Introduction
Skin wound healing is a sophisticated and sequential
physiological process, with three independent and simultaneously
overlapping stages, namely inflammation, proliferation, and
remolding. A skin wound heals depending on well-orchestrated
biological processes, including the production of cytokines,
synthesis and degradation of the extracellular matrix, cell
lineages, specifics of the wound, and age of the patients (1). In adult skin wound healing, wounds
are usually accompanied by scar formation, with a mature scar
forming a narrow, flat, and hypo-pigmented appearance, which may
depress with time (2).
Pathological or fibrotic scars are caused by dysregulation of the
wound healing mainly related to unregulated inflammation, abnormal
cell proliferation, excessive collagen deposition, abnormal
cytokine production, and imbalance between synthesis and
degradation of the extracellular matrix (3). Pathological or fibrotic scars can
lead to serious problems, such as physiological dysfunction, a
defective appearance, and an economic burden. Fetal skin is prone
to heal without scaring in a given period, due to intrinsic
abilities, such as minimal inflammation, special fibroblast
phenotypes, and certain gene expression profiles. To date,
researchers have made several efforts to find effective therapies
to heal aberrant scar formation in clinical cases.
Inflammation is a crucial part of the healing
process (4,5), defending against damage from
pathogenic microorganisms and regulating the production of
cytokines and angiogenesis. Diverse inflammatory cytokines and
cells were involved in wound healing process. Immediately after
injury, neutrophils are the first cells to infiltrate the wound
site and help to destroy foreign particles. Later, macrophages are
attracted to the wound site to phagocytize bacteria and tissue
debris (6), however, an excessive
macrophage presence can stimulate scarring. Some pro-inflammatory
cytokines, such as IL-1 (7), IL-6,
IL-8 (8), IL-17 (9), IL-18 (10), and IL-33 (11) have been reported to participate in
fibrosis.
MicroRNAs (miRNAs or miRs) are a class of
endogenous, highly conserved non-coding small RNAs that regulate
most eukaryotic protein-coding genes. Their ‘seed regions’ (located
on miRNA nucleotides 2–8 from the 5′ region) bind to the reverse
complement of the 3′-untranslated region (3′-UTR) of the target
mRNA, resulting in its degradation or repressed translation. miRNAs
participate in development, disease progression, cell
proliferation, differentiation, apoptosis, and many other
processes. miRNAs are also involved in inflammation, scar
formation, and angiogenesis during skin wound healing.
miR-149 has an important regulatory role in many
fields, it has been reported to regulate AKT1/mTOR and TGF-β/SMAD
signaling pathway (12–14). In addition, miR-149 is closely
associated with inflammation. miR-149 can regulate TNF-α, IL-6, and
iNOS, in order to regulate the pathogenesis of osteoarthritis
(15) and endothelial dysfunction
(16). As reported in the current
study, miR-149 is downregulated in the inflammatory phase in skin
tissues at 24 h compared to 0 h after wounding (17), and it shows a lower expression in
scars compared to normal skin tissue (18).
In a previous study, we performed small RNA
next-generation sequencing to uncover the dynamic changes between
miRNAs in mid- and late-gestational fetal skin keratinocytes
(19). We found that many miRNAs
have different expression patterns between mid- and late-gestation.
In total, 88 known miRNAs exhibit more than a two-fold change in
expression in fetal keratinocytes, fifteen of which are
significantly upregulated, whereas the others significantly
downregulated. Multiple miRNAs regulate the AKT/mTOR, TGF-β/SMAD,
and NF-κB pathways, which affect the skin wound healing process. We
also found that several interleukins are downregulated in
mid-gestation fetal keratinocytes, as compared to those in
late-gestation. We focused on miR-149, which is upregulated in
mid-gestation fetal keratinocytes, and whose predicted target mRNAs
are IL-1α, IL-1β and IL-6. We explored the anti-inflammatory and
anti-scarring effects of miR-149 in the skin wound healing process
through regulation of the expression of IL-1α, IL-1β, and IL-6.
Materials and methods
Cell culture and treatments
The human immortal keratinocyte cell line, HaCaT
cells and human dermal fibroblasts (both cells were obtained from
the Department of Stem Cell and Regenerative Medicine) used in this
study were cultured in Dulbecco's Modified Eagle's Medium
(DMEM)-High Glucose (HyClone, Logan, UT, USA) with 10% fetal bovine
serum (FBS; Invitrogen Life Technologies, Carlsbad, CA, USA) at
37°C and 5% CO2.
HaCaT cells were transfected with miR-149 mimic or a
negative control (50 mM; GenePharma, Shanghai, China) using the
Lipofectamine® 2000 reagent (Invitrogen Life Technologies)
according to the manufacturer's protocol.
To analyze the effect of miR-149 on inflammatory
cytokines, treatments with and without TNF-α were used to reflect
the inflammatory and basal environments, respectively. HaCaT cells
were transfected with miR-149 mimic or a negative control, treated
48 h later with or without TNF-α (20 ng/ml; Peprotech, Inc., Rocky
Hill, NJ, USA), and then cultured in an incubator for another 24
h.
The dynamic expression of miR-149 between mid- and
late-gestational fetal skin keratinocytes was measured by
next-generation sequencing (19).
Co-culture assay
To explore how HaCaT cells transfected with the
miRNA mimic or a negative control affected the behavior of dermal
fibroblasts, HaCaT cells and fibroblasts were co-cultured in
transwell polyester membrane inserts with a 0.4 µm pore size
(CoStar, Corning, NY, USA) for 48 h. Then the fibroblasts were
harvested to detect the mRNA and protein expression.
Migration assay
The migration of HaCaT cells from serum-free media
to standard culture media was measured in 24-well transwell culture
inserts with an 8.0 µm pore size (CoStar), as described previously
(20). HaCaT cells were
hematoxylin-eosin stained (Solarbio, Beijing, China) at 36 h and
counted from sixteen fields per well randomly.
