Introduction
Alzheimer's disease (AD) is a chronic
neurodegenerative disorder, that results in the death of nerve
cells, deterioration of cognitive function and eventual mortality
as a result of complications (1).
Furthermore, AD is the most common cause of dementia in older
adults with loss of cognitive functions and memory (2). The exact cause of AD is poorly
understood, although increasing age, family history and genetics,
head injuries and hypertension are recognized as primary risk
factors (3,4). In the past several decades, attempts
to uncover the underlying mechanism of AD pathogenesis and to
further translate these findings into the clinic have been made
(5). However, there are no
treatments that stop or reverse its progression, and the current
therapeutic strategies only temporarily improve symptoms (2,5).
Therefore, an improved understanding of AD may facilitate the
diagnosis or treatment of this devastating disease.
Aluminum (Al) is the third most abundant element in
the earth's crust (6). It has been
implicated as an etiologic factor in various neuronal diseases,
particularly in AD (7–9). Foci of Al have been detected in the
neurofibrillary tangle of AD patients (10) and Al has been reported as
neurotoxic in the central nervous system (11,12).
Maltolate (Malt) is a common component of the human diet, contained
in coffee, soybeans, baked cereals, and caramelized and browned
foods (13). Malt has been
reported to be a strong enhancer of Al accumulation in the brain.
Furthermore, numerous studies have demonstrated that,
aluminum-maltolate (Al-Malt), the complex of Al and Malt, is a
potent neurotoxin. Furthermore, in vitro and in vivo
investigations have elucidated that apoptosis induced by Al-Malt
was the major cause of neurotoxicity (12,14).
Therefore, inhibition of Al-Malt-induced apoptosis may present a
novel strategy to hinder the progression of AD.
MicroRNAs (miRNAs), a class of small non-coding
transcripts, regulate gene expression in various physiological and
pathological conditions (15).
Numerous studies have shown that miRNAs have been used to decode
different signaling pathways associated with various types of
disease (16). For example,
certain miRNAs, such as miR-9 and miR-125b, have been utilized as
biomarkers for the diagnosis of early AD (17). Furthermore, various miRNAs have
been found to be associated with the pathogenesis of AD, and to
affect the expression levels and functions of AD-relevant molecules
(18). The role of miRNAs in
neuronal apoptosis in AD has previously been investigated. Absalon
et al (19) demonstrated
that miR-26b activated apoptosis in post-mitotic neurons, and
inhibition of miR-26b was a novel strategy for neuroprotection.
Zhang et al (20) found
that inhibition of miR-16 in the cellular AD model with primary
hippocampal neurons increased apoptosis. However, whether various
miRNAs, including miR-322, are involved in Al-Malt-induced
apoptosis in neural cells, has not yet been well investigated.
Therefore, the aim of the present study was to
investigate the role of miR-322 in Al-Malt-induced apoptosis in
neural cells. The human SH-SY5Y neuroblastoma cell line was used to
induce apoptosis in vitro by treatment with eight
concentrations of Al-Malt. Cell viability, apoptosis and its
associated factors were determined to investigate the role of
Al-Malt in neural cell apoptosis and its associated mechanism.
Subsequently, cells were transfected with miR-322 mimics and/or
treated with Al-Malt, and the changes in cell viability, apoptosis
and the associated factors were measured again to reveal the role
of miR-322 in Al-Malt-induced apoptosis. These findings may provide
a basic understanding of the mechanism of Al-Malt-associated
neurodegenerative pathogenesis.
Materials and methods
Cell culture
The human SH-SY5Y neuroblastoma cell line, a cell
line that is commonly used for researching neurotoxicity and
neurodegenerative diseases (14),
was purchased from the American Type Culture Collection (Manassas,
VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; GE Healthcare Life Sciences) and
maintained in a humidified atmosphere at 37°C with 5%
CO2 (21).
Al-Malt preparation
Al-Malt was prepared as previously described in
detail (22). Al-Malt was
dissolved in DMEM containing 10% FBS (12) to establish eight concentrations of
Al-Malt (0, 0.01, 0.05, 0.1, 0.25, 0.5, 1 and 2 mM). Prior to
Al-Malt treatment, the eight Al-Malt concentrations were adjusted
to a pH of 7.4 and filtered through a 0.22-μm pore filter.
