Introduction
Bladder cancer remains one of the common urinary
system malignancies worldwide, with a high mortality rate (1). A previous study estimated that ~90%
of bladder cancers are transitional cell bladder carcinomas
(2). It has been reported that
accurate staging and precise pathologic evaluation are important
for optimization of the correct bladder cancer treatment regimens
(3). At present, diagnosis for
bladder cancer remains unsatisfactory, primarily due to the high
cost, high false-positive rate, poor sensitivity and the delay in
result availability (3,4). Therefore, the identification of
several biomarkers for the diagnosis of bladder cancer will greatly
contribute to the improvement of clinical diagnosis and treatment
of bladder cancer.
MicroRNAs (miRNAs) are highly conserved endogenous
non-coding RNAs of 20–22 nt in length that function in various
biological processes at the transcriptional or post-transcriptional
level by targeting the 3′-untranslated region of mRNA (5). Previous studies have demonstrated
that various miRNAs are involved in the progression and biology of
tumorigenesis (6,7). For example, miRNA (miR)-490-5p has
been reported to function as a novel tumor suppressor in human
bladder cancer by targeting c-Fos (8), and miR-27a downregulation may lead to
cisplatin resistance in bladder cancer (9). Previous studies have reported that
miR-497 has an important role in certain diseases, including tumor
and brain diseases, such as cerebral ischemia, through complex
mechanisms (10,11). Additionally, Yan et al
(12) previously reported that
miR-497 prevents angiogenesis and metastasis of hepatocellular
carcinoma by regulation of astrocyte elevated gene-1 and vascular
endothelial growth factor A (12).
Likewise, previous studies have revealed that there may be a
correlation between miR-497 expression levels and the development
and metastasis of bladder cancer (13,14).
The aim of the present study was to investigate the
role of miR-497 expression in patients with bladder cancer and to
determine the association between miR-497 expression and the
metastasis and invasion of bladder cancer cells using T24 and
BIU-87 cell lines. Various experimental methods were used to
analyze the effect of abnormal miR-497 expression on bladder cancer
cell migration and invasion and the expression of
metastasis-associated proteins. The present study may provide a
theoretical basis for the application of miR-497 in improving the
diagnosis of bladder cancer.
Materials and methods
Patients
A total of 50 patients diagnosed with bladder cancer
in Linyi People's Hospital (Linyi, China) between March 2013 and
July 2014 were enrolled in the present study. A total of 34 cases
were male and 16 cases were female and the mean age was 59.83±7.42.
All patients underwent surgery in Linyi People's Hospital; patients
were diagnosed with urothelial bladder cancer using biopsy samples
acquired prior to the surgery and postoperative pathology
detection. The patients had not received any chemotherapy,
radiation, or immunotherapy prior the surgery. The pathological
classification was performed according to the WH01973 criteria
(15) and tumor-node-metastasis
(TNM) staging was based on the International Union against Cancer
in 2002, version 6 of TNM stage (16). According to pathological
classification (15), 29 cases
were stage I (urothelium, high differentiation), 12 cases were
stage II (urothelium, middle differentiation) and 9 cases were
stage III (urothelium, low differentiation). The patient
characteristics are described in Table
I. Pathological tissues and the adjacent noncancerous tissues
were extracted, based on histological analysis. The adjacent
noncancerous tissues were taken 3 cm from the edge of the tumor and
served as the control group. Tissues were subsequently snap frozen
with liquid nitrogen and stored at −80°C until RNA extraction. The
procedures of the present study were approved by the Protection of
Human Ethics Committee of Linyi People's Hospital (Linyi, China),
and all patients provided signed written informed consent prior to
the collection of clinical samples.
 | Table I.Characteristics of patients enrolled
in the present study. |
Table I.