An in vitro scratch assay was used to detect
the migration of the HaCaT cells. After transfection for 48 h, a
200 µl tip was used to make a scratch. The cells were washed with
PBS to discard the cell debris, and then photographed under a
microscope at 0, 24 and 36 h.
RT-qPCR
We used RT-qPCR to detect gene expression. To detect
microRNAs, RNAs were prepared from cells using the mirVana miRNA
Isolation kit (Ambion Life Technologies, Carlsbad, CA, USA), and
was added a poly (A) tail to the RNA transcripts by the Poly (A)
Tailing kit (Ambion Life Technologies). Reverse transcription was
performed with an RT Reagent kit with gDNA Eraser (Takara Bio,
Inc., Otsu, Japan), and RT-qPCR was performed using a SYBR Premix
Ex Taq II kit (Takara Bio, Inc.) on the 7500 Real-Time PCR system
(Applied Biosystems Life Technologies, Foster City, CA, USA). To
detect mRNA expression, total RNA was prepared using the TRIzol
(Invitrogen Life Technologies) reagent, and reverse transcription
and RT-qPCR were performed as above. The RT-qPCR reaction
conditions were 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 60°C for 34 sec. U6 and GAPDH were used as endogenous
reference genes. The primers used in RT-qPCR were shown in Table I. The 2−ΔΔCq method was
used to calculate the change in gene expression.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence |
|---|
| TGF-β1 | F:
GGAGCTGTACCAGAAATACAGC | R:
AACCCGTTGATGTCCACTTG |
| TGF-β3 | F:
TGGCTGTTGAGAAGAGAGTCC | R:
TGTCCACGCCTTTGAATTTG |
| Collagen I | F:
GAGTGGAGAGTACTGGATTGACC | R:
GCCGCCATACTCGAACTG |
| Collagen III | F:
ACAACAGGAAGCTGTTGAAGG | R:
GGCGAGTAGGAGCAGTTGG |
| IL-1α | F:
GCTGCTGAAGGAGATGCC | R:
ACGCCTGGTTTTCCAGTATC |
| IL-1β | F:
GATGAAGTGCTCCTTCCAGG | R:
GGAAAGAAGGTGCTCAGGTC |
| IL-6 | F:
GGATTCAATGAGGAGACTTGCC | R:
GGTTATTGCATCTAGATTCTTTGCC |
| NF-κB1 | F:
CCAACAGATGGCCCATACCT | R:
AACCTTTGCTGGTCCCACAT |
| NF-κB2 | F:
AAACAGCTGATGGCCCCTAC | R:
TGGCTGGTCCCTCGTAGTTA |
| p65 | F:
TGTTCACAGACCTGGCATCC | R:
GGGTACTCCATCAGCATGGG |
| RelB | F:
GCTCTACTTGCTCTGCGACA | R:
CGGCGTCTTGAACACAATGG |
| Rel | F:
CCGGTGCGTATAACCCGTAT | R:
CGGTTGTTGTCTGTGCTGTG |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG | R:
AGGGGCCATCCACAGTCTTC |
| U6 | F:
GCTTCGGCAGCACATATACTAAAAT | R:
CGCTTCACGAATTTGCGTGTCAT |
| miR-149 |
TCTGGCTCCGTGTCTTCACTCCC |
|
Elisa analysis
To detect secretory protein levels affected by
miR-149, cell culture supernatants were collected from HaCaT cells
transfected with miR-149 mimic or a negative control and the
protein expression of IL-1α, IL-1β, and IL-6 were measured using
ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) according
to the manufacturer's instruction.
Validation of target mRNAs
The target genes of miR-149 were predicted on the
website TargetScan (http://www.targetscan.org/) and microRNA.org (http://www.microrna.org/). To validate the target
mRNAs of miR-149, dual-luciferase reporter vectors containing the
potential binding sites were constructed by amplifying the 3-UTR of
the IL-1α, IL-1β and IL-6 mRNAs and cloning them into the
pGL3-reporter vector (obtained from the Department of Stem Cell and
Regenerative Medicine). The primers used for IL-1α 3-UTR: Forward,
GCT CTA GAG TGT TGA CAG TTC ATA TGT AC and reverse, GCT CTA GAT GTA
ACA TTA TGG TCT GAT C; primers used for IL-1β 3-UTR: Forward, GCT
CTA GAC TGG ACT TTC CTG TTG and reverse, GCT CTA GAG AAG TTT ATT
TCA GAA CCA TTG; primers used for IL-6 3-UTR: Forward, GCT CTA GAC
ATT CCT TCT TCT GGT C and reverse, GCT CTA GAT TTG AGG TAA GCC TAC
AC. The pRL-TK luciferase vector was used as an endogenous
reference. HaCaT cells were co-transfected with the luciferase
reporter plasmid (1.2 µg) and pRL-TK (0.12 µg), together with the
miRNA mimic or a negative control using the Lipofectamine® 2000
transfection reagent. Luciferase activity was analyzed 24 h after
transfection using the Dual-Luciferase Reporter Assay System
(Promega Corp., Madison, WI, USA) according to the manufacturer's
instruction.
Western blot assay
To detect the protein levels regulated by miR-149 in
fibroblasts, cells were washed with pre-chilled PBS, and the
protein extracted with the Whole Cell Lysis Assay kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) according to the
manufacturer's instruction. Protein concentration was measured
using the BCA protein assay kit (Kaiji Biotechnology, Nanjing,
China). Western blot analysis was performed according to standard
protocols. Cell lysates containing loading buffer were boiled at
99°C for 5 min, equal amounts of protein were run on 10% SDS
polyacrylamide gels, and transferred to a nitrocellulose membrane.
