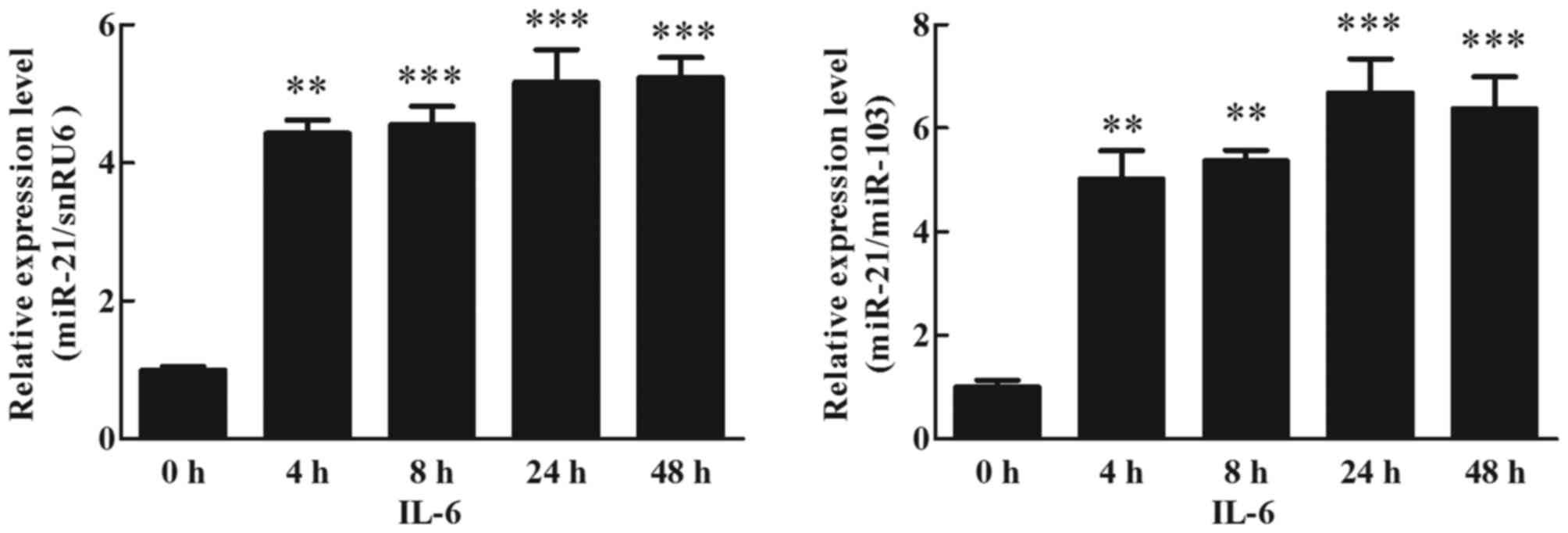
Effects of IL-6 on miR-21 expression
in human adipocytes
To detect the effects of IL-6 on miR-21 expression
in cultured human adipocytes, mature adipocytes were treated with
30 ng/ml IL-6 for 48 h, and the expression of miR-21 was analyzed
using RT-qPCR (TaqMan probe method). The results indicated that
IL-6 increased the expression of miR-21. At 4 h, this was 4-fold
higher than the treated cells (0 h), and this increase was
maintained up to 48 h (Fig. 2;
P<0.05).