Characteristics of patients enrolled
in the present study.
| Characteristic | n | miR-479 | P-value |
|---|
| Age (years) |
|
|
0.637 |
| ≤50 | 29 |
5.027±0.765 |
|
|
>50 | 21 |
4.926±0.711 |
|
| Diameter of tumor
(cm) |
|
|
0.948 |
| ≤2.5 | 37 |
4.822±0.651 |
|
|
>2.5 | 13 |
4.808±0.717 |
|
| Pathological
stage |
|
| <0.001 |
| I | 29 |
7.121±0.702 |
|
|
II/III | 21 |
4.988±0.547 |
|
| Lymph node
metastasis |
|
| <0.001 |
| No | 17 | 13.023±0.912 |
|
| Yes | 33 |
6.875±0.772 |
|
| Distant
metastasis |
|
| <0.001 |
| No | 12 | 14.159±0.512 |
|
| Yes | 38 |
7.485±0.661 |
Cell culture and transfection
T24 and BIU-87 human bladder cancer cell lines and
the SV-HUC-1 immortalized human bladder epithelium cells (all from
the American Type Culture Collection, Manassas, VA, USA) were
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) in a cell
culture incubator with 5% CO2 at 37°C. For the cell
transfection, miR-497 mimics (sense: 5′-CAGCAGCACACUGUGGUUUGU-3′;
antisense: 5′-AAACCACAGUGUGCUGCUGUU-3′) and scramble control mimics
(sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense:
5′-ACGUGACACGUUCGGAGAATT-3′) (GenePharma Co., Ltd., Shanghai,
China) were transfected into the cells at a final concentration of
50 nM using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.).
Migration and invasion assay
Cell migration and invasion were conducted using
Transwell migration chambers (8-µm pore size; Costar; Corning
Incorporated, Corning, NY, USA) (17). Bladder cancer cells were seeded at
a density of 5×104 cells/well in the upper portion of the chamber
with serum-free medium following the transfection. Medium
containing 10% fetal calf serum was used as a chemoattractant in
the lower chamber. Following incubation at 37°C for 24 h,
non-migrated cells on the top of the membrane were scraped and
removed with cotton swabs carefully. The migrated cells on the
bottom of the membrane were subsequently fixed with 4% formaldehyde
at room temperature for 15 min, stained with Diff-Quik (Siemens
Healthcare Diagnostics, Newark, DE, USA) and counted using a light
microscope. The membranes for the invasion assay were coated with a
diluted extracellular matrix solution (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany), the remaining processes for cell
treatment were the same as the aforementioned migration assay.
Reverse transcription-quantitative
polymerase chain reaction
Total RNA extraction from the treated cells was
conducted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) as previously described (18), the extracts were treated with
RNase-free DNase I (Promega Corporation, Madison, WI, USA).
Subsequently, the concentration and purity of the isolated RNA were
quantified using SMA400 UV-VIS (Merinton Instrument, Ltd.,
Shanghai, China). Purified RNA (0.5 µg/µl in nuclease-free water)
was used for cDNA synthesis with the PrimerScript First Strand cDNA
Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Expression levels of the targets were detected in an Eppendorf
Mastercycler (Brinkman Instruments, Westbury, NY, USA) using the
SYBR ExScript RT-qPCR kit (Takara Biotechnology Co., Ltd., Dalian,
China). Melting curve analysis of amplification products was
performed at the end of each PCR to confirm that only one product
was amplified and detected. Relative expression of targets was
calculated according to the 2−ΔΔCq method (19). GAPDH and U6 were chosen as the
internal controls. Primers used for miR-497 amplification were
designed by Biomics Biotechnology (Nantong, China), and primers for
the other targets amplification are presented in Table II.
 | Table II.Primers used for target amplification
in the present study. |
Table II.
Primers used for target amplification
in the present study.
| Target | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| GAPDH |
GGGTGGAGCCAAACGGGTC |
GGAGTTGCTGTTGAAGTCGCA |
| E-cadherin |
GAACTCAGCCAAGTGTAAAAGCC |
GAGTCTGAACTGACTTCCGC |
| Vimentin |
AAAGTGTGGCTGCCAAGAAC |
AGCCTCAGAGAGGTCAGCAA |
| α-SMA |
GGCCGAGATCTCACTGACTAC |
TTCATGGATGCCAGCAGA |
| U6 |
CGTTTTACTTCCTCATACAGCAC |
GCACCAAGAGACCTGTGACA |
Western blotting
Cells cultured for 48 h in each group were lysed
with radioimmunoprecipitation assay buffer (Sangon Biotech, Co.,
Ltd., Shanghai, China) containing phenylmethanesufonyl fluoride
(Sigma-Aldrich; Merck Millipore) and the lysates were centrifuged
at 14,000 × g for 10 min at 4°C. Supernatant was collected to
quantify the protein concentrations using bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal
quantity of protein (50 µg) per cell lysate was subjected to a 12%
sodium dodecylsulfate-polyacrylamide gel electrophoresis and
transferred onto a PVDF membrane (Merck Millipore). The PVDF
membranes were blocked with Tris-buffered saline Tween-20 (TBST)
containing 5% non-fat milk for 1 h at room temperature.