After blocking in 5% non-fat milk, membranes were incubated with
1:500 TGF-β1 rabbit anti-human polyclonal antibody (Abcam,
Cambridge, MA, USA), 1:500 TGF-β3 mouse anti-human monoclonal
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
1:500 collagen I mouse anti-human monoclonal antibody (Abcam), and
1:1,000 collagen III mouse anti-human monoclonal antibody
(Immunoway Biotechnology, Suzhou, China) for the detection of
fibrosis, and 1:10,000 GAPDH mouse anti-human monoclonal antibody
(ProteinTech Group, Inc., Chicago, IL, USA) for endogenous
reference protein. After overnight incubation at 4°C, the membranes
were washed three times in PBST and incubated with an
HRP-conjugated secondary antibody (1:10,000; Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China) for 1 h at
25°C. Membranes were washed three times in PBST and immunoreactive
bands were visualized by Amersham ECL Prime Western Blotting
Detection Reagent (GE Healthcare, Piscataway, NJ, USA) using a
Tanon 5200 detection system.
In vivo skin wound healing assay
Animal studies were performed in accordance with the
guidelinges of the China Medical University Review Committee. Nine
female Wistar rats were purchased from Beijing Weitong Lihua
(Beijing, China). Animals were separately housed in 12 h light/dark
cycles with ad libitum food and water. Twelve-week old animals
weighing approximately 250 g were anesthetized by intra-peritoneal
injection with pentobarbital sodium (40 mg/kg, Solarbio, China) and
surgery was conducted under standard conditions. Two circular 12 mm
full-thickness excisional wounds were created on the dorsal skin of
each rat. HaCaT cells transfected with miR-149 mimic or a negative
control were harvested from Nunc UpCell Surface dishes (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), which have a
temperature responsive cell culture surface and enable harvest of
cells with high vitality and intact surface proteins for cell
transplantation. These cells were transplanted to the wound
surface, followed by a coating with hydrogel (Hartmann AG,
Heidenheim, Germany), transparent dressing, and medical gauze.
Penicillin G sodium (80,000 units; Harbin Pharmaceutical Group Co.,
Ltd., Harbin, China) was used to prevent infection by
intra-peritoneal injection. Animals were euthanized with overdose
of pentobarbital sodium at days 1, 3 and 28 after wounding.
Complete wound tissues of them were excised with a 2 mm margin
around the wound edge. After fixation in 4% formaldehyde and
dehydration in a 30% sucrose solution, tissues were embedded in
Tissue-Tek O.C.T. compound (Sakura Fintek, Torrance, CA, USA) for
frozen sections. Hematoxylin and eosin staining and Masson's
trichrome staining (Solarbio, Beijing, China) were used for
immunohistochemical analysis according to the manufacturer's
instruction.
Statistical analysis
All results were performed at least in triplicate.
Data shown were mean ± standard deviation (SD). The differences
were calculated by using a Student's t-test according to SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Characterization of miR-149 expression
during mid- and late-gestational fetal skin keratinocytes
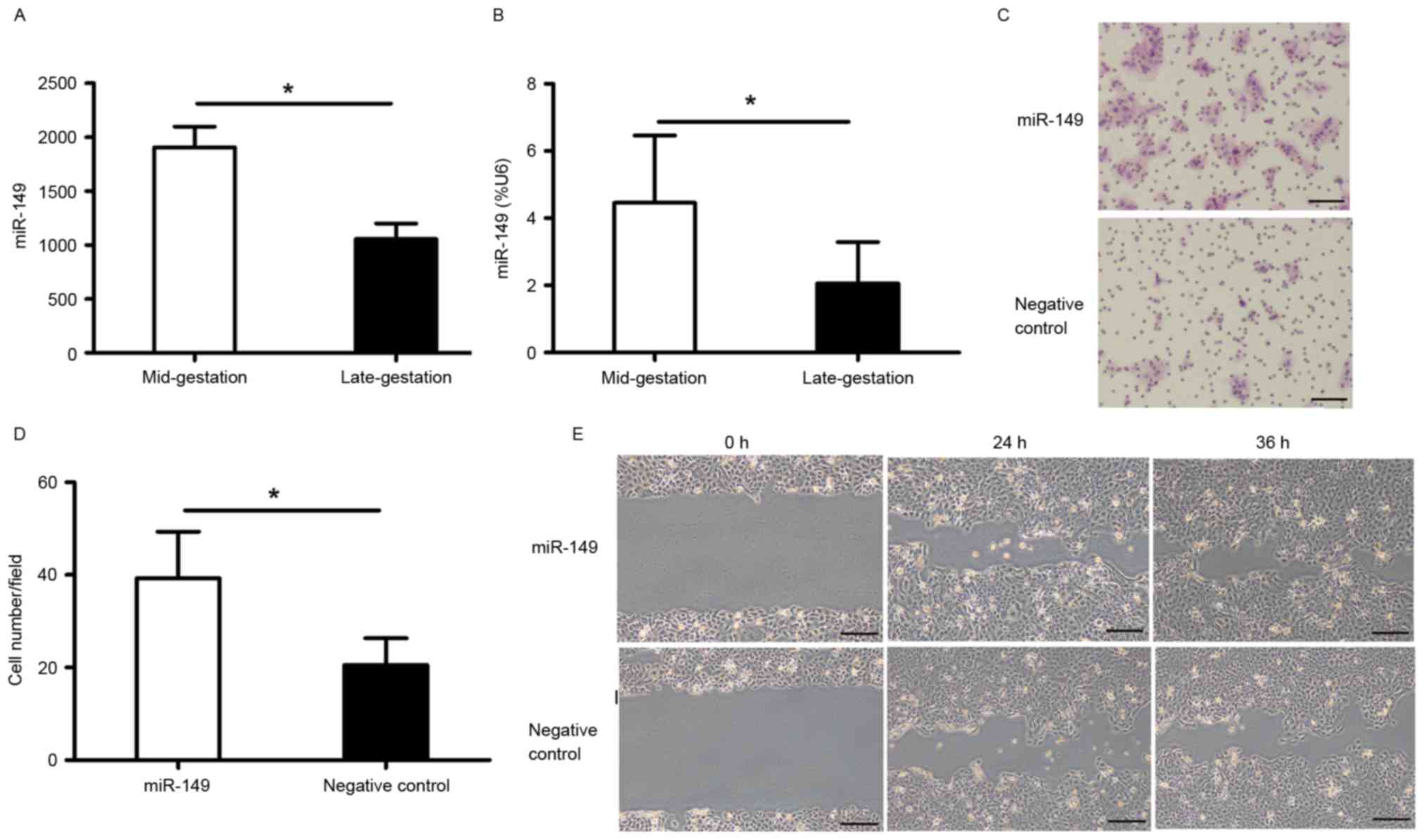
miR-149 was upregulated in the mid-gestational phase
with a 1.85-fold increase compared to that in the late-gestational
phase (Fig. 1A). RT-qPCR was
performed to validate this result (Fig. 1B).