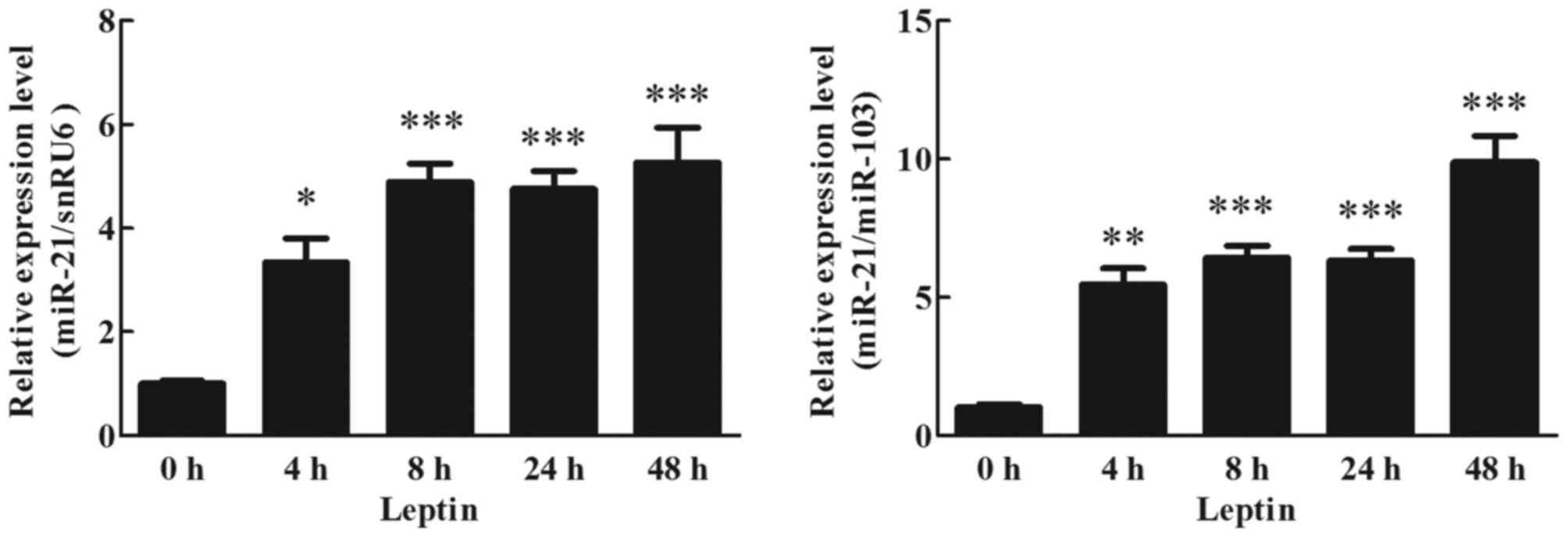
Effects of leptin on miR-21 expression
in human adipocytes
To detect the effects of leptin on miR-21 expression
in cultured human adipocytes, cultured adipocytes were treated with
60 ng/ml leptin. miR-21 expression at different time points was
analyzed by RT-qPCR. Expression of miR-21 was significantly
increased by 4 h following the initiation of leptin stimulation,
~4-fold higher than the treated cells (0 h), and this increase was
maintained up to 48 h (Fig. 3;
P<0.05).
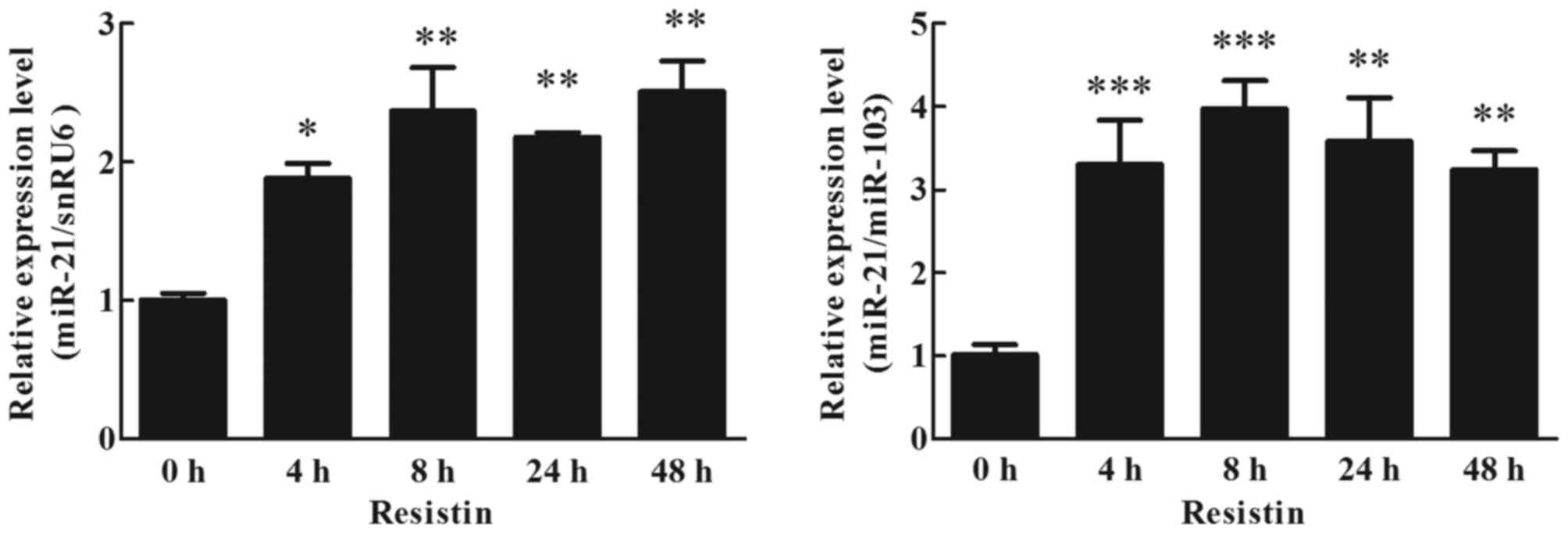
Effects of resistin on miR-21
expression in human adipocytes
To detect the effects of resistin on miR-21
expression in cultured human adipocytes, adipocytes were cultured
