Introduction
Currently, ischemic heart disease is the primary
cause of heart failure (HF) (1).
Ventricle remodeling and cardiac sympathetic hyperactivity
following myocardial infarction (MI) contribute to the development
of HF (2,3). Increased cardiac sympathetic nerve
activity causes sympathetic hyperinnervation and heterogeneous
nerve sprouting, also known as sympathetic neural remodeling, which
causes ventricular arrhythmia and sudden cardiac death (SCD)
(4,5). Oxidative stress has been demonstrated
to serve an important role in sympathetic innervation following MI,
which promotes sympathetic neural remodeling and arrhythmia via
increasing the expression of nerve growth factor (NGF) (6). Anti-oxidative stress therapy
significantly decreases the density of sympathetic nerve and the
protein expression of NGF following MI (7–9).
Intermedin (IMD) is a novel member of the calcitonin
gene-related peptide (CGRP) family, signaling via calcitonin
receptor-like receptor/receptor activity modifying protein
(CRLR/RAMP) complexes (10). It
was reported that IMD has beneficial effects on the cardiovascular
system (11). The expression of
IMD is increased in the failing heart and may have a certain
pathophysiological role in HF (12). A subsequent study demonstrated its
favorable haemodynamic, hormonal and renal actions in a sheep model
of experimental HF (13).
Furthermore, IMD protects against myocardial and renal
ischemia/reperfusion injury via inhibition of oxidative stress in
animal models (14–16). However, whether IMD may ameliorate
sympathetic neural remodeling via anti-oxidative effects following
MI remains unclear.
The present study investigated the effects of
long-term administration of exogenous IMD on cardiac function and
sympathetic neural remodeling in a rat model of post-MI HF.
Materials and methods
Peptide synthesis
Human IMD (IMD1-53) with the sequence
His-Ser-Gly-Pro-Arg-Arg-Thr-Gln-Ala-Gln-Leu-Leu-Arg-Val-Gly-Cys-Val-Leu-Gly-Thr-Cys-Gln-Val-Gln-Asn-Leu-Ser-His-Arg-Leu-Trp-Gln-Leu-Met-Gly-Pro-Ala-Gly-Arg-Gln-Asp-Ser-Ala-Pro-Val-Asp-Pro-Ser-Ser-Pro-His-Ser-Tyr-NH2
with an intramolecular disulfide bond between Cys16-Cys21 (17) was synthesized by ShineGene
Bio-Technologies (Shanghai, China).
Establishment of animal models
Adult male Sprague Dawley rats, (weight, 280–320 g;
n=60), were supplied by Sino-British Sippr/BK Lab Animal Ltd.
(Shanghai, China) The animal experiment was in compliance with the
National Research Council's protocol for the Care and Use of
Laboratory Animals, and was approved by the Animal Care Committee
of Shanghai General Hospital (Shanghai, China). The HF model was
induced in Sprague Dawley rats by ligation of the left anterior
descending (LAD) coronary artery. Briefly, all rats were
anesthetized with intraperitoneal injection of 1% sodium
pentobarbital (40 mg/kg; Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China), endotracheal intubated and mechanically
ventilated with a small animal ventilator. Then all rats underwent
thoracotomy and pericardiotomy, and the LAD coronary artery was
ligated by a 6–0 prolene suture at the origin. Successful
myocardium ischemia was verified by ST-segment elevation on an
electrocardiogram. The sham group rats underwent same procedure
without LAD coronary artery ligation.
Animal grouping and treatment
The rats that survived 24 h after surgery were
randomly assigned to the following 3 groups: Sham (n=10), where
rats were administrated subcutaneously with saline (0.6 µg/kg/h) by
a mini-osmotic pump (Alzet model 2004; DURECT Corporation,
Cupertino, CA, USA) for 4 weeks; HF (n=18), where rats were
administrated subcutaneously with saline (0.6 µg/kg/h) by a
mini-osmotic pump for 4 weeks; and HF rats with IMD treatment
(HF+IMD group; n=20), where rats were daily administrated
subcutaneously with IMD (0.6 µg/kg/h) (13) by a mini-osmotic pump for 4 weeks.
After 4 weeks, rats underwent echocardiographic examination,
haemodynamic measurement and ventricular fibrillation threshold
(VFT) determination. After that, animals were sacrificed and hearts
were excised for further study.
Echocardiography and haemodynamic
measurement
Rats were lightly anesthetized with 1% sodium
pentobarbital, (40 mg/kg; Sinopharm Chemical Reagent Co., Ltd.) and
transthoracic echocardiography was performed with a 30 MHz high
frequency transducer (VisualSonics Vevo770; VisualSonics, Inc.,
Toronto, ON, Canada) as previously described (18). End-diastolic and end-systolic left
ventricle diameters were measured by M-mode tracing. Left
ventricular ejection fraction (LVEF) and fractional shortening
(LVFS) were calculated. Following echocardiography study,
haemodynamic parameters were measured using the BL-420E Biological
system (Chengdu Tai-Meng Science and Technology Co., Ltd., Chengdu,
Sichuan, China). Briefly, a catheter was inserted into the right
common carotid artery to monitor the arterial blood pressure.
Subsequently the catheter was inserted into the left ventricle to
record the left ventricular end-diastolic pressure (LVEDP) and the
maximal rate of left ventricular pressure increase and decrease
(±LVdp/dtmax).
Determination of VFT
A total of 5 rats in the HF+IMD group and 7 rats in
the HF group succumbed during the haemodynamic measurement and
anesthesia prior to the second thoracotomy. Therefore, 10 rats in
each group were selected for the determination of VFT. VFT
measurement was performed as previously described (19). A bipolar needle pacing electrode
was penetrated into the peri-infarct zone, which was defined as the
zone with a <3-mm width located between the infarct zone and the
non-infarct area. A train of rectangular 4 ms pacing pulses was
performed for 5 sec at a frequency of 50 HZ using the BL-420E
Biological system. The interval between pulse trains was 1 min. The
pacing voltage of the first pulse train started at 4 V and
progressively augmented by a step of 1 V. The VFT was the minimum
voltage which induced ventricular fibrillation (sustained >2
sec).
