Introduction
Chronic obstructive pulmonary disease (COPD) is
chronic airway inflammation characterized by persistent airflow
limitation. Pulmonary hypertension is a pathophysiological status
which accompanies abnormally increased pulmonary artery pressure,
eventually leading to circulatory failure or mortality (1). The majority of patients with severe
COPD in intensive care units are in a hypoxic state. Hypoxic
environments may cause pulmonary artery vasoconstriction, pulmonary
artery smooth muscle cell (PASMC) proliferation and migration,
vascular matrix reconstruction and a series of pathophysiological
changes, which ultimately leads to the occurrence of hypoxic
pulmonary hypertension (HPH) (2).
Previous studies have indicated that proliferation and migration of
PASMCs serves a critical role in the pathological development
process of HPH (3).
Resveratrol is a phenolic compound extracted from a
plant, and has significant anti-inflammatory, antioxidant and
anti-aging biological effects (4,5).
Csiszar et al (3)
demonstrated that resveratrol may inhibit the development of
pulmonary hypertension induced by monocrotaline in rats; however,
the underlying mechanisms remain unknown. Previous studies have
used resveratrol to examine its anti-tumor effects and its specific
roles in abrogating cell proliferation and inducing apoptosis via
downregulation of signal transducer and activator of transcription
3 and nuclear factor-kB (6–10).
Protein kinase B (AKT) is a member of serine/threonine protein
kinase family and responds to a variety of stimuli, including
protein phosphatases, stress and growth factor stimulation. It is
additionally implicated in tumorigenesis, and is activated by
phosphoinositide 3-kinase (PI3K) (11). Resveratrol has been reported to
inhibit AKT activity and induce apoptosis in human uterine cancer
cells (12,13). Jiang et al (14) previously indicated downregulation
of PI3K/AKT/mammalian target of rapamycin (mTOR) signaling pathways
in human U251 glioma cells. Similar to its role in tumorigenesis,
activation of AKT is important for preventing apoptosis of PASMCs
(15), which may be induced by
hypoxia (16). Therefore, the
present study aimed to investigate the potential antiproliferative
effect of resveratrol on PASMCs under hypoxic conditions via
downregulation of the PI3K/AKT signaling pathway. The present study
investigated the role of resveratrol by examining alterations in
expression levels of genes associated with the PI3K/AKT pathways,
proliferation and migration, and comparing the viability and wound
healing rate between treated and control excised rat PASMCs.
Materials and methods
Experimental animals
Twenty-five Sprague-Dawley rats (age, 50 days;
weight, 150–180 g) of specific pathogen free level of either sex
were purchased from the Laboratory Animal Center of Zhejiang
Medical Academy (Hangzhou, China). Animals were housed with a
regular 12 h light/dark cycle at a controlled temperature (25±2°C),
with a humidity of 76% and free access to food and water. The
current study was reviewed and approved by the Institutional Animal
Care and Ethics Committee of The Third Affiliated Hospital of
Qiqihar Medical University (Qiqihar, China), and was conducted
according to the US National Institutes of Health and the European
Commission guidelines.
Experimental reagents
Ether, methanol, 75% and 95% ethanol, anhydrous
ethanol and anhydrous methanol were purchased from Hangzhou
Chemical Reagent Co., Ltd. (Hangzhou, Zhejiang, China). Dulbecco's
modified Eagle's medium (DMEM), PBS, 0.25%
trypsin-ethylenediaminetetraacetic acid (EDTA), penicillin and
streptomycin were obtained from Hangzhou Genom Biological
Pharmaceutical Technology Co., Ltd. (Hangzhou, Zhejiang, China).
Resveratrol and homocysteine were purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany); fetal bovine serum (FBS) was
obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA); MTT was purchased from Amresco, LLC (Solon, OH, USA); 4′,
6-diamidino-2-phenylindole (DAPI) was obtained from Roche
Diagnostics (Basel, Switzerland). The following primary antibodies
were purchased from Abcam (Cambridge, UK): Monoclonal rabbit
anti-rat SM-actin (cat. no. ab5694; 1:1,000), rabbit anti-rat p21
(cat. no. ab109520; 1:1 000), anti-p27 (cat. no. ab32034; 1:1,000),
anti-matrix metalloproteinase (MMP)-2 (cat. no. ab92536; 1:1,000)
and polyclonal anti-MMP-9 (cat. no. ab194314; 1:1,000). Rabbit
anti-AKT (cat. no. 4691; 1:1,000) and phosphorylated (p)-AKT
polyclonal primary antibodies (cat. no. 4060; 1:2,000) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit (cat. no.
111035003; 1:15,000) and fluorescein isothyocyanate-labelled goat
anti-rabbit IgG (cat. no. 111095003; 1:15,000) secondary antibodies
were purchased from Jackson ImmunoResearch Laboratories, Inc.
(Baltimore, PA, USA). Western blotting and gelatin zymography
relative regents were obtained from Beyotime Institute of
Biotechnology (Haimen, China).
Primary cell culture and
identification of rat PAMSCs
Rats were anesthetized and sacrificed by procedures
reviewed and approved by the Committee on Animal Resources of
Qiqihar Medical University (Qiqihar, China). Anesthesia was
produced using atropine (0.05 mg/kg; subcutaneous administration)
purchased from Sigma-Aldrich; Merck KGaA. A tissue explants
adherent method was used to culture rat PASMCs; morphological assay
and immunocytochemistry were used to detect SM-actin expression to
identify PASMCs, and purity of PASMCs was evaluated by the
association between SM-actin and DAPI in the nuclei. Cultured rat
PASMCs at passages 3–5 were used for further experiments. Culture
media containing 5% FBS and different concentrations (10, 30 and
100 µmol/l) of resveratrol (Tianjin Jianfeng Biotechnology Co.,
Ltd., Tianjin, China) was used in the experiments.
A hypoxic environment for PAMSCs was induced using
an autonomous plexiglass chamber supplied with 5% CO2
and 95% N2 at 20 ml/min. A CR-2 oxygen detector was
applied to monitor the oxygen concentration of the chamber and
maintained levels at 3%. Finally, the chamber was incubated at 37°C
for 3 days.
