Introduction
Skin is an essential natural barrier protecting body
from physical, chemical and microbial hazards, also a visual
indicator of body's aging process (1). 90%of the ultraviolet (UV) reaching
surface of the earth is long-wavelength irradiation (UVA, 320–400
nm), which can penetrate the epidermis into the dermis. So it is
well established that UVA is responsible for skin photoaging
induced by UV. Studies demonstrated UVA radiation can induce
cytokine expression in human epidermoid carcinoma cells. UVA
radiation caused an increased proportion of β-Gal positive cells
and reduced telomere length in human skin fibroblasts. In addition,
UVA radiation inhibited TGF-β 1 secretion, induced G1 phase arrest,
reduced SOD and GSH-Px levels, increased MDA levels and the
expression of MMP-1, TIMP-1, p66, p53 mRNA expression (2,3).
Human amnion-derived mesenchymal stem cells (HAMSCs)
obtained from human amniotic membrane (AM) are readily available
and high abundant tissue, with substantial benefits as seed cells
(3,4). Their low anti-flammatory properties
and fewer ethical concerns compared with other sources of stem cell
are clear advantage (5–7). It is proved that HAMSCs secrete a
variety of cytokines, which is essential to a series of basic
biological processes of cells (8).
Recent studies revealed that HAMSCs have important roles in cell
differentiation (9–12), promoting cell proliferation
(13–15), enhancing cell viability and
function (16–18), protecting cells from adverse
effects and inhibiting apoptosis (19,20)
in vivo or in vitro. The present study aimed to
determine whether HAMSCs involved in the protection of human dermal
fibroblasts (HDFs) from UVA-induced senescence.
In order to investigate the protective mechanisms of
HAMSCs against UVA-induced HDFs senescence, an in vitro
cell-senescence model was built through the exposure of
pre-HAMSCs-treated HDFs to UVA, and the effects of HAMSCs on ROS
contents and mitochondrial membrane potential (Δψm), HDFs
senescence marker genes p53 and MMP1 (21) expression were detected by reverse
transcription quantitative polymerase chain reaction analysis and
western blot. Furthermore, senescence-associated β-galactosidase
(SA-β-Gal) staining was performed to evaluate the senescence status
of HDFs. SA-β-Gal activity distinguishes senescent cells from those
terminally differentiated, therefore act as a senescence biomarker.
Our results showed that HAMSCs up-regulated MERK1/2 in UVA induced
senescence HDFs, which means skin senescence might related to
ERK1/2 MAPK signal pathway.
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS), α-minimum essential medium
(αMEM), trypsin-EDTA, phosphate-buffered saline (PBS) and
penicillin G-streptomycin sulfate were purchased from Gibco Life
Technologies (Carlsbad, CA, USA). 2,7-dichlorodihydro
fluoresceindiacetate (DCFH-DA) fom Sigma-Aldrich (St. Louis, MO,
USA). Transwells (6-well millicell Hanging Cell Culture Inserts,
0.4 µm, PET) and 6-well culture plates were purchased from
Millipore Corp. (Billerica, MA, USA). The goat anti rabbit IgG,
phosphor-p44/42 MAPK rabbit mAb (p-ERK1/2), JNK MAPK rabbit mAb,
p53 rabbit mAb, SIRT1 rabbit mAb and Senescence β-Galactosidase
Staining kit were purchased from Cell Signaling Technology, Inc. (3
Trask Lane; Danvers, MA, USA). Penicillin and streptomycin from
Gibco Life Technologies. Other reagents used were of the highest
commercial grade available.
Cell culture
Human amnion-derived mesenchymal stem cells were
prepared as described previously (3). Briefly, human amniotic membrane was
mechanically peeled off from the chorion of a placenta obtained
from an uncomplicated elective caesarean section with the informed
consent of the donor patient. The HAMSCs layer was thoroughly
scraped out from the underlying tissues such as the spongy and
fibroblast layers. Within 24 h AM layer was then treated with
0.125% trypsin three times each for 20 min to obtain dissociated
HAMSCs. The cells were cultured in α-MEM supplemented with 10% FBS,
penicillin (100 U/ml) and streptomycin (100 µg/ml), incubated in an
incubator at 37°C with 5% CO2 in a humidified
atmosphere. The culture medium was changed every 3 days.
Primary HDFs were purchased from Wuxi BioHermes
Bio&Medical Technology Co., Ltd. (Wuxi, China). Cultured in a
10-cm dish in α-MEM supplemented with 10% fetal bovine serum,
penicillin (100 U/ml) and streptomycin (100 µg/ml).
The Co-culture system
The transwell co-culture system was used to
investigate the effects of HAMSCs on HDFs. HDFs were seeded at an
initial density of 5×104 cells/cm2 in 6-well culture plates.
Transwells were placed in other 6-well culture plates and seed at
increasing HAMSCs (5×104 cells/transwell, 10×104 cells/transwell
and 15×104 cells/transwell). Immediately after 9 J/cm2 UVA on HDFs
to create UVA induced senescence, HAMSCs in transwells moved into
the appropriate well of 6-well plate to co-culturing with HDFs.
HDFs in wells with HAMSCs on transwells served as the treatment
groups, while HDFs without transwells were designated as the
control group.