Determination of infarct size and
tissue preparation
Following determination of VFT, all rats were
sacrificed by injecting a fatal dose of pentobarbital (200 mg/kg;
Sinopharm Chemical Reagent Co., Ltd.). The heart was excised and
rinsed in cold saline. The atria and right ventricle were trimmed
off, and the left ventricle was weighed. A transverse section
obtained from the papillary muscle level of the left ventricle was
fixed in 10% formalin and embedded in paraffin. The section was
then sectioned into 4-µm slices and stained with haematoxylin-eosin
for infarct size measurement. The infarct size was determined as
previously described (9). Rats
with infarct size <30% were excluded from analysis (n=2 from the
HF+IMD group; n=1 from the HF group) (20). Left ventricular tissues from the
peri-infarct zone were fixed in 10% formalin for 24 h and embedded
for immunohistochemical studies and frozen in liquid nitrogen for
western blot analysis.
Immunohistochemical studies
Paraffin-embedded tissues were sectioned into 4-µm
slices using a microtome (Leica Microsystems, Wetzlar, Germany).
Following deparaffinization and rehydration, slides were treated
with 3% H2O2 and subjected to heat mediated
antigen retrieval with citrate buffer. The slides were incubated
with primary antibodies against tyrosine hydroxylase (TH; 1:200,
cat no. ab75875) and growth associated protein 43 (GAP43; 1:500,
cat no. ab75810) (both from Abcam, Cambridge, UK) overnight at 4°C.
Following this, the slides were washed and incubated with a
horseradish peroxidase (HRP)-conjugated immunoglobulin G secondary
antibody (cat no. GK600705; Gene Tech Biological Technology, Inc.,
Shanghai, China) for 30 min at room temperature. The
immunoreactivity was developed with 3, 3′-diaminobenzidine
tetrahydrochloride (Biological Technology, Inc.). Slides were
counterstained with hematoxylin and analyzed under a light
microscope.
The densities of TH- and GAP43-positive nerve fibers
were determined using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA) and expressed as the nerve
area divided by the total area examined. For each heart, one slide
was taken to study. Each slide was divided into four quadrants at a
×400 magnification and one microscopic field with the highest nerve
density in each quadrant was selected for nerve counting. The mean
nerve density of four selected fields was used to represent the
nerve density of that slide.
Western blotting
Briefly, heart tissues were minced and then lysed
for 20 min on ice with lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The lysates were centrifuged at
10,000 × g for 15 min at 4°C and the supernatants were collected.
Total protein was extracted, the concentrations were determined
using a bicinchoninic acid assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Equal amounts of proteins (20 µg)
were separated by SDS-PAGE (10% separating gel and 5% stacking gel;
80 V; 120 min) and subsequently transferred onto PVDF membranes
(EMD Millipore, Billerica, MA, USA). After that, the membranes were
blocked with 5% non-fat milk for 2 h at room temperature. Membranes
were then incubated with rabbit monoclonal antibodies against TH
(1:2,000) and GAP43 (1:10,000), a rabbit polyclonal antibody
against NGF (1:2,000, cat no. ab6199; Abcam), and a mouse
monoclonal antibody against GAPDH (1:5,000, cat no. 60004–1-Ig;
ProteinTech Group, Inc., Chicago, IL, USA) overnight at 4°C. The
membranes were then washed and incubated with HRP-conjugated goat
anti-rabbit (1:5,000, 111–035-003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) and anti-mouse IgG
secondary antibodies (1:2,000, cat no. A0216; Beyotime Institute of
Biotechnology) at room temperature for 1 h. The bands were
visualized using an Enhanced Chemiluminescence kit (EMD Millipore).
The protein expressions were normalized to GAPDH protein
content.
Measurement of plasma B-type
natriuretic peptide (BNP) level
On the day of sacrifice, 1 ml fresh blood was
collected from all rats via the inferior vena cava and centrifuged
at 1,500 × g for 15 min at 4°C. Then supernatants were collected
and stored at −80°C. Plasma BNP levels were determined using an
enzyme-linked immunosorbent assay (ELISA) kit (cat no. WEA541Ra;
Wuhan USCN Business Co., Ltd., Wuhan, China).
Detection of malondialdehyde (MDA)
level and superoxide dismutase (SOD) activity
Prior to the detection of the MDA levels and SOD
activity the protein concentration was quantified using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology).
Then the remaining ventricle samples were homogenized in saline and
centrifuged at 1,000 × g for 15 min at 4°C. The supernatants were
collected and the level of MDA and the activity of SOD were
detected using commercial kits (Nanjing Jiancheng Bioengineering
Research Institute, Nanjing, China) according to the manufacturer's
protocol. The level of MDA is expressed as nmol/mgprot and the
activity of SOD is expressed as U/mgprot.