Cell viability
Cell viability was assessed using the
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, cells were seeded in 96-well plates at a density of
2×103 cells/well. After 12 h of incubation at 37°C,
cells were treated with the eight concentrations of Al-Malt (0–2
mM) for 3 days. Al-Malt with 0 mM served as a blank control.
Subsequently, 0.5 mg/ml MTT solution (Sigma Aldrich; Merck KGaA,
Darmstadt, Germany) was added to each well, and incubated for
another 3 h at 37°C. Finally, formazan was dissolved in dimethyl
sulfoxide (DMSO) and the absorbance was recorded with a Multiskan
EX (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a
wavelength of 570 nm (23).
Cell apoptosis
Cell apoptosis was evaluated using an Annexin
V/fluorescein isothiocyanate (FITC) and propidium iodide (PI)
apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA).
Briefly, cells were pre-treated with 0.25, 0.5 and 1 mM
concentrations of Al-Malt for 3 days at 37°C, and cells were
collected and suspended in Annexin-binding buffer. Cells were first
stained with Annexin V-FITC for 30 min, and subsequently stained
with PI for 10 min, in the dark at room temperature. The percentage
of apoptotic cells was immediately assessed by flow cytometry (BD
Biosciences).
Western blot analysis
Following 3 days of Al-Malt treatment, cellular
protein was extracted using lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and equal quantities of protein
samples (100 µg) were separated on 10–12% sodium dodecyl
sulfate-polyacrylamide gels and transferred to nitrocellulose
membranes (Whatman GmbH, Dassel, Germany). After blocking with 5%
skimmed milk for 1 h at room temperature, the membranes were
incubated with the appropriate primary antibodies (all at a
dilution of 1:1,000) overnight at 4°C. Anti-Actin (no. 8457) and
anti-Bcl-2-associated X protein (Bax) (no. 5023) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-V-Myc
avian myelocytomatosis viral oncogene homolog (c-Myc) (ab32072),
anti-caspase-3 (ab13585) and anti-cleaved caspase-3 (ab32042) were
obtained from Abcam (Cambridge, USA). Actin served as a loading
control. Subsequently, the membranes were incubated with a
horseradish peroxidase-conjugated secondary antibody (anti-mouse
IgG, ab6728; anti-rabbit IgG, ab6721) (Abcam) diluted 1:2,000, for
2 h at room temperature. Protein bands were visualized using the
WEST-ZOL-plus Western Blot Detection System (Intron Biotechnology,
Inc., Seongnam, Korea). Optical density was calculated by ImageJ
software version 1.49 (National Institutes of Health, Bethesda, MD,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol
reagent-phenol chloroform (Thermo Fisher Scientific, Inc.), and
synthesis of cDNA was performed using a Transcriptor First Strand
cDNA Synthesis kit (Roche Applied Science, Madison, WI, USA),
according to the manufacturer's instructions. FastStart Universal
SYBR-Green Master (ROX) (Roche Applied Science) was used for the
analysis, and 20-µl reaction mixtures were performed on an ABI
PRISM 7500 Real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The results were analyzed using the
2−ΔΔCq method (24),
and were normalized to U6 snRNA. The miRNA primers were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China).
Cell transfection
Cells were seeded in 6-well plates at a density of
5×104/well. After reaching ~60% confluence, cells were
transfected with miR-322 mimic or control (Shanghai GenePharma Co.,
Ltd.) using Lipofectamine RNAiMAX reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After a 48-h transfection, cells were collected for subsequent
experimentation.
Statistical analysis
All data are presented as the mean ± standard
deviation from three independent experiments and analyses.