with 60 ng/ml resistin. The effects of resistin on miR-21
expression in cultured human adipocytes were analyzed by RT-qPCR
(TaqMan probe method). The result indicated that resistin
upregulated the expression of miR-21, it was ~2-fold higher than
the treated cells (0 h) at 4 h, and this increase was maintained up
to 48 h (Fig. 4; P<0.05).
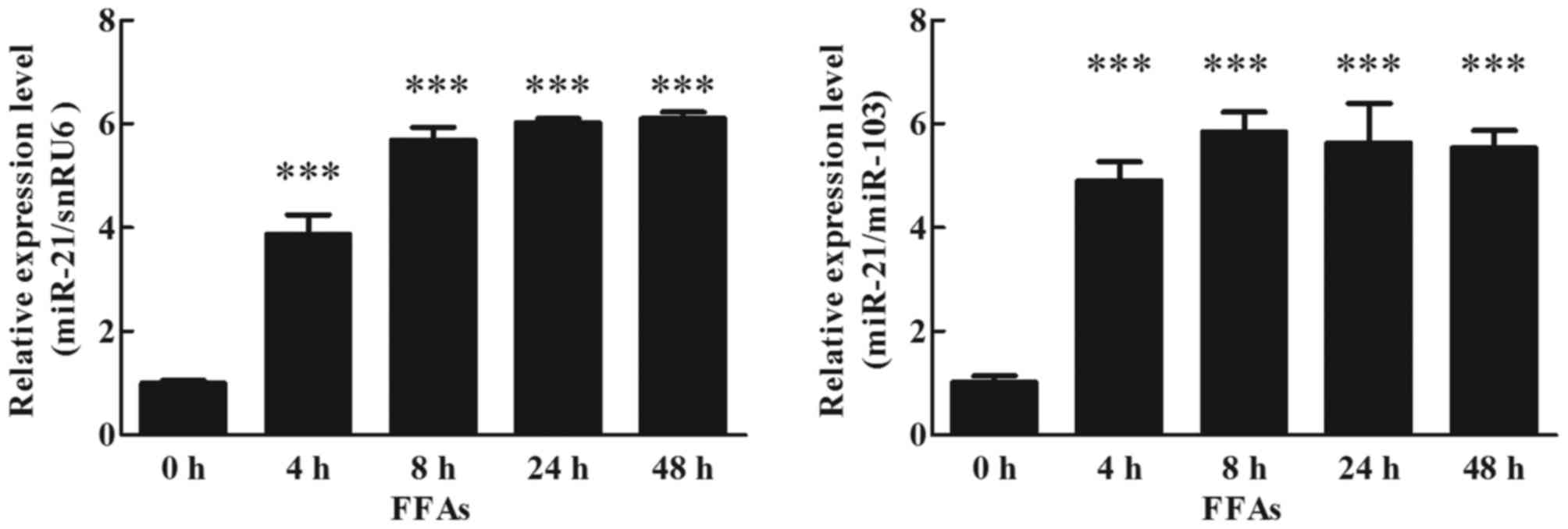
Effects of FFAs on miR-21 expression
in human adipocytes
To detect the effects of FFAs on miR-21 expression
in cultured human adipocytes, mature adipocytes were cultured with
1 mM FFAs, analyzed by RT-qPCR (TaqMan probe method). The result
indicated that FFAs increased expression of miR-21 at 4 h, and this
increase was maintained up to 48 h (Fig. 5; P<0.05).