PASMC MTT proliferation assay
PASMCs at passages 3–5 were digested with 0.25%
trypsin, suspended in DMEM supplemented with 10% FBS. Cells were
seeded into a 96-well plate at a density of 6×103 cells/well. When
the cultured cells grew against the wall of the plate, serum-free
medium was added for 24 h for cell synchronization. Resveratrol
(10, 30 or 100 µmol/l), or a combination of resveratrol with
LY-294002, a PI3K inhibitor, or insulin-like growth factor-1
(IGF-1), was added to the cells for 24, 48 or 72 h. For the
positive control, saline was added to cells in hypoxic conditions.
Cells not exposed to hypoxia and treated with saline served as the
negative control. Each group had three duplication wells, and two
blank wells without cells were reserved. Following culture, 20 µl
MTT was added to each culture well and cells were incubated at 37°C
for 4–6 h. Subsequently, the supernatant was discarded and 150 µl
dimethyl sulfoxide was added to each well for 10 min, with
agitation. The optical density (OD) value was measured at a
wavelength of 492 nm using an MTT enzyme-linked immunometric meter.
The growth curve was calculated using the time on the horizontal
axis and absorbance value on the vertical axis.
PASMC wound healing migration
assay
PASMCs at passages 3–5 were digested with 0.25%
trypsin, suspended in DMEM supplemented with 10% FBS. Cells were
seeded in a 6-well plate at a density of 1×105 cells/well. When the
cultured cells proliferated to form a full layer, serum-free medium
was added for 24 h for cell synchronization. Hydroxyurea (1.8
mmol/l; Sigma-Aldrich; Merck KGaA) was used to inhibit cell
proliferation. A scratch was made in the cell monolayer by drawing
a sterile P-200 pipette tip across the surface of the culture dish.
PBS was used to wash the plate three times. Cell migration into the
scratched area was assessed after 0, 12, 24, 48 and 72 h. The areas
of cells were measured using Image ProPlus software, version 6.0
(Media Cybernetics, Inc., Rockville, MD, USA). The migration rate
of the PASMCs was calculated as the ratio of the migration area to
the original scratch area.
PASMC Transwell migration assay
PASMCs at passages 3–5 were cultured in serum-free
medium for cell synchronization. PASMCs were incubated for 12 h
with 1.8 mmol/l hydroxyurea and digested with 0.25% trypsin.
Following this, cells were suspended in high-glucose DMEM
supplemented with 1% FBS and counted (4×104/ml). Each Transwell-24
plate, containing an upper compartment with 200 µl cell suspension
and a lower compartment with 500 µl DMEM supplemented with 10% FBS,
was cultured for 4, 8, 12, 24 and 48 h separately. The upper PASMCs
which did not penetrate the membrane were removed with a swab. The
Transwell semipermeable membrane was washed three times with PBS
and fixed with 3.7% paraformaldehyde at room temperature for 5 min,
followed by washing three times with PBS again. Nuclear DNA was
labelled with 3 µg/ml DAPI, following which cells were washed three
times with PBS. Cells were imaged in five random fields under a
fluorescence microscope, following which the number of cells
penetrating the semipermeable membrane was counted at ×100
magnification in triplicate wells of each group.
Protein expression levels of p21, p27,
MMP-2, MMP-9, AKT and p-AKT in PASMCs, detected by western
blotting
Total protein was extracted from PASMCs. Briefly,
the PASMCs were washed with 1X PBS once and each well in the
12-well cell culture plate was lysed with 100 µl of ice cold
radioimmunoprecipitation assay buffer (25 mM Tris-Cl, pH 7.4, 150
mM NaCl, 50 mM KCl, 1% SDS, 2 mM EDTA, 0.5% glycerol, 50 mM NaF)
with 1:100 (vol/vol) dilution of the proteinase inhibitor and
phosphotase I and II inhibitor mixture (Sigma-Aldrich; Merck KGaA).
This was then briefly vortexed and placed on ice for 15 min, spun
down at 8,000 × g for 15 min to pellet the debris and the
supernatant was collected for the western blotting. The protein
concentration was determined using a Bicinchoninic Acid Protein
Assay kit (Sigma-Aldrich; Merck KGaA), according to the
manufacturer's instructions. Then, 30 µg protein lysate was boiled
with 500 mM DTT in 2X sample buffer for 5 min, separated by 10%
SDS-PAGE and transferred onto a nitrocellulose membrane. The
membrane was blocked with Tris-buffered saline containing 5% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) at room temperature for
30 min, followed by incubation with the appropriate primary
antibody (1:1,000) overnight at 4°C. β-actin served as an internal
control. Following this, membranes were incubated with a secondary
antibody (1:5,000) at room temperature for 1 h. Proteins were
visualized by Enhanced Chemiluminescence (EMD Millipore, Billerica,
MA, USA).