miR-149 promotes migration of HaCaT
cells
The migration of keratinocytes is one of the
essential physiological process during skin wound healing. To
evaluate whether miR-149 has an effect on HaCaT cell migration, we
performed a transwell migration assay and an in vitro
scratch assay. The results showed that overexpression of miR-149
accelerated HaCaT cell migration 48 h after transfection (Fig. 1C-E).
miR-149 targets IL-1α, IL-1β, and IL-6
genes in HaCaT cells
MicroRNAs participate in various biological
processes by binding to their target mRNAs. Based on online
predictions, we identified IL-1α, IL-1β and IL-6 as the main target
genes of miR-149 in HaCaT cells. We synthesized dual-luciferase
reporter vectors containing the 3′-UTR of IL-1α, IL-1β, and IL-6 to
investigate whether miR-149 binds to these areas to regulate the
expression of IL-1α, IL-1β, and IL-6 in HaCaT cells. The results
showed (Fig. 2A) decreased
luciferase activity in HaCaT cells after transfection with miR-149
mimic, as compared to those transfected with the negative control.
Elisa and RT-qPCR results also showed that miR-149 suppressed both
mRNA and protein expression of IL-1α, IL-1β, and IL-6 in HaCaT
cells transfected with miR-149 mimic, as compared to the negative
control (Fig. 2B and C). Under
inflammatory conditions, miR-149 also decreased the mRNA expression
of IL-1α, IL-1β, and IL-6 (Fig.
2D).
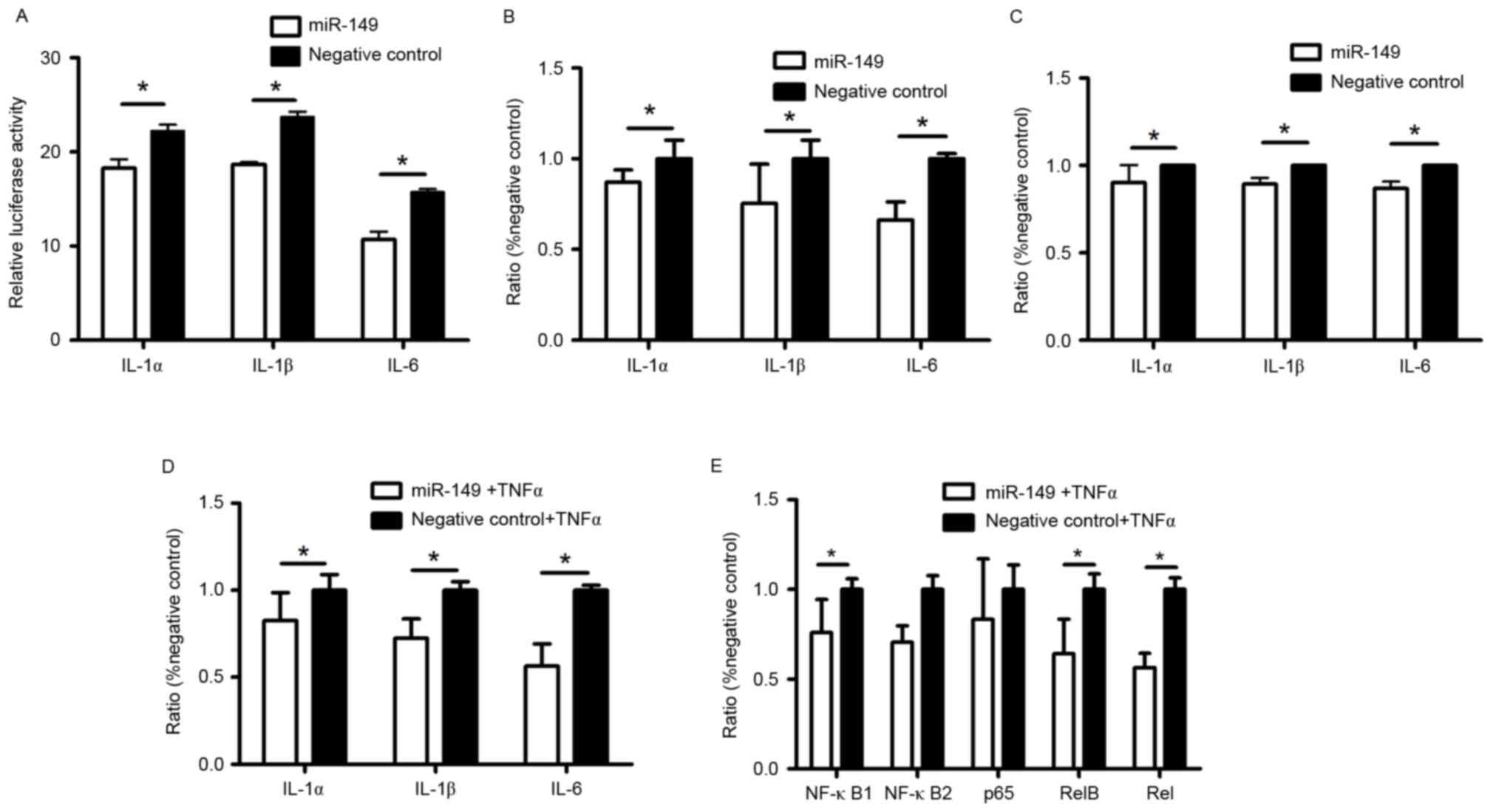 | Figure 2.Anti-inflammatory role of miR-149 in
HaCaT cells. HaCaT cells were transfected with luciferase reporter
plasmids containing (A) IL-1α, IL-1β, and IL-6 3′-UTR or pRL-TK
plasmids together with miR-149 mimic or a negative control.