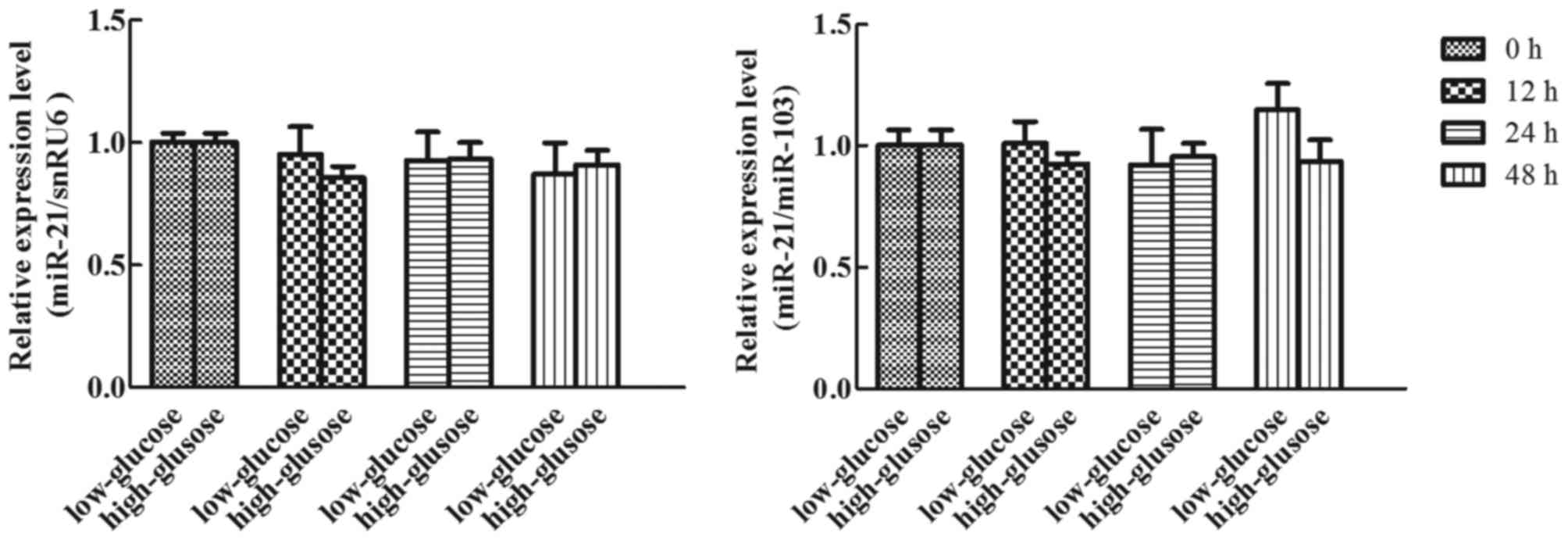
Effects of glucose on miR-21
expression in human adipocytes
To detect the effects of glucose on miR-21
expression in cultured human adipocytes, cultured adipocytes were
treated with 5 or 25 mM glucose for different durations (0, 12, 24
and 48 h), and the expression of miR-21 was analyzed using RT-qPCR
(TaqMan probe method). Following normalization using snRU6 and
miR-103, the expression of miR-21 presented no statistical
difference at each time-point in high or low glucose conditions
(Fig. 6; P>0.05).
Discussion
Obesity is a chronic metabolic disease, which
involves an excessive accumulation of adipose tissue, caused by a
variety of genetic, environmental and psychosocial factors
(14–16). Adipose tissue dysfunction that
increases cell number and volume serves a prominent role in the
development of obesity and obesity-associated insulin resistance
(17). Meanwhile, TNF-α, IL-6,
leptin, resistin, FFAs and glucose are associated with obesity and
obesity-associated insulin resistance (2,4,5).
However, the mechanisms underlying the impact of these molecules on
insulin-secreting cells have not been fully clarified.
MicroRNAs, which are short non-coding RNAs that
regulate gene expression at post-transcriptional level, possess
important roles in various diseases, including insulin resistance,
adipogenesis, obesity and diabetes (18–20).
Existing research indicates that some miRNAs have been implicated
in the specialized metabolic functions of mature adipocytes. For
example, miR-143 is a well-characterized miRNA involved in both
obesity and obesity-associated insulin resistance (21,22).