Statistical analysis
All statistical analysis was performed using SPSS
version 12.0 (SPSS, Inc., Chicago, IL, USA). Each assay was
performed in triplicate, and data are expressed as the mean ±
standard deviation. Multiple comparisons were evaluated by one-way
analysis of variance and Fisher's Least Significant Difference
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
Culture and identification of primary
PASMCs
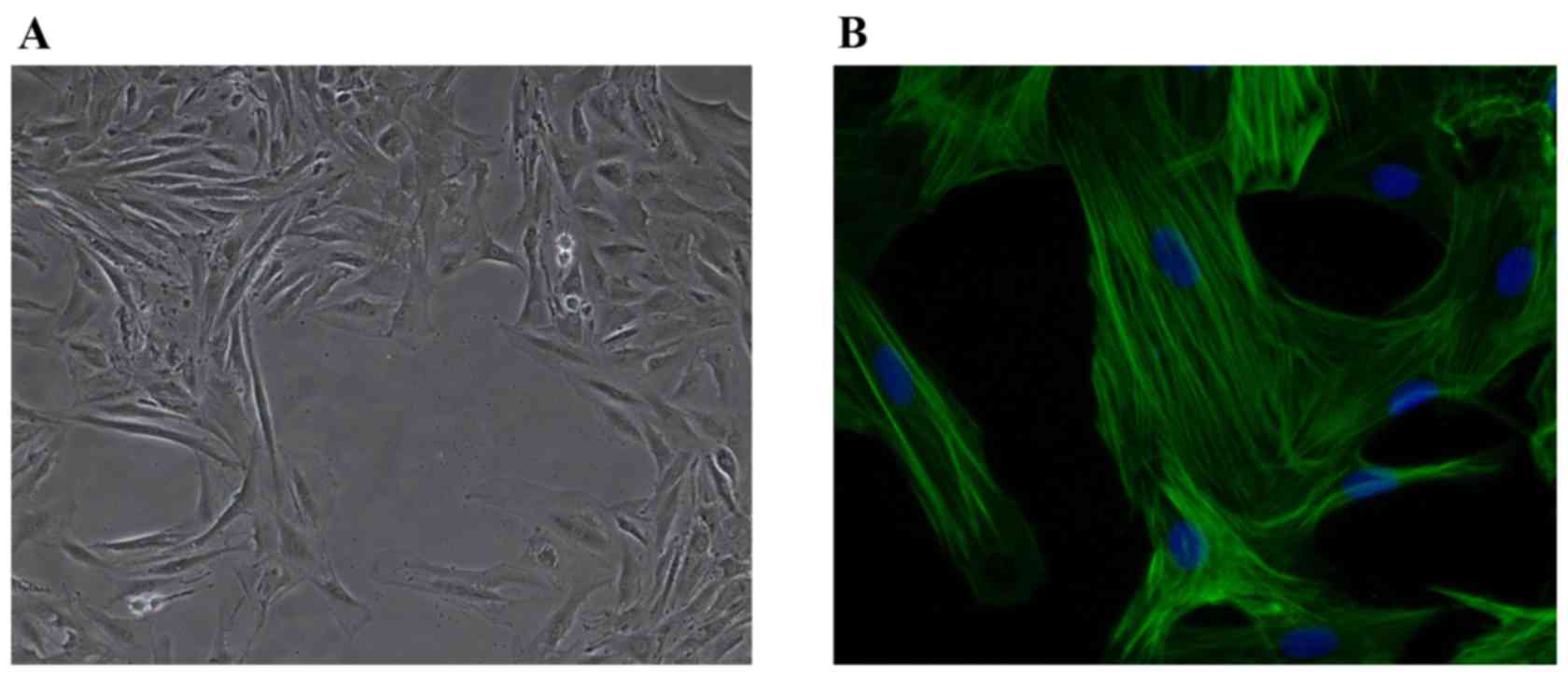
PASMCs were cultured using the tissue explant
method. On day 10, cells were observed around the tissue block.
Cell fusion occurred on day 15 (Fig.
1A). SM-actin immunofluorescence was identified in PASMCs, and
cell purity was detected to be >99% by DAPI nuclear staining
(Fig. 1B).
Resveratrol treatment inhibits
hypoxia-induced proliferation and migration of PASMCs
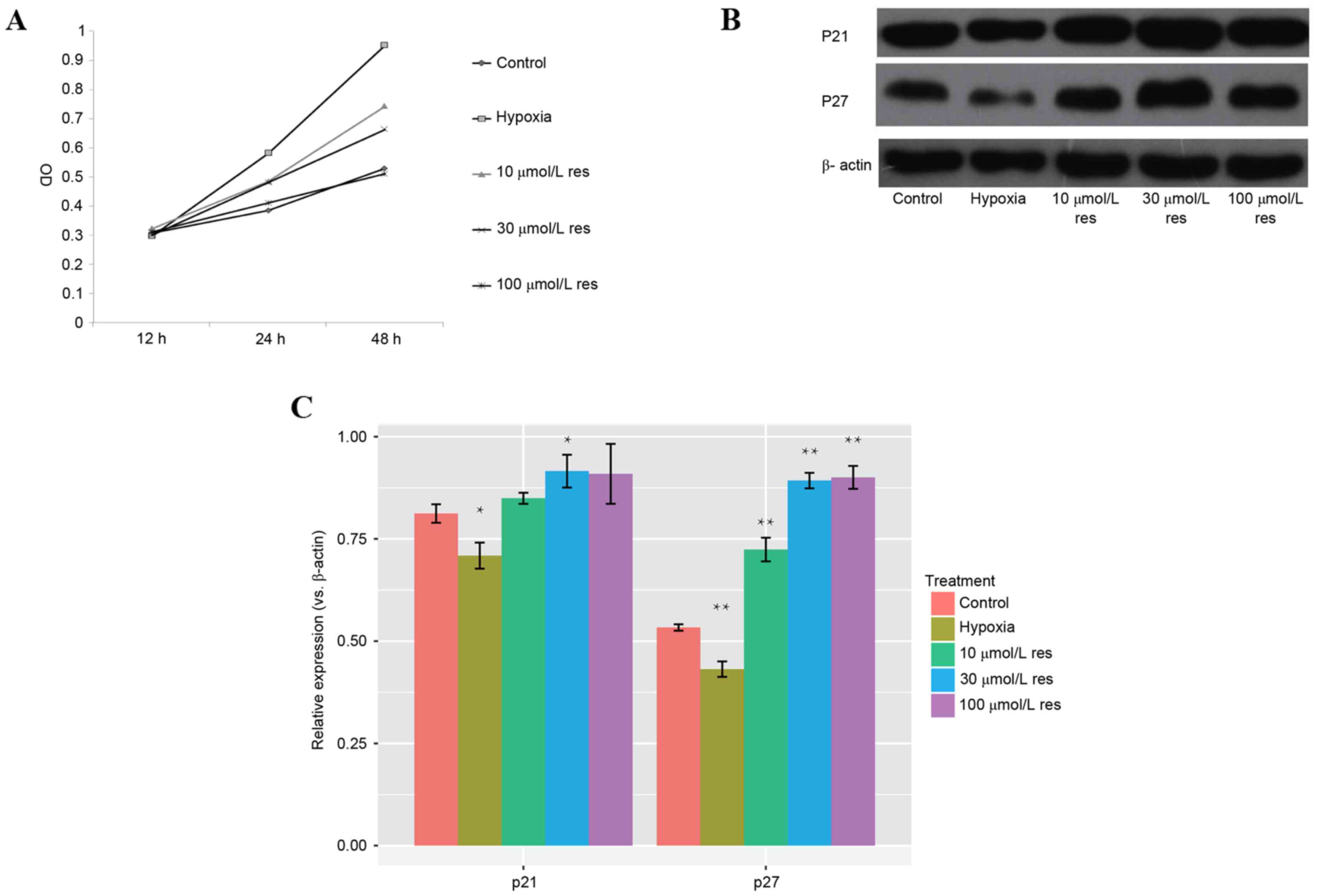
The results of the MTT assay indicated that the OD
value of the hypoxic group was significantly increased compared
with the control group (P=0.016), indicating that hypoxia may
promote PASMC migration. The OD value of resveratrol group markedly
decreased in a dose-dependent manner compared with the control
group (P=0.008; Fig. 2A).
