Introduction
Gemcitabine is a nucleoside antimetabolite that
inhibits DNA synthesis (1). It is
most commonly used in organ malignancies due to its functions in
the promotion of cell death in several cancers including non-small
cell lung cancer, colon squamous cell carcinoma, nasopharyngeal
carcinoma and ovarian, breast and pancreatic cancer (2–5).
Gemcitabine-induced antitumor therapy resistance is a widely used
model in studying chemotherapeutic resistance (6). Gemcitabine is used as a standard drug
treatment for human osteosarcoma and has a significant therapeutic
effect in osteosarcoma (7),
however, the underlying mechanism requires further
investigation.
Autophagy, a conserved pathway that involves the
degradation of aggregated proteins and damaged organelles, serves
an essential role in maintaining tissue homeostasis to support cell
growth and survival (8,9). In addition, autophagy is considered
to be a unique signaling pathway that influences a number of
pathological conditions such as oncogenesis and cancer therapy
resistance (10). Several
essential signaling pathways, including mechanistic target of
rapamycin, death-associated protein kinases, Beclin 1 and caspases
are reported to be involved in the process of autophagy (11,12).
Beclin 1 is considered to be related to the initiation and
progression of autophagy. Previous studies have demonstrated that
activation of autophagy may inhibit the ability of antitumor
chemotherapy drugs to induce apoptosis (13,14).
However, the underlying mechanism remains unknown.
The Wnt/β-catenin signaling pathway, which has an
important role in cell proliferation and tumorigenesis, was
previously reported to be aberrantly activated in the majority of
tumors, including breast cancer, colon cancer as well as renal
carcinomas (15,16). Evidence has also indicated that
activation of the Wnt/β-catenin signaling pathway suppresses
autophagy (17). As tumors often
exhibit reduced levels of autophagy, and drug resistance is
associated with abnormal apoptosis, but whether Wnt/β-catenin
pathway inhibition is associated with antitumor chemotherapy drug
resistance remains to be further determined.
The present study investigated the effect of
Wnt-β-catenin pathway activation and autophagy on drug resistance
in the MG63 human osteosarcoma cell line. The results will provide
novel insights into the mechanism by which the Wnt/β-catenin
signaling pathway may regulate chemotherapy drug-induced cancer
cell resistance.
Materials and methods
Cell culture
The MG63 human osteosarcoma cell line (American Type
Culture Collection, Manassas, VA, USA) was cultured in Dulbecco's
modified Eagle's medium (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin at 37°C in an atmosphere containing 5%
CO2.
Treatment
The cultured cells were randomly divided into the
following groups: Control (untreated); Beclin 1 overexpression
treatment; Gemcitabine treatment; XAV939 treatment; wnt3α
treatment; Beclin 1 + Gemcitabine treatment; Beclin 1 + Gemcitabine
+ XAV939 treatment; Beclin 1 + Gemcitabine + Wnt3α treatment. The
pcDNA3-Beclin 1 plasmid was purchased from Addgene Inc. (#21150;
Cambridge, MA, USA). XAV939, Wnt3α and gemcitabine were purchased
from Sigma-Aldrich (Merck-KGaA, Darmstadt, Germany).
Overexpression of Beclin 1 in MG63
cells
pcDNA3.1 (empty vector) and pcDNA3-Beclin 1
over-expressing Beclin 1 gene were transfected into MG63 cells
using FugeneHD (Promega Corporation, Madison, WI, USA), according
to the manufacturer's instructions. Following incubation for 2
days, the cell transfection efficiency was determined by western
blot analysis.
Confocal microscopy
To visualize the induction of autophagy, the MG63
cell line was transfected with the pQXI-DsRed-LC3-GFP
puromycin-encoding plasmid (#31183; Addgene, Inc.). MG63 cells
cultured in serum-free medium for 3 days were used as a positive
control. While DsRed was constitutively expressed in all treatment
groups, the induction of autophagy resulted in cleavage of the GFP
domain from DsRed-LC3-GFP (18).