In addition, studies have demonstrated that miR-21 is associated
with hASC differentiation and metabolic syndrome (11–13).
Therefore, further investigation of the correlation between
mediators and miR-21 may provide novel insights into the underlying
mechanisms of obesity and obesity-associated insulin
resistance.
One of the pathogenic mechanisms underlying obesity
is inflammation. TNF-α and IL-6 are major pro-inflammatory
cytokines, which are produced by adipocytes and macrophages in
adipose tissue (23,24). The increase of TNF-α and IL-6 serve
crucial roles in obesity-associated insulin resistance and diabetes
(25). In the present study,
treatment with high concentrations of TNF-α and IL-6 in human
mature adipocytes led to a positive effect on miR-21 expression.
The results indicated that miR-21 may be a key factor associated
with obesity and obesity-associated insulin resistance.
Leptin and resistin are adipocyte-derived hormones
and are abundantly expressed in adipose tissue, and are linked to
obesity-associated insulin resistance (26,27).
In the present study, the results indicated that leptin and
resistin strongly upregulated the expression of miR-21 in human
mature adipocytes. Therefore, leptin and resistin may affect
obesity-associated insulin resistance via promoting expression of
miR-21 in human mature adipocytes.
A previous study demonstrated that high FFA levels
contribute to insulin resistance in obese patients (4,28). A
potential underlying mechanism is that FFAs may inhibit the insulin
signaling pathway at the level of insulin-stimulated glucose
transport and phosphorylation. Glucose, one of energy-source
materials, has been strongly linked to obesity and its
complications (5). Obesity-induced
insulin resistance inhibits glucose uptake and utilization
efficiency, accompanied by hyperglycemia and hyperinsulinemia.
miR-21, which regulates adipocyte proliferation, differentiation
and metabolic syndrome, has been confirmed to be associated with
obesity (11,12). In the present study, to better
understand the molecular mechanisms underlying FFAs, glucose and
miR-21 with obesity and obesity-associated insulin resistance,
human mature adipocytes were treated with FFAs and glucose. The
expression of miR-21 was increased in FFAs treated human mature
adipocytes. Notably, miR-21 expression was not significantly
altered by either high or low levels of glucose stimulation. These
results indicated that miR-21 may be an intermediate factor between
FFAs and obesity-associated insulin resistance.
The present study indicated that miR-21 expression
is promoted by TNF-α, IL-6, leptin, resistin and FFAs in human
adipocytes, but is not promoted by glucose. Therefore, adipokines
and energy-source materials may affect obesity development and
obesity-associated insulin resistance via promoting miR-21
expression. However, the specific molecular mechanisms remain to be
further explored. These findings implicate miR-21 as a potential
therapeutic target for the treatment of obesity and
obesity-associated insulin resistance.
Acknowledgements
The present study was support by The Natural Science
Foundation of Jiangsu Province China (grant no. BK2012666).
References
|
1
|
Jeffery RW and Sherwood NE: Is the obesity
epidemic exaggerated? No. BMJ. 336:2452008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galic S, Oakhill JS and Steinberg GR:
Adipose tissue as an endocrine organ. Mol Cell Endocrinol.
316:129–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boden G: Obesity, insulin resistance and
free fatty acids. Curr Opin Endocrinol Diabetes Obes. 18:139–143.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parhofer KG: Interaction between glucose
and lipid metabolism: More than diabetic dyslipidemia. Diabetes
Metab J. 39:353–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deiuliis JA: Micrornas as regulators of
metabolic disease: Pathophysiologic significance and emerging role
as biomarkers and therapeutics. Int J Obes (Lond). 40:88–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pfeffer SR, Yang CH and Pfeffer LM: The
role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beckett EL, Martin C, Choi JH, King K,
Niblett S, Boyd L, Duesing K, Yates Z, Veysey M and Lucock M:
Folate status, folate-related genes and serum miR-21 expression:
Implications for miR-21 as a biomarker. BBA Clin. 4:45–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao W, Zhao JJ, Zhang L, Xu QF, Zhao YM,
Shi XY and Xu AG: Serum miR-21 level: A potential diagnostic and
prognostic biomarker for non-small cell lung cancer. Int J Clin Exp
Med. 8:14759–14763. 2015.PubMed/NCBI
|
|
10
|
Seeger T, Fischer A, Muhly-Reinholz M,
Zeiher AM and Dimmeler S: Long-term inhibition of miR-21 leads to
reduction of obesity in db/db mice. Obesity (Silver Spring).