Statistical analyses were performed using SPSS version 13.0 (SPSS,
Inc., Chicago, IL, USA), and differences were analyzed by one-way
analysis of variance followed by Dunnett's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
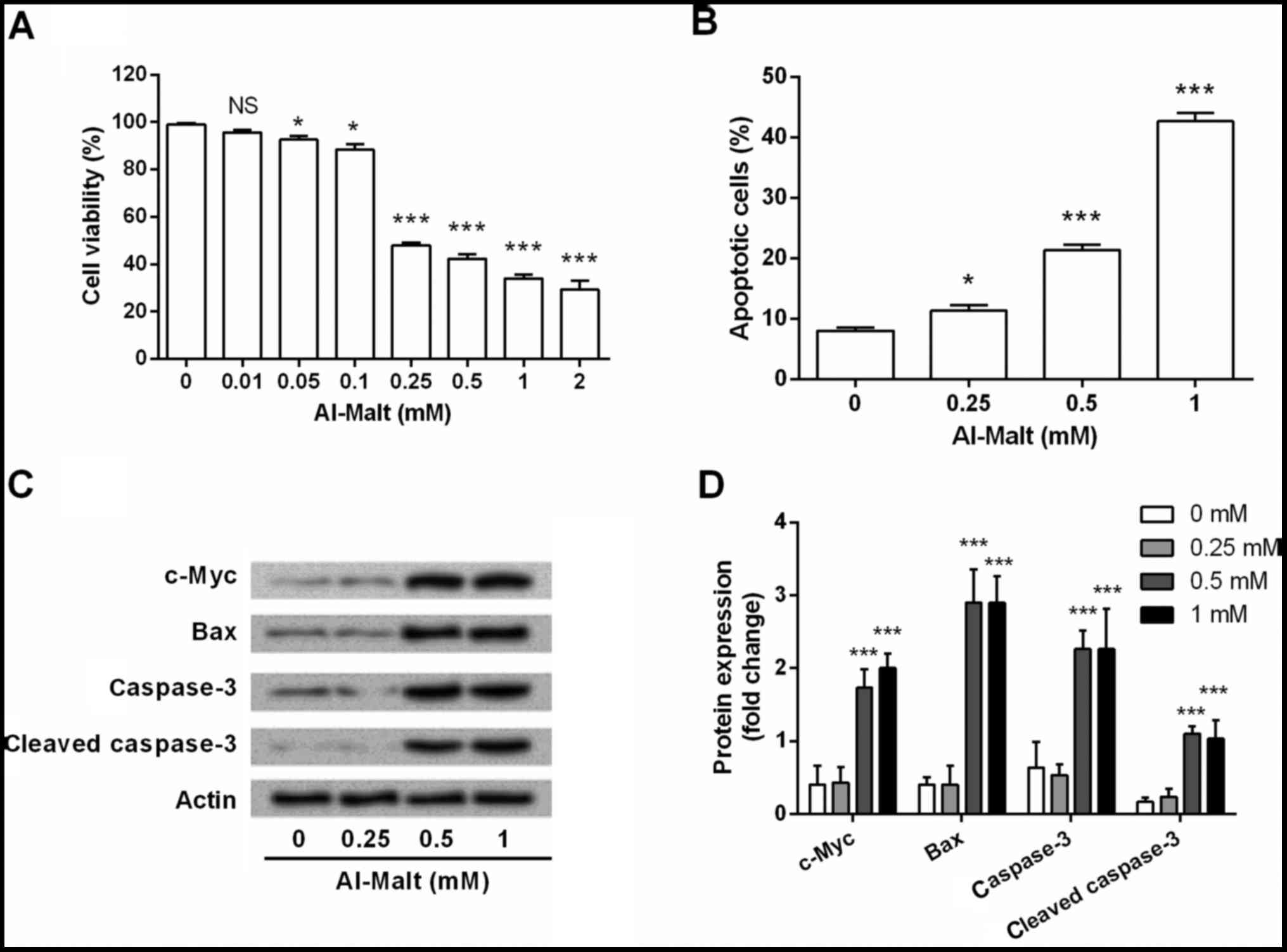
Al-Malt induced cell apoptosis by
regulating the expression levels of c-Myc
To investigate the functional role of Al-Malt in
neural cell apoptosis, eight concentrations of Al-Malt (0–2 mM)
were prepared and used for treating SH-SY5Y cells. After 3 days of
treatment, cell viability and apoptosis were determined by MTT
assay (Fig. 1A) and flow cytometry
(Fig. 1B). Al-Malt was
demonstrated to significantly decrease cell viability (P<0.05
and P<0.001) and increase apoptosis (P<0.05 and P<0.001),
compared with the control group. Higher concentrations of Al-Malt
led to stronger cell viability inhibitive and apoptosis inductive
effects. These results revealed that Al-Malt induced cell apoptosis
in a dose-dependent manner. In addition, three of the higher
concentrations of Al-Malt (0.25, 0.5 and 1 mM) were selected for
the further investigation.