Subsequently, the membranes were incubated with rabbit anti-human
primary antibodies for E-cadherin (ab15148), vimentin (ab45939) and
a-smooth muscle actin (α-SMA; ab5694) (all from Abcam, Cambridge,
UK) overnight at 4°C. The membranes were incubated with
horseradish-peroxidase labeled goat anti-rabbit secondary antibody
(1:5,000 dilution; ab205718; Abcam) at room temperature for 1 h.
The PVDF membranes were washed three times with 1X TBST buffer for
10 min. The signals were detected following incubation with a
chromogenic substrate using enhanced chemiluminescence (GE
Healthcare Life Sciences, Chalfont, UK). Additionally, GAPDH was
used as the internal control.
Statistical analysis
All data were expressed as the mean ± standard error
of mean. Clinicopathologic data were analyzed using Student's
t-test. Data presented in the figures were analyzed using one-way
or two-way analysis of variance followed by Tukey's significant
difference post hoc test. Analyses were performed using GraphPad
Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
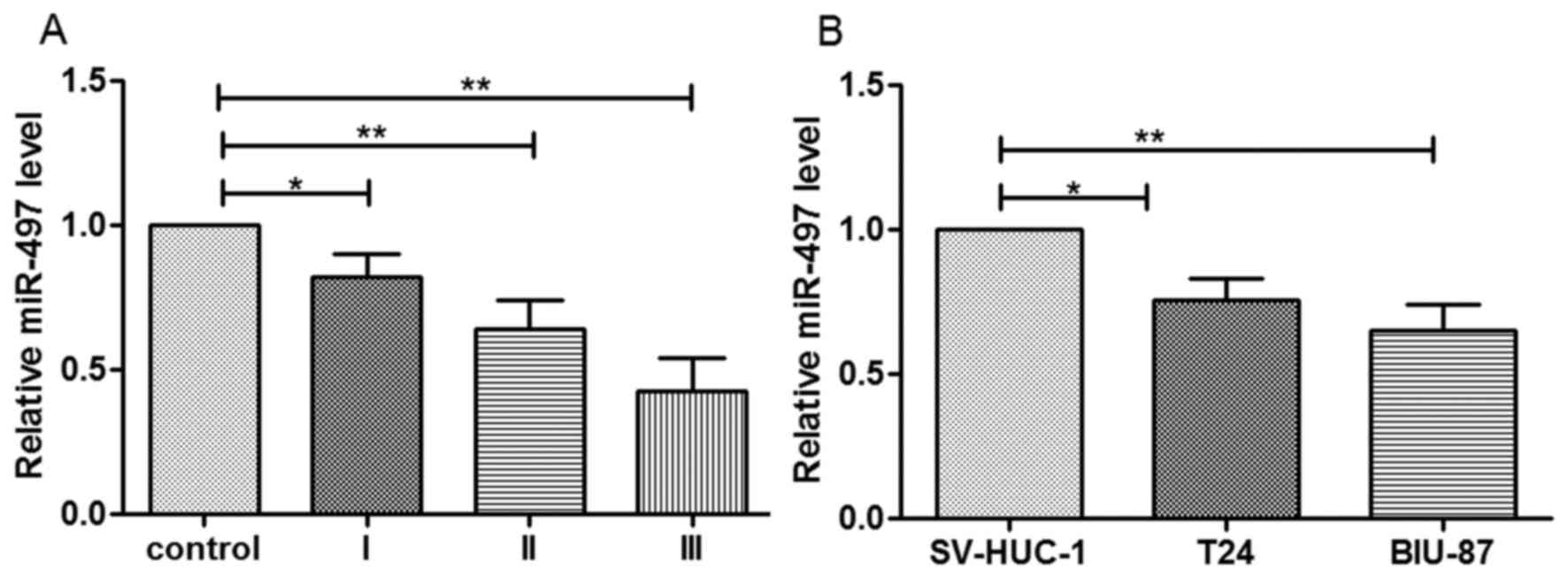
Expression of miR-497 in bladder
cancer cells
In order to analyze the expression of miR-497 in
bladder cancer cells, patients with bladder cancer and the adjacent
noncancerous tissues were collected. The findings of the present
study determined that miR-497 expression was significantly reduced
in the cancer tissues compared with the adjacent noncancerous
tissues. Additionally, its expression was reduced gradually from
cancer tissue stage I to stage III (Fig. 1A). Therefore, the expression of
miR-497 may be negatively associated with the stage of bladder
cancer. The expression level of miR-497 was significantly reduced
in the cancer cells compared to the SV-HUC-1 normal uroepithelium
cells (P<0.05 in T24 cells; P<0.01 in BIU-87 cells; Fig. 1B).