22:2352–2360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ling HY, Hu B, Hu XB, Zhong J, Feng SD,
Qin L, Liu G, Wen GB and Liao DF: miRNA-21 reverses high glucose
and high insulin induced insulin resistance in 3T3-L1 adipocytes
through targeting phosphatase and tensin homologue. Exp Clin
Endocrinol Diabetes. 120:553–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim YJ, Hwang SH, Cho HH, Shin KK, Bae YC
and Jung JS: MicroRNA-21 regulates the proliferation of human
adipose tissue-derived mesenchymal stem cells and high-fat
diet-induced obesity alters microrna 21 expression in white adipose
tissues. J Cell Physiol. 227:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YJ, Hwang SJ, Bae YC and Jung JS:
miR-21 regulates adipogenic differentiation through the modulation
of TGF-beta signaling in mesenchymal stem cells derived from human
adipose tissue. Stem Cells. 27:3093–3102. 2009.PubMed/NCBI
|
|
14
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wellen KE and Hotamisligil GS:
Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waterland RA: Epigenetic mechanisms
affecting regulation of energy balance: Many questions, few
answers. Annu Rev Nutr. 34:337–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abranches MV, Oliveira FC, Conceição LL
and Peluzio MD: Obesity and diabetes: The link between adipose
tissue dysfunction and glucose homeostasis. Nutr Res Rev.
28:121–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chakraborty C, Doss CG, Bandyopadhyay S
and Agoramoorthy G: Influence of miRNA in insulin signaling pathway
and insulin resistance: Micro-molecules with a major role in type-2
diabetes. Wiley Interdiscip Rev RNA. 5:697–712. 2014.PubMed/NCBI
|
|
19
|
Arner P and Kulyté A: MicroRNA regulatory
networks in human adipose tissue and obesity. Nat Rev Endocrinol.
11:276–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jinwei Z, Yi L, Yuhao W, Liujun H,
Mingzhou L and Xun W: MicroRNA regulates animal adipocyte
differentiation. Yi Chuan. 37:1175–1184. 2015.PubMed/NCBI
|
|
21
|
Kilic ID, Dodurga Y, Uludag B, Alihanoglu
YI, Yildiz BS, Enli Y, Secme M and Bostanci HE: MicroRNA-143 and
−223 in obesity. Gene. 560:140–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jordan SD, Krüger M, Willmes DM, Redemann
N, Wunderlich FT, Brönneke HS, Merkwirth C, Kashkar H, Olkkonen VM,
Böttger T, et al: Obesity-induced overexpression of miRNA-143
inhibits insulin-stimulated AKT activation and impairs glucose
metabolism. Nat Cell Biol. 13:434–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamada Y, Nagasaki H, Fujiya A, Seino Y,
Shang QL, Suzuki T, Hashimoto H and Oiso Y: Involvement of de novo
ceramide synthesis in pro-inflammatory adipokine secretion and
adipocyte-macrophage interaction. J Nutr Biochem. 25:1309–1316.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finucane OM, Reynolds CM, McGillicuddy FC
and Roche HM: Insights into the role of macrophage migration
inhibitory factor in obesity and insulin resistance. Proc Nutr Soc.
71:622–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiruvengadam V, Amperayani S, Babu RP and
Uppuluri R: Correlation of childhood obesity and related insulin
resistance with leptin and retinol binding protein 4. Indian J
Pediatr. 82:799–804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park HK, Qatanani M, Briggs ER, Ahima RS
and Lazar MA: Inflammatory induction of human resistin causes
insulin resistance in endotoxemic mice. Diabetes. 60:775–783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Medina-Urrutia A, Posadas-Romero C,
Posadas-Sánchez R, Jorge-Galarza E, Villarreal-Molina T,
González-Salazar Mdel C, Cardoso-Saldaña G, Vargas-Alarcón G,
Torres-Tamayo M and Juárez-Rojas JG: Role of adiponectin and free
fatty acids on the association between abdominal visceral fat and
insulin resistance. Cardiovasc Diabetol. 14:202015. View Article : Google Scholar : PubMed/NCBI
|