Significantly reduced protein expression levels of p21 and p27 were
observed in the hypoxic group by western blot analysis (P=0.023;
Fig. 2B). p21 and p27 protein
expression levels were increased in the resveratrol group compared
with the hypoxic group (P<0.05), and this effect was
dose-dependent (Fig. 2C). These
results indicated that resveratrol may inhibit hypoxia-induced
proliferation of PASMCs.
Resveratrol treatment inhibits
hypoxia-induced migration of PASMCs
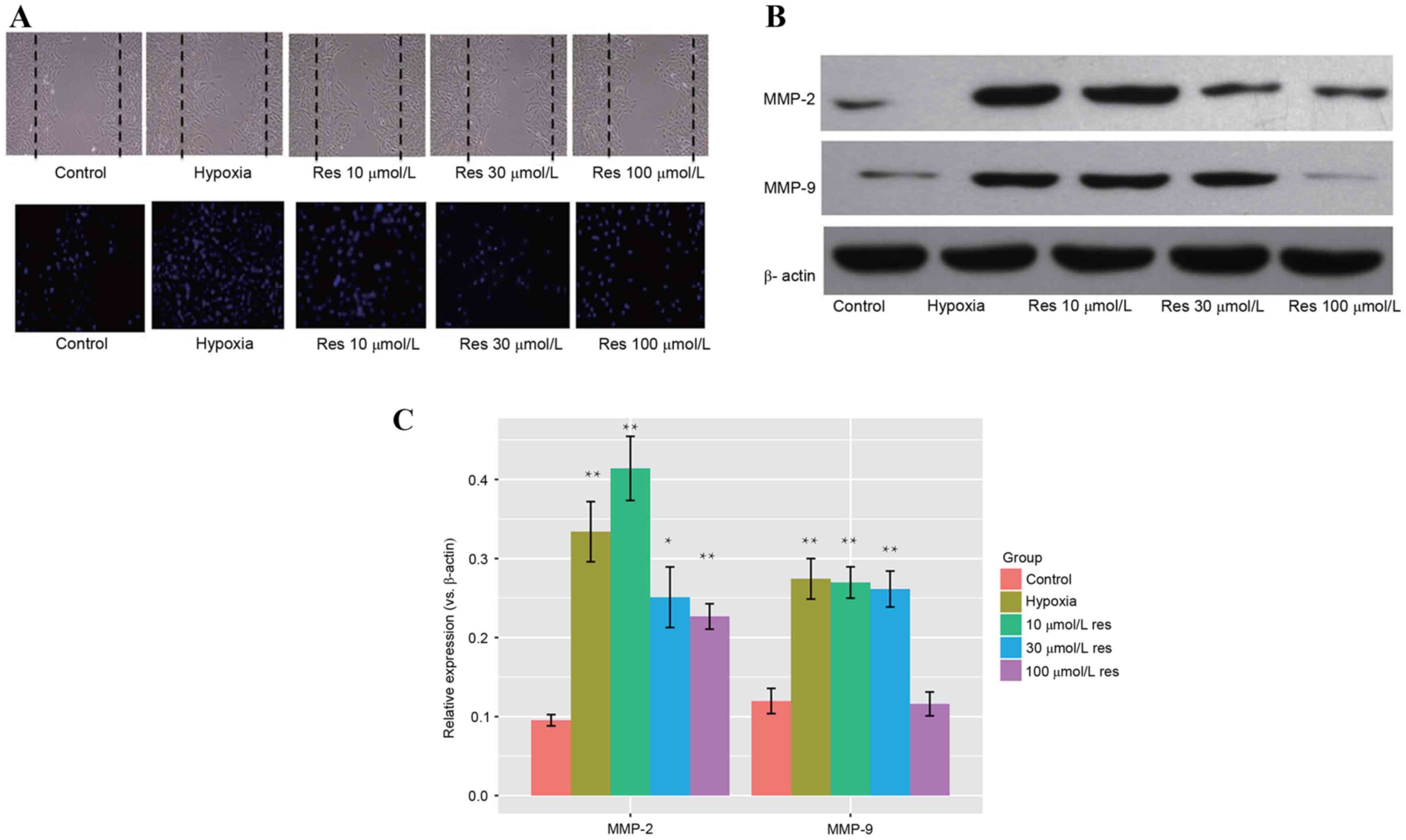
As assessed by Transwell and wound healing migration
assays, the migration rate of PASMCs in the hypoxic group was
increased compared with the control group when the intervention
factor was considered in each group (P=0.012, Hypoxia vs. Control).
Migration of PASMCs in the resveratrol-treated group was reduced
compared with the cells treated with hypoxia (P<0.05), and this
effect was dose-dependent (Fig.
3A). Western blot analysis (Fig.
3B) identified significantly increased protein expression
levels of MMP-2 and MMP-9 in the hypoxic group when compared with
control (P=0.002 for MMP-2; P=0.004 for MMP-9). Protein expression
levels of MMP-2 and MMP-9 in the resveratrol group were reduced
compared with the hypoxic group (MMP-2, P<0.001 hypoxia vs. 10
µmol/l resveratrol; MMP-9, P=0.056 hypoxia vs. 10 µmol/l
resveratrol), and this effect was dose-dependent (Fig. 3C). These results indicated that
resveratrol may inhibit hypoxia-induced migration of PASMCs.