Therefore, the percentage of autophagy-positive cells was
determined by the amount of GFP-negative cells under a Zeiss laser
scanning confocal fluorescence microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following incubation of cells with 10 µg/ml
Gemcitabine, 10 µg/ml Gemcitabine + 5 µM Beclin 1, 50 µg/ml
Gemcitabine, 50 µg/ml Gemcitabine + 5 µM Beclin 1, 20 µM XAV939 or
15 µM Wnt3α for 2 days, the expression of autophagy-related (ATG)4,
ATG5, ATG12, Beclin 1, B-cell lymphoma 2 (BCL-2), cellular
FLICE-inhibitory protein (c-FLIP), caspase-3, caspase-7, cyclin D1
and lysosomal-associated membrane protein 1 (LAMP1) was determined
by RT-qPCR. Total cellular RNA was extracted using TRIzol® reagent
(Invitrogen, Thermo Fisher Scientific, Inc.) and cDNA was
synthesized using AMV reverse transcriptase according to the
manufacturer's protocol (Promega Corporation). qPCR was performed
using SYBR-Green Real-Time PCR in the 7900HT Fast Real-Time PCR
system (Applied Biosystems, Thermo Fisher Scientific, Inc.) The PCR
program included initial denaturation for 10 min at 95°C, followed
by 40 cycles of denaturation for 15 sec at 95°C, annealing for 1min
at 55°C and extension for 40 sec at 72°C. Expression levels were
measured by calculating the threshold cycle (Cq) of target genes
following normalization against the Cq value of GAPDH using the
2−ΔΔCq method (19).
Experiments were repeated three times. The primers were designed by
Primer Premier 5.0 software (Premier Biosoft International, Palo
Alto, CA, USA) and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). Primer sequences were as follows: GAPDH forward,
5′-CACCATCTTCCAGGAGCGAG-3′ and reverse, 5′-TCACGCCACAGTTTCCCGGA-3′;
BCL-2 forward, 5′-CCGATCAGTGGAGCTGAAGAA-3′ and reverse,
5′-GCCACAGGATGTTCTCGTCA-3′; cyclin D1 forward,
5′-CAAGGCCTGAACCTGAGGAG-3′ and reverse, 5′-CTTGGGGTCCATGTTCTGCT-3′;
c-FLIP forward, 5′-GAGTGCCGGCTATTGGACTT-3′ and reverse,
5′-GCGCTTCTCTCCTACACCTC-3′; caspase-3 forward,
5′-GCGGTTGTAGAAGTTAATAAAGGT-3′ and reverse,
5′-TACCAGACCGAGATGTCATTCC-3′; caspase-7 forward,
5′-CGTGGGAACGGCAGGAAGT-3′ and reverse, 5′-CGGGTGGTCTTGATGGATCG-3′;
ATG4 forward, 5′-TACAGCATTTTCACAGAGAAGGACG-3′ and reverse,
5′-CTCCAGCAGGGAACCCATTAC-3′; ATG5 forward,
5′-TGGGCCATCAATCGGAAACTC-3′ and reverse,
5′-TGCAGCCACAGGACGAAACTC-3′; ATG12 forward,
5′-TTGGAGGCATAGACAGACAC-3′ and reverse, 5′-TATGTGTATTCCGTGCCATC-3′;
Beclin 1 forward, 5′-GTTGCCGTTATACTGTTCGT-3′ and reverse,
5′-CCTCCAGTGTCTTCAATG-3′; LAMP1 forward, 5′-GTGTCAGCTGGACGAGAACA-3′
and reverse, 5′-GCGCTAGATGGTCTGGTAGC-3′.
Western blotting
Cells (1×106) were washed twice with PBS and
incubated with pyrolysed solution containing 0.1 M
phenylmethylsulfonyl fluoride. Cell lysates were centrifuged at
13,000 × g for 20 min at 4°C. Proteins were quantified by Bradford
assay and 10 µg were separated by 12% SDS-PAGE and transferred onto
a polyvinylidene difluoride membrane. Following blocking with
5%non-fat dry milk in TBS for 1 h at room temperature, membranes
were incubated with rabbit anti-Beclin 1 (1:500 dilution, ab55878),
anti-GAPDH antibody (1:1,000 dilution, ab9485) or rabbit
anti-microtubule-associated protein 1A/1B-light chain 3 (LC3)B
(1:1,000 dilution, ab81785) (all from Abcam, Cambridge, UK) primary
antibodies at 4°C overnight. Following fives washes with TBS with
0.5% Tween-20, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:2,000 dilution, ab6717; Abcam) for 30 min at room temperature.
Specific bands were observed using enhanced chemiluminescene (ECL;
Thermo Fisher Scientific, Inc.) and detected using a Bio-Rad
ChemiDoc XRS image system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Flow cytometry
To determine cell apoptosis, 1×106 MG63 cells were
treated with Beclin 1, Gemcitabine, Beclin 1 + Gemcitabine, Beclin
1 + Gemcitabine + XAV939, or Beclin 1 + Gemcitabine + Wnt3α for 2
days. Cells were then fixed with 2% paraformaldehyde and
permeabilized by PBS containing 0.5% Triton X-100 (both from Sigma
Aldrich; Merck KGaA, Darmstadt, Germany). Cells were subsequently
resuspended in 1 ml Annexin V binding buffer. A fluorescein
isothiocyanate-conjugated Annexin V antibody at 1:500 (5 µl;
RUO-556419; BD Biosciences, San Diego, CA, USA) was added and
incubated for 20 min at room temperature. The cells were washed 3
times with PBS for 5 min, and at 20 min prior to flow cytometry
analysis, 5 µl propidium iodide was added to visualize DNA and
washed 3 times with PBS for 5 min. Data was acquired by BD
FACSCalibur™ 3C (BD Biosciences, Franklin Lakes, NJ, USA) and
analyzed by FlowJo software 7.6.1 (Tree Star, Inc., Ashland, OR,
USA).