Statistical analyses
Data are presented as the mean ± standard deviation
and data analysis was performed by SPSS 19.0 software (IBM SPSS,
Armonk, NY, USA). Statistical differences between groups were
analyzed using one-way analysis of variance followed by the
Students Newman-Keuls post-hoc test after normality distribution
was evaluated. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of IMD on cardiac
function
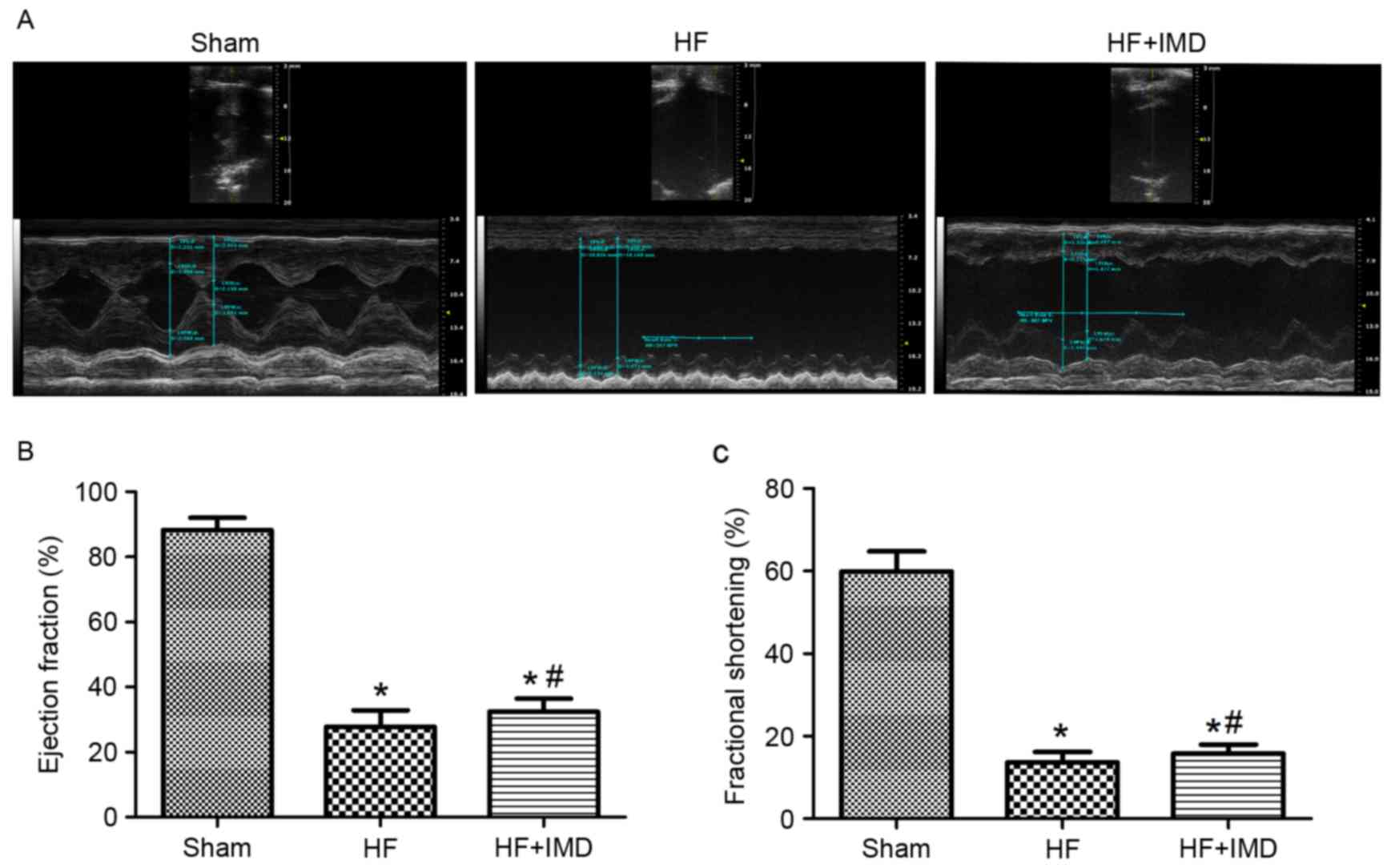
Representative echocardiograms are presented in
Fig. 1A. Compared with the HF
group, IMD treatment significantly increased LVEF (32.5±4.0 vs.
27.8±5.1%; P<0.05; Fig. 1B) and
LVFS (15.8±2.2 vs. 13.6±2.6%; P<0.05; Fig. 1C). A significant increase in
±LVdp/dtmax and a decrease in LVEDP were observed in IMD treatment
group in comparison with the HF group (Table I). In addition, IMD treatment
significantly decreased arterial blood pressure compared with the
sham group and the HF group (Table
I).
 | Table I.Body weight and haemodynamics at the
end of the study. |
Table I.
Body weight and haemodynamics at the
end of the study.
|
| Sham | HF | HF+IMD |
|---|
| BW (g) | 384±8 | 384±11 | 389±13 |
| HR (bpm) | 371±8 | 373±9 | 373±7 |
| SBP (mmHg) | 133±11 | 134±9 | 112±9a,b |
