Introduction
Autism is a complex neurodevelopmental disorder
characterized by three central features: Social interaction
deficits, language retardation and aberrant stereotypic behavior
(1,2). In 2016, the Centers for Disease
Control and Prevention reported that 1 in 68 children is diagnosed
with autism spectrum disorder in the United States (3). Currently, no effective therapeutic
strategies are available to ameliorate the key deficits associated
with autism Therefore, the need for the development of novel
targeted treatments is urgent for the management of autism, and for
improvement of the patients' quality of life.
Previous studies have suggested that oxidative
stress may be involved in the development of autism (4,5).
N-acetylcysteine (NAC) is a source of the amino acid L-cysteine,
and is a precursor in the formation of glutathione (GSH) (6), which is a major intracellular
antioxidant within the central nervous system (CNS) (7). In addition to providing cysteine for
GSH production, NAC has been revealed to directly scavenge oxygen
free radicals through its thiol-reducing group, and has been
suggested as a promising neuroprotectant in the CNS (8,9). NAC
has been reported to cross the blood brain barrier (10,11),
decrease oxidative stress and inflammatory cytokine production,
replenish GSH levels, and thus ameliorate autism-associated
irritability, including self-injurious behavior (12). Previous placebo-controlled human
trials demonstrated that, following administration of NAC,
autism-associated irritability was attenuated in pediatric patients
with autism (12,13). In addition, two clinical trials
demonstrated that the combination of NAC with risperidone
significantly alleviated irritability in young patients with autism
(14,15). Furthermore, NAC has been suggested
to ameliorate additional features of autism, as it was demonstrated
to improve social interaction (7,16)
and reduce anxiety-like behaviors (16). However, to the best of our
knowledge, the effects of NAC on repetitive/stereotypic activity
and oxidative stress in autism models, and the molecular mechanisms
underlying the effects of NAC in the treatment of autism, have yet
to be elucidated.
The canonical Wnt pathway includes the major
effector β-catenin and glycogen synthase kinase (GSK)-3β. In the
absence of Wnt ligands, β-catenin associates with a cytoplasmic
degradation protein complex, leading to proteasomal degradation
(17). With secreted Wnt ligands,
the canonical Wnt pathway is activated, and the cytoplasmic
degradation protein complex is prevented from forming. As a result,
β-catenin accumulates in cytoplasm and translocates to the nucleus
(18). GSK-3β destabilizes
β-catenin by phosphorylating its inhibitory sites, such as Ser33,
Ser37, and Thr41 (19). Previous
studies have reported that oxidative stress activated the canonical
Wnt intracellular signaling pathway during the pathogenesis of
various diseases, including preeclampsia (20), diabetic nephropathy (21) and gastric cancer (22). The canonical Wnt pathway has also
been reported to be involved in the development of autism (23–25).
Therefore, elucidating the molecular mechanisms that are involved
in the modulation of oxidative processes, and the roles of the Wnt
pathway in the pathogenesis of autism, is of critical
importance.
The present study aimed to investigate the effects
of NAC on autism-like behavioral phenotypes, and to examine the
molecular mechanisms underlying its actions in a valproic acid
(VPA)-induced animal model of autism. NAC was demonstrated to
reverse behavioral abnormalities, including stereotypic behaviors,
and its therapeutic effects were associated with a reduction in
oxidative stress. However, the molecular mechanisms involved in the
actions of NAC did not appear to be associated with the canonical
Wnt signaling pathway. The Wnt/β-catenin pathway may be indirectly
regulated by NAC through the activation of alternative signaling
pathways or upstream factors, and further studies are required to
elucidate the mechanisms that may be involved in its actions.
Materials and methods
Animals and experimental groups
Experimental procedures were approved by the
Laboratory Animal Care and Use Committee of Xinxiang Medical
University, and were in accordance with the guidelines for animal
experimentation of the Institutes of Health of Xinxiang Medical
University (Xinxiang, China). A total of 60 Sprague-Dawley rats
(age, 12 weeks; 40 female, 220–250 g; 20 male, 350–390 g) were
purchased from Vital River Laboratories Co., Ltd. (Beijing, China)
and were allowed to breed. The rats were kept in a cage with 40–80%
relative humidity and a controlled temperature of 24±1°C on a 12-h
light/dark cycle (lights off at 19:00). Rats had free access to
food and water. The presence of vaginal plugs was used to indicate
gestation. For the establishment of the autism model (26,27),
female rats were intraperitoneally injected with 600 mg/kg VPA
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on gestation day
12.5, vehicle-treated rats received physiological saline. The pups
were weaned on postnatal day 23 and housed separately. Offspring
(23 days old) of VPA-treated mothers were randomly assigned into
the following 2 groups: VPA and VPA + NAC (Sigma-Aldrich; Merck
KGaA) groups. The 23-day-old-offspring of saline-treated mothers
were randomly divided into the following 2 groups: Control and NAC
groups. NAC (150 mg/kg) was intraperitoneally administered to rats
in the NAC and VPA + NAC groups once daily for 4 weeks.
Subsequently, male offspring were used for
repetitive/stereotypic-like behavioral testing and then sacrificed
by decapitation. Prefrontal cortex (PFC) and hippocampus (HC)
samples were isolated and frozen at −80°C for further analysis.
Behavioral testing
Behavioral testing was conducted using an open field
test, as previously described (28). Briefly, rats were placed
individually in a plywood apparatus (100×100×40 cm). Testing was
performed between 9:00 am and 3:00 pm. Two rats were observed
simultaneously, with one animal per chamber. The animals were
individually placed in the field and allowed to explore it freely
for 60 min. Movements were recorded using a digital video camera
linked to a computer (Ethovision 3.0; Noldus Information Technology
BV, Wageningen, The Netherlands); the mean activity during the
60-min testing session, divided into 6 time bins (10 min each), was
then analyzed (EthoVision 3.0; Noldus). Repetitive/stereotypic-like
movements (for example run and rotate, nose picking, lip biting,
repeated sucking) were measured and analyzed by an observer blind
to the treatments.
Oxidative stress assays
Malondialdehyde (MDA) assay
As a marker of lipid peroxidation, the MDA contents
were evaluated in brain tissue samples isolated from rats using a
commercially available MDA assay kit (cat no. S0131; Beyotime
Institute of Biotechnology, Haimen, China), according to the
manufacturer's protocol. Briefly, brain tissue sections were
homogenized in 0.15 ml thiobarbituric acid (TBA) diluent, 0.05 ml
TBA and 3 µl antioxidant then heated and boiled for 15 min.
Following centrifugation at 1,000 × g for 10 min at room
temperature, the supernatants were collected, and the absorbance of
the samples was measured at 532 nm using a microplate reader. MDA
contents were expressed as µmol/mg protein.
GSH assay
5,5′-Dithiobis-(2-nitrobenzoic acid) was used to
detect intracellular GSH levels with a Total GSH assay kit (cat no.
S0052; Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Briefly, brain tissue sections were
homogenized with glass homogenizer and incubated with the assay
solution for 5 min at 25°C. The concentration of GSH was measured
spectrophotometrically at 412 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). GSH contents were expressed
as µmol/mg protein.