Luciferase activity was measured 24 h after transfected. Expression
of (B) IL-1α, IL-1β, and IL-6 in supernatants was detected by ELISA
after transfected with miR-149 mimic or a negative control 48 h.
Expression of (C) mRNAs IL-1α, IL-1β, and IL-6 in HaCaT cells was
detected by RT-qPCR after transfected with miR-149 mimic or a
negative control 48 h. Addition of TNF-α in HaCaT cells with
transfected miR-149 mimic or a negative control was to reflect
inflammatory condition, and the expression of (D) mRNAs IL-1α,
IL-1β, and IL-6 in TNF-α-treated HaCaT cells was detected by
RT-qPCR. Expression of (E) NF-κB1, NF-κB2, p65, RelB, and Rel in
HaCaT cells was detected under the inflammatory condition by
RT-qPCR. (P<0.05). RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; miR, microRNA; TGF-β, transforming
growth factor-β; IL, interleukin; NF, nuclear factor. |
Regulation of miR-149 in NF-κB
pathway
NF-κB pathway can be activated by TNF-α through
phosphorylating IκB, an inhibitor of NF-κB. Under inflammatory
condition, RT-qPCR shows that miR-149 significantly decreased the
expression of NF-κB1, RelB, and Rel, which are subunits of NF-κB
family (Fig. 2E).
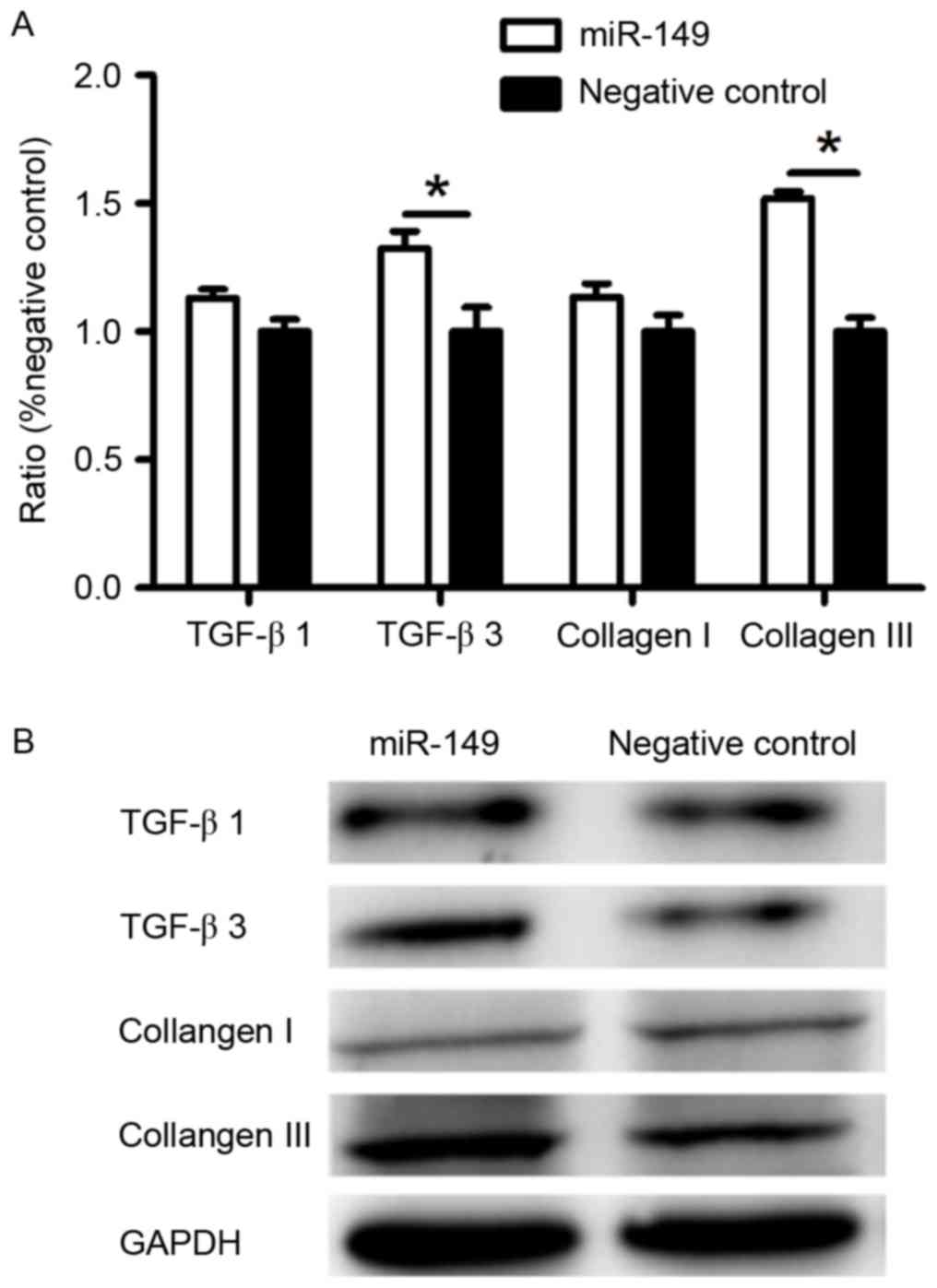
Expression of TGF-β3 and collagen III
was upregulated at the mRNA and protein level by miR-149 in
fibroblasts
To further investigate how HaCaT cells transfected
with miR-149 mimic affect the protein expression of dermal
fibroblasts, we performed RT-qPCR and western blotting. HaCaT cells
transfected with miR-149 mimic promoted the expression of TGF-β3
and collagen III of fibroblasts, but had no significant effect on
the expression of TGF-β1 and collagen I (Fig. 3), as compared to the negative
control.