UVA irradiation
24 h after HDFs seeded in the six-well plate, HDFs
were exposed to 9 J/cm2 (30 min) UVA irradiation. Cells were washed
with phosphate-buffered saline (PBS) and covered with a thin layer
of PBS prior to UVA exposure. The culture plate lid was removed,
and the 6-well plate was placed on a brass block embedded on ice,
in order to reduce any evaporation, at a distance of 15 cm from the
UVA light source. As the UVA irradiation source, an Ultraviolet
phototherapy instrument (SS-04A; Shanghai Sigma High-tech Co.,
Ltd., Shanghai, China) equipped with a 15-W ozone-free UVA lamp
(CEL015 W; Philips, Groningen, The Netherlands) was used. The
incidence dose of UVA was measured with a UVA/UVB-ultraviolet meter
(Factory affiliated to Beijing Normal University, Beijing, China).
After UVA irradiation, PBS was replaced with culture medium and
transwells seeded with HAMSCs were placed in wells of the
co-culture group, then they were incubated under standard
conditions for 72 h prior to analysis.
Analysis of cellular
proliferation
HDFs accepted UVA irradiation then co-cultured with
HAMSCs after 72 h, transwells containg HAMSCs were removed and HDFs
were harvested. After fixed with 75% ice-cold ethanol at 4°C in the
dark, cell cycle fractions (G0, G1, and G2, M phase) were
determined by flow cytometry.
SA-β-Gal staining
SA-β-Gal activity was evaluated using a
β-galactosidase staining kit (Beyotime Institute of Biotechnology,
Haimen, China). Cells were washed with PBS and fixed for 15 min at
room temperature with fixative solution. The HDFs cells were then
incubated at 37°C overnight. SA-β-Gal-positive staining was
expressed as a percentage of the total number of cells; cell
numbers were counted in four continuous visual fields using a
microscope (Olympus CX51; Olympus, Tokyo, Japan; total
magnification, ×20).
Assessment of ROS production
The level of ROS induced by UVA in HDFS was measured
using DCFH-DA as a fluorescent probe. After irradiation and
co-culturing with HAMSCs, transwells were removed and HDFs were
washed three times with PBS, incubated with DCFH-DA (10 mM) for 30
min at 37°C, washed three times with PBS. Macrographs of DCFDA
fluorescence were immediately.
Flow cytometry analysis of
mitochondrial membrane potential
Mitochondrial membrane potential (Δψm) was analyzed
by a fluorescent dye JC-1 (Beyotime Institute of Biotechnology),
following manufactur's protocol. JC-1 is capable of selectively
entering mitochondria where it forms monomers and emits green
fluorescence (530 nm) when Δψm is relatively low. At high Δψm, JC-1
aggregates and gives a red fluorescence (590 nm). Assays were
initiated by incubating HDFs with JC-1 for 30 min at 37°C in the
dark and the fluorescence of separated cells was detected with a
flow cytometer (FAC-SCalibur; BD Biosciences, San Diego, CA, USA).
Δψm was determined by a ratio of fluorescence intensity at 590 nm
to that at 530 nm. A minimum of 10,000 cells per sample was
acquired and analyzed.
Assessment of senescence related RNA
and protein
Total RNA was extracted from the cells using TRIzol
reagent (Promega Corp., Madison, WI, USA). RNA concentration and
purity were determined with a Nanodrop 2000-UV spectrophotometer
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Ribosomal RNA
band integrity was evaluated using conventional denaturing agarose
gel electrophoresis using the SDS-PAGE gel quick preparation kit
(Beyotime Institute of Biotechnology). Equal amounts of RNA (500
ng) from each sample were reverse transcribed using a PrimeScript™
RT Reagent kit with gDNA Eraser (Takara Bio, Dalian, China)
according to the manufacturer's instructions. qPCR was performed
using SYBR-Green dye method (Premix Ex Taq; Takara Bio) using an
ABI700 Real-Time PCR detection system (Applied Biosystems; Life
Technologies, Thermo Fisher Scientific, Inc.). The following
standard cycling conditions for qPCR were applied: 95°C for 3 min
to activate polymerase, 40 cycles of denaturation at 95°C for 15
sec and annealing-extension at 60°C for 30 sec. Melting curve
analysis was performed following every run by defined heating up to
95°C to assess the presence of unspecific PCR products. Specific
primers for the RT-qPCR reactions were as follows: MMP1 forward,
5′-TTGGAGGGGATGCTCATT-3′ and, reverse,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′; p53, forward,
5′-AGAATCTCCGCAAGAAAGG-3′, reverse, 5′-GCTGGTATGTCCTACTCCC-3′;
β-actin, forward, 5′-TGGAATCTTGCTCTTATTTTCACA-3′ and reverse,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. All primers were synthesized by
Sangon Biotech, Co., Ltd. (Shanghai, China) and used at 400 nM
expect for β-actin at 300 nM. All PCR efficiencies were between 90
and 110%.