Cell viability
An MTT assay was used to measure cell viability
according to the manufacturer's protocol. Cells were plated at a
density of 5×104/well into 96-well plates and treated with Beclin
1, Beclin 1 + XAV939 or Beclin 1 + wnt3α for 24 h, and then
stimulated with 20, 40 or 60 µg/ml Gemcitabine for 2 days.
Subsequently, 10 µl MTT solution (5 mg/ml; Sigma-Aldrich; Merck
KGaA) was added to each well and cells were incubated at 37°C for
4–6 h. Formazan crystals dissolved in 150 µl DMSO were added for 10
min with agitation. Absorbance was measured at a wavelength of 490
nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. One-way analysis of
variance was performed to determine statistical significance for
multiple comparisons, followed by a least-significant difference or
Tamhane test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Beclin 1 gene overexpression induces
autophagy in MG63 human osteosarcoma cell line
Previous reports have demonstrated that Beclin 1 is
required for initiating autophagosome formation (20). To validate the autophagic role of
Beclin 1, it was over-expressed in the MG63 cell line, and
DsRed-LC3-GFP reporters were used to monitor autophagy under a
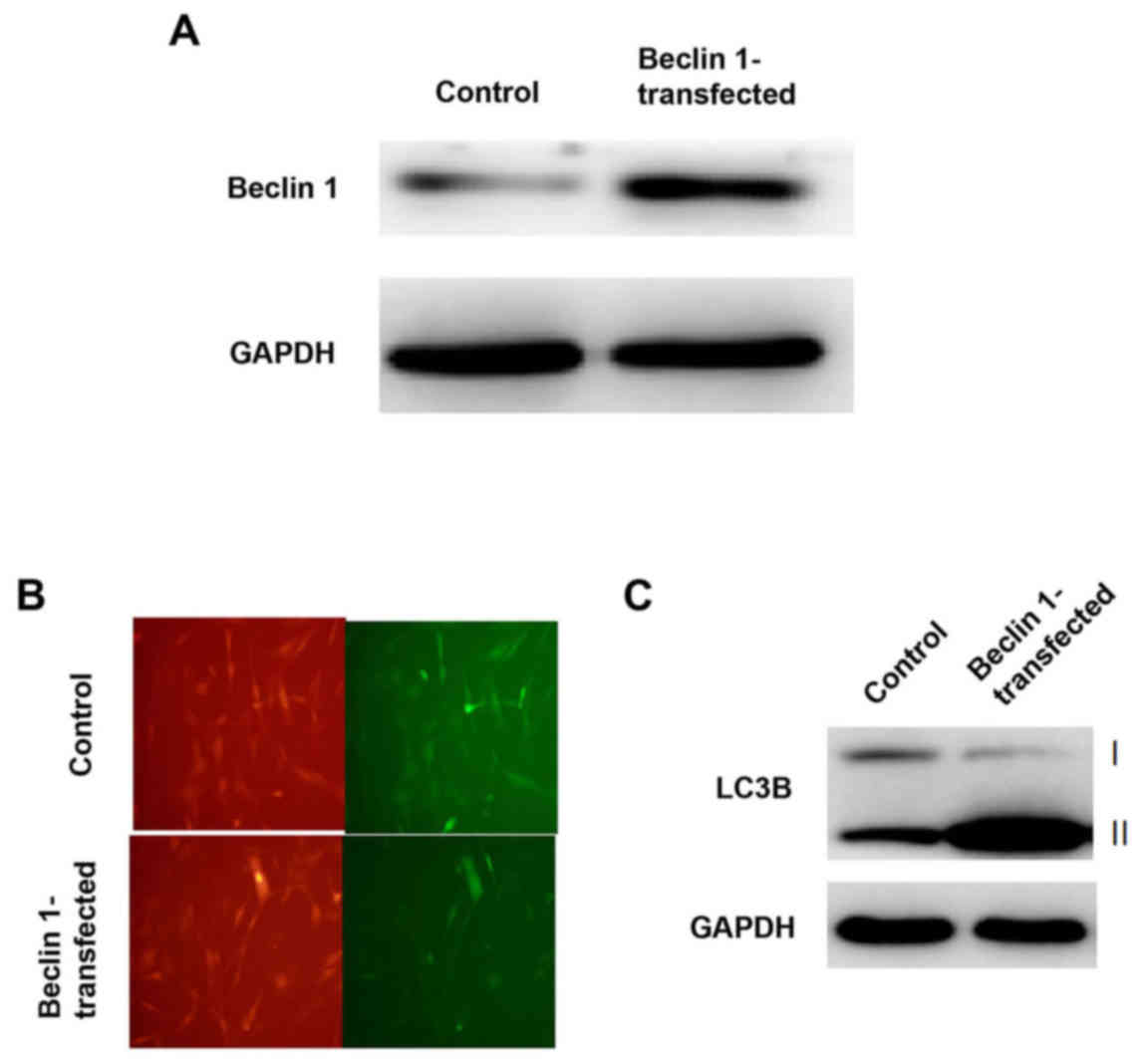
fluorescence microscope. Western blotting demonstrated that the
Beclin 1 gene was successfully over-expressed in MG63 cells
(Fig. 1A). Furthermore, Beclin 1
overexpression was associated with enhanced autophagy, which was
identified by dampened green fluorescence observed in MG63 cells
transfected with Beclin 1 compared with the control cells treated
with empty vector (Fig. 1B). LC3B
is a specific marker of the steady-state levels of autophagosomes.