Western blot analysis
Cytoplasmic and nuclear proteins were isolated from
brain tissue samples using a Nuclear and Cytoplasmic Protein
Extraction kit (cat no. P0028; Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Tissue
samples (60 mg) were lysed in a 200 µl solution containing
cytoplasmic protein extraction agent A and B (20:1) supplemented
with phenylmethylsulfonyl fluoride (PMSF). Following tissue
homogenization, the samples were incubated for 15 min on ice. Then
the lysates were centrifuged for 5 min at 1,500 × g at 4°C, and the
supernatants, consisting of the cytoplasmic fraction, were
immediately frozen at −80°C for subsequent experiments. The pellets
were resuspended in nuclear protein extraction agent B supplemented
with PMSF. Following vortexing for 5 sec, the mixture was incubated
on ice for 1 min and centrifuged for 10 min at 12,000 × g at 4°C to
obtain supernatants containing nuclear proteins. Proteins were
quantified with a bicinchoninic acid assay kit (cat no. P0010;
Beyotime Institute of Biotechnology). Equal amounts of extracted
protein samples (20–50 µg) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
bovine serum albumin (cat no. A7030; Sigma-Aldrich; Merck KGaA) or
fat-free milk in TBS containing 0.1% Tween-20 for 1 h at room
temperature. Membranes were then incubated with primary antibodies
overnight at 4°C. Following incubation with horseradish
peroxidase-conjugated goat anti-rabbit antibodies (cat no.
111035003; Jackson Immunoresearch Laboratories, Inc., West Grove,
PA, USA) at a 1:5,000 dilution for 1 h at room temperature, the
protein bands were visualized using an enhanced chemiluminescence
kit (EMD Millipore). Blots were semi-quantified using densitometric
analysis with ImageJ software (version 1.44p; National Institutes
of Health, Bethesda, MD, USA). GAPDH (1:5,000; cat no. HRP-60004;
ProteinTech Group, Inc., Chicago, IL, USA) or histone H3 (1:1,000;
cat no. AF0009; Beyotime Institute of Biotechnology,) were used as
the internal control for cytoplasmic and nuclear proteins,
respectively. The following primary antibodies were used in the
present study: Anti-glycogen synthase kinase (GSK)-3β (1:1,000; cat
no. 9315), anti-phosphorylated (p)-GSK-3β (Ser-9; 1:500; cat no.
9336) (both from Cell Signaling Technology, Inc., Danvers, MA,
USA), anti-β-catenin (1:1,000; cat no. sc-7199; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-p-β-catenin
(Ser-33/37/Thr41; 1:500; cat no. 9561; Cell Signaling Technology,
Inc.).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean, for 5 repeated experiments. The statistical significance
of the differences between groups was assessed using a one-way
analysis of variance (ANOVA) followed by Fisher's least-significant
difference post hoc correction for multiple comparisons. The
statistical significance of the differences between correlated
samples was assessed using a repeated measures two-way ANOVA
followed by Fisher's least-significant difference post hoc test for
multiple comparisons. Statistical analyses were performed using
SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of NAC administration on
repetitive/stereotypic behavior in VPA-exposed rats
Previous studies have reported that treatment with
NAC ameliorated behavioral disorders in children with autism
(13,15,29,30).
Therefore, the present study investigated the effects of NAC on
autism-associated behavioral disorders in a VPA-induced rat model
of autism.
An open field test was used to assess
repetitive/stereotypic behavior in VPA-exposed male rats. The
present results demonstrated that the distance traveled by rats in
the VPA group was significantly increased compared with the control
group between 0 and 40 min of testing (Fig. 1A). The distance traveled by rats in
the NAC and NAC + VPA groups was comparable to that of rats in the
control group, and was significantly decreased compared with the
VPA group across the 0–20, 30–40 and 50–60 min testing durations
(Fig. 1A). In addition,
VPA-treated rats spent significantly more time engaged in
repetitive/stereotypic activities, and their number of