At the end of 72 h after UVA irradiation and
co-culture, transwells containg HAMSCs were removed and HDFs in
each group were lysed in RIPA buffer containg 1 mM phenylmethane
sulfonylfuoride according to the manufacturer's instructions. The
total protein concentration was determined using a bicinchoninic
acid (BCA) assay kit. Protein lysates (20 µg) were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto 0.22 µm polyvinylidene
difluofide membranes (Millipore Corp.). After blocking, membranes
were incubated overnight at 4°C with specific antibodies for the
detection of p53 (1:1,000), p38 (1:1,000), SIRT1 (1:1,000),
p-ERK1/2 (1:500), ERK1/2 (1:500). After three washes with PBST
(0.5% Tween-20 in PBS), the membranes were incubated with the
relevant secondary antibodies (1:2,000) for 1 h at 37°C, washed and
visualized with an ECL detection kit (Amersham Pharmacia Biotech,
Piscataway, NJ, USA). The GAPDH (1:500) served as internal
control.
Statistical analysis
Analyses were performed using GraphPad Prism
software (GraphPad Inc., La Jolla, CA, USA). Values are presented
as the mean ± standard deviation. The one-way analysis of variance
was used for comparisons involving more than two groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
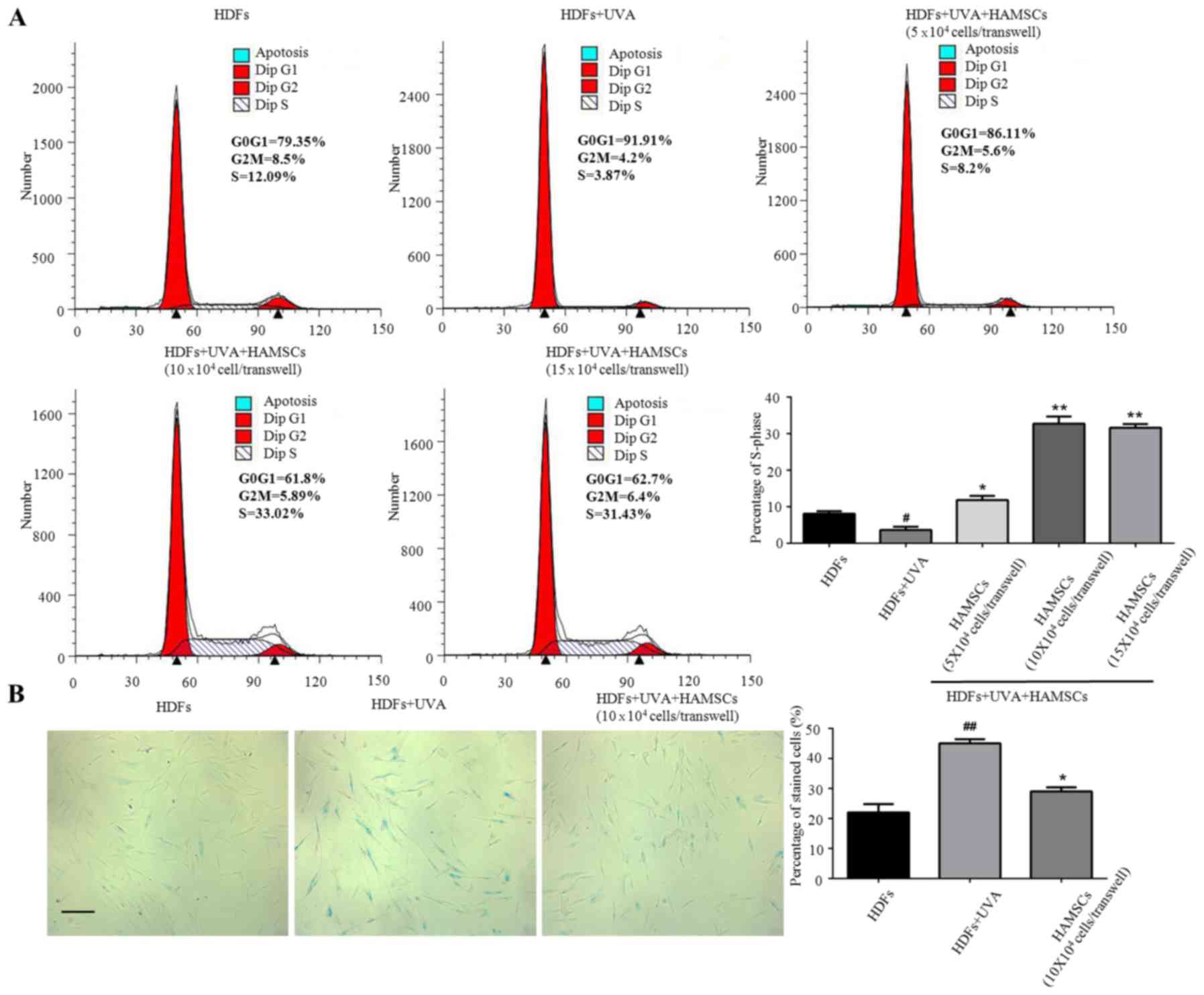
HAMSCs promoted UVA induced HDFs
proliferation and reduce UVA induced HDFs senescence
Flow cytometry were used to measure the
proliferation of UVA treated HDFs seeded in the 6-well plates
co-culture with HAMSCs. Cell cycle fractions (G0, G1, S, and G2, M
phase) were determined by flow cytometry at 72 h after UVA and
HAMSCs treatment. The S phase showed significant inhibited treated
by UVA, after co-culture with HAMSCs the S-phase checkpoints
increased (Fig. 1A). So we chose
10×104 cells/transwell HAMSCs in following experiment. Our results
further demonstrated that co-culturing with HAMSCs accelerated
UVA-induced HDFs proliferation.