Therefore, western blot analysis was performed to detect protein
expression levels of LC3B conversion and progression of autophagy.
Consistent with the fluorescence microscope results, increased
conversion of LC3B was detected following Beclin-1 overexpression
compared with control cells, which indicated increased autophagy in
cells over-expressing Beclin 1 (Fig.
1C).
Beclin 1 gene overexpression reduces
gemcitabine-induced apoptosis
To confirm whether activation of autophagy may
inhibit the induction of apoptosis by antitumor chemotherapy drugs,
the apoptosis of cells overexpressing Beclin 1 following treatment
with gemcitabine was analyzed by investigating the mRNA expression
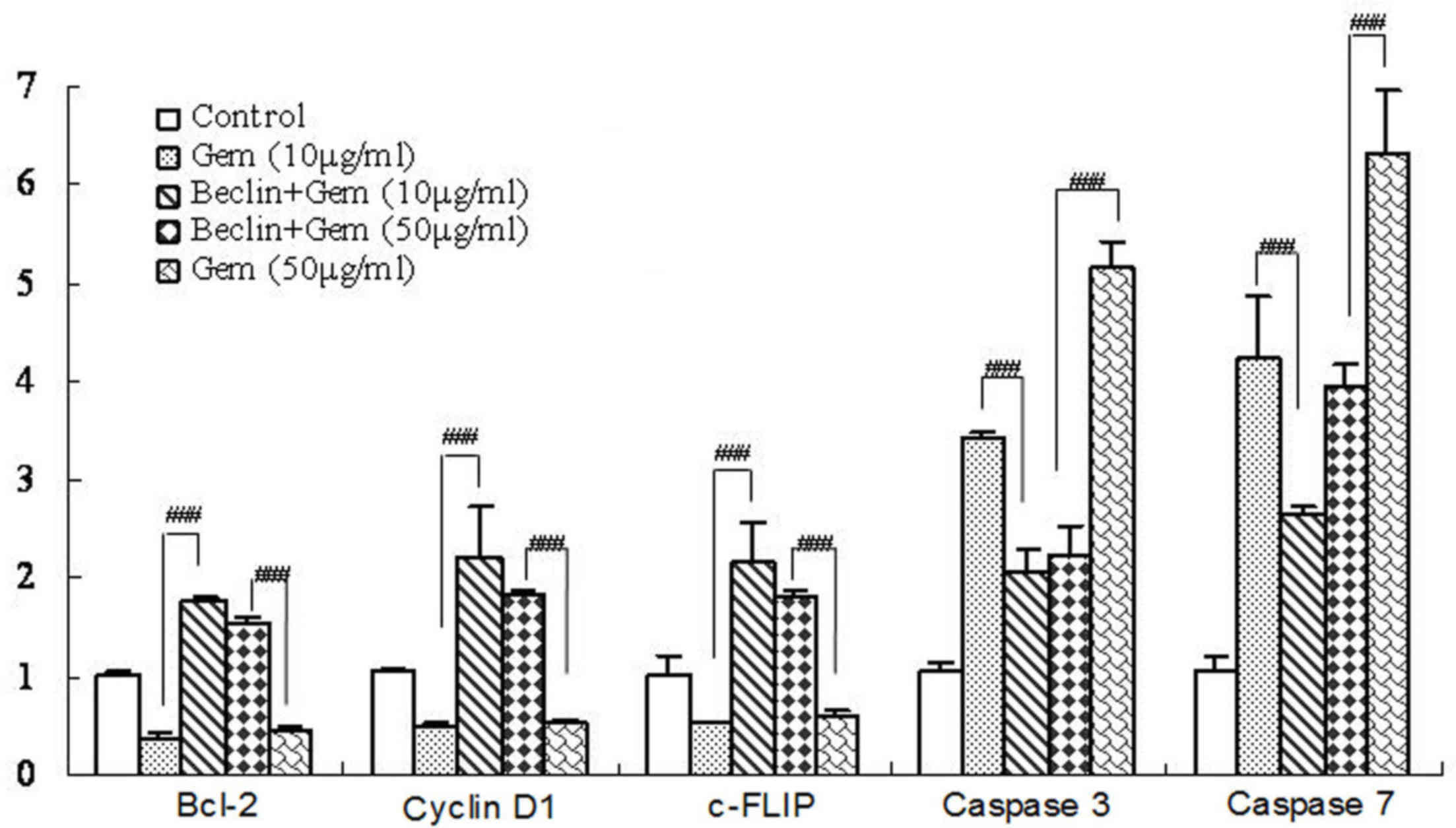
levels of pro- and anti-apoptotic genes. It was revealed that
gemcitabine alone induced apoptosis, which was determined by the
increased expression of apoptosis-associated genes caspase-3
(P<0.001) and 7 (P<0.001), and the reduced expression of
anti-apoptotic Bcl-2 (P<0.005), cyclin D1 (P<0.005) and
c-FLIP (P<0.005) (Fig. 2). As
expected, Beclin 1 overexpression significantly reduced the
expression of pro-apoptotic caspase-3 (P<0.001) and caspase-7
(P<0.001) induced by 50 µg/ml gemcitabine compared with
gemcitabine-treated cells without Beclin 1 stimulation. The results
demonstrated that overexpression of Beclin 1 was associated with a
downregulation in gemcitabine-induced apoptosis.
Activation of the Wnt/β-catenin