Association between miR-497 expression
and patient characteristics
The association between miR-497 expression and
pathological parameters of patients with bladder cancer was
evaluated and presented in Table
I. It was determined that there was no association between
miR-497 expression and age or tumor diameter (P>0.05). However,
miR-497 expression was lower in patients at stage II and III
compared with patients at stage I (P<0.05), indicating that
miR-497 expression may be associated with tumor stage. Furthermore,
miR-497 expression was downregulated in patients with lymph node
metastasis or distant metastasis, compared with patients without
metastasis (P<0.05), suggesting that miR-497 expression may be
associated with the occurrence of metastasis.
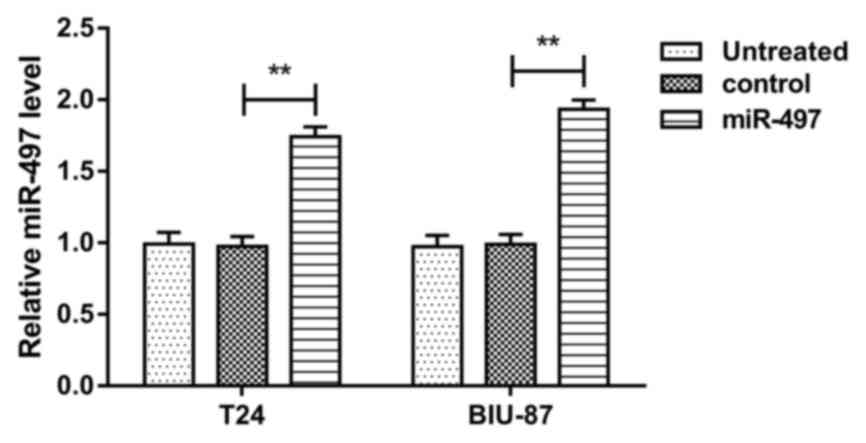
Aberrant expression of miR-497 in
bladder cancer cells after transfection
The T24 and BIU-87 cells were transfected with
miR-497 mimic or scramble control mimics, followed by estimation of
miR-497 expression. MiR-497 levels in T24 and BIU-87 cells were
both markedly upregulated by transfection with miR-497 mimics,