X-gal staining results showed that the percentage of
cells stained by X-gal following 9 J/cm2 UVA irradiation
was markedly increased compared with that of the control group
(10.8 and 22.6%, respectively; P<0.05), while HAMSCs attenuated
the ratio of positive staining compared with that of the
UVA-treated only cells (15.3 and 22.6%, respectively; P<0.05)
(Fig. 2B).
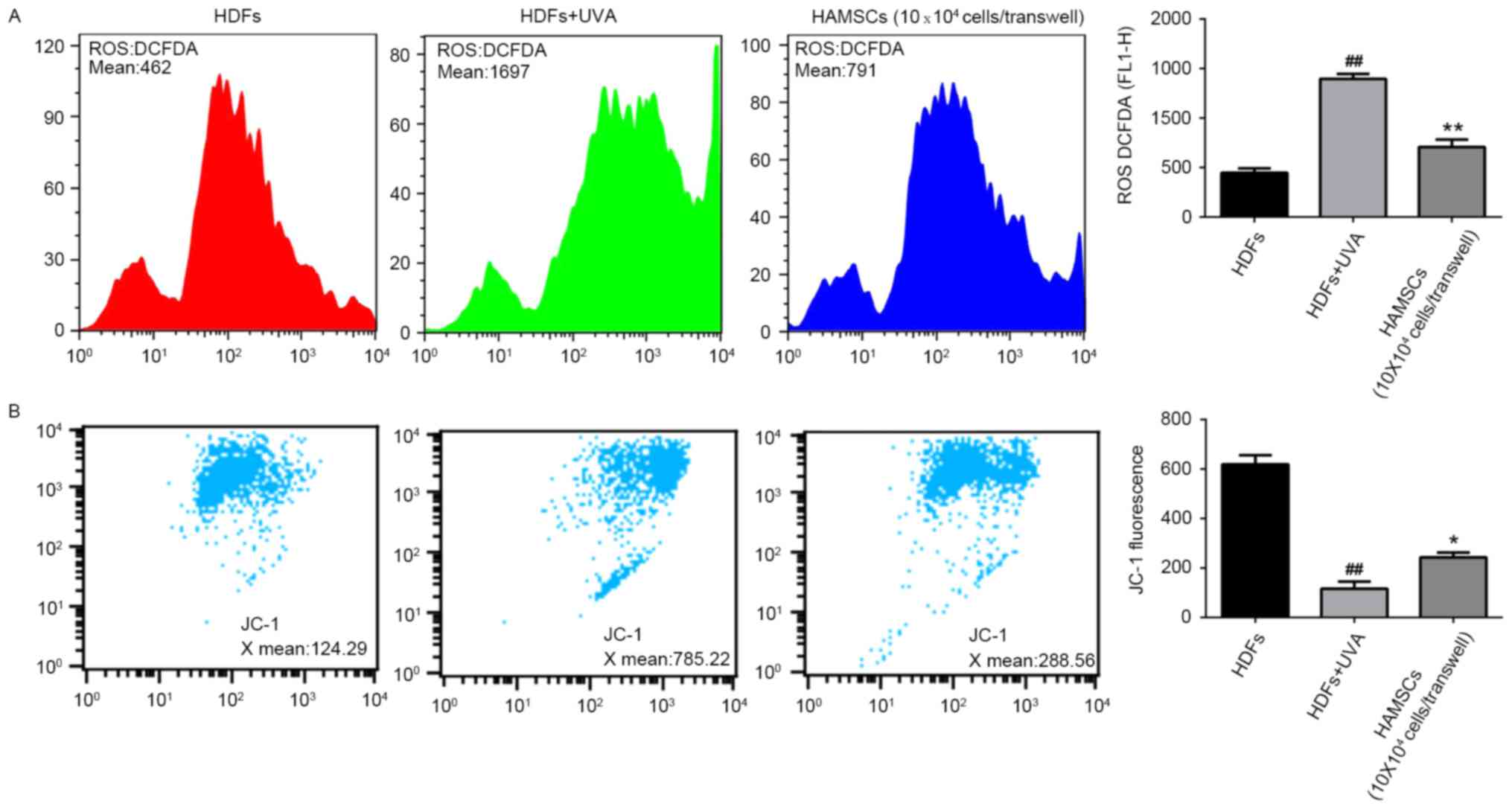
HAMSCs inhibited ROS generation and
mitochondria depolarization in UVA induced HDFs
To elucidate whether the beneficial effects of
HAMSCs were linked to their antioxidant properties, the ROS
generation in UVA-induced HDFs were measured. Subsequently, the
intensity of fluorescence was determined by flow cytometry.
As shown in Fig.
2A, after UVA irradiation, intracellular ROS generation
increased significantly. The level of ROS in UVA treated cells was
much higher than the level of ROS in control cells throughout the
experiment. Co-culture with HAMSCs significantly inhibited the
elevated intracellular concentration of ROS.