signaling pathway attenuates autophagy
The present study also investigated the potential
mechanisms of how Beclin 1 overexpression may reduce
gemcitabine-induced apoptosis. It has been previously reported that
suppression of the Wnt/β-catenin signaling pathway enhanced breast
cancer stem-like cell autophagy (21). The autophagic role of the
Wnt/β-catenin signaling pathway in the MG63 human osteosarcoma cell
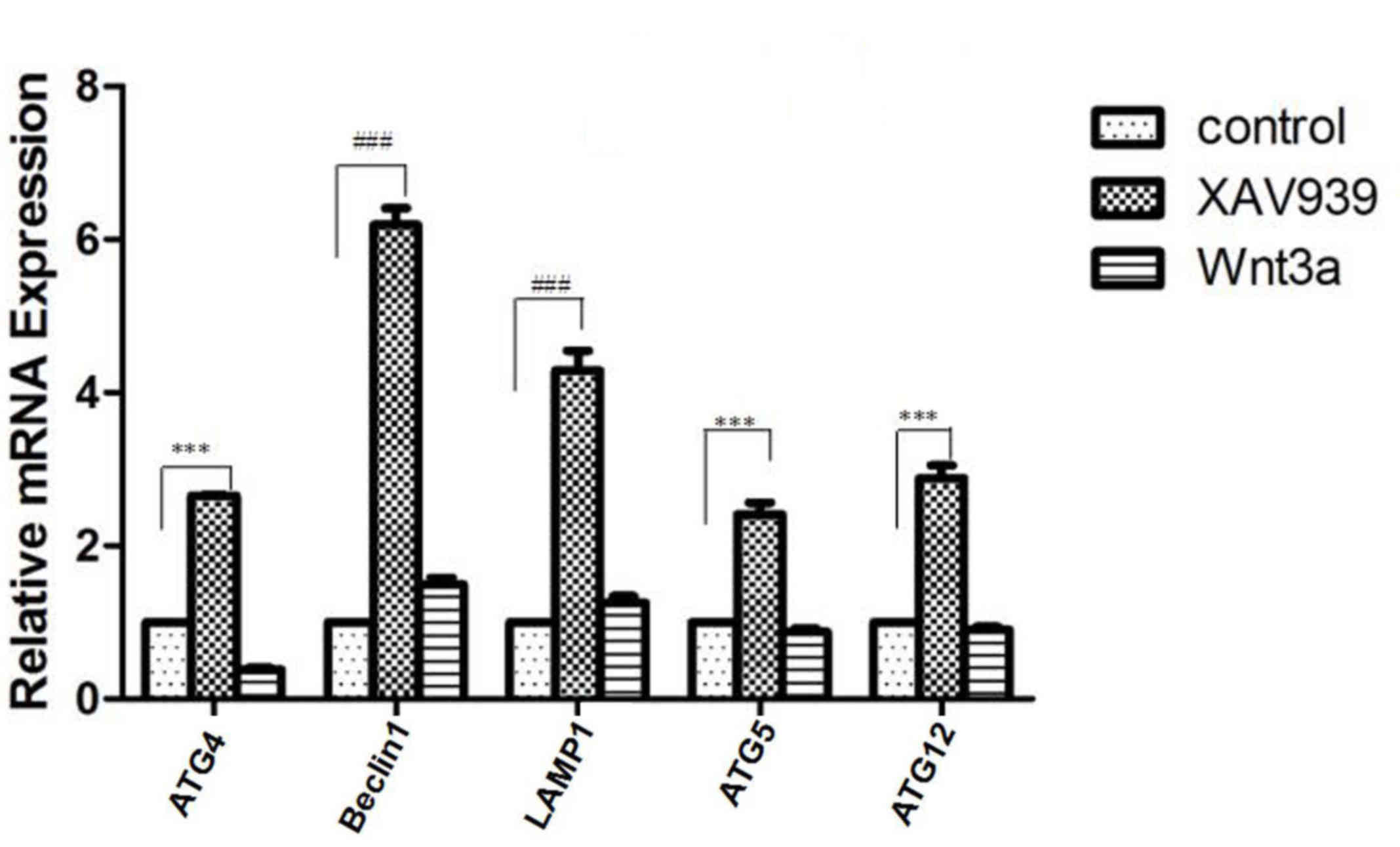
line was investigated using RT-qPCR. As demonstrated in Fig. 3, very little change was observed in
the relative mRNA expression of several autophagy-associated genes
including ATG4, Beclin 1, ATG5, ATG12 and LAMP1 in Wnt3a-treated
cells (activator of the Wnt/β-catenin signaling pathway) compared
with the control (P>0.05). However, the expression of ATG4
(P<0.05), ATG5 (P<0.05), ATG12 (P<0.05), Beclin 1
(P<0.01), and LAMP1 (P<0.01) was increased in XAV939-treated
cells (inhibitor of the Wnt/β-catenin signaling pathway) compared
with the control. Particularly, Beclin 1 expression was
significantly increased following the inhibition of the
Wnt/β-catenin signaling pathway by XAV939, indicating that altered
Beclin 1 expression may be involved in the Wnt/β-catenin signaling
pathway-attenuated autophagy.
Activation of the Wnt/β-catenin
signaling pathway inhibits autophagy and reduces the resistance of
Beclin 1-overexpressing MG63 cells to gemcitabine-induced
apoptosis
To further investigate the role of the Wnt/β-catenin