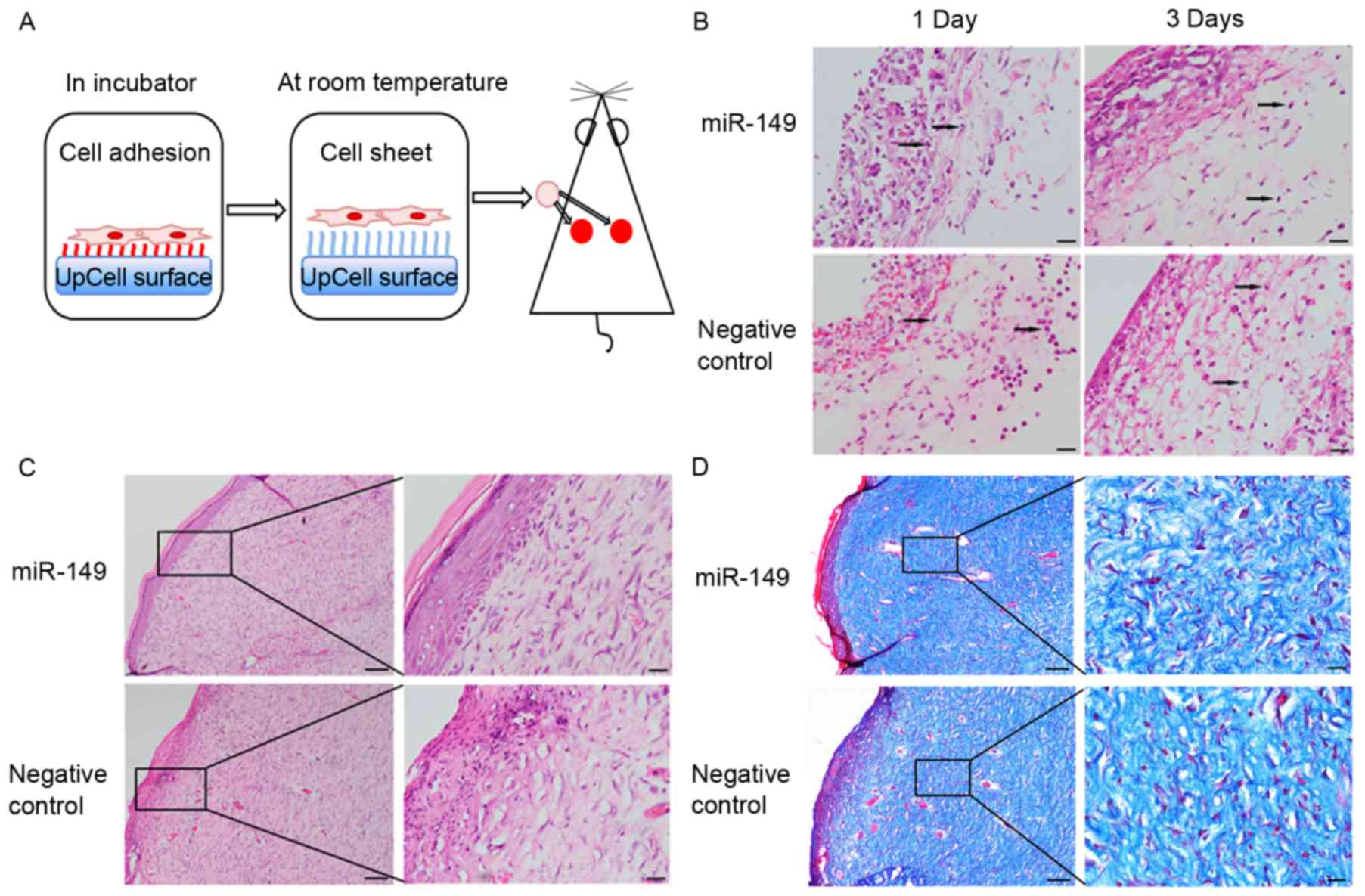
HaCaT cells transfected with miR-149
mimic improve skin wound healing in a rat model
To determine whether miR-149 could indirectly
improve skin wound healing through transfected HaCaT cells, we used
a rat full-thickness skin excisional model (Fig. 4A). There were no obvious
differences in these two groups in healing rate. The
hematoxylin-eosin staining results showed that the wounds of the
experimental group showed lower levels of inflammatory infiltration
and more continuous epidermis and organized tissue, as compared to
those in the negative control group (Fig. 4B and C). Masson's trichrome
staining was used to assess the overall quality of the scarred
areas and showed that collagen fibers within the wound areas of the
experimental group were more compact and arranged, as compared to
those in the negative control group (Fig. 4D).
