Introduction
Major depressive disorder (MDD) is a common distress
disorder that is characterized by anhedonia, depressed mood and
altered cognitive functions (1).
According to previous studies, depression will become the leading
cause of disability to work and social contact by 2030 (2), which may have serious consequences
for individuals and their families (3). Although genetic and environmental
factors have been reported to be involved in the etiopathogenesis
of depression, the precise mechanisms are still unknown (4). Among the hypotheses regarding the
pathogenesis of depression, there is more evidence that implicates
hypothalamic-pituitary-adrenal (HPA) axis hyperactivity and
neuroendocrine disorders; as well as the involvement of monoamine
neurotransmitters (5,6). Current pharmacologic antidepressant
therapies target monoamine neurotransmitters in the central nervous
system to alleviate depressive symptoms (7). However, the majority of currently
available clinical antidepressants have a high risk of adverse
effects (8–10), and more than 30% of patients with
depression fail to respond to antidepressant treatment, which not
only reflects our incomplete understanding of the etiology of
depression, but also indicates a need to discover effective and
reliable treatment strategies.
Owing to the safety and effectiveness in alleviating
the symptoms of depression, traditional Chinese medicines have been
considered as complementary and alternative therapies (11). The well-known medicinal fungus
Paecilomyces tenuipes has been used as a crude drug and/or a folk
tonic food for antitumor and immunomodulatory therapy (12). The antidepressant-like effects of
P. tenuipes and its compounds have been reported in animal
models (13). One study revealed
that P. tenuipes treatment regulated the function of the HPA
axis in rats, particularly in modulating the serum levels of
cortisol and adrenocorticotropic hormone (ATCH) (14). However, the antidepressant-like
effects of P. tenuipes and the possible mechanisms of action
have rarely been examined.
Based on previous research, the present study aimed
to analyze the antidepressant-like effects of P. tenuipes in
chronic unpredictable mild stress (CUMS)-induced model rats.
Following 4 weeks of treatment with P. tenuipes N45 water
extract (PTNE), behavioral tests were conducted to examine its
effects on depression-like behaviors in CUMS rats; in addition,
hormone and neurotransmitter levels were measured in serum and
hypothalamus. The present data may provide experimental evidence to
verify whether P. tenuipes possesses the potential for use
as an adjuvant therapy for depression.
Materials and methods
PTNE preparation
P. tenuipes N45 (China Center for Type
Culture Collection, Wuhan, China) was cultured using a submerged
fermentation process on a rotary shaker using a Biostat B fermenter
(10 l flask; 150 rpm; Biostat B; Sartorius AG, Göttingen, Germany)
for 5 days at 26°C. The cultured medium contained glucose (40 g/l),
peptone (10 g/l) and yeast extract powder (10 g/l), and was made in
water up to 7 l. Cultured mycelia were extracted twice in 1,000 ml
double distilled water (ddH2O) at 80°C for 3 h.
Following centrifugation of the water extract at 3,550 × g and 4°C
for 10 min, the supernatant was concentrated using an R1002B Rotary
Evaporator for 4 h (pressure, 0.09 mPa; temperature, 80°C; Shanghai
Senco Technology Co., Ltd., Shanghai, China) and freeze-dried using
a Genesis Pilot Lyophilizer 25ES for further testing. Preliminary
determination indicated that PTNE contains 3.9% polysaccharides,
12.7% cordycepic acid and 0.3% adenosine, which were detected via
the phenolsulfuricacid method (15) and high performance liquid
chromatography (16).
Depression-like rat model
establishment and drug treatment procedure
Male Sprague-Dawley rats (n=60; age, 6-weeks-old;
weight, 180–220 g) were purchased from the Laboratory Animal Center
of Jilin University (Changchun, China) and maintained on a 12 h
light/dark cycle (lights on 07:00-19:00 h) at 23±1°C with water and
food available ad libitum. The experimental protocol was
approved by The Institution Animal Ethics Committee of Jilin
University (Changchun, China).
CUMS rats were established by exposing them to
random stressors over a 1 week period. Stressors included: Forced
swimming for 5 min at 4°C, 24 h wet litter, 12 h food and water
deprivation, 90 sec tail pinch, overnight illumination and 24 h
cage tilt (cages were tilted to 45°C from the horizontal). To
prevent habituation, all stressors were randomly scheduled every
week and repeated for eight weeks; rats were exposed to a different
stressor each day of each week, and this was repeated for eight
weeks. Rats housed in separated cages that did not receive any
treatments served as the control (CTRL) group.