signaling pathway in Beclin 1 overexpression-induced autophagy,
Beclin 1-transfected MG63 human osteosarcoma cells were treated
with either a Wnt/β-catenin signaling activator (Wnt3a) or a
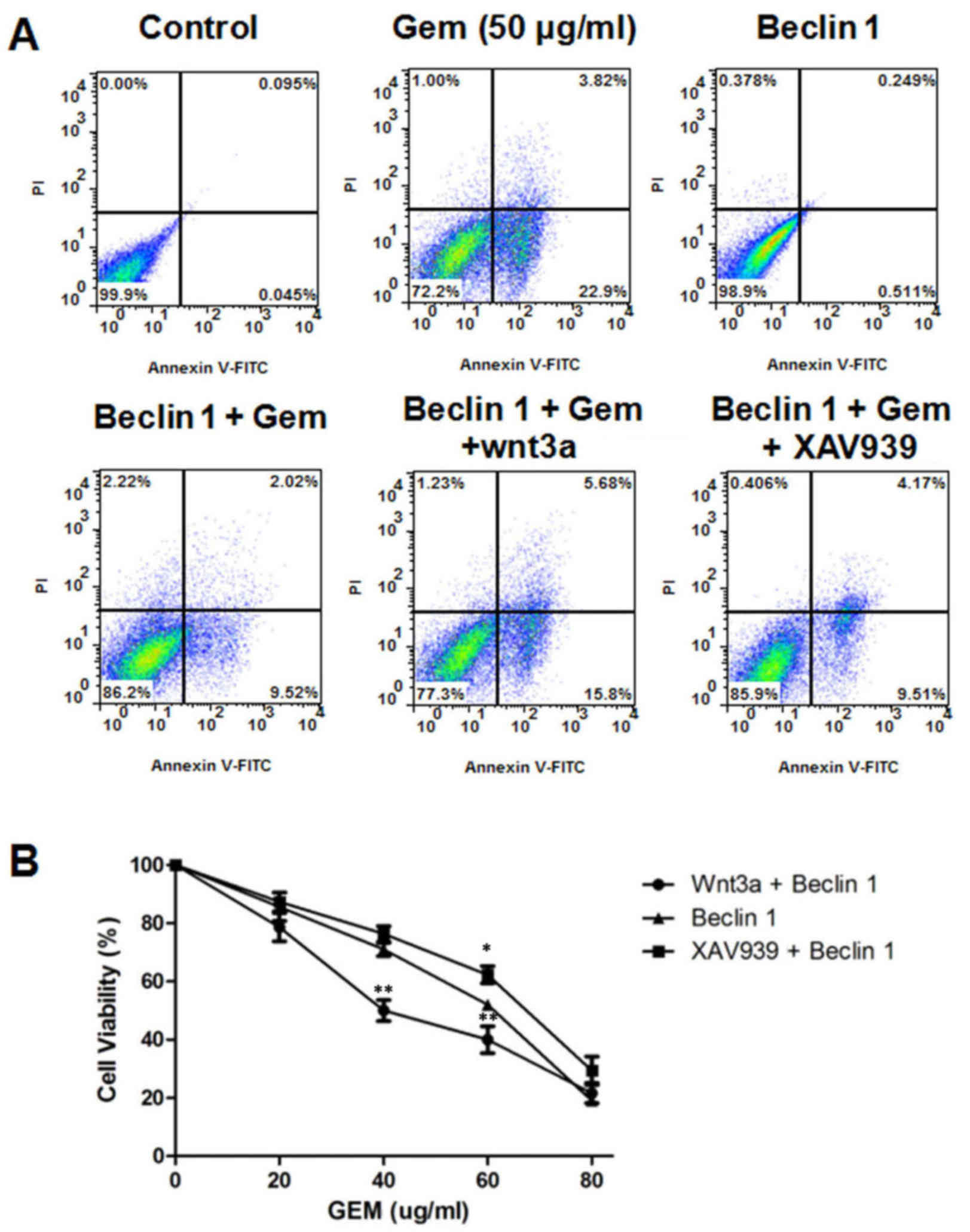
inhibitor (XAV939). The rate of apoptosis was measured by flow
cytometry, and the percentage of Annexin V-positive cells presented
in Fig. 4A. It was suggested that
Beclin 1 overexpression reduced apoptosis induced by gemcitabine,
and the activation of the Wnt/β-catenin signaling pathway by Wnt3a
reversed this reduction. Furthermore, Beclin 1 significantly
reduced cell viability induced by gemcitabine following the
activation of the Wnt/β-catenin signaling pathway, whereas Beclin 1
promoted cell viability induced by gemcitabine after the inhibition
of the Wnt/β-catenin signaling pathway (P<0.01; Fig. 4B). The results demonstrated that
the Wnt/β-catenin signaling pathway may inhibit autophagy and
increase gemcitabine-induced apoptosis in the MG63 cell line by
downregulating the expression of Beclin 1.
Discussion
In addition to the roles in development,
oncogenesis, cardiovascular, metabolic and neurodegenerative
diseases, autophagy is an important tumor resistance mechanism that
eliminates damaged proteins and organelles, and protects cells from
apoptosis (14). However, the
mechanisms by which autophagy affects drug-induced resistance in
tumor cells requires further investigation.