To investigate the possible underlying mechanism of
Al-Malt-induced apoptosis, the expression changes of c-Myc, Bax,
caspase-3 and cleaved caspase-3 in cells were evaluated by western
blotting. As shown in Fig. 1C and
D, the protein expression levels of c-Myc, Bax, caspase-3 and
cleaved caspase-3 were markedly upregulated by 0.5 and 1 mM
concentrations of Al-Malt (all P<0.001). Therefore, it was
inferred that Al-Malt induced SH-SY5Y cell apoptosis via regulating
c-Myc, Bax and caspase-3.
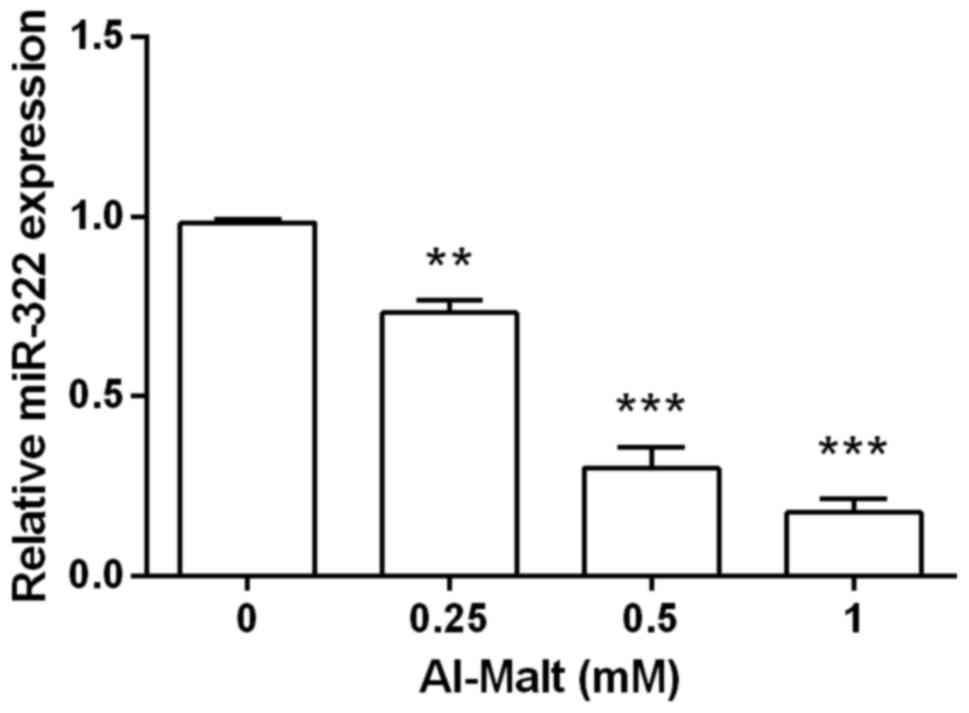
MiR-322 was negatively regulated by
Al-Malt
To establish whether miR-322 was involved in the
apoptosis induced by Al-Malt, SH-SY5Y cells were pre-treated with
Al-Malt, and the level of miR-322 mRNA expression was evaluated by
RT-PCR analysis (Fig. 2). Compared
with the control group, the mRNA level of miR-322 expression was
significantly downregulated by Al-Malt (P<0.01 and P<0.001).
Furthermore, a higher concentration of Al-Malt exerted a stronger
suppressive effect on miR-322. Thus, it was inferred that miR-322
may be a pivotal gene in Al-Malt-induced apoptosis, and miR-322 may
be negatively regulated by Al-Malt. In addition, a 1-mM
concentration of Al-Malt was selected for the following
investigation.
MiR-322 attenuated Al-Malt-induced
apoptosis by recovering the expression changes of c-Myc
To determine the role of miR-322 in Al-Malt-induced
apoptosis, cells were transfected with miR-322 mimic and/or treated
with 1 mM Al-Malt. Cell viability and apoptosis were subsequently
measured by MTT assay (Fig. 3A)
and flow cytometry (Fig. 3B). As
expected, Al-Malt significantly inhibited cell viability
(P<0.001) and promoted apoptosis (P<0.001), whereas miR-322
overexpression exhibited the completely opposite effect (All
P<0.05). Notably, miR-322 overexpression significantly
attenuated the inhibitive cell viability and inductive apoptotic
effects of Al-Malt (P<0.01 and P<0.001). Therefore, it was
proposed that miR-322 attenuated Al-Malt-induced apoptosis.