Drug treatments began from the fifth week of CUMS
exposure, and were performed 1 h prior to the daily administration
of CUMS. After 8 weeks of exposure to stressors, CUMS-induced
depression-like rats were randomly separated into the following 5
groups (n=10/group; all treatments were administered orally for 4
weeks): i) Model group, which received physiological saline (10
ml/kg); ii) fluoxetine hydrochloride (Flu) group, which received
Flu (3 mg/kg; Shanghai Zhongxi Pharmaceutical Co., Ltd., Shanghai,
China); and three PTNE treatment groups, which were treated with
iii) 0.04 g/kg, iv) 0.2 g/kg, or v) 1.0 g/kg of PTNE. CTRL rats
were treated with 10 ml/kg of sterile saline (n=10). The bodyweight
of each experimental rat was measured once per week.
Behavioral assessments
Forced running test (FRT)
The FRT was performed as previously described
(17). Before formal testing, rats
were allowed to run at 20 mph on an FT-200 treadmill (Chengdu
Taimeng Science and Technology, Ltd., Chengdu, China) 3 times for 1
min each. The time until exhaustion was recorded to evaluate the
running performance of each rat.
Hotplate test
The hotplate test was performed as reported
previously (18). Briefly, rats
were placed on a 55±0.5°C surface and paw withdrawal latency time
(licking the hind paw of the injured side or jumping at the plate)
was measured.
Forced swim test (FST)
Rats were placed individually in a Plexiglas
cylinder (height, 50 cm; diameter, 20 cm) filled with 24±0.5°C
water to 40±1.5 cm. Immobility, which was defined as the lack of
motion of the whole body except for the small movements that were
necessary to keep the rats head above the water, was recorded
during the last 5 min of the 6 min test.
Hormone and neurotransmitter detection
Upon completion of the aforementioned experiments,
blood was collected from the caudal vein, and the rats were
sacrificed by injection of pentobarbital (200 mg/kg). The
hypothalamus was collected, weighed and homogenized with
ddH2O. Samples were purified by centrifugation at 4°C
and 10,000 × g for 5 min. For detection of serum levels, 1 ml blood
was centrifuged at 25°C and 2,000 × g for 5 min and the serum was
collected. The levels of 5-hydroxytryptamine (5-HT; cat no.
CK-E30326), 5-hydroxyindoleacetic acid (5-HIAA; cat no.
CK-E92141R), dopamine (DA; cat no. CK-E30237),
3,4-dihydroxyphenylacetic acid (DOPAC; cat no. CK-E93592R),
glucocorticoid receptor (GR; cat no. CK-E30214), norepinephrine
(NE; cat no. CK-E30189R), adrenocorticotropic hormone (ACTH; cat
no. CK-E30596), acetylcholine (Ach; cat no. CK-E30422) and
histamine (His; cat no. CK-E30476) in the hypothalamus and/or serum
were measured by enzyme-linked immune sorbent assay (ELISA) kits
(Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China),
following the manufacturer's protocol. His and Ach levels were
measured in the serum but not in the hypothalamus. Experiments were
performed twice independently.
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. Statistical significance was determined by one-way
analysis of variance followed by Dunn's test using SPSS software
version 16.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Antidepressant-like activities of
PTNE
CUMS treatment significantly reduced final
bodyweights (P<0.001; Fig. 1A),
increased immobility time in FST (P<0.001; Fig. 1B) and the paw withdrawal latency in
hotplate test (P<0.001; Fig.
1C), and reduced exhaustive time in FRT (P<0.001; Fig. 1D), compared with CTRL rats.
Compared with untreated CUMS model rats, rats in the PTNE and Flu
groups exhibited significantly higher final bodyweights (P<0.01;
Fig. 1A), reduced immobility time
in FST (P<0.01; Fig. 1B),
suppressed paw withdrawal latency in hotplate test (P<0.05;
Fig. 1C) and enhanced exhaustive
time in FRT (P<0.01; Fig. 1D),
which suggested antidepressant-like activities of PTNE in
CUMS-induced depression-like rats.