Loss of mitochondrial membrane potential in cells
has been estimated using JC-1 assay kit. In normal cells, JC-1
aggregated in mitochondria and the ratio was 124.29. UVA
irradiation treated cells showed the higher ratio 785.22, which
indicated the dissipation of Δψm. HDFs treatedwith UVA and
co-cultured with HAMSCs demonstrated attenuation of the dissipation
of Δψm 288.56 (Fig. 2B). Above
results uggested that HAMSCs protect mitochondria depolarization
induced by UVA irradiation.
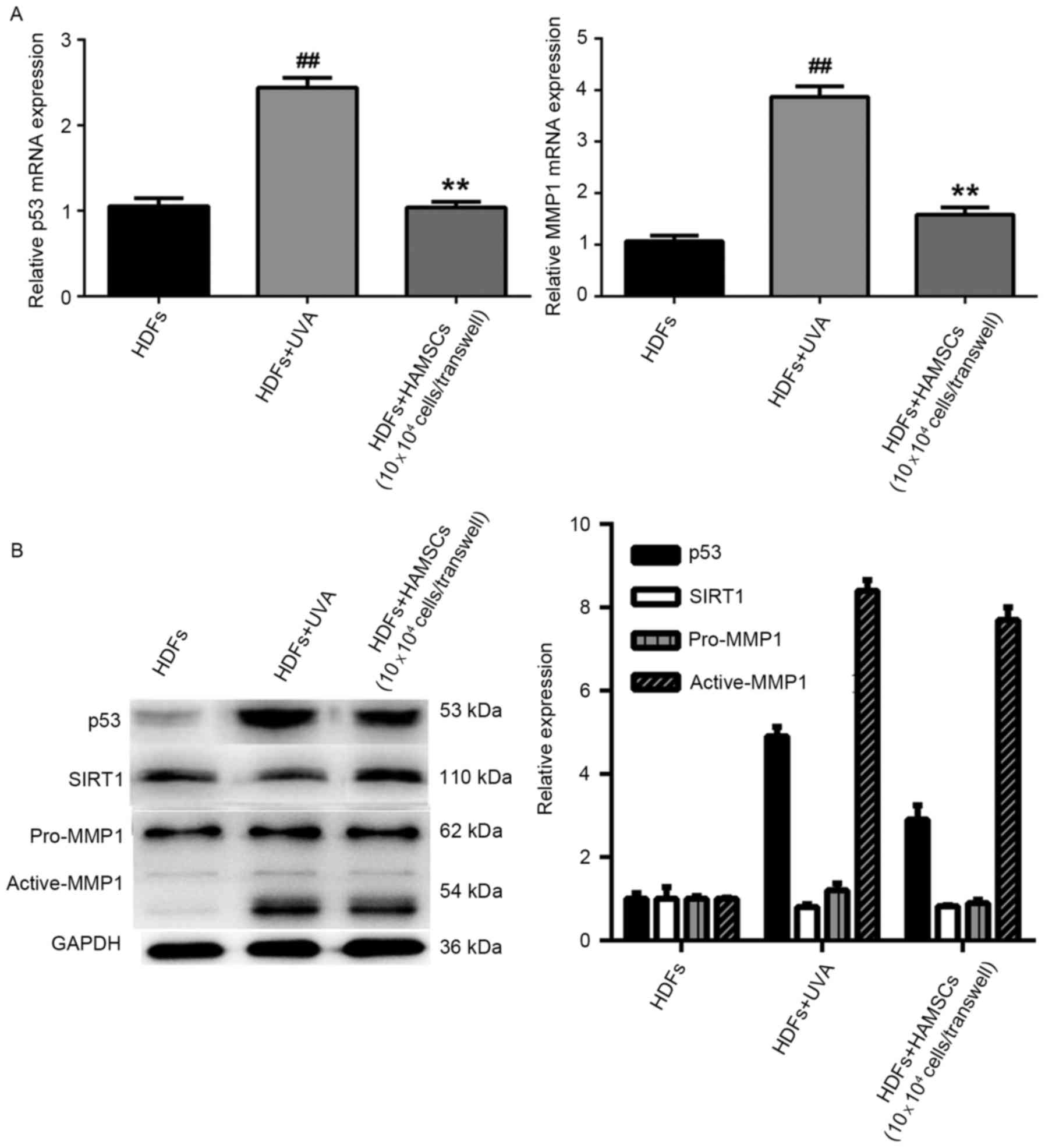
HAMSCs reduced the expression of
senescence related markers
Reverse transcription quantitative polymerase chain
reaction analysis showed mRNA expression levels of p53 and MMP1
were significantly reduced in UVA-treated HDFs co-cultured with
HAMSCs than that of the UVA-treated only group (P<0.05)
(Fig. 3A). In order to further
study the efficacy of HAMSCs, western blot analysis was to evaluate
the protein expression in co-culture with UVA and HAMSCs or with
UVA only of HDFs. The result showed that HAMSCs had a significant
effect on p53, active-MMP1 and SIRT1 (Fig. 2B).