In the present study, Beclin 1 overexpression
enhanced autophagy and reduced gemcitabine-induced apoptosis in the
MG63 human osteosarcoma cell line, and the activation of the
Wnt/β-catenin pathway attenuated autophagy by downregulating Beclin
1 gene expression. The results of the current study also
demonstrated that Beclin 1 overexpressing MG63 cells were more
resistant to gemcitabine-induced apoptosis. However, Wnt/β-catenin
activation reversed the inhibition. The results revealed the
potential novel mechanisms of how autophagy may regulate
drug-induced resistance in antitumor chemotherapy. A number of
studies have demonstrated that Beclin 1 expression regulates
autophagy, and that Beclin 1 upregulation was associated with the
increased autophagosome numbers (22,23).
In addition, knockdown of Beclin 1 in tumor cell lines or in mice
was observed to inhibit autophagy (24,25).
Downregulated Beclin 1 expression has been previously demonstrated
in a number of tumor types, including human breast carcinoma, brain
tumors and prostatic carcinoma (26,27).
In the present study, to confirm the autophagic role of Beclin 1, a
Beclin 1 gene plasmid was transfected into the MG63 human
osteosarcoma cell line and the process of autophagy was monitored.
As expected, enhanced expression of LC3B and activity of autophagy
following overexpression of Beclin 1 was observed, indicating that
Beclin 1 functions promoted the autophagy process in MG63 tumor
cell lines.