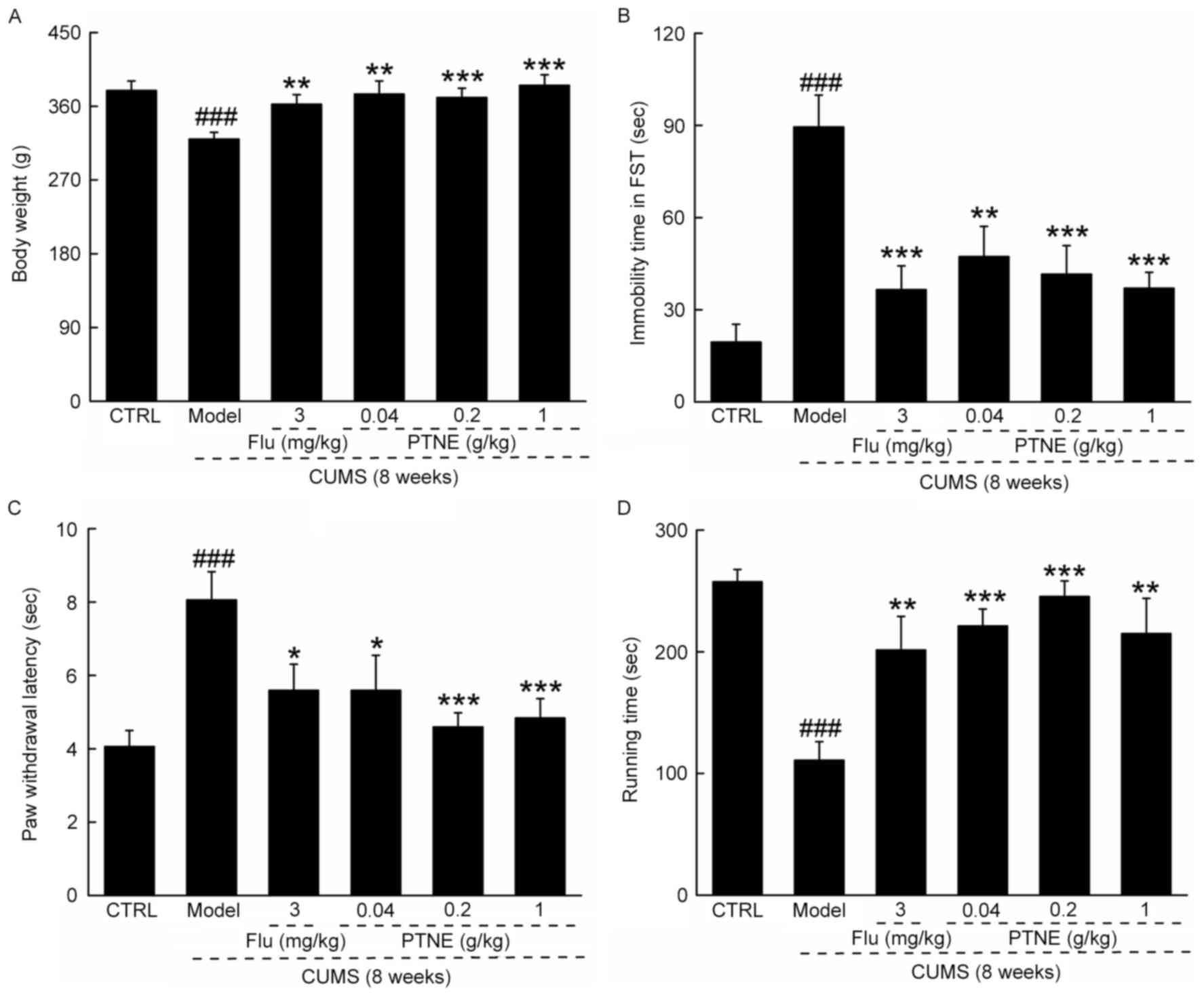 | Figure 1.PTNE exhibited anti-depression-like
effects in a CUMS-induced rat model. CUMS-induced depression-like
rats were treated with PTNE (0.04, 0.2 and 1.0 g/kg) and Flu (3
mg/kg) orally for four weeks. Compared with untreated CUMS model
rats, those treated with Flu or the various concentrations of PTNE
exhibited (A) higher bodyweight, (B) reduced immobility time in
FST, (C) reduced paw withdrawal latency time in the hotplate test,
and (D) increased running time in the forced running test compared
with untreated CUMS rats. Data are presented as the mean ± standard
deviation of the mean (n=10) and analyzed by a one-way analysis of
variance followed by Dunn's test. ###P<0.001 vs. CTRL
rats; *P<0.05, **P<0.01 and ***P<0.001 vs. untreated CUMS
rats. CTRL, control; CUMS, chronic unpredictable mild stress; Flu,
fluoxetine hydrochloride; FST, forced swimming test; PTNE,
Paecilomyces tenuipes N45 water extract. |
PTNE regulates neurotransmitter and
metabolite levels in serum and hypothalamus
Significant reductions in the serum levels of 5-HT,
5-HIAA, DA, DOPAC, NE, Ach and His were observed in the untreated
CUMS model rats compared with CTRL rats (P<0.05; Table I). Flu treatment resulted in a
significant increase in the serum concentrations of the
neurotransmitters and metabolites in depression-like rats
(P<0.05, Table I). CUMS model
rats treated with PTNE exhibited similar results as rats treated
with Flu: PTNE treatment, particularly at doses of 0.2 and 1.0
g/kg, increased the serum levels of the neurotransmitters and the
metabolites to healthy CTRL-like conditions (P<0.05; Table I). However, PTNE at 0.04 g/kg
failed to significantly influence the levels of DA, DOPAC, Ach and
His, while PTNE at 0.2 g/kg failed to significantly reverse the
abnormal levels of 5-HIAA and Ach in the serum of CUMS rats
(Table I).
 | Table I.The effects of Flu and PTNE treatment
on neurotransmitter and metabolite levels in the serum of
CUMS-induced depression-like rats. |
Table I.