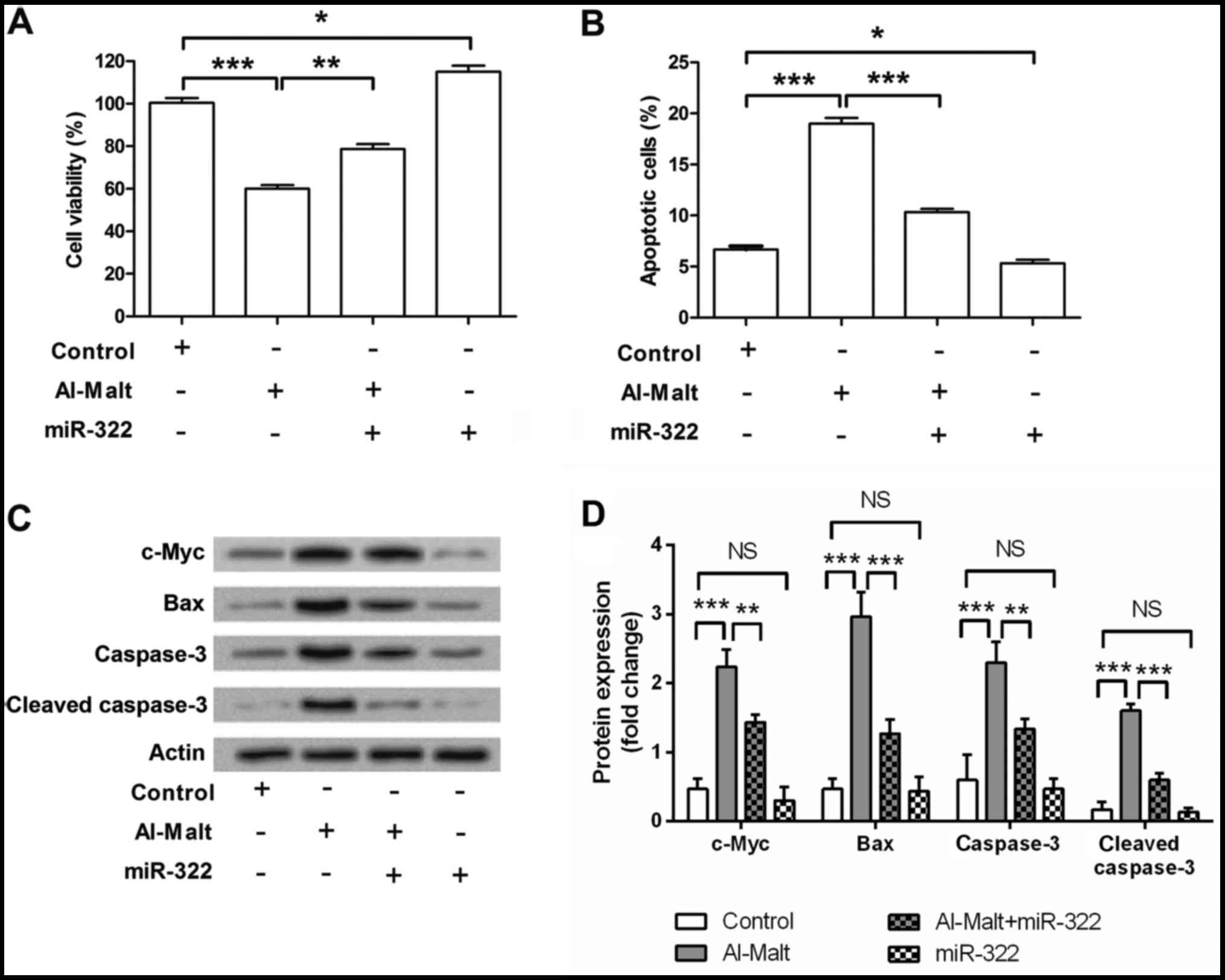 | Figure 3.miR-322 attenuated Al-Malt-induced
apoptosis by recovering the expression level changes of c-Myc.