Resveratrol inhibits activation of the
PI3K/AKT signaling pathway
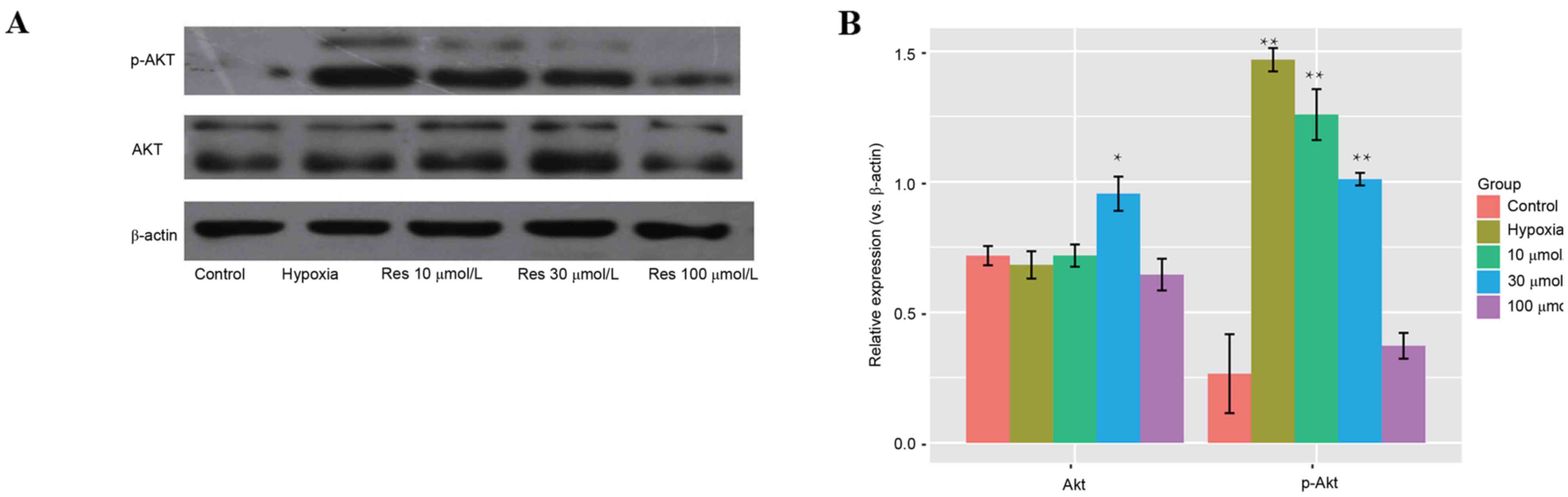
No significant differences in protein expression
levels of AKT were observed between the different groups. However,
protein expression levels of p-AKT were increased significantly
(P=0.0021) in the hypoxic group compared with the 10 and 30 µmol/l
resveratrol-treated groups, and this effect was dose-dependent
(Fig. 4).
Resveratrol inhibits hypoxia-induced
proliferation and migration of PASMCs by inhibiting the PI3K/AKT
signaling pathway
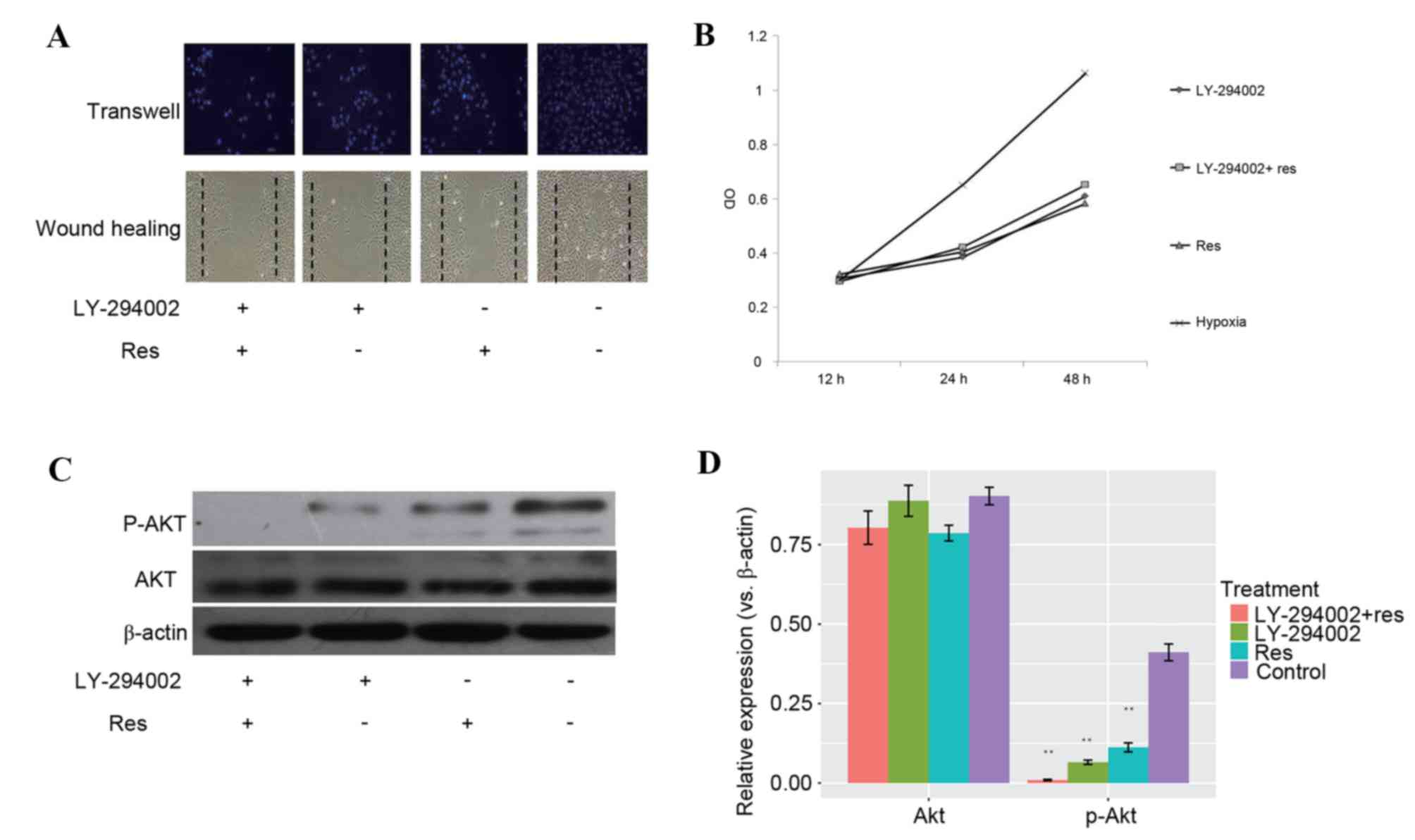
LY-294002 is a specific inhibitor of PI3K and
significantly inhibits the expression of p-AKT. Following 20 nmol/l
LY-294002 treatment, proliferation and migration of PASMCs were
markedly decreased. No significant differences were observed in
migration (Fig. 5A) and
proliferation (Fig. 5B) between
the resveratrol group treated with LY-294002 and the hypoxic group
treated with LY-294002. PASMCs treated with resveratrol alone
seemed to produce the same effect as LY-294002 only treatment on
inhibiting hypoxia-induced proliferation, while a combined
treatment of resveratrol and LY-294002 did not increase the potency
of antiproliferation (Fig. 5B).