The effects of Flu and PTNE treatment
on neurotransmitter and metabolite levels in the serum of
CUMS-induced depression-like rats.
| Group | Conc. | 5-HT (ng/ml) | 5-HIAA (ng/ml) | DA (pg/ml) | DOPAC (ng/ml) | NE (ng/ml) | Ach (pg/ml) | His (ng/ml) |
|---|
| CTRL | N/A | 12.2±1.2 | 4.8±0.5 | 122.1±11.5 | 29.0±1.9 | 8.8±0.7 | 628.4±52.4 | 39.9±2.7 |
| CUMS | N/A |
8.32±1.1a |
3.3±0.3a |
89.3±6.0a |
20.1±1.9a |
6.9±0.2a |
461.7±27.8a |
26.5±3.3a |
| Flu (mg/kg) | 3.0 |
13.2±1.1c |
4.7±0.4b |
127.3±14.6b |
27.9±1.2b |
8.8±0.4c |
584.7±38.7b |
34.4±2.9b |
| PTNE (g/kg) | 0.04 |
13.8±1.5c |
4.3±0.3b | 76.0±13.3 | 25.2±2.2 |
8.4±0.3b | 428.9±26.9 | 32.8±4.9 |
|
| 0.2 |
11.8±1.2b | 3.4±0.2 |
119.3±6.2b |
29.7±3.1b |
8.4±0.5b | 581.9±81.9 |
41.5±3.2b |
|
| 1.0 | 11.3±1.3 |
4.2±0.24b |
121.5±10.3b |
30.8±2.4b |
9.9±1.0b |
680.3±75.4b |
40.5±2.6b |
Consistent with the effects on serum levels, a
significant reduction was observed in the levels of 5-HT, 5-HIAA,
DA, DOPAC and NE in the hypothalami of CUMS-induced depression-like
rats (P<0.05; Table II),
compared with CTRL rats. Certain Flu and PTNE treatments
significantly increased the CUMS-inhibited neurotransmitter and
metabolite levels in the hypothalamus of depression-like rats
(P<0.05; Table II). PTNE at a
dose of 1.0 g/kg was the only treatment for which a significant
increase in the level of hypothalamic DA concentration was
identified (P<0.01; Table
II).
 | Table II.The effects of Flu and PTNE on
neurotransmitter and metabolite levels in the hypothalamus of
CUMS-induced depression-like rats. |
Table II.
The effects of Flu and PTNE on
neurotransmitter and metabolite levels in the hypothalamus of
CUMS-induced depression-like rats.
| Group | Conc. | 5-HT (ng/g) | 5-HIAA (ng/g) | DA (pg/g) | DOPAC (ng/g) | NE (ng/g) |
|---|
| CTRL | N/A | 183.0±8.4 | 35.2±4.4 | 2,003.2±99.9 | 348.3±27.2 | 145.5±3.0 |
| CUMS | N/A |
148.2±12.6a |
23.7±2.2a |
1,726.4±61.8a |
255.9±25.1a |
105.6±2.9b |
| Flu (mg/kg) | 3 |
187.5±11.7c |
33.3±2.9c | 1,710.4±145.5 |
323.8±14.8c |
131.6±5.0e |
| PTNE (g/kg) | 0.04 |
190.3±15.1c | 19.3±2.0 | 1,596.1±180.9 | 258.1±22.2 |
146.6±4.6e |
|
| 0.2 |
203.0±13.3c |