repetitive/stereotypic movements was increased compared with the
control group. VPA-treated rats were overactive between 0 and 40
min, and between 50 and 60 min of testing (Fig. 1B), and they spent more time
occupied in repetitive/stereotypic movements between 0 and 40 min
of testing compared with the control rats (Fig. 1C). Conversely, rats in the VPA +
NAC group were not overactive and spent significantly less time
occupied in repetitive/stereotypic movements between 0 and 40 min
of testing (Fig. 1C). Furthermore,
they exhibited significantly fewer repetitive/stereotypic movements
throughout the duration of the experiment compared with rats in the
VPA group (Fig. 1B). VPA +
NAC-treated rats exhibited a similar behavior to rats in the
control group throughout the 60-min duration of the experiment.
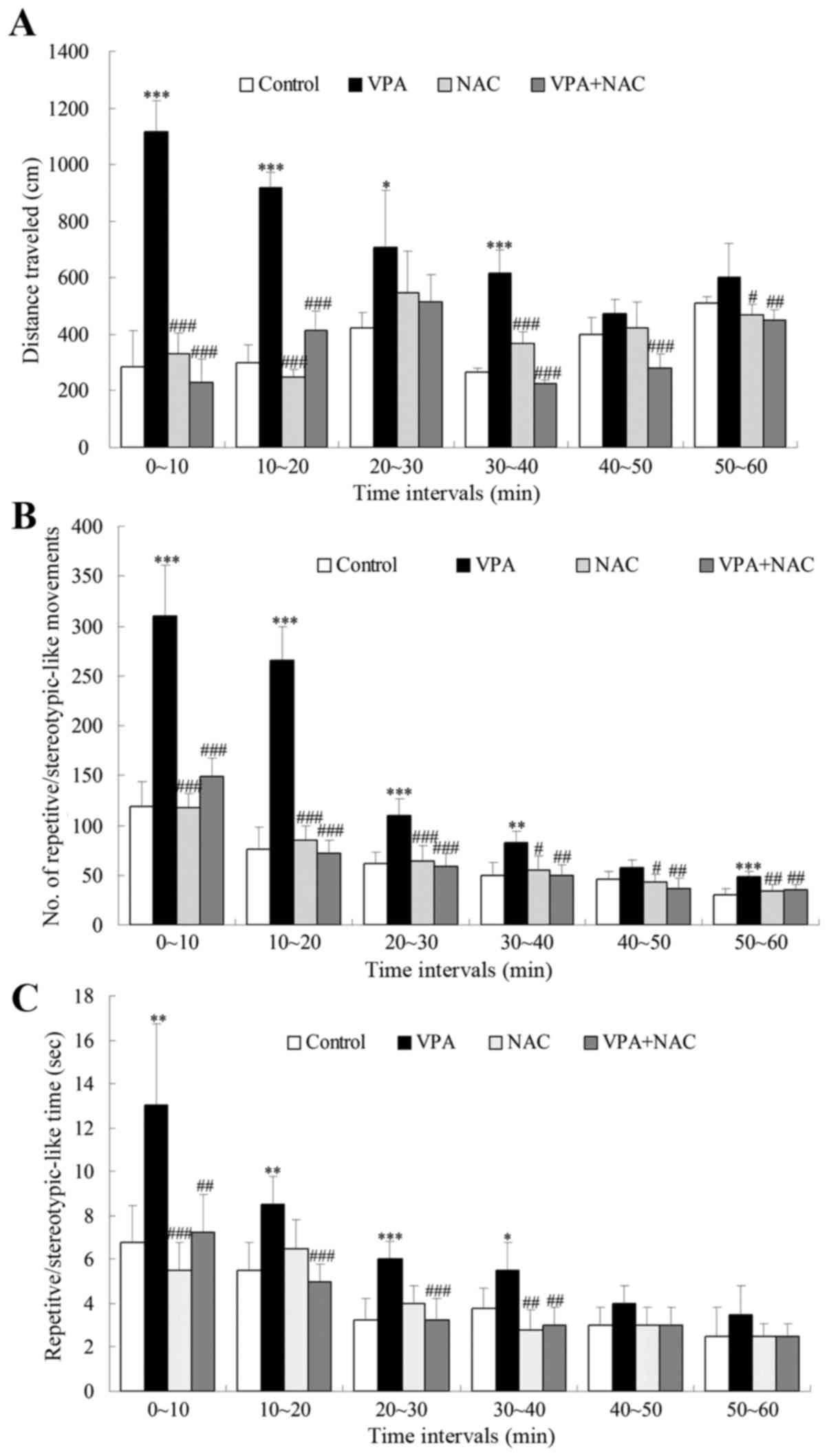 | Figure 1.Effects of NAC on
repetitive/stereotypic behaviors in VPA-exposed rats during an open
field test. (A) The distance traveled, (B) the number of
repetitive/stereotypic movements and (C) the time spent engaged in
repetitive/stereotypic behaviors were detected for 60 min. Control,
rats received no treatment; VPA, rats were prenatally exposed to
VPA for the establishment of an autism model; NAC, rats received
daily treatment with NAC for 4 weeks; VPA + NAC, rats were
prenatally exposed to VPA for the establishment of an autism model,
and subsequently received daily treatment with NAC for 4 weeks.
Data are expressed as the mean ± standard error. Control, n=8; VPA
group, n=10; NAC group, n=7; VPA + NAC group, n=12. *P<0.05,
**P<0.01, ***P<0.001 vs. control; #P<0.05,
##P<0.01, ###P<0.001 vs. VPA. NAC,
N-acetylcysteine; VPA, valproic acid. |
Effects of NAC on oxidative stress in
VPA-exposed rats
NAC has previously been demonstrated to ameliorate
autism-associated social interaction deficits and anxiety-related
behaviors (16), and the present
results suggested it could also attenuate repetitive/stereotypic
movements. Therefore, the molecular mechanisms underlying the
effects of NAC on autism-like phenotypes were further investigated,
and its restorative actions on abnormal oxidative homeostasis were
revealed. MDA and GSH contents were measured in brain tissue
samples isolated from VPA-exposed rats, in order to examine the
effects of NAC on lipid peroxidation and total antioxidative
capacity in the brain. As demonstrated in Fig. 2, MDA levels were significantly
increased, whereas GSH levels were significantly decreased, in the
prefrontal cortex and hippocampus of VPA-treated rats compared with
controls. Notably, treatment with NAC was revealed to significantly
downregulate cortical and hippocampal MDA levels, and upregulate
GSH levels compared with VPA-exposed rats (Fig. 2). No significant differences were
detected in MDA and GSH levels between rats in the VPA + NAC and
control groups (Fig. 2).
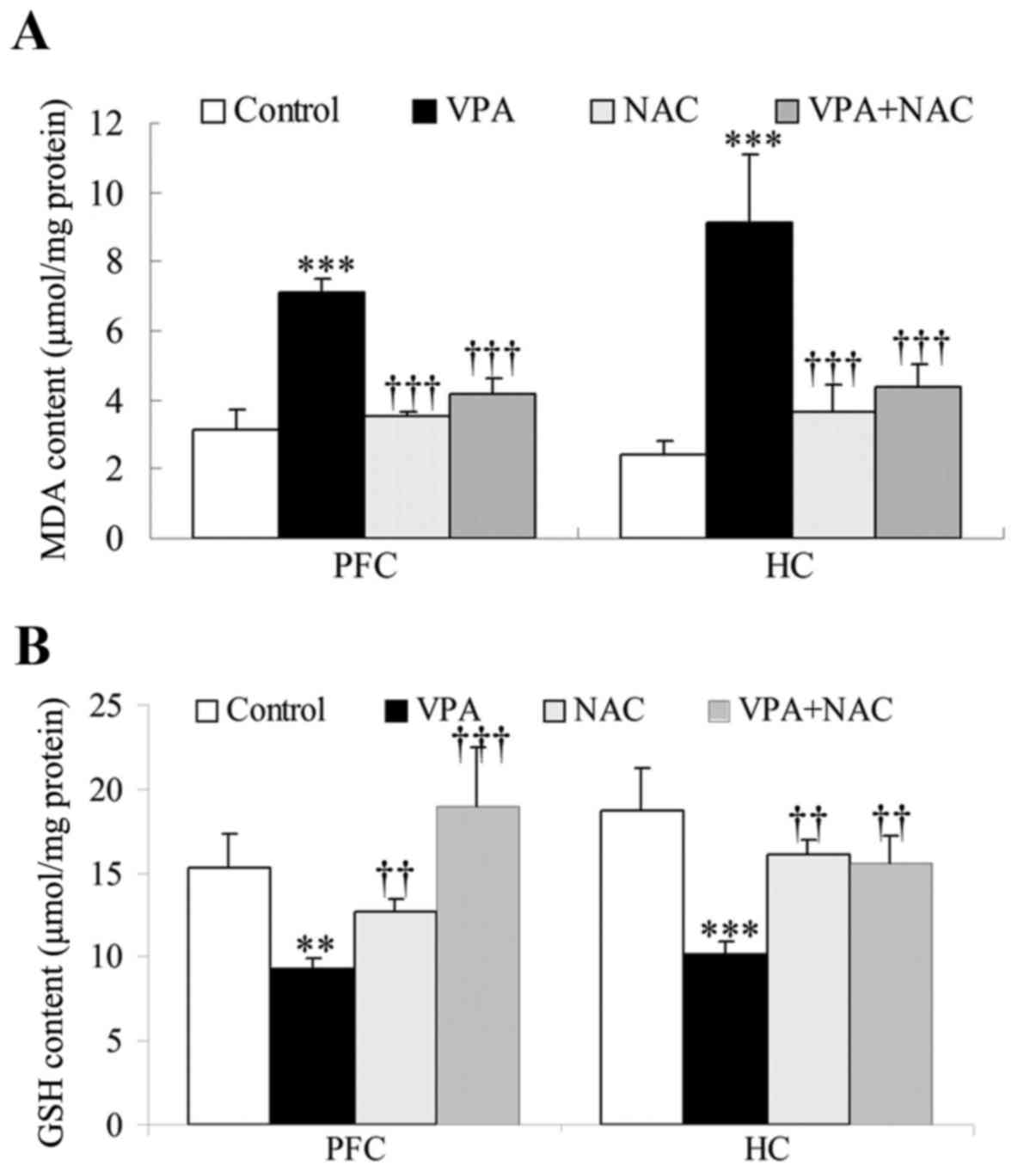 | Figure 2.Effects of NAC on MDA and GSH levels
in rat brain tissue samples. Intracellular total (A) MDA and (B)
GSH levels were measured using commercially available kits in PFC
and HC tissue samples. Control, rats received no treatment; VPA,
rats were prenatally exposed to VPA for the establishment of an
autism model; NAC, rats received daily treatment with NAC for 4
weeks; VPA + NAC, rats were prenatally exposed to VPA for the
establishment of an autism model, and subsequently received daily
treatment with NAC for 4 weeks. Data are expressed as the mean ±
standard error (n=5). **P<0.01, ***P<0.001 vs. control;
††P<0.01, †††P<0.001 vs. VPA. NAC,
N-acetylcysteine; MDA, malondialdehyde; GSH, glutathione; PFC,
prefrontal cortex; HC, hippocampus; VPA, valproic acid. |
Effects of NAC on the canonical Wnt
signaling pathway
Oxidative stress has been suggested to be involved
in the pathogenesis of autism (4,5), and
the canonical Wnt signaling pathway has been implicated in
oxidative processes (20–22). Therefore, the present study
investigated the involvement of the Wnt pathway in the beneficial
effects of NAC in VPA-exposed rats.