SH-SY5Y cells were transfected with miR-322 mimic and/or treated
with 1 mM Al-Malt, and the cell viability, apoptosis and expression
level changes of its associated factors were measured by (A)
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide assay,
(B) flow cytometry and (C) western blot analysis. (D) Quantitative
analysis of western blot results. Values represent the mean ±
standard deviation. *P<0.05, **P<0.01 and ***P<0.001. miR,
microRNA; Al-Malt, aluminum-maltolate; c-Myc, V-Myc avian
myelocytomatosis viral oncogene homolog; Bax, Bcl-2-associated X
protein; NS, no significance. |
To further investigate the possible underlying
mechanism of miR-322 attenuated Al-Malt-induced apoptosis, and
expression changes of c-Myc, Bax, caspase-3 and cleaved caspase-3
in SH-SY5Y cells were detected by western blotting. Al-Malt
markedly upregulated the expression levels of c-Myc, Bax, caspase-3
and cleaved caspase-3 (all P<0.001, Fig. 3C and D). miR-322 slightly
downregulated these four factors, however the downregulatory impact
of miR-322 on these proteins did not reach statistical significance
(P>0.05). However, miR-322 overexpression was able to ameliorate
Al-Malt-induced changes in the expression levels of these factors.
Therefore, miR-322 attenuated Al-Malt-induced apoptosis by
recovering the expression changes of these factors.
Discussion
miRNA research has become popular in AD studies,
although the role of miRNAs in Al-Malt-induced apoptosis remains
unclear. In the current study, the human neuroblastoma cell line,
SH-SY5Y was used to induce apoptosis by treatment with different
concentrations of Al-Malt for 3 days. It was identified that
Al-Malt induced cell apoptosis by regulating the expression levels
of c-Myc, Bax and caspase-3. Furthermore, Al-Malt negatively
regulated the expression level of miR-322. miR-322 attenuated the
apoptosis Al-Malt-induced by recovering the expression changes of
c-Myc, Bax and caspase-3.
As a potent neurotoxin, Al-Malt has been widely
investigated for its neurotoxicity in various types of nervous
system disease. Increasing evidence has demonstrated that Al-Malt
exerted its neurotoxic effects by inducing neural cell apoptosis.
Savory et al (25)
demonstrated that, in the hippocampus of rabbits, Al-Malt induced
apoptosis by perturbing the ratio of Bcl-2: Bax. More recently,
experiments in rat brain tissue samples showed that Al-Malt induced
neural cell apoptosis by altering the AKT/p53 signaling pathway
(14). Partly consistent with
these previous studies, the present findings indicate that Al-Malt
is a powerful neurotoxin for inducing neural cell apoptosis.
However, the current study provides a novel insight into the
mechanism by which Al-Malt induced apoptosis, demonstrating that
the progression of apoptosis was associated with expression level
changes of c-Myc.
The proto-oncogene, c-Myc is a Myc family member,
which performs a pivotal function in growth control,
differentiation and apoptosis (26). In the past several decades, the
role of c-Myc in apoptosis has been extensively investigated. c-Myc
has been reported as a vital factor in cell apoptosis induction.
For example, in interleukin (IL)-3-dependent myeloid cells, c-Myc
was demonstrated to induce or sensitize cells to apoptosis
(27). In rat fibroblasts,
deregulating the expression of c-Myc resulted in cell death by
apoptosis (28). Furthermore,
studies have established that the mitochondrial apoptotic pathway
participates in c-Myc-mediated apoptosis, and the pro-apoptotic
factor, Bax is also involved (26). Using a switchable mouse model of
c-Myc-induced apoptosis in pancreatic β-cells, c-Myc induced
apoptosis in vivo by regulating Bax (29). Notably, c-Myc amplifies apoptosis
signaling at the mitochondria by controlling the caspase feedback
amplification loop; a process of effector caspases, caspase-3 and
caspase-7, which activate caspase-8 (26). Taken together, c-Myc exerts a
pivotal role in apoptosis induction by regulating Bax and caspases.
In the current study, the expression levels of c-Myc, Bax,
caspase-3 and cleaved caspase-3 were markedly upregulated by
Al-Malt. Therefore, it was hypothesized that Al-Malt induced
apoptosis by regulating the expression level of c-Myc.
miR-322 is an miRNA located on chromosome X, which
is abundantly expressed in various types of species (30). miR-322 has previously been
investigated as a vital regulator in the cell cycle, and cell
proliferation, formation and differentiation (31,32).