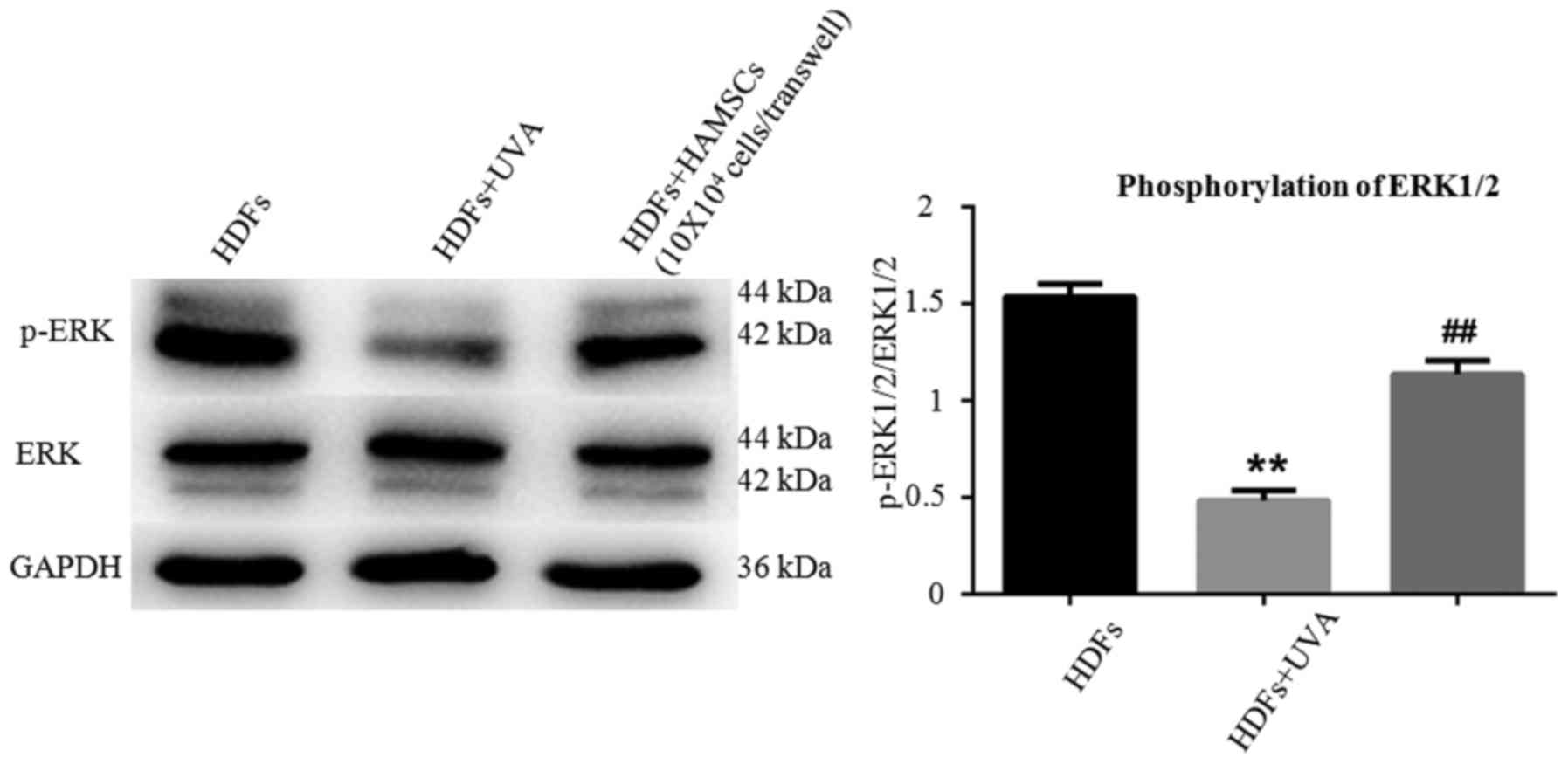
HAMSCs activated ERK1/2 in UVA induced
HDFs
EEK1/2 are important members of the MAPK signal
pathway, which regulates the differentiation, mineralization and
proliferation of HDFs. Fig. 4
showed ERK1/2 in HDFs in respective of UVA treatment after 72 h
with or without co-culture with HAMSCs. Higher level of
phosohorylated ERK1/2 were observed compared with UVA irradiated
HDFs co-cultured with HAMSCs than without. These results suggested
that HAMSCs enhance UVA-induced inhibition of ERK1/2, which might
play a role in regulating UVA induced HDF senescence.
Discussion
HAMSCs has been the shining star in cell-based
therapy in recent years, which appear to have several advantages
over other stem cell lineages as a cell therapy. Studies showed
that HAMSCs can maintain mouse spermatogonial stem cells in an
undifferentiated state when cultured long term due to high leukemia
inhibitor factor (LIF) expression. HAMSCs had an increased
proliferative capacity, higher colony-forming efficiency, fewer
apoptotic cells, and similar cell-junction formation capabilities
and pump functionality compared with primary HCECs (21,22).
Amniotic membrane can restrict dedifferentiation of human retinal
pigment epithelial cells (RPE cells) in culture, promoting RPE65,
CRALBP, VEGF, CD68, and tyrosinase gene expression in RPE cells
(23). Experiments have
demonstrated the ability of HAMSCs to migrate into brain, prevents
the degeneration of nigral dopmineneurons in rats with
6-hydroxydopami-ne lesions (24).