compared with cells transfected with scramble control mimics (both
P<0.01) (Fig. 2). MiR-497
expression level was not altered following transfection with
scramble control mimics, compared with untreated cells. The data
confirmed that miR-497 was upregulated following transfection with
miR-497 mimics.
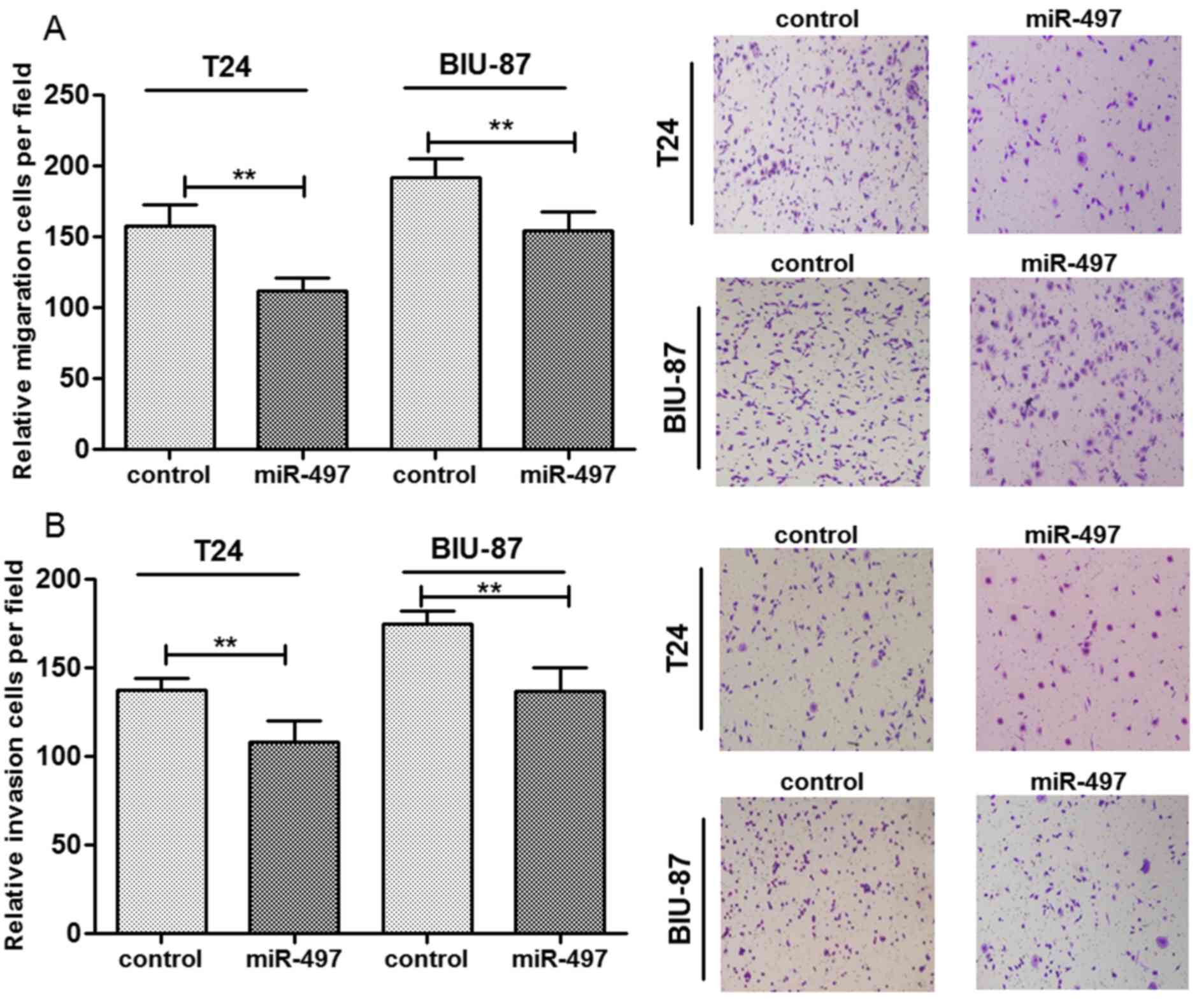
miR-497 overexpression suppresses cell
migration and invasion
Based on the aforementioned findings, miR-497
expression may be associated with metastasis of bladder cancer.
Therefore, the effect of miR-497 overexpression on cell migration
and invasion was investigated (Fig.
3). The findings revealed that overexpression of miR-497
significantly reduced the number of migrated cells in T24 and the
BIU-87 cells compared with the control miRNA (P<0.01; Fig. 3A). The number of invasive cells of
both cell lines were also significantly reduced in the groups
overexpressing miR-497 (P<0.01; Fig. 3B). These findings indicated that
overexpressed miR-497 may be associated with reduced migration and
invasion of bladder cancer cells.