| DBP (mmHg) | 89±5 | 85±6 | 66±7a,b |
| LVEDP (mmHg) | 5±2 | 20±4a | 11±2a,b |
| +LVdp/dtmax
(mmHg/s) | 7721±553 |
3270±544a |
4469±39a,b |
| −LVdp/dtmax
(mmHg/s) | 5971±443 |
2959±507a |
3908±326a,b |
Effects of IMD on VFT
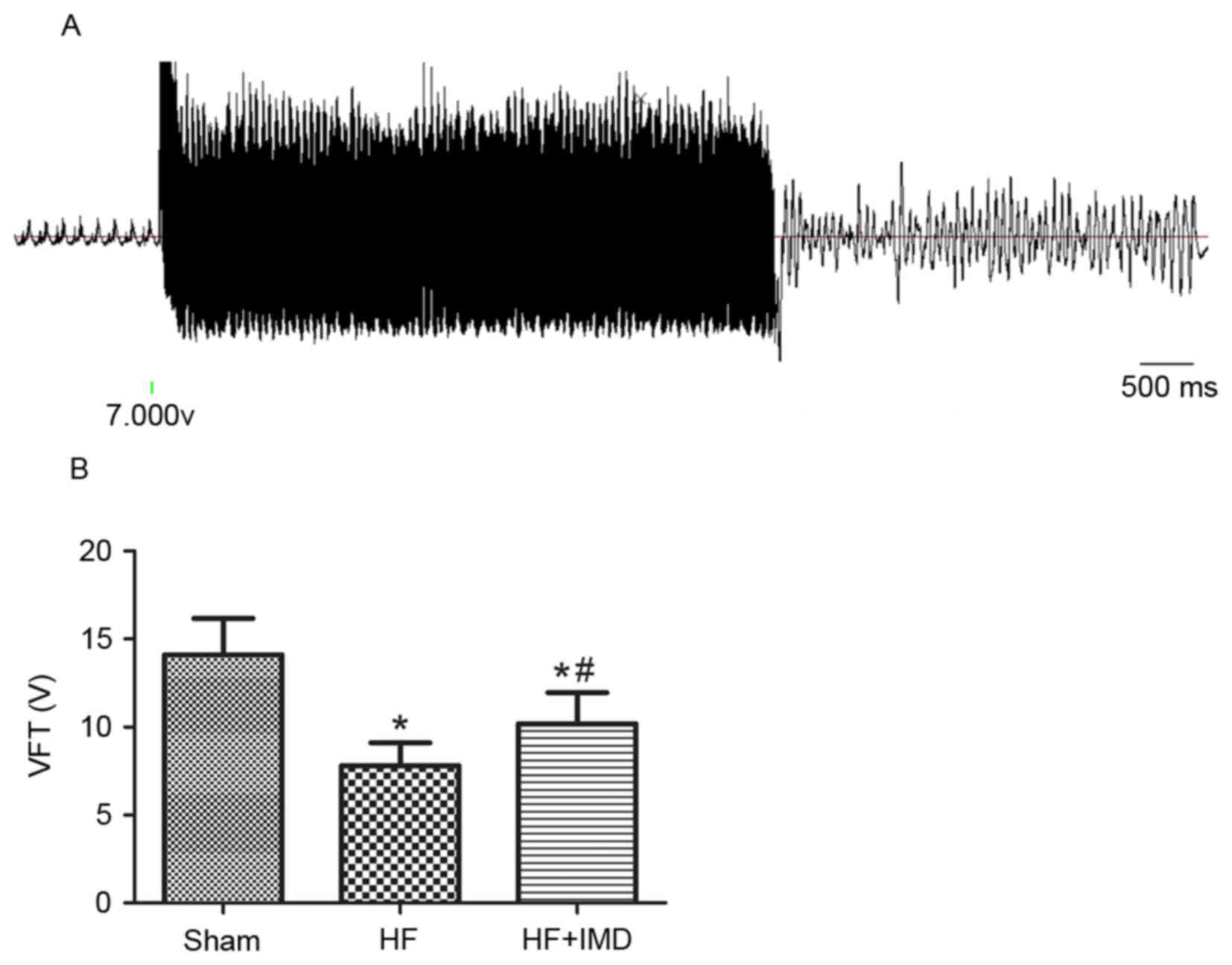
A representative ventricle fibrillation induced by
ventricle electric stimulation in an HF rat treated with saline is
presented in Fig. 1A. The VFT of
the HF group was significantly reduced compared with the sham group
(7.8±1.3 vs. 14.1±2.1 V; P<0.05; Fig. 2B). IMD induced a rise of VFT
compared with the HF group (10.2±1.8 vs. 7.8±1.3 V; P<0.05;
Fig. 2B).
Effects of IMD on cardiac
morphology
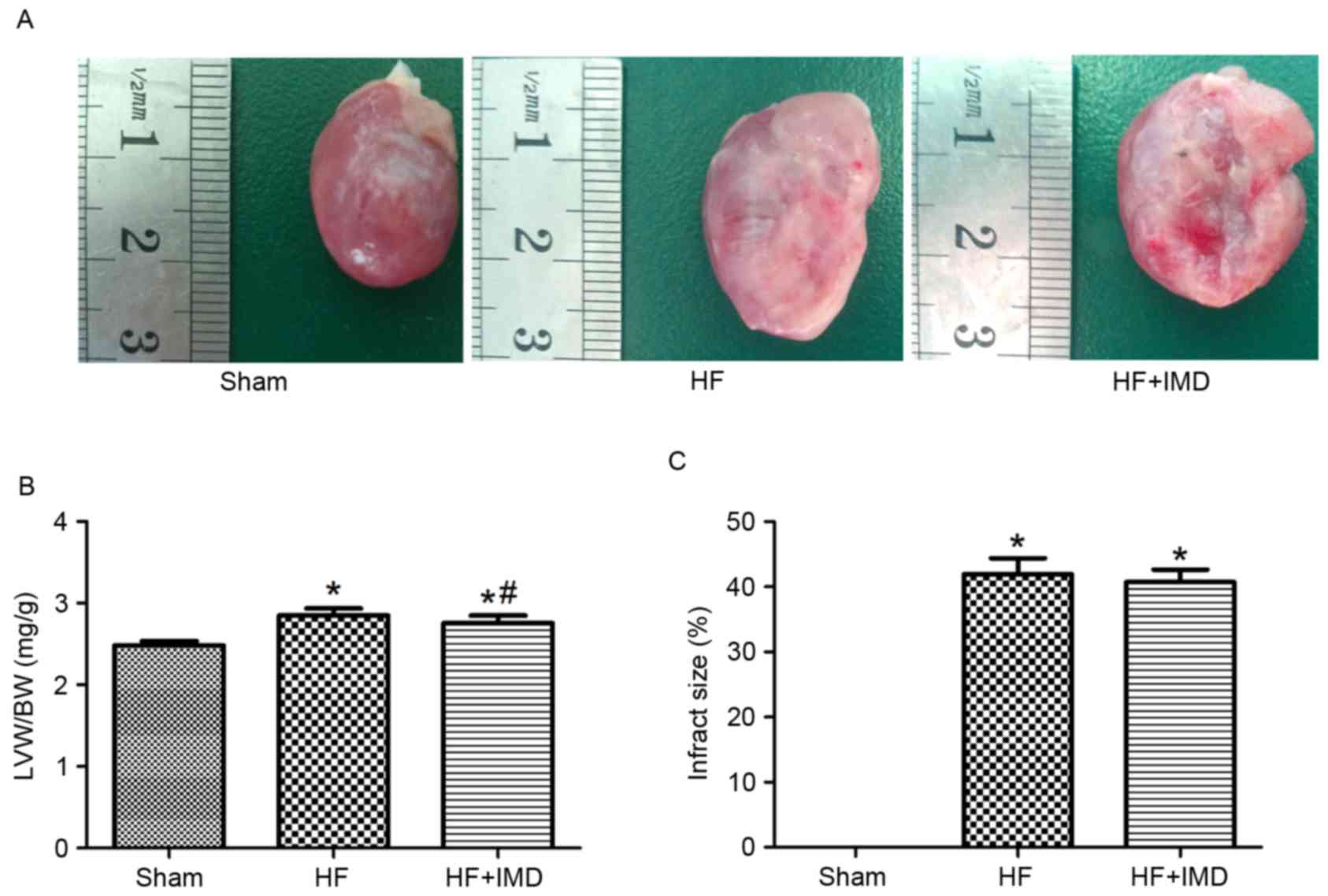
There were no significant differences in body weight
between the three groups at the end of experiment (Table I). However, the left ventricle
weight/body weight (LVW/BW) value was significantly increased in
the HF group compared with the sham group (2.85±0.08 vs. 2.48±0.05;
P<0.05; Fig. 3A and B). A
significant decrease in LVW/BW was observed in the IMD treatment
group compared with the HF group (2.76±0.09 vs. 2.85±0.08;
P<0.05; Fig. 3B). However, the
infarct size between the HF group and IMD treatment group was not
significantly different (Fig.