HAMSCs transplantation promotes ovarian function by inhibiting
tumor necrosis factor-alpha-mediated cell apoptosis and reducing
inflammation in chemotherapy-induced premature ovarian failure
(25). HAMSCs are able to
produce/release a number of biologically active cytokines/growth
modulators, such as basic fibroblast growth factor (bFGF),
epithelial growth factor (EGF), insulin growth factor-1 (IGF-1),
stem cell factor (SCF), IL-1a, IL-10, insulin, tumor necrosis
factor-a, IFN-g, and leukemia inhibitory factor (LIF), some of
which could constitute crucial components in maintaining/enhancing
the survival/anti-senescence/apoptosis of progenitor/adult cells
(26). HAMSCs can secrete several
cytokines and growth factors, promoting the survival of the
surrounding cells. The cytokines and the growth factors, such as
IL-6, M-CSF, IL-10, HGF, TGF-β and PGE2 contribute to preventing
apoptosis of injured pancreatic β-cells and enhancing regeneration
of endogenous progenitor cells via angiogenic, cytoprotective,
anti-inflammatory, mitogenic and anti-apoptotic effects (21).
It was reported that mitochondria use oxidative
phosphorylation to convert dietary intake into ATP; in the process,
they generate ROS, which can damage mitochondrial DNA, impair
respiratory chain function, and cause nuclear DNA damage and
cellular checkpoint activation (27). p53 is a transcription factor that
plays a key role in both cell cycle arrest and apoptosis. p53 has
many anticancer mechanisms and plays a role in apoptosis, genetic
stability, and inhibition of senescence/apoptosis (28).
Present study was to investigate the potential
molecular signaling pathways of UVA-induced HDF senescence engaged
by HAMSCs. We found that HAMSCs promoted proliferation in UVA
induced HDFs which confirmed by flow cytometry. SA-β-gel staining
revealed that senescence of UVA irradiated HDFs co-cultured with
HAMSCs decreased compared with HDFs accepted UVA irradiation only.
ROS generation in UVA induced HDFs was determined to measure the
anti-oxidant properties. The excessive production of ROS, such as
superoxides and H2O2 severely damages the
DNA, protein and lipids. Our findings suggest that HAMSCs inhibited
ROS generation in UVA-induced HDFs. Expression of MMP, p53, p38,
SIRT1 were also significantly increased in the co-culture group. By
improving senescence against oxidative stress, HAMSCs might
represent an appropriate therapeutic alternative against UVA
induced skin aging.
Signal pathways involved in oxidative stress-induced
inhibition of senescence consist of MAPK, Akt/mTOR/4EBP1, p53 and
NFκB [Sreedhar et al (29),
2016]. The present study highlights the antioxidant role of HAMSCs
in promoting UVA-induced proliferation and senescence. We found
that activation of the ERK/MAPK signaling pathway is essential for
protective effect against oxidative stress induced cell injury in
HDFs. These data shed light on the molecular mechanism the
signaling cascade mediated by HAMSCs and identify the potential
role of HAMSCs in tissue engineering.
Acknowledgements
The present study was support by the Nanjing Medical
Science and Technology Development Fund Project (grant no.
2015NJMUZD088).
References
|
1
|
He YY, Council SE, Feng L and Chignell CF:
UVA-induced cell cycle progression is mediated by a disintegrin and
metalloprotease/epidermal growth factor receptor/AKT/Cyclin D1
pathways in keratinocytes. Cancer Res. 68:3752–3758. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morita A, Grewe M, Grether-Beck S,
Olaizola-Horn S and Krutmann J: Induction of proinflammatory
cytokines in human epidermoid carcinoma cells by in vitro
ultraviolet A1 irradiation. Photochem Photobiol. 65:630–635. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Min W, Liu X, Qian Q, Lin B, Wu D, Wang M,
Ahmad I, Yusuf N and Luo D: The Effects of baicalin against
UVA-induced photoaging in skin fibroblasts. Am J Chin Med.
42:709–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ilancheran S, Michalska A, Peh G, Wallace
EM, Pera M and Manuelpillai U: Stem cells derived from human fetal
membranes display multilineage differentiation potential. Biol
Reprod. 77:577–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miki T, Lehmann T, Cai H, Stolz DB and
Strom SC: Stem cell characteristics of amniotic epithelial cells.
Stem Cells. 23:1549–1559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song YS, Joo HW, Park IH, Shen GY, Lee Y,
Shin JH, Kim H, Shin IS and Kim KS: Transplanted human amniotic
epithelial cells secrete paracrine proangiogenic cytokines in rat
model of myocardial infarction. Cell Transplant. 24:2055–2064.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YT, Li W, Hayashida Y, He H, Chen SY,
Tseng DY, Kheirkhah A and Tseng SC: Human amniotic epithelial cells
as novel feeder layers for promoting ex vivo expansion of limbal
epithelial progenitor cells. Stem Cells. 25:1995–2005. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai D, Wang Y, Sun J, Chen Y, Li T, Wu Y,
Guo L and Wei C: Derivation and characterization of human embryonic
stem cells on human amnion epithelial cells. Sci Rep. 5:100142015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banas R, Miller C, Guzik L and Zeevi A:
Amnion-derived multipotent progenitor cells inhibit blood monocyte
differentiation into mature dendritic cells. Cell Transplant.