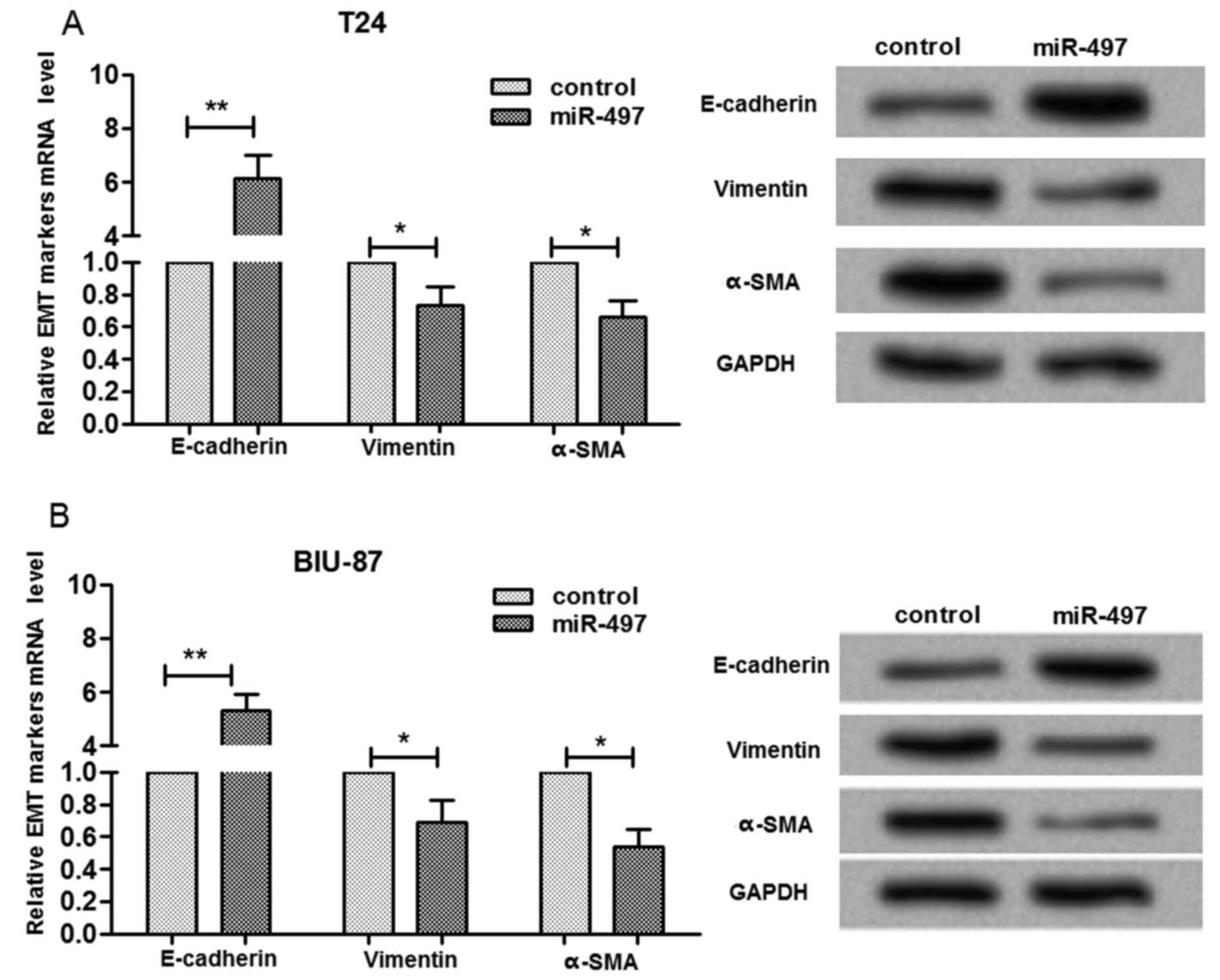
miR-497 expression was associated with
migration and invasion-associated proteins
In order to investigate the underlying molecular
mechanism for the influences of miR-497 expression on bladder
cancer cell migration and invasion, the expression levels of
migration and invasion-associated proteins was determined (Fig. 4). When miR-497 was overexpressed in
T24 cells, the mRNA and protein expression level of E-cadherin was
significantly increased (P<0.01), whereas vimentin and α-SMA
expression levels were significantly reduced (P<0.05, Fig. 4A). The present study also
determined that the alterations in E-cadherin, vimentin and α-SMA
expression levels were similar in BIU-87 and in T24 cells (Fig. 4B). These findings indicated that
miR-497 expression is associated with migration and invasion
through affecting the protein expression of epithelial-mesenchymal
transition markers in bladder cells.
Discussion
Previous studies have demonstrated that miRNAs are
important for the biology and tumorigenesis of various types of
cancer. Previous research reported that miR-497 is involved in
breast cancer malignancy (11),
and another study demonstrated that miR-497 may inhibit
angiogenesis and metastasis of hepatocellular carcinoma (20). However, investigations focusing on
the relationship between miR-497 and bladder cancer are limited.
The present study analyzed the expression of miR-497 in bladder
cancer tissues and investigated the association between miR-497
expression and the metastasis of bladder cancer. The findings of
the present study revealed that miR-497 expression level was
reduced in bladder cancer tissues compared with the adjacent
noncancerous tissues and its expression was gradually reduced with
increasing pathological classification stage, which was consistent
with a previous study (21). In
addition, the present study evaluated the association between
miR-497 expression and patient characteristics, such as age,
diameter of tumor, TNM stage, and pathological classification
stage. Our results in Table I
demonstrated that miR-497 expression was negatively associated with
TNM stage, pathological classification stage and metastasis.