The western blot of p-Akt displayed a similar pattern (Fig. 5C). Quantification of the protein
level of Akt and p-Akt demonstrated that LY-294002 and resveratrol
reduced the levels of p-Akt (Fig.
5D), suggesting that resveratrol and LY-294002 may function via
a similar mechanism.
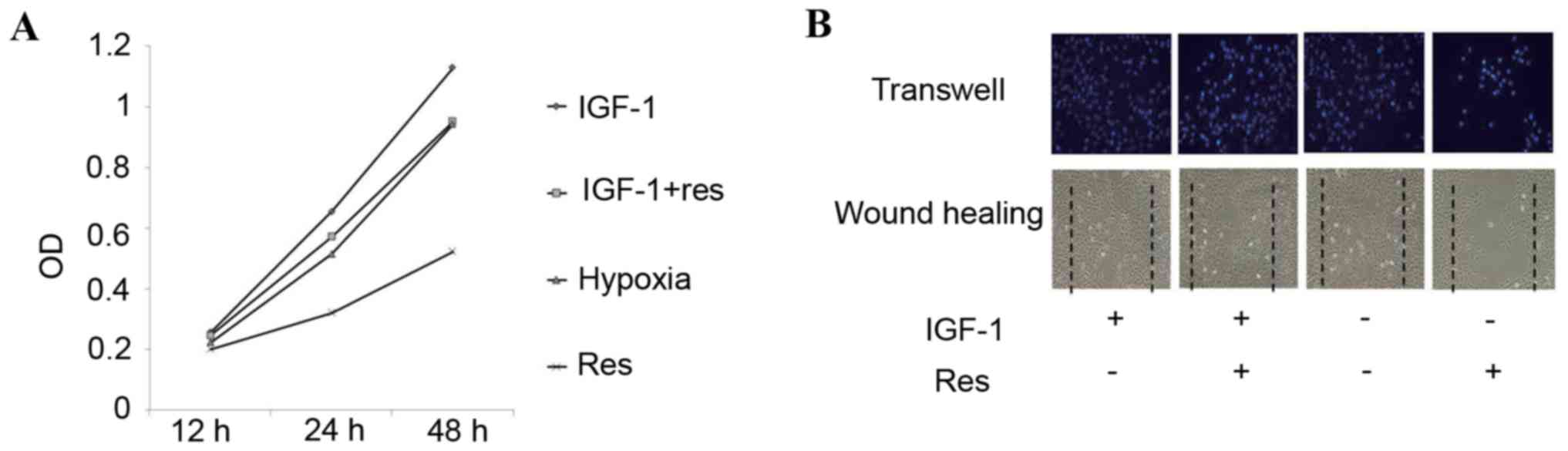
IGF-1 is an agonist of PI3K and significantly
increases the expression of p-AKT. Following 3 ng/ml IGF-1
treatment, the inhibitory effect on proliferation and migration of
PASMCs was reversed and markedly increased. No significant
differences were observed in proliferation and migration between
the resveratrol group treated with +IGF-1 and the hypoxic group
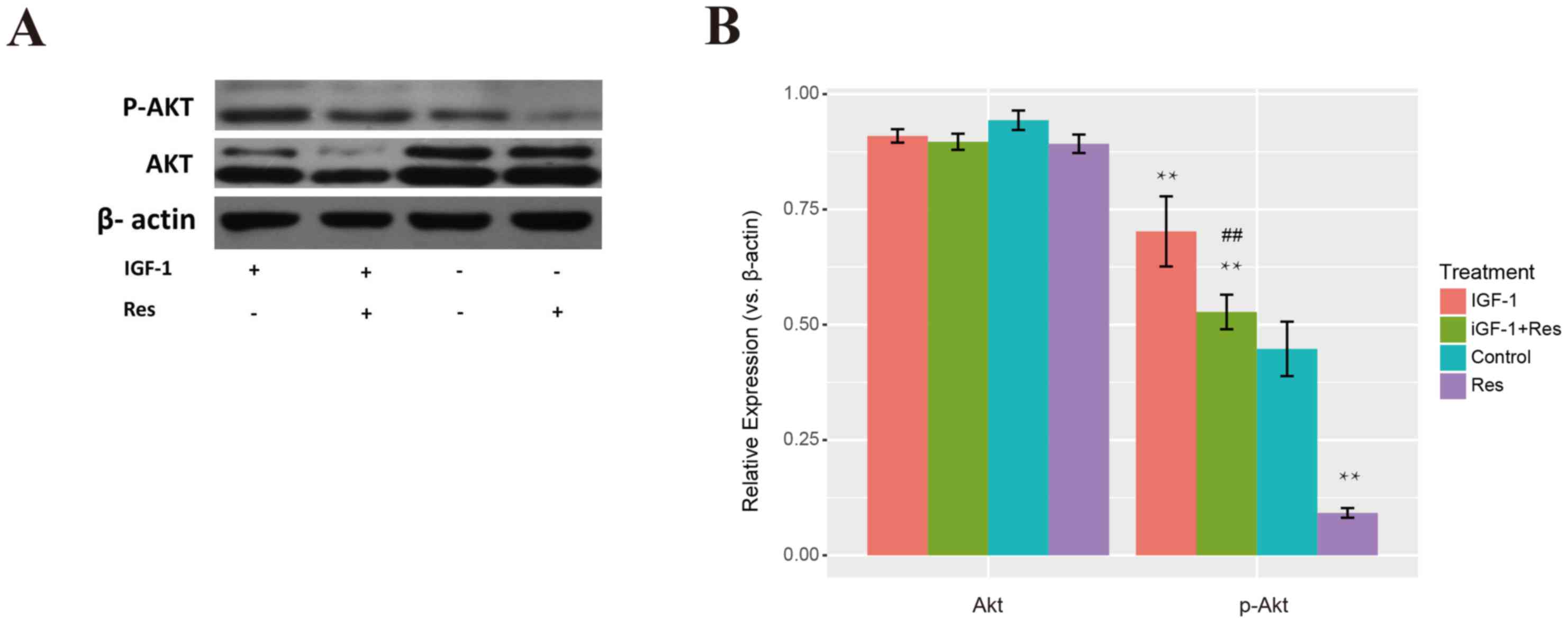
treated with +IGF-1 in proliferation and migration (Fig. 6A and B). The protein level of p-Akt
was significantly suppressed by resveratrol, however, it was
markedly increased by IGF-1. The protein level of p-Akt with
IGF-1+Res treatment was significantly higher than that of control,
while the Res treatment group exhibited significantly lower levels
than the control. The protein level of p-Akt following IGF-1+Res
treatment was also significantly increased when compared with Res
treatment alone (Fig. 7A and B;
P<0.01). Thus, treatment with IGF-1 seemed to counteract the
inhibition of resveratrol on p-Akt expression, suggesting that
p-Akt may be the pharmacological effector of.