23:1111–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz-Prado S, Muiños-López E,
Hermida-Gómez T, Cicione C, Rendal-Vázquez ME, Fuentes-Boquete I,
de Toro FJ and Blanco FJ: Human amniotic membrane as an alternative
source of stem cells for regenerative medicine. Differentiation.
81:162–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Díaz-Prado S, Muiños-López E,
Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ
and Blanco FJ: Multilineage differentiation potential of cells
isolated from the human amniotic membrane. J Cell Biochem.
111:846–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han K, Lee JE, Kwon SJ, Park SY, Shim SH,
Kim H, Moon JH, Suh CS and Lim HJ: Human amnion-derived mesenchymal
stem cells are a potential source for uterine stem cell therapy.
Cell Prolif. 41:705–725. 2008. View Article : Google Scholar
|
|
13
|
Onishi R, Onishi S, Higashi R, Yamahara K,
Yoshimatsu J, Katsurada T, Okubo N, Nakagawa K, Takeda H and
Sakamoto N: The anti-inflammatory effect of human annion-derived
mesenchymal stem cells. Placenta. 35:A232014. View Article : Google Scholar
|
|
14
|
Lee JH, Ryu IH, Kim EK, Lee JE, Hong S and
Lee HK: Induced expression of insulin-like growth factor-1 by
amniotic membrane-conditioned medium in cultured human corneal
epithelial cells. Invest Ophthalmol Vis Sci. 47:864–872. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohno-Matsui K, Ichinose S, Nakahama K,
Yoshida T, Kojima A, Mochizuki M and Morita I: The effects of
amniotic membrane on retinal pigment epithelial cell
differentiation. Mol Vis. 11:1–10. 2005.PubMed/NCBI
|
|
16
|
Akrami H, Soheili ZS, Sadeghizadeh M,
Khalooghi K, Ahmadieh H, Kanavi MR, Samiei S and Pakravesh J:
Evaluation of RPE65, CRALBP, VEGF, CD68, and tyrosinase gene
expression in human retinal pigment epithelial cells cultured on
amniotic membrane. Biochem Genet. 49:313–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan ZJ, Zhang P, Hu YQ, Zhang HT, Hong SQ,
Zhou HL, Zhang MY and Xu RX: Neural stem like cells derived from
human amnion tissue are effective in treating traumatic brain
injury in rat. Nerochem Res. 38:1022–1033. 2013. View Article : Google Scholar
|
|
18
|
Nogami M, Tsuno H, Koike C, Okabe M,
Yoshida T, Seki S, Matsui Y, Kimura T and Nikaido T: Isolation and
characterization of human amniotic mesenchymal stem cells and their
chondrogenic differentiation. Transplantation. 93:1221–1228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim H, Han K, Lee J, Shim S, Moon J, Suh
C, Kim J and Kim H: Human amnion derived mesenchymal stem cells may
have potential to contribute and differentiate endometrial cells in
vivo. Hum Reprod. 22:i1712007.
|
|
20
|
Tamagawa T, Ishiwata I, Ishikawa H and
Nakamura Y: IInduced in-vitro differentiation of neural-like cells
from human amnion-derived fibroblast-like cells. Hum Cell.
21:38–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niknejad H, Yazdanpanah G and Ahmadiani A:
Induction of apoptosis, stimulation of cell-cycle arrest and
inhibition of angionenesis make human amnion-derived cells
promising sources for cell therapy of cancer. Cell Tissue Res.
363:599–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei LT, Chen JB, Zhao YL, Yang SP and He
L: Resveratrol attenuates senescence of adipose-derived mesenchymal
stem cells and restores their paracrine effects on promoting
insulin secretion of INS-1 cells through Pim-1. Eur Rev Med
Pharmacol Sci. 20:1203–1213. 2016.PubMed/NCBI
|
|
23
|
Kawakubo K, Ohnishi S, Fujita H, Kuwatani
M, Onishi R, Masamune A, Takeda H and Sakamoto N: Effect of fetal
membrane-derived mesenchymal stem cell transplantation rats with
acute and chronic pancreatitis. Pancreas. 45:707–713. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ono M, Ohnishi S, Honda M, Ishikawa M,
Hosono H, Onishi R, Nakagawa K, Takeda H and Sakamoto N: Effects of
human amnion-derived mesenchymal stromal cell transplantation in
rats with radiation proctitis. Cytotherapy. 17:1545–1559. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Koike-Soko C, Sugimoto J, Yoshida T,
Okabe M and Nikaido T: Human amnion-derived stem cells have
immunosuppressive properties on NK cells and monocytes. Cell
Transplant. 24:2065–2076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sahin E, Colla S, Liesa M, Moslehi J,
Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al:
Telomere dysfunction induces metabolic and mitochondrial
compromise. Nature. 470:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sreedhar R, Giridharan W, Arumugam S,
Karuppagounder V, Palaniyandi SS, Krishnamurthy P, Quevedo J,
Watanabe K, Konishi T and Thandavarayan RA: Role of MAPK-mediated
endoplasmic reticulum stress signaling in the heart during aging in
senescence-accelerated prone mice. Biofactors. 42:368–375. 2016.
View Article : Google Scholar : PubMed/NCBI
|