β-catenin is a key signaling molecule involved in
the canonical Wnt pathway, whereas GSK-3β negatively regulates
β-catenin via phosphorylation of its inhibitory sites, including
Ser-33, Ser-37 and Thr-41 (19).
The present results demonstrated that GSK-3β Ser-9 phosphorylation
was enhanced in brain tissue samples isolated from VPA-exposed rats
compared with control rats (Fig. 3A
and B), thus suggesting that GSK-3β activity may be suppressed
in VPA-induced autism. In addition, VPA-exposed rats exhibited
significantly decreased β-catenin phosphorylation levels
(Ser-33/37/Thr-41) compared with the controls (Fig. 3C and D), thus suggesting that VPA
may inhibit GSK-3β to stabilize β-catenin. However, no
statistically significant differences were detected in GSK-3β and
β-catenin phosphorylation levels between rats in the VPA + NAC and
VPA groups (Fig. 3).
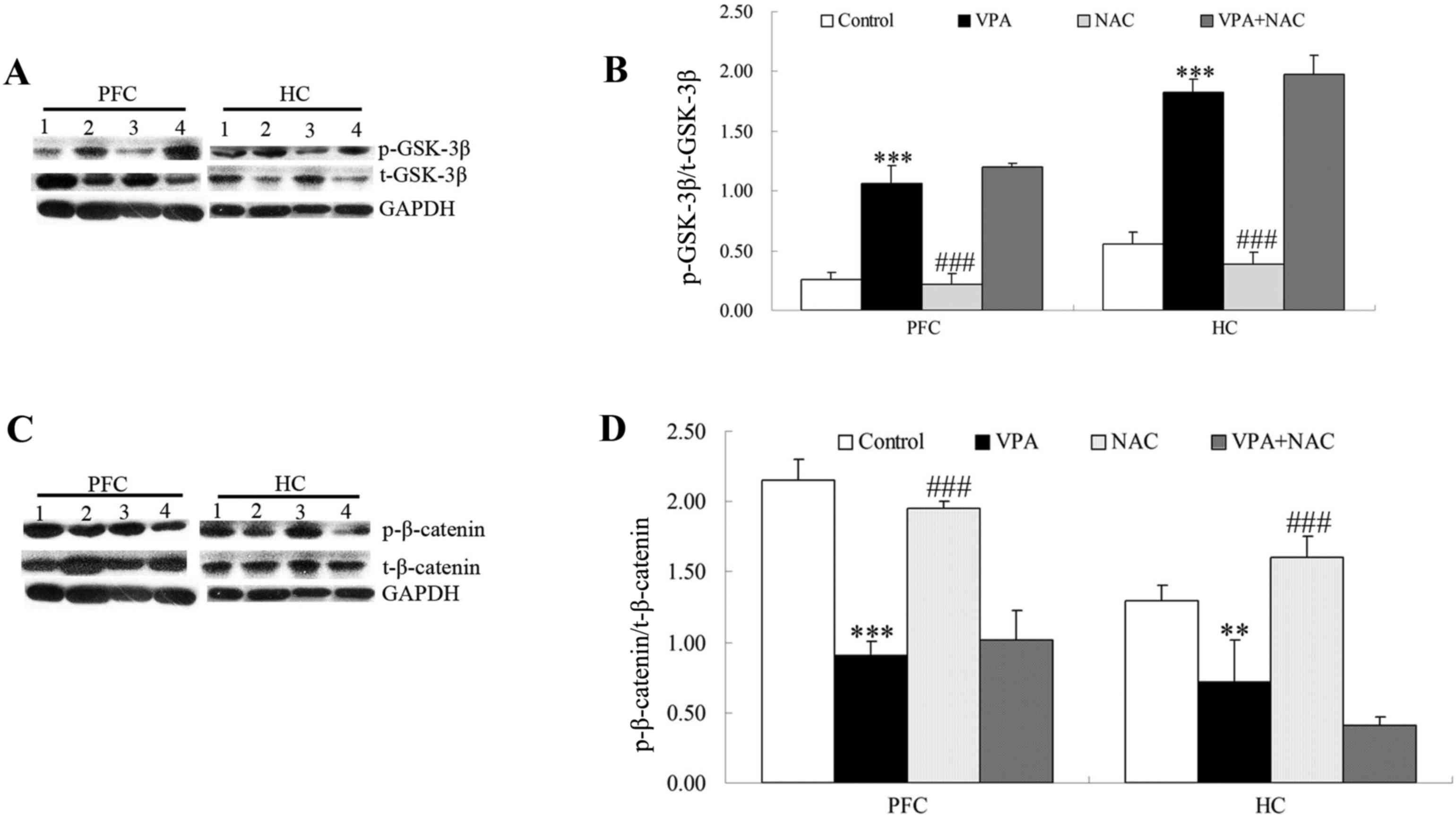 | Figure 3.Effects of NAC on the Wnt/β-catenin
signaling pathway. Protein expression levels of (A and B) GSK-3β
and p-GSK-3β, and (C and D) β-catenin and p-β-catenin were assessed
using western blot analysis in PFC and HC tissue samples. GAPDH was
used as an internal control. 1, Control; 2, VPA; 3, NAC; 4, VPA +
NAC groups. Control, rats received no treatment; VPA, rats were
prenatally exposed to VPA for the establishment of an autism model;
NAC, rats received daily treatment with NAC for 4 weeks; VPA + NAC,
rats were prenatally exposed to VPA for the establishment of an
autism model, and subsequently received daily treatment with NAC
for 4 weeks. Data are expressed as the mean ± standard error (n=5).