34.4±3.7c | 1,944.3±137.2 |
350.2±28.6c |
144.3±6.1e |
|
| 1.0 |
193.1±7.3c |
32.7±3.4c |
2,105.0±97.7d |
315.5±7.2c |
140.6±6.2e |
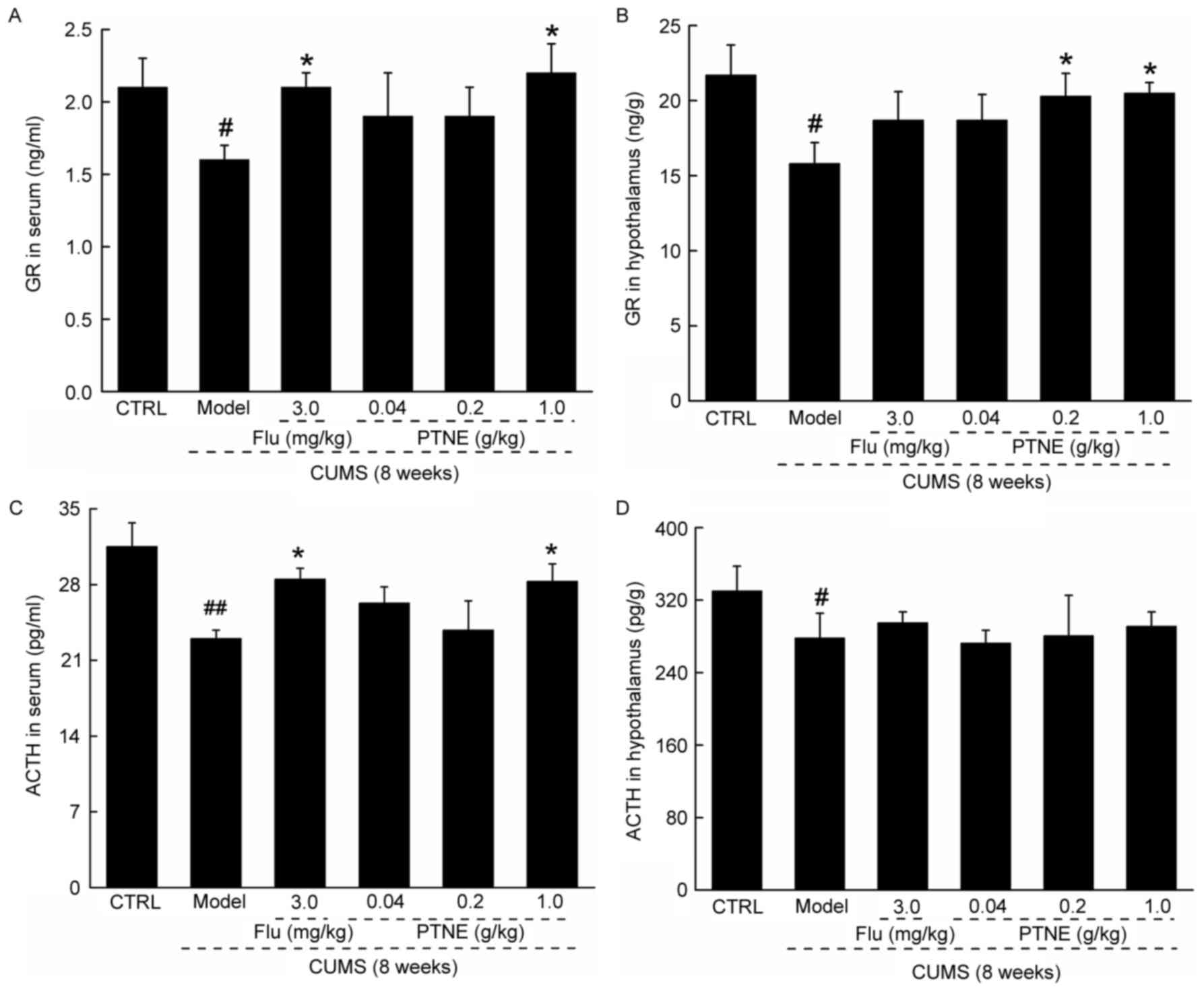
PTNE regulates hormone levels in serum
and hypothalamus
Untreated CUMS model rats exhibited significantly
reduced levels of GR and ACTH in both serum and hypothalamus
compared with CTRL rats (P<0.05; Fig. 2). Flu and 1.0 g/kg PTNE treatments
resulted in an increase in the levels of GR and ACTH in the serum
compared with untreated CUMS rats (P<0.05; Fig. 2A and C). However, only 0.2 and 1.0
g/kg PTNE treatment was able to increase GR levels in the
hypothalamus (P<0.05; Fig. 2B);
whereas neither Flu nor PTNE treatments were able to affect ACTH
levels in the hypothalamus in CUMS rats (Fig. 2D).
Discussion
Depression has been recognized as one of the most
prevalent disorders worldwide and results in significant social
burden (4). Depression-like
behaviors can be successfully produced in rats through
environmental manipulations or by genetic and/or pharmacological
factors (19). When exposed to
CUMS, rats exhibit behavioral disturbances and neurobiological
changes that are similar to the symptoms of patients with
depression (20); thus,
CUMS-induced depression-like model rats have been widely used to
evaluate the antidepressive effects of a wide range of drug
treatments (21). The present
study investigated the antidepressant-like effects of PTNE
treatment on CUMS model rats. Exercise promotes physical health,
and has been reported to be an efficient antidepressant in patients
with depression (22). A previous
study associated depression-like phenotypes in rats with mechanical
allodynia and transient thermal hyperalgesia in the hotplate test,
and suggested that nociceptive responding may be used as an
alternative method to assess depression-like behaviors (23). FST is a classical model that is
used for measuring behavioral despair and antidepressant response
(24). In the present study,
PTNE-treated CUMS rats exhibited observable antidepressant effects
in FST, hotplate test and forced running test, which led to the
exploration of the underlying mechanisms of PTNE on depression-like
behavior.