Discussion
In this study, we focused on miR-149, which is
upregulated in mid-gestational fetal skin keratinocytes, and
studied its function in cutaneous wound healing in vitro and
in vivo. Our results suggest that miR-149 restricts
inflammation by downregulating the expression of IL-1α, IL-1β, and
IL-6, and promotes scarless wound healing by upregulating TGF-β3
and collagen III in fibroblasts co-cultured with
miR-149-transfected HaCaT cells. We believe that miR-149 may act as
a positive regulator during the skin wound healing process.
It was first determined in the 1970s that fetal skin
wounds can heal without the formation of scar tissue (21). Different from adult skin, fetal
skin is associated with an attenuated inflammatory response during
the wound healing process. Fetal skin wounds have lower expression
of the pro-inflammatory cytokines IL-6 and IL-8 (22) and increased production of the
anti-inflammatory cytokine IL-10 (23), in contrast to adult skin wounds.
The reduction in inflammatory molecules can reduce scarring in
postnatal wounds, thus, emphasizing the role of inflammation in
scarless healing. Dermal fibroblasts are key effector cells during
the skin wound healing process. Fetal fibroblasts are intrinsically
different from adult fibroblasts in collagen synthesis,
extracellular matrix production, and contractility (24). We performed small RNA sequencing
analysis and uncovered the dynamic changes of miRNAs between mid-
and late-gestational fetal skin keratinocytes. We first determined
a group of miRNAs that targeted interleukins and were associated
with the inflammatory response during skin wound healing. As
reported, miR-149 is downregulated in osteoarthritis chondrocytes
(15), in 24 h wounds relative to
0 h wounds (17), and in
hypertrophic scar tissue (25). In
this study, miR-149 was upregulated in mid-gestational fetal skin
keratinocytes relative to late-gestational fetal skin
keratinocytes. As mid-gestational fetal skin heals without
scarring, we conclude that overexpression of miR-149 could
contribute to scarless healing by dampening an excessive
inflammatory response. But, miR-149 has no effect on skin wound
healing rate.
Importantly, miR-149 can regulate the expression of
IL-1α, IL-1β, and IL-6 in HaCaT cells. Keratinocytes play an
important role in innate immunity (26). Appropriate inflammation is critical
in skin wound healing, but excessive and persistent inflammation
may result in scar formation. In some studies, IL-1β and IL-6 have
been validated as target genes of miR-149 in chondrosarcoma cells
(15) and a human embryonic kidney
cell line 293T (18). In this
study, miR-149 suppressed the production of IL-1α, IL-1β, and IL-6,
which are essential pro-inflammatory cytokines in the inflammatory
response. As reported, excessive infiltrating pro-inflammatory
cytokines can lead to the formation of scar tissue. Our study
suggests that miR-149 can suppress expression of pro-inflammatory
cytokines IL-1α, IL-1β, and IL-6 both at basal levels and in
inflammatory conditions, thus reducing scar tissue formation. To
validate the pathway involving in the anti-inflammatory role of
miR-149, should be involved in NF-κB pathway, we used RT-qPCR to
detect the expression of NF-κB family. Results showed that miR-149
can suppress expression of pro-inflammatory cytokines through NF-κB
pathway. After degradation of phosphorylating IκB, activated dimer
of NF-κB translocated into the nucleus and activated inflammatory
pathway (27). The exact
regulatory way of miR-149 needs more studies.
miR-149 can indirectly regulate gene expression of
TGF-β3 and collagen III in fibroblasts. Studies have shown that
fetal skin wounds express higher levels of anti-fibrotic TGF-β3 and
decreased levels of pro-fibrotic TGF-β1 and TGF-β2 (24). The collagen deposited in the fetal
skin wound is in a reticular pattern, unlike that in fibrotic
tissues and hypertrophic scars, where collagen deposition appears
as thick bundles parallel to the epidermal surface. Collagen III
accounts for a higher ratio of the total collagen in fetal skin
wounds than that in adult skin wounds and is regarded as a positive
element in the healing process. Fibroblasts are the cells most
involved in the synthesis and degradation of the extracellular
matrix, and our results showed that TGF-β3 and collagen III were
upregulated in fibroblasts co-cultured with miR-149 mimic.
Skin wound healing is a complex biological process,
thus, it may be difficult to improve markedly the quality of wound
healing with a single factor. Our study suggests that miR-149
overexpression may reduce the expression of pro-inflammatory
cytokines and improve the arrangement of collagen fibers. miR-149
may be a potential regulator in the skin wound healing process,
though we are still researching other miRNAs that may have a
synergistic effect with miR-149 to improve skin wound healing more
effectively.
Acknowledgements
This study was supported by the Program for National
Basic Research Program of China (no. 2012CB518103), the National
Science Foundation of China (no. 81450017), the Shenyang Key
Laboratory Project (no. F15-157-1-00), the Science and Technology
Planning Project of Shenyang (no. F14-201-4-00), and the Science
and Technology Planning Project of Liaoning Province (no.
2012225080).
References
|
1
|
Leavitt T, Hu MS, Marshall CD, Barnes LA,
Lorenz HP and Longaker MT: Scarless wound healing: Finding the
right cells and signals. Cell Tissue Res. 365:483–493. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poetschke J and Gauglitz GG: Current
options for the treatment of pathological scarring. J Dtsch
Dermatol Ges. 14:467–477. 2016. View Article : Google Scholar
|
|
3
|
Wilgus TA, Ferreira AM, Oberyszyn TM,
Bergdall VK and DiPietro LA: Regulation of scar formation by
vascular endothelial growth factor. Lab Invest. 88:579–590. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kryczka J and Boncela J: Leukocytes: The
double-edged sword in fibrosis. Mediat Inflamm. 2015:6520352015.