**P<0.01, ***P<0.001 vs. control; ###P<0.001
vs. VPA. NAC, N-acetylcysteine; GSK, glycogen synthase kinase; p-,
phosphorylated; PFC, prefrontal cortex; HC, hippocampus; VPA,
valproic acid. |
Active β-catenin is translocated into the nucleus to
activate the transcription of target genes of the Wnt/β-catenin
pathway (31). Therefore, the
protein expression levels of β-catenin were further investigated in
the cytoplasm and nucleus. VPA-exposed rats exhibited significantly
increased nuclear and cytoplasmic protein expression levels of
β-catenin compared with rats in the control group (Fig. 4). Compared with the VPA group, NAC
alone decreased the protein levels of β-catenin in the cytoplasm
and nucleus (Fig. 4). However, no
statistically significant differences were detected in nuclear and
cytoplasmic β-catenin levels between rats in the VPA + NAC and VPA
groups (Fig. 4).
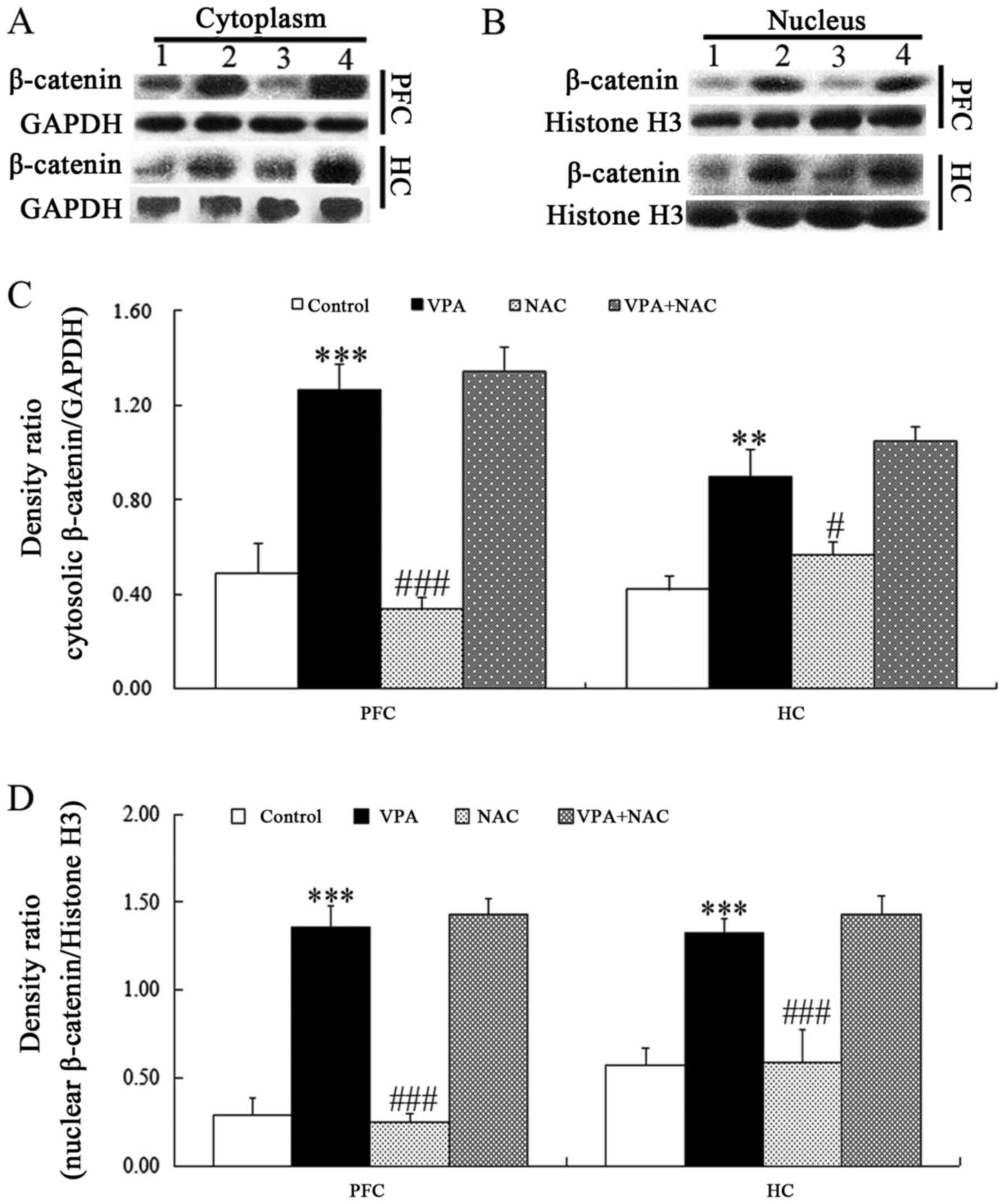 | Figure 4.Effects of NAC on β-catenin protein
expression levels in the cytoplasm and nucleus of rat brain tissue
samples. Western blot analysis was used to assess β-catenin protein
expression levels in the (A and C) cytoplasm and (B and D) nucleus
in PFC and HC tissue samples. GAPDH and histone H3 were used as
internal controls. 1, Control; 2, VPA; 3, NAC; 4, VPA + NAC.
Control, rats received no treatment; VPA, rats were prenatally
exposed to VPA for the establishment of an autism model; NAC, rats
received daily treatment with NAC for 4 weeks; VPA + NAC, rats were
prenatally exposed to VPA for the establishment of an autism model,
and subsequently received daily treatment with NAC for 4 weeks.
Data are expressed as the mean ± standard error (n=5).
***P<0.001 vs. control; ##P<0.01,
###P<0.001 vs. VPA. NAC, N-acetylcysteine; PFC,
prefrontal cortex; HC, hippocampus; VPA, valproic acid. |
Discussion
The present study demonstrated that NAC exerted
beneficial effects in a VPA-induced rat model of autism. Treatment
with NAC was demonstrated to reverse abnormal locomotor behaviors,
including repetitive/stereotypic activity in rats prenatally
exposed to VPA, compared with untreated VPA-exposed offspring. The
beneficial effects of NAC in autism-induced deficits were
associated with its antioxidative properties, resulting in the
restoration of redox homeostasis in the brain. However, its actions
did not appear to be associated with the canonical Wnt signaling
pathway, as demonstrated by the unaltered GSK-3β and β-catenin
phosphorylation levels, and the β-catenin protein levels in the
cytoplasm and nucleus. These results suggested that the
Wnt/β-catenin signaling pathway may be indirectly regulated by NAC
through the activation of alternative signaling pathways or
upstream factors. NAC alters glutathione metabolism and improves
mitochondrial dysfunction in a subset of autistic lymphoblastoid
cell lines (32). In accordance
with these results, NAC has been reported to ameliorate autism
phenotypes, associated with altered GSH levels (7,30).