Therefore, abnormal miR-497 expression may be associated with the
tumor stage and metastasis of bladder cancer.
The effect of miR-497 expression on bladder cancer
cell migration and invasion was determined using T24 and BIU-87
cells. The findings of the present study revealed that the
overexpression of miR-497 significantly reduced the number of
migrated and invasive cells in both cell lines. The results of the
present study are consistent with previous studies performed in
other cell types. Ruan et al (22) previously determined that miR-497
expression was reduced in malignant tumors, and its upregulation
may inhibit cell migration in human osteosarcoma (22). Additionally, miR-497 expression
modulates breast cancer invasion by targeting cyclin E1 (23). However, the association between
miR-497 expression and the migration and invasion of bladder cancer
cells has not been fully elucidated. Our study revealed that
miR-497 upregulation may serve a suppressive role in bladder cancer
metastasis through inhibition of migration and invasion.
Additionally, the present study determined the
influence of abnormal miR-497 expression on metastasis-associated
protein expression and the findings revealed that E-cadherin
expression was increased, whereas vimentin and α-SMA expression
levels were reduced in cells overexpressing miR-497 for both cell
lines. Vimentin is a cytoskeleton protein that is expressed in
fibroblasts and endothelial cells. Previous studies have
demonstrated that vimentin may function as a tumor marker, having
an important role in cell proliferation, invasion and migration and
high expression levels of vimentin induce the migration and
invasion of tumor cells (24,25).
E-cadherin expression was low in bladder cancer cells and has been
identified to be associated with tumor development and prognosis of
bladder cancer (26). α-SMA is a
key downstream factor of the serum response factor (SRF) and the
combination of SRF and α-SMA may promote the translation of mRNA
(27). A previous study determined
that α-SMA participates in the development of various tumors, such
as squamous cell carcinoma (28).
It has been previously demonstrated that miR-133 modulates α-SMA
expression in bladder cancer (29). Additionally, E-cadherin
overexpression and vimentin downregulation are markers of reduced
migration and invasion in bladder cancer (30,31).
The findings of the present study revealed that miR-497 may inhibit
bladder cancer cell migration and invasion through upregulation of
E-cadherin and downregulations of vimentin and α-SMA.
In conclusion, the data presented in the present
study suggested that miR-497 is downregulated in bladder cancer
tissues. The abnormal expression of miR-497 was associated with the
metastasis of bladder cancer and miR-497 upregulation limited the
metastasis of bladder cancer by affecting the expression levels of
metastasis-associated proteins, such as E-cadherin, vimentin and
α-SMA. The present study may provide the theoretical basis for the
application of miR-497 in diagnosing bladder cancer metastasis.
Further studies are required in order to fully elucidate the
underlying molecular mechanism.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang X and Zhang Y: Bladder cancer and
genetic mutations. Cell Biochem Biophys. 73:65–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harshman LC, Preston MA, Bellmunt J and
Beard C: Diagnosis of bladder carcinoma: A clinician's perspective.
Surg Pathol Clin. 8:677–685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheung G, Sahai A, Billia M, Dasgupta P
and Khan MS: Recent advances in the diagnosis and treatment of
bladder cancer. BMC Med. 11:132013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aigner A: MicroRNAs (miRNAs) in cancer
invasion and metastasis: Therapeutic approaches based on
metastasis-related miRNAs. J Mol Med (Berl). 89:445–457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng N, Yang P, Wang Z and Zhou Q:
OncomicroRNAs-mediated tumorigenesis: Implication in cancer
diagnosis and targeted therapy. Curr Cancer Drug Targets. 17:40–47.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Medina PP and Slack FJ: MicroRNAs and
cancer: An overview. Cell Cycle. 7:2485–2492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lan G, Yang L, Xie X, Peng L and Wang Y:
MicroRNA-490-5p is a novel tumor suppressor targeting c-FOS in
human bladder cancer. Arch Med Sci. 11:561–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drayton RM, Dudziec E, Peter S, Bertz S,
Hartmann A, Bryant HE and Catto JW: Reduced expression of miRNA-27a
modulates cisplatin resistance in bladder cancer by targeting the
cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 20:1990–2000.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin KJ, Deng Z, Huang H, Hamblin M, Xie C,
Zhang J and Chen YE: miR-497 regulates neuronal death in mouse
brain after transient focal cerebral ischemia. Neurobiol Dis.