Discussion
PASMCs are located in pulmonary artery media and
serve an important role in vasoconstriction under healthy
conditions. The pulmonary arteries of patients with COPD in anoxic
conditions are associated with persistent airflow limitation, which
induces and activates proliferation and migration of PASMCs
(17). Consequently, proliferating
and migrating PASMCs secrete inflammatory factors, causing cascade
amplification of the inflammatory reaction and increasing levels of
components of the extracellular matrix simultaneously. These
factors lead to remodeling of the pulmonary arteries and luminal
stenosis, resulting in development of pulmonary hypertension
(18,19).
Resveratrol is a type of polyphenol with pleiotropic
biology that is extracted from red wine, and has gained increasing
attention due to its protective properties for cardiac-cerebral
vessels (20). Follow-up studies
have identified that resveratrol has cardioprotective effects, and
may inhibit tumor cell growth and attenuate diabetes-associated
complications by its anti-inflammatory and antioxidant properties
(21–23). Csiszar et al (3) first identified that resveratrol may
inhibit the development of monocrotaline-induced pulmonary
hypertension; however, the underlying mechanisms are not clear
(3). Previous studies demonstrated
that resveratrol may improve the function of rat pulmonary artery
endothelium by increasing the expression of NO to decrease the
effect of oxidative stress, inhibit inflammatory reactions and
inhibit cell proliferation and vascular remodeling (3,24).
Resveratrol may reverse the dysfunction of rat pulmonary artery
vessels and inhibit cardiomyocyte hypertrophy (25,26).
The present study demonstrated that resveratrol may inhibit
proliferation and migration of PASMCs, which may be the mechanism
underlying the inhibitory effect on the development of pulmonary
hypertension. In addition, resveratrol inhibited cell proliferation
of PASMCs induced by hypoxia in a dose-dependent manner. Protein
expression levels of p21 and p27, which are established
cyclin-dependent kinase inhibitors (27,28),
were rescued from hypoxia following treatment with resveratrol,
suggesting the potential role of resveratrol in regulating cell
cycles. MMPs serve important roles in proliferation and metastasis
of smooth muscle cells, and provide space for PASMCs to migrate and
proliferate around surrounding tissues. On the other hand, MMPs may
increase cell migration and proliferation by activating the
PI3K/AKT signaling pathway (29,30).
The results of the present study demonstrated that resveratrol
significantly inhibits protein expression levels of MMP-2 and
MMP-9, which may be one of the underlying mechanisms inhibiting
proliferation and migration of PASMCs.
The PI3K/AKT signaling pathway serves a key role in
proliferation and migration of PASMCs and phenotype switch
(31). p-AKT may further activate
the mTOR/P70S6K signaling pathway, increase the expression of
p-P70S6K and increase proliferation and migration of PASMCs
(32). Chen et al (33) demonstrated that angiotensin II may
increase the expression of p-AKT in rat pulmonary artery tissues,
leading to the formation of pulmonary hypertension. Goncharova
et al (34) further
demonstrated that activation of the PI3K/AKT signaling pathway in
PASMCs via formation of the mTOR complex led to increased
transcription and translation of genes associated with cell
proliferation, eventually leading to proliferation of PASMCs. These
results were consistent with those of the present study, where
resveratrol was demonstrated reduce p-AKT and AKT protein
expression levels, thereby inhibiting proliferation and migration
of PASMCs. In addition, previous studies have demonstrated that
resveratrol has synergistic functions with the AKT phosphorylation
inhibitor LY-294002; however, IGF-1 may counterbalance the
activation of AKT (24–26). This was observed in the present
study as protein expression levels of p-Akt were downregulated
despite considerable basal expression of AKT in each treatment
group, indicating that resveratrol may reduce the expression of AKT
and abrogate its phosphorylation.
In conclusion, the present study was based on
previous studies which have demonstrated that resveratrol may
inhibit proliferation and migration of PASMCs. It was demonstrated
that resveratrol may inhibit proliferation and migration of PASMCs
by blocking the PI3K/AKT signaling pathway. The present study
provided a novel perspective of the underlying mechanisms of
resveratrol treatment on resistance to HPH.
Acknowledgements
The present study was supported by the Instructive
Research Fund of Qiqihar (grant no. SFZD-2015164).
References
|
1
|
Ogawa A, Yamadori I, Matsubara O and
Matsubara H: Pulmonary tumor thrombotic microangiopathy with
circulatory failure treated with imatinib. Intern Med.
52:1927–1930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Afonso AS, Verhamme KM, Sturkenboom MC and
Brusselle GG: COPD in the general population: Prevalence, incidence
and survival. Respir Med. 105:1872–1884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Csiszar A, Labinskyy N, Olson S, Pinto JT,
Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, et al:
Resveratrol prevents monocrotaline-induced pulmonary hypertension
in rats. Hypertension. 54:668–675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He X, Wang Y, Zhu J, Orloff M and Eng C:
Resveratrol enhances the anti-tumor activity of the mTOR inhibitor
rapamycin in multiple breast cancer cell lines mainly by
suppressing rapamycin-induced AKT signaling. Cancer Lett.
301:168–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J and Li Q: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soto BL, Hank JA, Van de Voort TJ,
Subramanian L, Polans AS, Rakhmilevich AL, Yang RK, Seo S, Kim K,
Reisfeld RA, et al: The anti-tumor effect of resveratrol alone or
in combination with immunotherapy in a neuroblastoma model. Cancer
Immunology Immunotherapy. 60:731–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brody H: Chronic obstructive pulmonary
disease. Nature. 489:S12012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torpy JM, Goodman DM, Burke AE and
Livingston EH: JAMA patient page. CHronic obstructive pulmonary
disease. JAMA. 308:12812012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuder RM and Petrache I: Pathogenesis of
chronic obstructive pulmonary disease. J Clin Invest.