Autism is a complex neurodevelopmental disorder, and
its etiology has yet to be fully elucidated. Previous studies have
suggested that oxidative stress may be involved in the pathogenesis
of autism (30,33–36).
NAC is a potent antioxidant that is widely used for the treatment
of an acetaminophen overdose (37), as a renal protectant in
contrast-induced nephropathy (38), and as a preventive agent for atrial
fibrillation (39). NAC is a
membrane-permeable L-cysteine precursor, and is intracellularly
reduced to L-cysteine, a precursor of the endogenous antioxidant,
GSH (40). In the brain, the
oxidation of NAC-derived L-cysteine to cystine has been reported,
which can subsequently modulate extracellular glutamate levels
(41). The regulatory effects of
NAC on extracellular glutamate levels, and its restorative actions
on intracellular GSH levels, in addition to its well-established
safety profile, suggest a potential for NAC as an alternative
therapeutic strategy for the treatment of patients with autism.
Clinical studies have reported that NAC alone, or in combination
with risperidone improved some of the core symptoms of autism,
including irritability or aggressive behavior (7,12,14,15),
self-injurious behavior (13),
social interaction impairments (7,16),
and anxiety-like behavior (16).
In the present study, NAC was demonstrated to ameliorate
stereotypic/repetitive locomotor activity in a VPA-induced rat
model of autism.
During open field behavioral testing, NAC was
revealed to attenuate repetitive/stereotypic activity in
VPA-exposed rats, possibly through a decrease in hyper-reactivity
to stress. A previous study from our laboratory demonstrated that
VPA-treated rats have reduced sensitivity to stress and exhibited
repetitive/stereotypic activity (28). Conversely, when stress sensitivity
is restored to physiological levels, repetitive/stereotypic
activities are abolished (28).
Therefore, it may be hypothesized that the frequent
repetitive/stereotypic movements in VPA-exposed rats are a result
of hyper-reactivity to stress. However, a previous study reported
contradictory results, demonstrating that the distance traveled in
the open field was similar between VPA + NAC-treated and
VPA-treated rats (16). This
discrepancy may be due to the different doses of VPA used and the
variation in NAC treatment duration. However, further studies are
required in order to examine the complex interactions between
neuropeptides and neuroendocrine factors that modulate stress
reactions, and to determine their effects on movement impairments
in VPA-treated rats.
The present study investigated the involvement of
the antioxidative properties of NAC in the amelioration of
autism-like behavioral abnormalities. In order to assess the
antioxidative effects of NAC, the levels of MDA, a marker of lipid
peroxidation, and thus of oxidative stress, were assessed in rat
brain tissue samples. GSH is an intracellular antioxidant that
participates in the endogenous mechanisms of defense against
reactive oxygen species (42). The
present results demonstrated that prenatal exposure to VPA induced
oxidative stress in rats, as demonstrated by the increased MDA and
decreased GSH levels in brain tissue samples. Notably, following
treatment with NAC, MDA levels were suppressed, whereas GSH levels
were enhanced in VPA-exposed rats compared with the untreated VPA
group. These findings are in accordance with a previous study
reporting that NAC significantly enhanced GSH levels in diabetic
rats (43).
Further studies are required to elucidate the
molecular mechanisms underlying the effects of NAC in the
prevention of abnormal locomotor behaviors in autism. NAC is a GSH
precursor and augments intracellular GSH levels. Wnt and its
associated signaling proteins have been identified as targets for
GSH (44), whereas NAC has been
demonstrated to inhibit the activation of the canonical Wnt pathway
in the retinas of diabetic rats (43). Furthermore, previous studies have
reported a critical role for the Wnt pathway in the pathogenesis of
autism (23,28,45).
In a previous study by the present authors, VPA was revealed to
inhibit the phosphorylation, and thus the inactivation, of
β-catenin, which is a key effector during canonical Wnt signaling,
possibly through the upregulation of WNT1 and WNT2
expression, thereby activating the canonical Wnt pathway (46). Notably, following treatment with
the specific Wnt pathway inhibitor sulindac, Wnt/β-catenin
signaling was suppressed and autism-like behavioral abnormalities
in a VPA-induced rat model of autism were attenuated (28). These results suggested that
increased Wnt-mediated signaling may contribute to the
susceptibility to autism.
Oxidative stress and the Wnt signaling pathway have
been implicated in numerous pathological conditions (21,47,48);
however, further studies are required to elucidate the modulatory
mechanisms that affect oxidative processes and implicate the Wnt
pathway in the pathogenesis of autism. As a critical effector of
Wnt signaling, β-catenin can activate the transcription of various
target genes, following its cytoplasmic accumulation and subsequent
nuclear translocation (18,49).
In the present study, VPA was revealed to induce oxidative stress,
as demonstrated by the increased levels of the oxidative stress
marker, MDA, and the downregulation in the levels of the endogenous
antioxidant, GSH, in VPA-exposed rats. Furthermore, prenatal
exposure to VPA enhanced β-catenin levels in the cytoplasm and
nucleus, whereas it was revealed to inhibit the phosphorylation of
β-catenin, and promote the phosphorylation of GSK-3β. These results
indicated that VPA activated the canonical Wnt signaling pathway in
the rat autism model. However, treatment with NAC did not appear to
affect the nuclear and cytoplasmic β-catenin levels, or the
phosphorylation of β-catenin and GSK-3β, thus suggesting that the
Wnt pathway may not be involved in NAC-mediated amelioration of
autism-like behavior in VPA-treated rats. These results are in
accordance with our previous in vitro study, which
demonstrated that NAC reduced oxidative stress in primary neuronal
cultures following pre-treatment with VPA without affecting the
activity of the Wnt pathway (45).
These findings suggested that the unaltered activity of the
canonical Wnt pathway may be an indirect consequence, of reduced
oxidative stress in VPA-exposed offspring followed by NAC
administration.
The association between the Wnt signaling pathway
and oxidative stress under various pathological conditions remains
controversial. A previous study from our group reported that
treatment with sulindac, a small-molecule inhibitor of the
canonical Wnt pathway, reduced the levels of 4-hydroxynonenal, a
marker of lipid peroxidation, in a VPA-induced rat model of autism,
thus suggesting that oxidative stress may be involved in the
regulation of Wnt signaling (28).
However, it may be hypothesized that sulindac directly suppressed
oxidative stress due to its antioxidative properties, and these
effects may not be mediated by the Wnt pathway. Additionally,
sulindac, a nonsteroidal anti-inflammatory drug, which has also
been examined in cancer, neurodegenerative disease, and age-related
macular degeneration, is able to reduce the levels of lipid
peroxidation in an N-nitrosamine-induced mouse model of lung
tumorigenesis (50), to reduce the
generation of superoxide anions in Alzheimer's disease (51), and to lower ROS levels in a retinal
pigmented epithelial cell line (52). VPA and sulindac reduce the activity
of the canonical Wnt signaling pathway, compared with VPA exposure
alone (28), however, sulindac has
anti-inflammatory properties. Therefore, VPA may induce
inflammatory status by possibly, though not exclusively, affecting
the Wnt pathway. Further studies are required to investigate the
association between oxidative stress and inflammation in the
pathogenesis of autism. The molecular mechanisms underlying the
direct and indirect implication of the canonical Wnt pathway in
oxidative stress during the pathogenesis of autism require further
investigation.