3C).
Effects of IMD on TH- and
GAP43-positive nerve fiber density
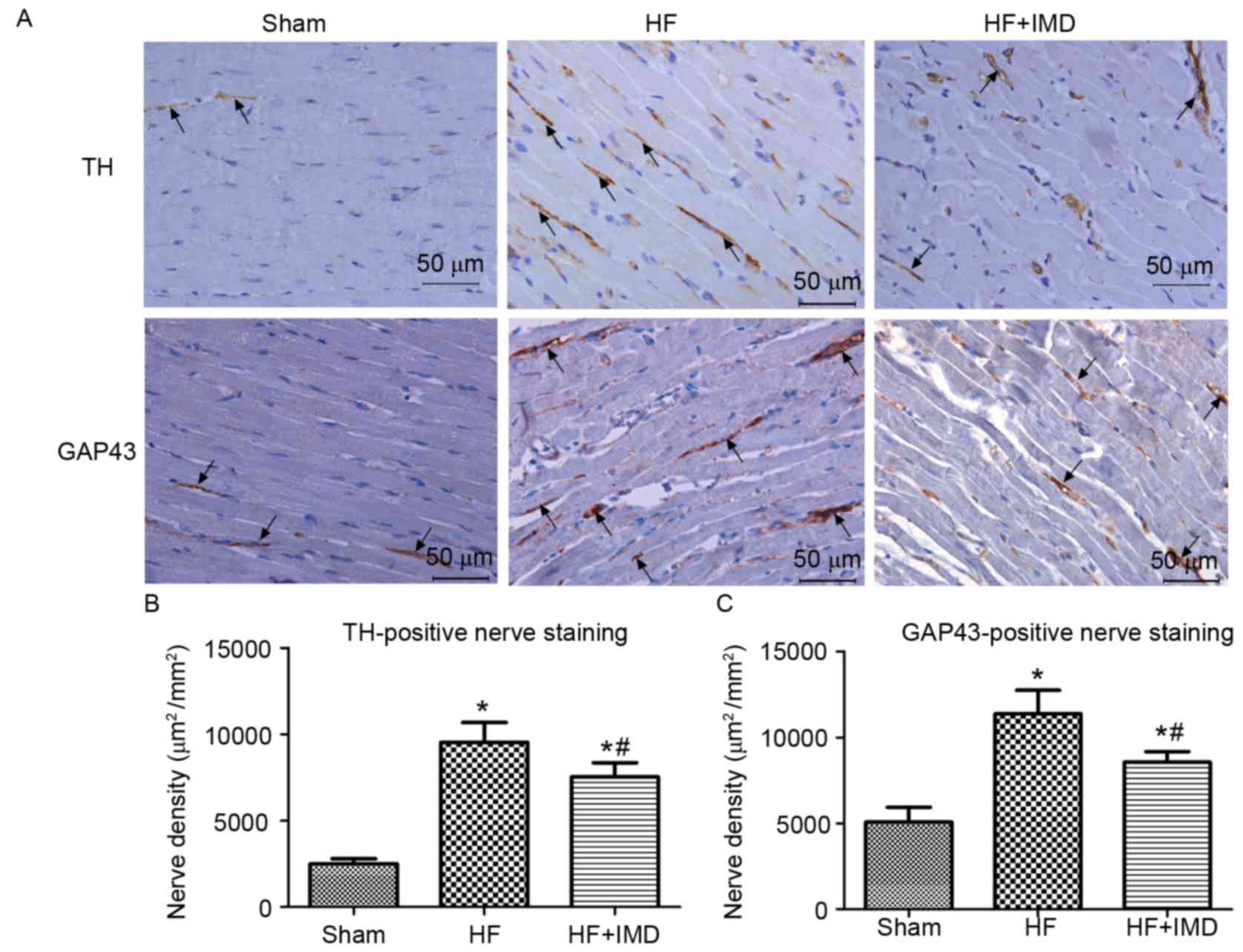
Representative immunostaining for TH- and
GAP43-positive nerve fibers in the peri-infarct zone are presented
in Fig. 4A. The densities of TH-
and GAP43-positive nerve fibers in the peri-infact zone of the HF
group were significantly increased compared with the sham group
(TH, 9534±1150 vs. 2509±281 µm2/mm2; GAP43,
11367±1375 vs. 5073±854 µm2/mm2; all
P<0.05; Fig. 4B and C,
respectively). However, a significant decrease in the densities of
TH- and GAP43-positive nerve fibers was observed in the IMD
treatment group compared with the HF group (TH, 7532±825 vs.
9534±1150 µm2/mm2; GAP43, 8554±615 vs.
11367±1375 µm2/mm2; all P<0.05; Fig. 4B and C).