38:17–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang
Y, Zou C, Zhang X, Liu S, Wang X, et al: Analysis of MiR-195 and
MiR-497 expression, regulation and role in breast cancer. Clin
Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: MiR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015.PubMed/NCBI
|
|
13
|
Zhang Y, Zhang Z, Li Z, Gong D, Zhan B,
Man X and Kong C: MicroRNA-497 inhibits the proliferation,
migration and invasion of human bladder transitional cell carcinoma
cells by targeting E2F3. Oncol Rep. 36:1293–1300. 2016.PubMed/NCBI
|
|
14
|
Du M, Shi D, Lin Y, Yuan L, Li P, Chu H,
Qin C, Yin C, Zhang Z and Wang M: Circulating miR-497 and miR-663b
in plasma are potential novel biomarkers for bladder cancer. Sci
Rep. 5:104372015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mostofi FK, Davis CJ and Sesterhenn IA:
WHO histological typing of urinary bladder tumors. Springer;
Berlin: 1973
|
|
16
|
Union for International Cancer Control
(UICC), . TNM Classification of Malignant Tumours. Sobin LH and
Wittekind CH: 6th. John Wiley & Sons; Hoboken, NJ: 2002
|
|
17
|
Brackenbury WJ and Djamgoz MB: Nerve
growth factor enhances voltage-gated Na+ channel activity and
transwell migration in Mat-LyLu rat prostate cancer cell line. J
Cell Physiol. 210:602–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin Y, Yue B, Xiang H, Liu Y, Ma X and
Chen B: Survivin is expressed in degenerated nucleus pulposus cells
and is involved in proliferation and the prevention of apoptosis in
vitro. Mol Med Rep. 13:1026–1032. 2016.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: MiR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015.PubMed/NCBI
|
|
21
|
Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang
B, Shu Y and Liu P: miR-497 modulates multidrug resistance of human
cancer cell lines by targeting BCL2. Med Oncol. 29:384–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ruan WD, Wang P, Feng S, Xue Y and Zhang
B: MicroRNA-497 inhibits cell proliferation, migration, and
invasion by targeting AMOT in human osteosarcoma cells. Onco
Targets Ther. 9:303–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Q, Li X, Gao Y, Long Y, Chen L, Huang
Y and Fang L: MiRNA-497 regulates cell growth and invasion by
targeting cyclin E1 in breast cancer. Cancer Cell Int. 13:952013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu QS, Rosenblatt K, Huang KL, Lahat G,
Brobey R, Bolshakov S, Nguyen T, Ding Z, Belousov R, Bill K, et al:
Vimentin is a novel AKT1 target mediating motility and invasion.
Oncogene. 30:457–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McInroy L and Määttä A: Down-regulation of
vimentin expression inhibits carcinoma cell migration and adhesion.
Biochem Biophys Res Commun. 360:109–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng RY, Zhou GH and Chun LI: Expression
of E-cadherin in Bladder Cancer. Prac J Cancer. 15:87–88. 2000.
|
|
27
|
Kumar CC, Kim JH, Bushel P, Armstrong L
and Catino JJ: Activation of smooth muscle alpha-actin promoter in
ras-transformed cells by treatments with antimitotic agents:
Correlation with stimulation of SRF:SRE mediated gene
transcription. J Biochem. 118:1285–1292. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kojc N, Zidar N, Vodopivec B and Gale N:
Expression of CD34, alpha-smooth muscle actin, and transforming
growth factor beta1 in squamous intraepithelial lesions and
squamous cell carcinoma of the larynx and hypopharynx. Hum Pathol.
36:16–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duan LJ, Qi J, Kong XJ, Huang T, Qian XQ,
Xu D, Liang JH and Kang J: miR-133 modulates TGF-β1-induced bladder
smooth muscle cell hypertrophic and fibrotic response: Implication
for a role of microRNA in bladder wall remodeling caused by bladder
outlet obstruction. Cell Signal. 27:215–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang M, Shan LP and Zhang H: Expression
of Snail and E-cadherin in bladder urothelial carcinoma and their
relation with tumor recurrence. J Mod Oncol. 18:2422–2425.
2010.
|
|
31
|
Lewis SA, Traub P and Spilker CM: The
N-terminal domain of vimentin alters bladder permeability. J Urol.
170:2091–2094. 2003. View Article : Google Scholar : PubMed/NCBI
|