Compared with untreated CUMS rats, PTNE treatment
was able to restore the levels of the neurotransmitters in the
serum and hypothalamus to a normal standard, particularly the
monoamines. In the monoamine hypothesis, depression is connected
with the metabolic turnover of DA and 5-HT in the brain (25). 5-HT was reported to regulate
various functions within the central nervous system, including
impulsivity and mood (26). A
recent clinical trial suggested that the concentration of platelet
5-HT1A receptors may serve as a diagnostic biomarker for depression
(27). Another study reported that
a depression-like mouse model was successfully established through
the genetic deletion of 5-HT transporter in mice (28). Notably, in the present study, PTNE
treatment increased the low levels of 5-HT and 5-HIAA in serum and
hypothalamus of depression-like rats. In addition, DA was revealed
to be the most abundant catecholaminergic neurotransmitter in the
brain, and was implicated in the development of stress-related
disorders (29). Consistent with
the monoamine hypothesis, a deficit of DA and/or DA metabolites in
the brain has been noted in patients with depression (30). Certain antidepressant treatments
have been reported to exhibit pharmacological effects related to
the modulation of DA functions by increasing the expression of DA
receptors or DA levels in the serum and hypothalamus (31,32).
In the present study, PTNE treatment not only increased the levels
of DA in the serum and hypothalamus of CUMS model rats, but also
induced an increase in the levels of NE and DOPAC. NE is another
catecholaminergic neurotransmitter that has previously been
reported to be involved in mood control (33). Based on the present data, 5-HT and
DA may be involved in the antidepressant-like effects of PTNE
treatment in CUMS-induced model rats; however, further
experimentation is required. The neurotransmitters Ach and His were
also examined in the present study, and PTNE treatment was noted to
increase their expression levels in CUMS rats. One reported
hypothesis suggested that learning difficulties may be explained by
the lack of Ach expression in patients with Alzheimer's disease
(34). His was demonstrated to act
as a modulator in the brain that affects the action of other
neurotransmitters (35).
Extracellular recordings revealed that His can either excite or
depress neuronal activity in different regions of the brain
(36). The present study
investigated the roles of Ach and His associated with the
anti-depressant-like effects of PTNE treatment in CUMS rats, and it
was demonstrated that PTNE (1 g/kg) treatment significantly
increased the levels of Ach and His in the serum of rats compared
with CUMS-induced model rats.
Preclinical and clinical studies have revealed that
hyperactivity of the HPA axis may be another potential component in
the pathophysiology of depression (37). In patients with depression,
increased levels of serum ACTH and cortisol indicated an important
role for the HPA axis (38).
Antidepressants have been reported to ameliorate neurobiological
disturbances, including HPA axis hyperactivity, partly by restoring
GR function (39). Restraint
stress affects the HPA axis and regulates monoamine
neurotransmitters in brain (40).
Icariin, extracted from Herba Epimedii, displayed
antidepressant-like activity in the social defeat mouse model by
regulating the function of the HPA axis, particularly by
upregulating the levels of GR (41). Similarly, PTNE enhanced the levels
of ACTH and GR in serum and hypothalamus of CUMS rats, which
indicates that its antidepressant-like effect may be associated
with the normalization of HPA axis activity. Compared with other
synthetic chemical antidepressants, PTNE is an herbal medicine that
contains multi-effective components (42), which may target many molecules such
as neurotransmitters. The ‘systemic targeting’ of PTNE, including
action in the modulation of neurotransmitters, metabolites and
hormones, may relieve depression-like behaviors in a more natural
way compared with synthetic antidepressants, so that less adverse
side effects would be expected.
In conclusion, the present study demonstrated that
PTNE possesses antidepressant-like activities, which may function
by regulating the levels of neurotransmitters, metabolites and
hormones. The data provided experimental evidence in support of the
potential use of PTNE against major depression.
Acknowledgements
The present study was supported by the ‘Twelfth
Five-Year’ Science and Technology Planning Project of Jilin
Province in China (grant no. 2014B033), and The Science and
Technology Development Program of Jilin Province in China (grant
no. 20160520036JH).
References
|
1
|
Kessler RC: The costs of depression.
Psychiat Clin N Am. 35:12012. View Article : Google Scholar
|
|
2
|
Mathers CD and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PLoS Med.