Effects of IMD on the protein
expression levels of TH, GAP43 and NGF
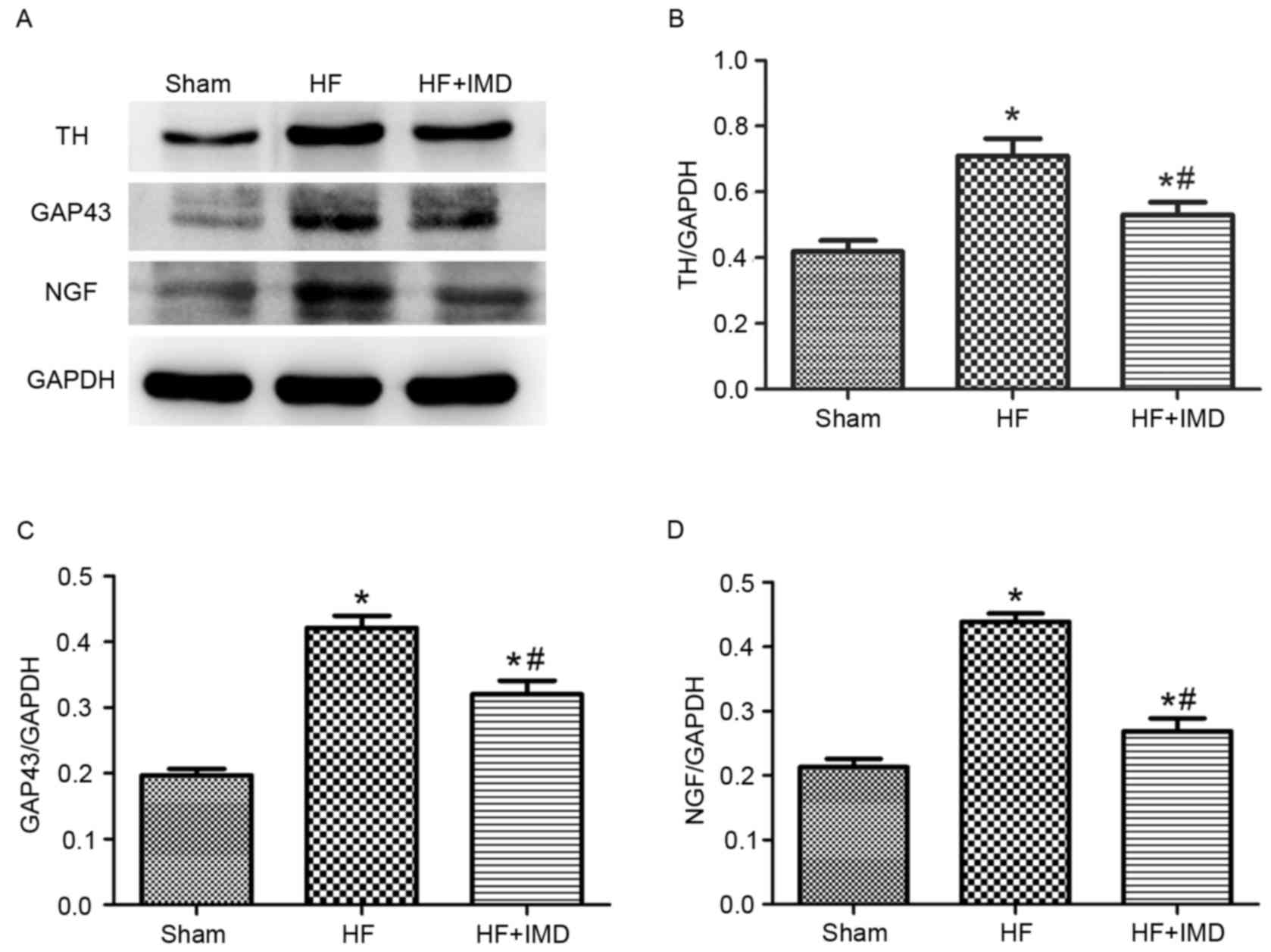
Representative western blot images are presented in
Fig. 5A. The protein expression
levels of TH, GAP43 and NGF were significantly increased in the HF
group compared with the sham group (TH, 0.710±0.053 vs.
0.419±0.034; GAP43, 0.422±0.018 vs. 0.197±0.010 and NGF:
0.439±0.013 vs. 0.213±0.013; all P<0.05; Fig. 5B-D, respectively). IMD treatment
significantly decreased the protein expression levels of TH, GAP43
and NGF compared with the HF group (TH, 0.530±0.038 vs.
0.710±0.053; GAP43, 0.320±0.021 vs. 0.422±0.018 and NGF,
0.270±0.021 vs. 0.439±0.013; all P<0.05; Fig. 5B-D, respectively).
Effects of IMD on plasma BNP levels
and oxidative stress in the heart
The plasma BNP level in the HF group was
significantly increased compared with the sham group. IMD treatment
significantly decreased the plasma BNP level compared with the HF
group (Table II). The MDA level
in the peri-infarct zone of the HF group was significantly
increased, while the SOD activity was significantly decreased
compared with the sham group. IMD significantly decreased the MDA
level and increased SOD activity in comparison to the HF group
(Table II).
 | Table II.Plasma BNP level, MDA content and SOD
activity in the myocardium. |
Table II.
Plasma BNP level, MDA content and SOD
activity in the myocardium.
|
| Sham | HF | HF+IMD |
|---|
| BNP (pg/ml) | 154.16±33.34 |
1089.75±124.22a |
784.51±190.81a,b |
| MDA
(nmol/mgprot) | 0.98±0.25 |
1.47±0.09a |
1.29±0.18a,b |
| SOD (U/mgprot) | 404.10±21.95 |
296.37±60.90a |
354.66±16.96a,b |
Discussion
The present study demonstrated that long-term
treatment with IMD improves cardiac function and attenuates
sympathetic neural remodeling in a rat model of post-MI HF.
Furthermore, the present study revealed that IMD significantly
increased the VFT in ischemic rat hearts, which may reduce the
incidence of ventricular arrhythmia. Notably, the effect of IMD on
sympathetic innervation was associated with its anti-oxidative
property.
As a member of CGRP family, IMD had hypotensive and
positive inotropic effects via activation of the CRLR/RAMP
complexes (21–23). These findings suggested that IMD
may be beneficial in treating HF. Intravenous infusion of IMD to HF
sheep for 90 min decreased arterial blood pressure and enhanced
cardiac contractility and output (13). The BNP level in the IMD treatment
group was decreased in comparison with the HF group at a low dose
(0.6 µg/kg/h); however, the difference did not reach statistical
significance (13). The present
study observed that long-term administration of IMD significantly
decreased the arterial blood pressure, enhanced cardiac
contractility, and elevated LVEF and LVFS. Furthermore, it was
demonstrated that low dose (0.6 µg/kg/h) IMD decreases the BNP
level, which mirrored the improvement of cardiac function.
Angiotensin II (Ang II) is hypothesized to serve a
major role in ventricle remodeling in heart failure, which promotes
myocardium hypertrophy (24).
Blockade of the renin-angiotensin system attenuates cardiomyocyte
hypertrophy in post-MI HF (18).