In conclusion, the results of the present study
demonstrated that NAC reversed abnormal locomotor behaviors,
including repetitive/stereotypic activity, in a VPA-induced rat
model of autism, possibly due to its antioxidative properties.
Notably, the beneficial effects of NAC in autism-like behavior did
not appear to be associated with the canonical Wnt signaling
pathway, suggesting that Wnt/β-catenin signaling may be indirectly
regulated by NAC through alternative signaling pathways or upstream
mediators. However, the results of the present study should be
interpreted with caution, as autism is a complex and highly
heterogeneous group of pathologies, and a VPA-induced rat model is
not representative of the wide spectrum of disorders that autism
encompasses.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301174), the
support project for the Disciplinary group of Psychology and
Neuroscience, Xinxiang Medical University (grant no.
2016PN-KFKT-18), the Doctoral Scientific Research Foundation of
Xinxiang Medical University (grant no. DOC2012-07) and the National
College Student Innovation Project (grant no. 201310472070).
References
|
1
|
Baird G, Simonoff E, Pickles A, Chandler
S, Loucas T, Meldrum D and Charman T: Prevalence of disorders of
the autism spectrum in a population cohort of children in South
Thames: The special needs and autism project (SNAP). Lancet.
368:210–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levitt P and Campbell DB: The genetic and
neurobiologic compass points toward common signaling dysfunctions
in autism spectrum disorders. J Clin Invest. 119:747–754. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christensen DL, Baio J, Van Naarden Braun
K, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS,
Fitzgerald RT, Kurzius-Spencer M, et al: Prevalence and
characteristics of autism spectrum disorder among children aged 8
years-autism and developmental disabilities monitoring network, 11
sites, United States, 2012. MMWR Surveill Summ. 65:1–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghezzo A, Visconti P, Abruzzo PM, Bolotta
A, Ferreri C, Gobbi G, Malisardi G, Manfredini S, Marini M, Nanetti
L, et al: Oxidative stress and erythrocyte membrane alterations in
children with autism: Correlation with clinical features. PLoS One.
8:e664182013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghanizadeh A, Akhondzadeh S, Hormozi M,
Makarem A, Abotorabi-Zarchi M and Firoozabadi A:
Glutathione-related factors and oxidative stress in autism, a
review. Curr Med Chem. 19:4000–4005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dean O, Giorlando F and Berk M:
N-acetylcysteine in psychiatry: Current therapeutic evidence and
potential mechanisms of action. J Psychiatry Neurosci. 36:78–86.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghanizadeh A and Derakhshan N:
N-acetylcysteine for treatment of autism, a case report. J Res Med
Sci. 17:985–987. 2012.PubMed/NCBI
|
|
8
|
Paintlia MK, Paintlia AS, Barbosa E, Singh
I and Singh AK: N-acetylcysteine prevents endotoxin-induced
degeneration of oligodendrocyte progenitors and hypomyelination in
developing rat brain. J Neurosci Res. 78:347–361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Svedin P, Nie C, Lapatto R, Zhu C,
Gustavsson M, Sandberg M, Karlsson JO, Romero R, Hagberg H and
Mallard C: N-acetylcysteine reduces lipopolysaccharide-sensitized
hypoxic-ischemic brain injury. Ann Neurol. 61:263–271. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farr SA, Poon HF, Dogrukol-Ak D, Drake J,
Banks WA, Eyerman E, Butterfield DA and Morley JE: The antioxidants
alpha-lipoic acid and N-acetylcysteine reverse memory impairment
and brain oxidative stress in aged SAMP8 mice. J Neurochem.
84:1173–1183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holmay MJ, Terpstra M, Coles LD, Mishra U,
Ahlskog M, Öz G, Cloyd JC and Tuite PJ: N-acetylcysteine boosts
brain and blood glutathione in gaucher and parkinson diseases. Clin
Neuropharmacol. 36:103–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hardan AY, Fung LK, Libove RA, Obukhanych
TV, Nair S, Herzenberg LA, Frazier TW and Tirouvanziam R: A
randomized controlled pilot trial of oral N-acetylcysteine in
children with autism. Biol Psychiatry. 71:956–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marler S, Sanders KB and
Veenstra-VanderWeele J: N-acetylcysteine as treatment for
self-injurious behavior in a child with autism. J Child Adolesc
Psychopharmacol. 24:231–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghanizadeh A and Moghimi-Sarani E: A
randomized double blind placebo controlled clinical trial of
N-Acetylcysteine added to risperidone for treating autistic
disorders. BMC Psychiatry. 13:1962013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nikoo M, Radnia H, Farokhnia M, Mohammadi
MR and Akhondzadeh S: N-acetylcysteine as an adjunctive therapy to
risperidone for treatment of irritability in autism: A randomized,
double-blind, placebo-controlled clinical trial of efficacy and
safety. Clin Neuropharmacol. 38:11–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YW, Lin HC, Ng MC, Hsiao YH, Wang CC,
Gean PW and Chen PS: Activation of mGluR2/3 underlies the effects
of N-acetylcystein on amygdala-associated autism-like phenotypes in
a valproate-induced rat model of autism. Front Behav Neurosci.
8:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimelman D and Xu W: Beta-catenin
destruction complex: Insights and questions from a structural
perspective. Oncogene. 25:7482–7491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sierra J, Yoshida T, Joazeiro CA and Jones
KA: The APC tumor suppressor counteracts beta-catenin activation
and H3K4 methylation at Wnt target genes. Genes Dev. 20:586–600.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toledo EM, Colombres M and Inestrosa NC:
Wnt signaling in neuroprotection and stem cell differentiation.
Prog Neurobiol. 86:281–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang B, Luo X, Rao H, Li Q, Shan N, Liu
X and Qi H: Oxidative stress-induced C/EBPβ inhibits β-catenin
signaling molecule involving in the pathology of preeclampsia.
Placenta. 36:839–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu YC, Lee PH, Lei CC, Ho C, Shih YH and
Lin CL: Nitric oxide donors rescue diabetic nephropathy through
oxidative-stress-and nitrosative-stress-mediated Wnt signaling
pathways. J Diabetes Investig. 6:24–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida GJ and Saya H: Inversed
relationship between CD44 variant and c-Myc due to oxidative
stress-induced canonical Wnt activation. Biochem Biophys Res
Commun. 443:622–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hormozdiari F, Penn O, Borenstein E and
Eichler EE: The discovery of integrated gene networks for autism
and related disorders. Genome Res. 25:142–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Yuan X, Wang Z and Li R: The
canonical Wnt signaling pathway in autism. CNS Neurol Disord Drug
Targets. 13:765–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marui T, Funatogawa I, Koishi S, Yamamoto
K, Matsumoto H, Hashimoto O, Jinde S, Nishida H, Sugiyama T, Kasai
K, et al: Association between autism and variants in the
wingless-type MMTV integration site family member 2 (WNT2) gene.