122:2749–2755. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lahm T, Albrecht M, Fisher AJ, Selej M,
Patel NG, Brown JA, Justice MJ, Brown MB, van Demark M, Trulock KM,
et al: 17β-Estradiol attenuates hypoxic pulmonary hypertension via
estrogen receptor-mediated effects. Am J Respir Crit Care Med.
185:965–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SJ, Smith A, Guo L, Alastalo TP, Li M,
Sawada H, Liu X, Chen ZH, Ifedigbo E, Jin Y, et al: Autophagic
protein LC3B confers resistance against hypoxia-induced pulmonary
hypertension. Am J Respir Crit Care Med. 183:649–658. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sexton E, van Themsche C, LeBlanc K,
Parent S, Lemoine P and Asselin E: Resveratrol interferes with AKT
activity and triggers apoptosis in human uterine cancer cells. Mol
Cancer. 5:452006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang H, Shang X, Wu H, Gautam SC,
Al-Holou S, Li C, Kuo J, Zhang L and Chopp M: Resveratrol
downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma
cells. J Exp Ther Oncol. 8:25–33. 2009.PubMed/NCBI
|
|
15
|
Wu J, Yu Z and Su D: BMP4 protects rat
pulmonary arterial smooth muscle cells from apoptosis by
PI3K/AKT/Smad1/5/8 signaling. Int J Mol Sci. 15:13738–13754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi B, Cui J, Ning JN, Wang GS, Qian GS and
Lu KZ: Over-expression of PKGIα inhibits hypoxia-induced
proliferation, Akt activation, and phenotype modulation of human
PASMCs: The role of phenotype modulation of PASMCs in pulmonary
vascular remodeling. Gene. 492:354–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alexander MR and Owens GK: Epigenetic
control of smooth muscle cell differentiation and phenotypic
switching in vascular development and disease. Annu Rev Physiol.
74:13–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng L, Blanco FJ, Stevens H, Lu R,
Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, et
al: MicroRNA-143 activation regulates smooth muscle and endothelial
cell crosstalk in pulmonary arterial hypertension. Circ Res.
117:870–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu A, Zuo C, He Y, Chen G, Piao L, Zhang
J, Xiao B, Shen Y, Tang J, Kong D, et al: EP3 receptor deficiency
attenuates pulmonary hypertension through suppression of Rho/TGF-β1
signaling. J Clin Invest. 125:1228–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wallerath T, Deckert G, Ternes T, Anderson
H, Li H, Witte K and Förstermann U: Resveratrol, a polyphenolic
phytoalexin present in red wine, enhances expression and activity
of endothelial nitric oxide synthase. Circulation. 106:1652–1658.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jimenez-Gomez Y, Mattison JA, Pearson KJ,
Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM,
Lewis K, Allard JS, et al: Resveratrol improves adipose insulin
signaling and reduces the inflammatory response in adipose tissue
of rhesus monkeys on high-fat, high-sugar diet. Cell Metab.
18:533–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andreadi C, Britton RG, Patel KR and Brown
K: Resveratrol-sulfates provide an intracellular reservoir for
generation of parent resveratrol, which induces autophagy in cancer
cells. Autophagy. 10:524–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mattison JA, Wang M, Bernier M, Zhang J,
Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, et
al: Resveratrol prevents high fat/sucrose diet-induced central
arterial wall inflammation and stiffening in nonhuman primates.
Cell Metab. 20:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chicoine LG, Stewart JA Jr and Lucchesi
PA: Is resveratrol the magic bullet for pulmonary hypertension?
Hypertension. 54:473–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang DL, Zhang HG, Xu YL, Gao YH, Yang XJ,
Hao XQ and Li XH: Resveratrol inhibits right ventricular
hypertrophy induced by monocrotaline in rats. Clin Exp Pharmacol
Physiol. 37:150–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paffett ML, Lucas SN and Campen MJ:
Resveratrol reverses monocrotaline-induced pulmonary vascular and
cardiac dysfunction: A potential role for atrogin-1 in smooth
muscle. Vascul Pharmacol. 56:64–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Wang L, Chen L, Zhang Y and Shi
P: Induction of cell cycle arrest via the p21, p27-cyclin E, A/Cdk2
pathway in SMMC-7721 hepatoma cells by clioquinol. Acta Pharm.
65:463–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yadav V, Sultana S, Yadav J and Saini N:
Gatifloxacin induces S and G2-phase cell cycle arrest in pancreatic
cancer cells via p21/p27/p53. PLoS One. 7:e477962012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shivakrupa R, Bernstein A, Watring N and
Linnekin D: Phosphatidylinositol 3′-kinase is required for growth
of mast cells expressing the kit catalytic domain mutant. Cancer
Res. 63:4412–4419. 2003.PubMed/NCBI
|
|
30
|
Roche S, Koegl M and Courtneidge SA: The
phosphatidylinositol 3-kinase alpha is required for DNA synthesis
induced by some, but not all, growth factors. Proc Natl Acad Sci
USA. 91:9185–9189. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alessi DR, Andjelkovic M, Caudwell B, Cron
P, Morrice N, Cohen P and Hemmings BA: Mechanism of activation of
protein kinase B by insulin and IGF-1. EMBO J. 15:6541–6551.
1996.PubMed/NCBI
|
|
32
|
Rachid O and Alkhalaf M: Resveratrol
Regulation of PI3K-AKT Signaling Pathway Genes in MDA-MB-231 Breast
Cancer Cells. Cancer Genomics Proteomics. 3:383–388. 2006.
|
|
33
|
Chen B, Xue J, Meng X, Slutzky JL, Calvert
AE and Chicoine LG: Resveratrol prevents hypoxia-induced arginase
II expression and proliferation of human pulmonary artery smooth
muscle cells via Akt-dependent signaling. Am J Physiol Lung Cell
Mol Physiol. 307:L317–L325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goncharova EA, Ammit AJ, Irani C, Carroll
RG, Eszterhas AJ, Panettieri RA and Krymskaya VP: PI3K is required
for proliferation and migration of human pulmonary vascular smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 283:L354–L363.
2002. View Article : Google Scholar : PubMed/NCBI
|