View Article : Google Scholar
|
|
5
|
Xu N, Meisgen F, Butler LM, Han G, Wang
XJ, Söderberg-Nauclér C, Ståhle M, Pivarcsi A and Sonkoly E:
MicroRNA-31 is overexpressed in psoriasis and modulates
inflammatory cytokine and chemokine production in keratinocytes via
targeting serine/threonine kinase 40. J Immunol. 190:678–688. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brant JO, Lopez MC, Baker HV, Barbazuk WB
and Maden M: A comparative analysis of gene expression profiles
during skin regeneration in mus and acomys. Plos One.
10:e01429312015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Yan JW, Wang YJ, Wan YN, Wang BX,
Tao JH, Chen B, Li BZ, Yang GJ and Wang J: Association of
interleukin 1 family with systemic sclerosis. Inflammation.
37:1213–1220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003.PubMed/NCBI
|
|
9
|
Takagi N, Kawakami K, Kanno E, Tanno H,
Takeda A, Ishii K, Imai Y, Iwakura Y and Tachi M: IL-17A promotes
neutrophilic inflammation and disturbs acute wound healing in skin.
Exp Dermatol. 26:137–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Do DV, Ong CT, Khoo YT, Carbone A, Lim CP,
Wang S, Mukhopadhyay A, Cao X, Cho DH, Wei XQ, et al:
Interleukin-18 system plays an important role in keloid
pathogenesis via epithelial-mesenchymal interactions. Brit J
Dermatol. 166:1275–1288. 2012. View Article : Google Scholar
|
|
11
|
Yin H, Li X, Hu S, Liu T, Yuan B, Gu H, Ni
Q, Zhang X and Zheng F: IL-33 accelerates cutaneous wound healing
involved in upregulation of alternatively activated macrophages.
Mol Immunol. 56:347–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin L, Zhang YD, Chen ZY, Chen YX and Ren
CP: The clinicopathological significance of miR-149 and PARP-2 in
hepatocellular carcinoma and their roles in chemo/radiotherapy.
Tumor Biol. 37:12339–12346. 2016. View Article : Google Scholar
|
|
13
|
Yang C, Sun C, Liang X, Xie S, Huang J and
Li D: Integrative analysis of microRNA and mRNA expression profiles
in non-small-cell lung cancer. Cancer Gene Ther. 23:90–97. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pfeffer SR, Grossmann KF, Cassidy PB, Yang
CH, Fan M, Kopelovich L, Leachman SA and Pfeffer LM: Detection of
exosomal miRNAs in the plasma of melanoma patients. J Clin Med.
4:2012–2027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Santini P, Politi L, Vedova P Dalla,
Scandurra R and d'Abusco AS: The inflammatory circuitry of miR-149
as a pathological mechanism in osteoarthritis. Rheumatol Int.
34:711–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palmieri D, Capponi S, Geroldi A, Mura M,
Mandich P and Palombo D: TNFα induces the expression of genes
associated with endothelial dysfunction through p38MAPK-mediated
down-regulation of miR-149. Biochem Biophys Res Commun.
443:246–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li DQ, Wang AX, Liu X, Meisgen F, Grünler
J, Botusan IR, Narayanan S, Erikci E, Li X, Blomqvist L, et al:
MicroRNA-132 enhances transition from inflammation to proliferation
during wound healing. J Clin Invest. 125:3008–3026. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu G, Zhang Z, Xing Y, Wei J, Ge Z, Liu X,
Zhang Y and Huang X: MicroRNA-149 negatively regulates
TLR-triggered inflammatory response in macrophages by targeting
MyD88. J Cell Biochem. 115:919–927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao F, Wang Z, Lang H, Liu X, Zhang D,
Wang X, Zhang T, Wang R, Shi P and Pang X: Dynamic expression of
novel miRNA candidates and miRNA-34 family members in early- to
mid-gestational fetal keratinocytes contributes to scarless wound
healing by targeting the TGF-β pathway. Plos One.
10:01260872015.
|
|
20
|
Liu XY, Wang Z, Wang R, Zhao F, Shi P,
Jiang Y and Pang X: Direct comparison of the potency of human
mesenchymal stem cells derived from amnion tissue, bone marrow and
adipose tissue at inducing dermal fibroblast responses to cutaneous
wounds. Int J Mol Med. 31:407–415. 2013.PubMed/NCBI
|
|
21
|
Burrington JD: Wound healing in the fetal
lamb. J Pediatr Surg. 6:523–528. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zgheib C, Xu J and Liechty KW: Targeting
inflammatory cytokines and extracellular matrix composition to
promote wound regeneration. Adv Wound Care (New Rochelle).
3:344–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balaji S, King A, Marsh E, LeSaint M,
Bhattacharya SS, Han N, Dhamija Y, Ranjan R, Le LD, Bollyky PL, et
al: The role of interleukin-10 and hyaluronan in murine fetal
fibroblast function in vitro: Implications for recapitulating fetal
regenerative wound healing. Plos One. 10:e01243022015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Larson BJ, Longaker MT and Lorenz HP:
Scarless fetal wound healing: A basic science review. Plast
Reconstr Surg. 126:1172–1180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li P, He QY, Luo CQ and Qian LY:
Differentially expressed miRNAs in acute wound healing of the skin:
A pilot study. Medicine (Baltimore). 94:e4582015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strbo N, Yin N and Stojadinovic O: Innate
and adaptive immune responses in wound epithelialization. Adv Wound
Care (New Rochelle). 3:492–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pasparakis M: Regulation of tissue
homeostasis by NF-kappaB signalling: Implications for inflammatory
diseases. Nat Rev Immunol. 9:778–788. 2009. View Article : Google Scholar : PubMed/NCBI
|