Int J Neuropsychopharmacol. 13:443–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schneider T, Turczak J and Przewłocki R:
Environmental enrichment reverses behavioral alterations in rats
prenatally exposed to valproic acid: Issues for a therapeutic
approach in autism. Neuropsychopharmacology. 31:36–46.
2006.PubMed/NCBI
|
|
27
|
Silvestrin R Bristot, Bambini-Junior V,
Galland F, Bobermim L Daniele, Quincozes-Santos A, Abib R Torres,
Zanotto C, Batassini C, Brolese G, Gonçalves CA, et al: Animal
model of autism induced by prenatal exposure to valproate: Altered
glutamate metabolism in the hippocampus. Brain Res. 1495:52–60.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Sun Y, Wang F, Wang Z, Peng Y and
Li R: Downregulating the canonical Wnt/β-catenin signaling pathway
attenuates the susceptibility to autism-like phenotypes by
decreasing oxidative stress. Neurochem Res. 37:1409–1419. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slattery J Deepmala, Kumar N, Delhey L,
Berk M, Dean O, Spielholz C and Frye R: Clinical trials of
N-acetylcysteine in psychiatry and neurology: A systematic review.
Neurosci Biobehav Rev. 55:294–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wink LK, Adams R, Wang Z, Klaunig JE,
Plawecki MH, Posey DJ, McDougle CJ and Erickson CA: A randomized
placebo-controlled pilot study of N-acetylcysteine in youth with
autism spectrum disorder. Mol Autism. 7:262016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rose S, Frye RE, Slattery J, Wynne R,
Tippett M, Melnyk S and James SJ: Oxidative stress induces
mitochondrial dysfunction in a subset of autistic lymphoblastoid
cell lines. Transl Psychiatry. 4:e3772014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gvozdjáková A, Kucharská J, Ostatníková D,
Babinská K, Nakládal D and Crane FL: Ubiquinol improves symptoms in
children with autism. Oxid Med Cell Longev. 2014:7989572014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gu F, Chauhan V and Chauhan A: Glutathione
redox imbalance in brain disorders. Curr Opin Clin Nutr Metab Care.
18:89–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raymond LJ, Deth RC and Ralston NV:
Potential role of selenoenzymes and antioxidant metabolism in
relation to autism etiology and pathology. Autism Res Treat.
2014:1649382014.PubMed/NCBI
|
|
36
|
James SJ, Rose S, Melnyk S, Jernigan S,
Blossom S, Pavliv O and Gaylor DW: Cellular and mitochondrial
glutathione redox imbalance in lymphoblastoid cells derived from
children with autism. FASEB J. 23:2374–2383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Green JL, Heard KJ, Reynolds KM and Albert
D: Oral and intravenous acetylcysteine for treatment of
acetaminophen toxicity: A systematic review and meta-analysis. West
J Emerg Med. 14:218–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Quintavalle C, Donnarumma E, Fiore D,
Briguori C and Condorelli G: Therapeutic strategies to prevent
contrast-induced acute kidney injury. Curr Opin Cardiol.
28:676–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu XH, Xu CY and Fan GH: Efficacy of
N-acetylcysteine in preventing atrial fibrillation after cardiac
surgery: A meta-analysis of published randomized controlled trials.
BMC Cardiovasc Disord. 14:522014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shahripour R Bavarsad, Harrigan MR and
Alexandrov AV: N-acetylcysteine (NAC) in neurological disorders:
Mechanisms of action and therapeutic opportunities. Brain Behav.
4:108–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Berk M, Malhi GS, Gray LJ and Dean OM: The
promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol
Sci. 34:167–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baker MS, Feigan J and Lowther DA:
Chondrocyte antioxidant defences: The roles of catalase and
glutathione peroxidase in protection against H2O2 dependent
inhibition of proteoglycan biosynthesis. J Rheumatol. 15:670–677.
1988.PubMed/NCBI
|
|
43
|
Zhou T, Zhou KK, Lee K, Gao G, Lyons TJ,
Kowluru R and Ma JX: The role of lipid peroxidation products and
oxidative stress in activation of the canonical wingless-type MMTV
integration site (WNT) pathway in a rat model of diabetic
retinopathy. Diabetologia. 54:459–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gong SP, Lee EJ, Lee ST, Kim H, Lee SH,
Han HJ and Lim JM: Improved establishment of autologous stem cells
derived from preantral follicle culture and oocyte parthenogenesis.
Stem Cells Dev. 17:695–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y, Yang C, Yuan G, Wang Z, Cui W and
Li R: Sulindac attenuates valproic acid-induced oxidative stress
levels in primary cultured cortical neurons and ameliorates
repetitive/stereotypic-like movement disorders in Wistar rats
prenatally exposed to valproic acid. Int J Mol Med. 35:263–270.
2015.PubMed/NCBI
|
|
46
|
Wang Z, Xu L, Zhu X, Cui W, Sun Y, Nishijo
H, Peng Y and Li R: Demethylation of specific Wnt/β-catenin pathway
genes and its upregulation in rat brain induced by prenatal
valproate exposure. Anat Rec (Hoboken). 293:1947–1953. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Strakovsky RS and Pan YX: A decrease in
DKK1, a WNT inhibitor, contributes to placental lipid accumulation
in an obesity-prone rat model. Biol Reprod. 86:812012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen JR, Lazarenko OP, Shankar K,
Blackburn ML, Badger TM and Ronis MJ: A role for ethanol-induced
oxidative stress in controlling lineage commitment of mesenchymal
stromal cells through inhibition of Wnt/beta-catenin signaling. J
Bone Miner Res. 25:1117–1127. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
McGrew LL, Takemaru K, Bates R and Moon
RT: Direct regulation of the Xenopus engrailed-2 promoter by the
Wnt signaling pathway, and a molecular screen for Wnt-responsive
genes, confirm a role for Wnt signaling during neural patterning in
Xenopus. Mech Dev. 87:21–32. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bilodeau JF, Wang M, Chung FL and
Castonguay A: Effects of nonsteroidal antiinflammatory drugs on
oxidative pathways in A/J mice. Free Radic Biol Med. 18:47–54.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dairam A, Müller AC and Daya S:
Non-steroidal anti-inflammatory agents, tolmetin and sulindac
attenuate quinolinic acid (QA)-induced oxidative stress in primary
hippocampal neurons and reduce QA-induced spatial reference memory
deficits in male Wistar rats. Life Sci. 80:1431–1438. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sur A, Kesaraju S, Prentice H, Ayyanathan
K, Baronas-Lowell D, Zhu D, Hinton DR, Blanks J and Weissbach H:
Pharmacological protection of retinal pigmented epithelial cells by
sulindac involves PPAR-α. Proc Natl Acad Sci USA. 111:16754–16759.
2014. View Article : Google Scholar : PubMed/NCBI
|


