It has been reported that the cell size of H9c2 cells was increased
by Ang II treatment, while IMD significantly attenuates hypertrophy
in these cells (25). The
underlying mechanism involved its activation of both cyclic
adenosine monophosphate/protein kinase A and mitogen-activated
protein kinase/extracellular signal-regulated kinase 1/2
(MAPK/ERK1/2) signaling pathways, which further activated autophagy
in hypertrophic cardiomyocytes (25). The present study established an
ischemia-induced HF rat model and observed that long-term
administration of IMD prevented cardiac hypertrophy, as assessed by
cardiac morphology measurements and echocardiography. Therefore,
IMD may ameliorate cardiac hypertrophy, at least in part, via
abolishing the adverse effects of Ang II in ischemic rat hearts.
However, the more specific mechanism and the downstream signaling
pathway between IMD and Ang II requires further investigation. In
respect to infarct size, there was no significant difference
between the HF and IMD treatment groups in this study.
To the best of our knowledge, the effects of IMD on
sympathetic innervation after MI have not previously been studied.
The present study demonstrated that long-term treatment with IMD
suppresses sympathetic neural remodeling and increased the VFT
after MI.
TH is a rate-limiting enzyme of norepinephrine,
which reflects the activity of sympathetic nerves (26). GAP43, which is expressed at the
nerve growth cones, is a marker of new sprouting axons (26). Sympathetic neural remodeling
following MI was observed in MI patients and animal models, which
exhibited increased densities of TH- and GAP43-positive nerve
fibers (5,27). Subsequent studies demonstrated that
the new sprouting nerves were more obvious in the peri-infarct zone
(28,29). In accordance with a previous study,
the present study revealed that the densities of TH- and
GAP43-positive nerve fibers were significantly increased in the
peri-infarct zone, together with an increase in protein expression
levels of TH and GAP43. Compared with the HF group, treatment with
IMD decreased sympathetic nerve density and downregulated the
protein expression levels of TH and GAP43.
It has been demonstrated that increased sympathetic
nerve density accounts for the incidence of ventricular arrhythmia
and SCD in patients with severe HF (27). Furthermore, catheter-based mapping
techniques confirmed that the original site of most ventricular
arrhythmia is the peri-infarct zone in patients with MI (30). Therefore, to investigate whether
IMD could reduce the incidence of ventricular arrhythmia, the
present study determined the VFT, an indicator to evaluate the
incidence of ventricular arrhythmia. It was observed that IMD
treatment increased the VFT compared with the HF group, which
suggested that IMD could decrease the incidence of ventricular
arrhythmia.
The underlying mechanisms of sympathetic
hyperinnervation following MI remain to be clearly elucidated. It
is generally agreed that NGF, which is essential for sympathetic
nerve growth and survival (31),
is involved in the neural regeneration process. A previous study
demonstrated that the expression of NGF was increased in the
peri-infarct zone (29).
Furthermore, infusion of NGF to the left stellate ganglion elevated
the densities of TH- and GAP-43 positive nerve fibers and increased
the incidence of SCD in MI dogs (5). Similarly, overexpression of NGF in
transgenic mouse heart resulted in sympathetic hyperinnervation
(32).
Oxidative stress is characterized by increased
oxidant production and impaired anti-oxidant defenses. It has been
demonstrated that oxidative stress is increased and has a critical
role in sympathetic neural remodeling following MI (6). A further study revealed that
oxidative stress augmented the expression of NGF in infarcted rat
hearts (6). Inhibiting the
generation of superoxide decreased the expression of NGF and
attenuated sympathetic neural remodeling following MI (7–9). IMD
exerted an anti-oxidative effect via activating the MAPK/ERK1/2
pathway, which protected the heart from ischemia/reperfusion injury
(14,33). The present study observed that IMD
increased the activity of SOD, decreased the level of MDA and
downregulated the expression of NGF in the peri-infarct zone, which
suggested that IMD attenuates sympathetic neural remodeling via
inhibiting oxidative stress.
In conclusion, the present study demonstrated that
with favorable haemodynamic and anti-oxidative effects, long-term
administration of IMD improves cardiac function, prevents
sympathetic neural remodeling and reduces the incidence of
ventricular arrhythmia in rats with post-MI HF. These results
implicate IMD as a potential therapeutic agent for the treatment of
HF.
Acknowledgements
The present study was partially supported by the
National Nature Science Foundation of China (grant no. 30971265)
and the Shanghai Rising-Star Program (grant no. 08QA1404100).
References
|
1
|
Felker GM, Benza RL, Chandler AB,
Leimberger JD, Cuffe MS, Califf RM and Gheorghiade M: OPTIME-CHF
Investigators: Heart failure etiology and response to milrinone in
decompensated heart failure: Results from the OPTIME-CHF study. J
Am Coll Cardiol. 41:997–1003. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurrelmeyer K, Kalra D, Bozkurt B, Wang F,
Dibbs Z, Seta Y, Baumgarten G, Engle D, Sivasubramanian N and Mann
DL: Cardiac remodeling as a consequence and cause of progressive
heart failure. Clin Cardiol. 21 12 Suppl 1:I14–I19. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colucci WS, Sawyer DB, Singh K and
Communal C: Adrenergic overload and apoptosis in heart failure:
Implications for therapy. J Card Fail. 6 2 Suppl 1:S1–S7. 2000.
|
|
4
|
Swissa M, Zhou S, Gonzalez-Gomez I, Chang
CM, Lai AC, Cates AW, Fishbein MC, Karagueuzian HS, Chen PS and
Chen LS: Long-term subthreshold electrical stimulation of the left
stellate ganglion and a canine model of sudden cardiac death. J Am
Coll Cardiol. 43:858–864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao JM, Chen LS, KenKnight BH, Ohara T,
Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC and
Chen PS: Nerve sprouting and sudden cardiac death. Circ Res.