3:e4422006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luppa M, Heinrich S, Angermeyer MC, König
HH and Riedel-Heller SG: Cost-of-illness studies of depression: A
systematic review. J Affect Disord. 98:29–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krishnan V and Nestler EJ: The molecular
neurobiology of depression. Nature. 455:894–902. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Li L, Shen LL, Qian Y, Cao YX and
Zhu DN: Changes of adrenomedullin and its receptor components mRNAs
expression in the brain stem and hypothalamus-pituitary-adrenal
axis of stress-induced hypertensive rats. Sheng Li Xue Bao.
56:723–729. 2004.PubMed/NCBI
|
|
6
|
Sartori SB, Whittle N, Hetzenauer A and
Singewald N: Magnesium deficiency induces anxiety and HPA axis
dysregulation: Modulation by therapeutic drug treatment.
Neuropharmacology. 62:304–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou XJ, Liu M, Yan JJ, Cao Y and Liu P:
Antidepressant-like effect of the extracted of Kai Xin San, a
traditional Chinese herbal prescription, is explained by modulation
of the central monoaminergic neurotransmitter system in mouse. J
Ethnopharmacol. 139:422–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Z, Ji W, Qu R, Wang M, Yang W, Zhan Z,
Fu Q and Ma S: Metabonomic study on the antidepressant-like effects
of banxia houpu decoction and its action mechanism. Evid Based
Complement Alternat Med. 2013:2137392013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang A, Hao H, Zheng X, Liang Y, Xie Y,
Xie T, Dai C, Zhao Q, Wu X, Xie L and Wang G: Peripheral
anti-inflammatory effects explain the ginsenosides paradox between
poor brain distribution and anti-depression efficacy. J
Neuroinflammation. 8:1002011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui M, Li Q, Zhang M, Zhao YJ, Huang F and
Chen YJ: Long-term curcumin treatment antagonizes masseter muscle
alterations induced by chronic unpredictable mild stress in rats.
Arch Oral Biol. 59:258–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thachil AF, Mohan R and Bhugra D: The
evidence base of complementary and alternative therapies in
depression. J Affect Disorders. 97:23–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DH, Park T and Kim HW: Induction of
apoptosis by disturbing mitochondrial-membrane potential and
cleaving PARP in Jurkat T cells through treatment with
acetoxyscirpenol mycotoxins. Biol Pharm Bull. 29:648–654. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kan H, Ming L, Li C, Kan H, Sun B and
Liang Y: Antidepressant effect of bioactive compounds from
Paecilomyces tenuipes in mice and rats. Neural Regen Res.
5:1568–1572. 2010.
|
|
14
|
Yin YY, Ming L, Zheng LF, Kan HW, Li CR
and Li WP: Bioactive compounds from Paecilomyces tenuipes
regulating the function of the hypothalamo-hypophyseal system axis
in chronic unpredictable stress rats. Chin Med J (Engl).
120:1088–1092. 2007.PubMed/NCBI
|
|
15
|
Jiang Y, Qi X, Gao K, Liu W, Li N, Cheng
N, Ding G, Huang W, Wang Z and Xiao W: Relationship between
molecular weight, monosaccharide composition and immunobiologic
activity of Astragalus polysaccharides. Glycoconj J. 33:755–761.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li SP, Yang FQ and Tsim KW: Quality
control of Cordyceps sinensis, a valued traditional Chinese
medicine. J Pharm Biomed Anal. 41:1571–1584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakagawasai O, Yamada K, Nemoto W,
Fukahori M, Tadano T and Tan-No K: Liver hydrolysate assists in the
recovery from physical fatigue in a mouse model. J Pharmacol Sci.
123:328–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Espejo EF and Mir D: Structure of the
rat's behaviour in the hot plate test. Behav Brain Res. 56:171–176.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Overstreet DH and Steiner M: Genetic and
environmental models of stress-induced depression in rats. Stress
Med. 14:261–268. 1998. View Article : Google Scholar
|
|
20
|
Ji WW, Li RP, Li M, Wang SY, Zhang X, Niu
XX, Li W, Yan L, Wang Y, Fu Q and Ma SP: Antidepressant-like effect
of essential oil of Perilla frutescens in a chronic, unpredictable,
mild stress-induced depression model mice. Chin J Nat Med.
12:753–759. 2014.PubMed/NCBI
|
|
21
|
Yan WJ, Tan YC, Xu JC, Tang XP, Zhang C,
Zhang PB and Ren ZQ: Protective effects of silibinin and its
possible mechanism of action in mice exposed to chronic
unpredictable mild stress. Biomol Ther (Seoul). 23:245–250. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bjørnebekk A, Mathé AA and Brené S:
Running has differential effects on NPY, opiates, and cell
proliferation in an animal model of depression and controls.