However, the role of miR-322 in cell apoptosis has not been well
investigated. In a study by Cao et al (30) miR-322 served as an apoptosis
inductor in intestinal epithelial cells; silencing miR-322 together
with miR-503 resulted in the repression of apoptosis. However, Gu
et al (33) demonstrated
miR-322 as an apoptosis inhibitor in neural stem cells. miR-322 was
negatively regulated by maternal diabetes and high glucose, which
was implicated in high glucose-induced apoptosis by activating
caspases (33). The current
findings were partly consistent with the study by Gu et al
(33) that miR-322 overexpression
inhibited cell apoptosis. In addition, miR-322 was negatively
regulated by Al-Malt, and attenuated the apoptosis induced by
Al-Malt via recovering the expression level change of c-Myc.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that Al-Malt induced cell
apoptosis by upregulating protein expression levels of c-Myc.
miR-322 attenuates the apoptosis induced by Al-Malt, thus miR-322
may be involved in the pathogenesis of Al-Malt-associated AD.
Acknowledgements
The present study was supported by the fund for the
science and technology activities of excellent overseas students in
2015 (Ministry of Human Resources and Social Security; grant no.
008-0064) and the Basic and Clinical Research Project Foundation of
Capital Medical University (grant no. 303-01-007-0132).
References
|
1
|
Chopra K, Misra S and Kuhad A:
Neurobiological aspects of Alzheimer's disease. Expert Opin Ther
Targets. 15:535–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung HY, Choi BO, Jeong JH, Kong KA, Hwang
J and Ahn JH: Amyloid beta-mediated hypomethylation of heme
oxygenase 1 correlates with cognitive impairment in Alzheimer's
disease. PLoS One. 11:e01531562016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Puthiyedth N, Riveros C, Berretta R and
Moscato P: Identification of differentially expressed genes through
integrated study of Alzheimer's disease affected brain regions.
PLoS One. 11:e01523422016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scheltens P, Blennow K, Breteler MM, de
Strooper B, Frisoni GB, Salloway S and Van der Flier WM:
Alzheimer's disease. Lancet. 388:505–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng C, Zhou XW and Wang JZ: The dual
roles of cytokines in Alzheimer's disease: update on interleukins,
TNF-α, TGF-β and IFN-γ. Transl Neurodegener. 5:72016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lévesque L, Mizzen CA, McLachlan DR and
Fraser PE: Ligand specific effects on aluminum incorporation and
toxicity in neurons and astrocytes. Brain Res. 877:191–202. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wills MR and Savory J: Aluminium
poisoning: Dialysis encephalopathy, osteomalacia, and anaemia.
Lancet. 2:29–34. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garruto RM: Pacific paradigms of
environmentally-induced neurological disorders: Clinical,
epidemiological and molecular perspectives. Neurotoxicology.
12:347–377. 1991.PubMed/NCBI
|
|
9
|
Khachaturian ZS: Diagnosis of Alzheimer's
disease. Arch Neurol. 42:1097–1105. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perl DP and Brody AR: Alzheimer's disease:
X-ray spectrometric evidence of aluminum accumulation in
neurofibrillary tangle-bearing neurons. Science. 208:297–299. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sánchez-Iglesias S, Méndez-Alvarez E,
Iglesias-González J, Muñoz-Patiño A, Sánchez-Sellero I,
Labandeira-García JL and Soto-Otero R: Brain oxidative stress and
selective behaviour of aluminium in specific areas of rat brain:
Potential effects in a 6-OHDA-induced model of Parkinson's disease.
J Neurochem. 109:879–888. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson VJ, Kim SH and Sharma RP:
Aluminum-maltolate induces apoptosis and necrosis in neuro-2a
cells: Potential role for p53 signaling. Toxicol Sci. 83:329–339.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gralla EJ, Stebbins RB, Coleman GL and
Delahunt CS: Toxicity studies with ethyl maltol. Toxicol Appl
Pharmacol. 15:604–613. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu M, Huang C, Ma X, Wu R, Zhu W, Li X,
Liang Z, Deng F, Zhu J, Xie W, et al: Modulation of miR-19 in
aluminum-induced neural cell apoptosis. J Alzheimers Dis.
50:1149–1162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin W, Xie W, Yang X, Xia N and Yang K:
Inhibiting microRNA-449 attenuates cisplatin-induced injury in
NRK-52E cells possibly via regulating the SIRT1/P53/BAX pathway.