86:816–821. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee TM, Chen CC and Hsu YJ: Differential
effects of NADPH oxidase and xanthine oxidase inhibition on
sympathetic reinnervation in postinfarct rat hearts. Free Radic
Biol Med. 50:1461–1470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee TM, Chen WT, Yang CC, Lin SZ and Chang
NC: Sitagliptin attenuates sympathetic innervation via modulating
reactive oxygen species and interstitial adenosine in infarcted rat
hearts. J Cell Mol Med. 19:418–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee TM, Lin SZ and Chang NC:
Antiarrhythmic effect of lithium in rats after myocardial
infarction by activation of Nrf2/HO-1 signaling. Free Radic Biol
Med. 77:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xin P, Pan Y, Zhu W, Huang S, Wei M and
Chen C: Favorable effects of resveratrol on sympathetic neural
remodeling in rats following myocardial infarction. Eur J
Pharmacol. 649:293–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roh J, Chang CL, Bhalla A, Klein C and Hsu
SY: Intermedin is a calcitonin/calcitonin gene-related peptide
family peptide acting through the calcitonin receptor-like
receptor/receptor activity-modifying protein receptor complexes. J
Biol Chem. 279:7264–7274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bell D and McDermott BJ: Intermedin
(adrenomedullin-2): A novel counter-regulatory peptide in the
cardiovascular and renal systems. Br J Pharmacol. 153 Suppl
1:S247–S262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirose T, Totsune K, Mori N, Morimoto R,
Hashimoto M, Nakashige Y, Metoki H, Asayama K, Kikuya M, Ohkubo T,
et al: Increased expression of adrenomedullin 2/intermedin in rat
hearts with congestive heart failure. Eur J Heart Fail. 10:840–849.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rademaker MT, Charles CJ, Nicholls MG and
Richards AM: Hemodynamic, hormonal and renal actions of
adrenomedullin 2 in experimental heart failure. Circ Heart Fail.
1:134–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, Peng DQ, Zhang J, Song JQ, Teng X,
Yu YR, Tang CS and Qi YF: Extracellular signal-regulated kinase 1/2
activation is involved in intermedin1-53 attenuating myocardial
oxidative stress injury induced by ischemia/reperfusion. Peptides.
33:329–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Bian Y, Zhang N, Guo J, Wang C, Lau
WB and Xiao C: Intermedin protects against myocardial
ischemia-reperfusion injury in diabetic rats. Cardiovasc Diabetol.
12:912013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao X, Li RS, Li H, Zhu GZ, Huang XG,
Shao S and Bai B: Intermedin protects against renal
ischemia-reperfusion injury by inhibition of oxidative stress. Am J
Physiol Renal Physiol. 304:F112–F119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong Y, Hay DL, Quirion R and Poyner DR:
The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol.
166:110–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Connelly KA, Advani A, Advani S, Zhang Y,
Thai K, Thomas S, Krum H, Kelly DJ and Gilbert RE: Combination
angiotensin converting enzyme and direct renin inhibition in heart
failure following experimental myocardial infarction. Cardiovasc
Ther. 31:84–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vracko R, Thorning D and Frederickson RG:
Nerve fibers in human myocardial scars. Hum Pathol. 22:138–146.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfeffer MA and Braunwald E: Ventricular
remodeling after myocardial infarction. Experimental observations
and clinical implications. Circulation. 81:1161–1172. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hagner S, Stahl U, Knoblauch B, McGregor
GP and Lang RE: Calcitonin receptor-like receptor: Identification
and distribution in human peripheral tissues. Cell Tissue Res.
310:41–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong F, Taylor MM, Samson WK and Ren J:
Intermedin (adrenomedullin-2) enhances cardiac contractile function
via a protein kinase C- and protein kinase A-dependent pathway in
murine ventricular myocytes. J Appl Physiol (1985). 101:778–784.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujisawa Y, Nagai Y, Miyatake A, Miura K,
Nishiyama A, Kimura S and Abe Y: Effects of adrenomedullin 2 on
regional hemodynamics in conscious rats. Eur J Pharmacol.
558:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Zhang J, Zhang JQ and Weber KT:
Renin expression at sites of repair in the infarcted rat heart. J
Mol Cell Cardiol. 33:995–1003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen H, Wang X, Tong M, Wu D, Wu S, Chen
J, Wang X, Wang X, Kang Y, Tang H, et al: Intermedin suppresses
pressure overload cardiac hypertrophy through activation of
autophagy. PLoS One. 8:e647572013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen PS, Chen LS, Cao JM, Sharifi B,
Karagueuzian HS and Fishbein MC: Sympathetic nerve sprouting,
electrical remodeling and the mechanisms of sudden cardiac death.
Cardiovasc Res. 50:409–416. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao JM, Fishbein MC, Han JB, Lai WW, Lai
AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, et al:
Relationship between regional cardiac hyperinnervation and
ventricular arrhythmia. Circulation. 101:1960–1969. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li W, Knowlton D, Van Winkle DM and
Habecker BA: Infarction alters both the distribution and
noradrenergic properties of cardiac sympathetic neurons. Am J
Physiol Heart Circ Physiol. 286:H2229–H2236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hasan W, Jama A, Donohue T, Wernli G,
Onyszchuk G, Al-Hafez B, Bilgen M and Smith PG: Sympathetic
hyperinnervation and inflammatory cell NGF synthesis following
myocardial infarction in rats. Brain Res. 1124:142–154. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verma A, Marrouche NF, Schweikert RA,
Saliba W, Wazni O, Cummings J, Abdul-Karim A, Bhargava M, Burkhardt
JD, Kilicaslan F, et al: Relationship between successful ablation
sites and the scar border zone defined by substrate mapping for
ventricular tachycardia post-myocardial infarction. J Cardiovasc
Electrophysiol. 16:465–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lindsay RM, Thoenen H and Barde YA:
Placode and neural crest-derived sensory neurons are responsive at
early developmental stages to brain-derived neurotrophic factor.
Dev Biol. 112:319–328. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hassankhani A, Steinhelper ME, Soonpaa MH,
Katz EB, Taylor DA, Andrade-Rozental A, Factor SM, Steinberg JJ,
Field LJ and Federoff HJ: Overexpression of NGF within the heart of
transgenic mice causes hyperinnervation, cardiac enlargement and
hyperplasia of ectopic cells. Dev Biol. 169:309–321. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Shen YH, Utama B, Wang J, LeMaire
SA, Coselli JS, Vercellotti GM and Wang XL: HCMV infection
attenuates hydrogen peroxide induced endothelial
apoptosis-involvement of ERK pathway. FEBS Lett. 580:2779–2787.
2006. View Article : Google Scholar : PubMed/NCBI
|