Neuropsychopharmacology. 31:256–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burke NN, Hayes E, Calpin P, Kerr DM,
Moriarty O, Finn DP and Roche M: Enhanced nociceptive responding in
two rat models of depression is associated with alterations in
monoamine levels in discrete brain regions. Neuroscience.
171:1300–1313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Detke MJ, Rickels M and Lucki I: Active
behaviors in the rat forced swimming test differentially produced
by serotonergic and noradrenergic antidepressants.
Psychopharmacology (Berl). 121:66–72. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steru L, Chermat R, Thierry B and Simon P:
The tail suspension test: A new method for screening
antidepressants in mice. Psychopharmacology (Berl). 85:367–370.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohammad-Zadeh LF, Moses L and
Gwaltney-Brant SM: Serotonin: A review. J Vet Pharmacol Ther.
31:187–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang ZJ, Wang D, Man SC, Ng R, McAlonan
GM, Wong HK, Wong W, Lee J and Tan QR: Platelet 5-HT(1A) receptor
correlates with major depressive disorder in drug-free patients.
Prog Neuropsychopharmacol Biol Psychiatry. 53:74–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gobbi G, Murphy DL, Lesch KP and Blier P:
Modifications of the serotonergic system in mice lacking serotonin
transporters: An in vivo electrophysiological study. J Pharmacol
Exp Ther. 296:987–995. 2001.PubMed/NCBI
|
|
29
|
Chrousos GP: Stress and disorders of the
stress system. Nat Rev Endocrinol. 5:374–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lambert G, Johansson M, Agren H and
Friberg P: Reduced brain norepinephrine and dopamine release in
treatment-refractory depressive illness: Evidence in support of the
catecholamine hypothesis of mood disorders. Arch Gen Psychiatry.
57:787–793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Giovanni G, Strac D Svob, Sole M,
Unzeta M, Tipton KF, Mück-Šeler D, Bolea I, Corte L Della, Perkovic
M Nikolac, Pivac N, et al: Monoaminergic and histaminergic
strategies and treatments in brain diseases. Front Neurosci.
10:5412016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ainsworth K, Smith SE, Zetterström TS, Pei
Q, Franklin M and Sharp T: Effect of antidepressant drugs on
dopamine D1 and D2 receptor expression and dopamine release in the
nucleus accumbens of the rat. Psychopharmacology (Berl).
140:470–477. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pirke KM: Central and peripheral
noradrenalin regulation in eating disorders. Psychiat Res.
62:43–49. 1996. View Article : Google Scholar
|
|
34
|
Gareri P, Castagna A, Cotroneo AM,
Putignano D, Conforti R, Santamaria F, Marino S and Putignano S:
The Citicholinage Study: Citicoline plus cholinesterase inhibitors
in aged patients affected with Alzheimer's disease study. J
Alzheimers Dis. 56:557–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brown RE, Stevens DR and Haas HL: The
physiology of brain histamine. Prog Neurobiol. 63:637–672. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geller HM, Springfield SA and Tiberio AR:
Electrophysiological actions of histamine. Can J Physiol Pharmacol.
62:715–719. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deuschle M, Bode L, Schnitzler P,
Meyding-Lamadé U, Plesch A, Ludwig H, Hamann B and Heuser I:
Hypothalamic-pituitary-adrenal (HPA) system activity in depression
and infection with Borna disease virus and Chlamydia pneumoniae.
Mol Psychiatry. 8:469–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Varghese FP and Brown ES: The
hypothalamic-pituitary-adrenal axis in major depressive disorder: A
brief primer for primary care physicians. Prim Care Companion J
Clin Psychiatry. 3:151–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mason BL and Pariante CM: The effects of
antidepressants on the hypothalamic-pituitary-adrenal axis. Drug
News Perspect. 19:603–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bao L, Yao XS, Zhao L, Lu YQ and Kurihara
H: Correlation between changes of central neurotransmitter
expression and stress response in mice-A restraint time-course
analysis. Neural Regeneration Res. 3:167–171. 2008.
|
|
41
|
Wu J, Du J, Xu C, Le J, Xu Y, Liu B and
Dong J: Icariin attenuates social defeat-induced down-regulation of
glucocorticoid receptor in mice. Pharmacol Biochem Behav.
98:273–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song J, Xing G, Cao J and Teng L, Li C,
Meng Q, Lu J, Zhou Y, Liu Y, Wang D and Teng L: Investigation of
the antidepressant effects of exopolysaccharides obtained from
Marasmius androsaceus fermentation in a mouse model. Mol Med Rep.
13:939–946. 2016.PubMed/NCBI
|