Med Sci Monit. 22:818–823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ardekani AM and Naeini MM: The role of
microRNAs in human diseases. Avicenna J Med Biotechnol. 2:161–179.
2010.PubMed/NCBI
|
|
17
|
Lukiw WJ: Micro-RNA speciation in fetal,
adult and Alzheimer's disease hippocampus. Neuroreport. 18:297–300.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Lu J, Liu B, Cui Q and Wang Y:
Primate-specific miR-603 is implicated in the risk and pathogenesis
of Alzheimer's disease. Aging (Albany NY). 8:272–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Absalon S, Kochanek DM, Raghavan V and
Krichevsky AM: MiR-26b, upregulated in Alzheimer's disease,
activates cell cycle entry, tau-phosphorylation, and apoptosis in
postmitotic neurons. J Neurosci. 33:14645–14659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Chen CF, Wang AH and Lin QF:
MiR-16 regulates cell death in Alzheimer's disease by targeting
amyloid precursor protein. Eur Rev Med Pharmacol Sci. 19:4020–4027.
2015.PubMed/NCBI
|
|
21
|
Modi PK, Jaiswal S and Sharma P:
Regulation of neuronal cell cycle and apoptosis by MicroRNA 34a.
Mol Cell Biol. 36:84–94. 2015.PubMed/NCBI
|
|
22
|
Bertholf RL, Herman MM, Savory J,
Carpenter RM, Sturgill BC, Katsetos CD, Vandenberg SR and Wills MR:
A long-term intravenous model of aluminum maltol toxicity in
rabbits: Tissue distribution, hepatic, renal, and neuronal
cytoskeletal changes associated with systemic exposure. Toxicol
Appl Pharmacol. 98:58–74. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu L, Li C, Li D, Wang Y, Zhou C, Shao W,
Peng J, You Y, Zhang X and Shen X: Cryptotanshinone inhibits human
glioma cell proliferation by suppressing STAT3 signaling. Mol Cell
Biochem. 381:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Savory J, Rao JK, Huang Y, Letada PR and
Herman MM: Age-related hippocampal changes in Bcl-2: Bax ratio,
oxidative stress, redox-active iron and apoptosis associated with
aluminum-induced neurodegenerati: Increased susceptibility with
aging. Neurotoxicology. 20:805–817. 1999.PubMed/NCBI
|
|
26
|
Hoffman B and Liebermann DA: Apoptotic
signaling by c-MYC. Oncogene. 27:6462–6472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Askew DS, Ashmun RA, Simmons BC and
Cleveland JL: Constitutive c-myc expression in an IL-3-dependent
myeloid cell line suppresses cell cycle arrest and accelerates
apoptosis. Oncogene. 6:1915–1922. 1991.PubMed/NCBI
|
|
28
|
Evan GI, Wyllie AH, Gilbert CS, Littlewood
TD, Land H, Brooks M, Waters CM, Penn LZ and Hancock DC: Induction
of apoptosis in fibroblasts by c-myc protein. Cell. 69:119–128.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dansen TB, Whitfield J, Rostker F,
Brown-Swigart L and Evan GI: Specific requirement for Bax, not Bak,
in Myc-induced apoptosis and tumor suppression in vivo. J Biol
Chem. 281:10890–10895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao S, Xiao L, Rao JN, Zou T, Liu L, Zhang
D, Turner DJ, Gorospe M and Wang JY: Inhibition of Smurf2
translation by miR-322/503 modulates TGF-β/Smad2 signaling and
intestinal epithelial homeostasis. Mol Biol Cell. 25:1234–1243.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gámez B, Rodríguez-Carballo E, Bartrons R,
Rosa JL and Ventura F: MicroRNA-322 (miR-322) and its target
protein Tob2 modulate osterix (Osx) mRNA stability. J Biol Chem.
288:14264–14275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Merlet E, Atassi F, Motiani RK, Mougenot
N, Jacquet A, Nadaud S, Capiod T, Trebak M, Lompré AM and Marchand
A: miR-424/322 regulates vascular smooth muscle cell phenotype and
neointimal formation in the rat. Cardiovasc Res. 98:458–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu H, Yu J, Dong D, Zhou Q, Wang JY and
Yang P: The miR-322-TRAF3 circuit mediates the pro-apoptotic effect
of high glucose on neural stem cells. Toxicol Sci. 144:186–196.
2015. View Article : Google Scholar : PubMed/NCBI
|