The purpose of chemotherapy in tumors is to trigger
the apoptosis of tumor cells. Osteosarcoma, a cancerous tumor of
the bone, is an aggressive malignant neoplasm (28). Surgery plus chemotherapy is the
primary clinical treatment choice for osteosarcoma (29). Gemcitabine, an antitumor
chemotherapy drug that functions by inhibiting DNA synthesis, has
demonstrated significant clinical benefits in osteosarcoma
(30). A previous study revealed
that enhanced autophagy was associated with the resistance to
gemcitabine-induced tumor cell apoptosis (31). In the current study, consistent
with previous conclusions, the increased expression of
apoptosis-associated genes caspase-3 and −7, and downregulated
expression of anti-apoptotic genes BCL-2, cyclin D1 and c-FLIP by
gemcitabine treatment was observed in MG63 cell lines. However,
Beclin 1 overexpression reversed gemcitabine-induced resistance by
upregulating anti-apoptotic genes. The results demonstrated that
Beclin 1-induced autophagy may affect chemotherapy-induced
apoptosis in tumor cells.
Aberrant activation of the Wnt/β-catenin signaling
pathway is frequently observed in cancer cases. It was previously
revealed that the Wnt/β-catenin pathway also serves as a negative
regulator of autophagy (12,32,33).
To further examine the role of the Wnt/β-catenin signaling pathway
in chemotherapy resistance in tumor cells, the MG63 cell line was
treated with either a Wnt/β-catenin activator (Wnt3a) or a
inhibitor (XAV939), and the expression of autophagy-associated
genes and the apoptosis of gemcitabine-treated tumor cells were
investigated. Significantly enhanced autophagy-associatedgene
expression was observed following the inhibition of Wnt/β-catenin
pathway. Furthermore, the results demonstrated that Wnt/β-catenin
signaling pathway activation increased the percentage of
gemcitabine-induced apoptotic cells in the MG63 human osteosarcoma
cell line compared with cells only transfected with Beclin 1.
Beclin 1 mRNA expression levels were greatly inhibited following
Wnt/β-catenin activator treatment, compared with inhibitor XAV939
treatment. The results demonstrated that Wnt/β-catenin signaling
reversed gemcitabine resistance by attenuating Beclin 1-mediated
autophagy.
In conclusion, the results of the current study
provided a novel insight into resistance to antitumor chemotherapy
drugs. Aberrant Wnt/β-catenin signaling may enhance Beclin
1-mediated autophagy, thus preventing chemotherapy drug-induced
cell apoptosis. These results may provide evidence for enhancing
the sensitivity of tumors to apoptosis induced by chemotherapy
drugs, and by modulating aberrant Wnt/β-catenin
signaling-associated autophagy and autophagy proteins.
References
|
1
|
Plunkett W, Huang P, Xu YZ, Heinemann V,
Grunewald R and Gandhi V: Gemcitabine: Metabolism, mechanisms of
action, and self-potentiation. Semin Oncol. 22 4 Suppl 11:S3–S10.
1995.
|
|
2
|
Ahn DH, Krishna K, Blazer M, Reardon J,
Wei L, Wu C, Ciombor KK, Noonan AM, Mikhail S and Bekaii-Saab T: A
modified regimen of biweekly gemcitabine and nab-paclitaxel in
patients with metastatic pancreatic cancer is both tolerable and
effective: A retrospective analysis. Ther Adv Med Oncol. 9:75–82.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Y, Fu JT, Shi D, Feng B and Shi Z:
Clinical efficacy and safety of gemcitabine plus nedaplatin in the
treatment of advanced nasopharyngeal carcinoma. J Cancer Res Ther.
12:C252–C255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan MF, Gottesman S, Boyella R and
Juneman E: Gemcitabine-induced cardiomyopathy: A case report and
review of the literature. J Med Case Rep. 8:2202014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao S, Guo J, Sun L, Lv J and Qiu W:
Gemcitabine-based chemotherapy in colon squamous cell carcinoma: A
case report and literature review. Mol Clin Oncol. 6:561–565.
2017.PubMed/NCBI
|
|
6
|
Lee HS, Park SB, Kim SA, Kwon SK, Cha H,
Lee DY, Ro S, Cho JM and Song SY: A novel HDAC inhibitor, CG200745,
inhibits pancreatic cancer cell growth and overcomes gemcitabine
resistance. Sci Rep. 7:416152017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei MY, Zhuang YF and Wang WM: Gemcitabine
for the treatment of patients with osteosarcoma. Asian Pac J Cancer
Prev. 15:7159–7162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:1845–1846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muzes G and Sipos F: Anti-tumor immunity,
autophagy and chemotherapy. World J Gastroenterol. 18:6537–6540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong JT, Xu Y, Yi HW, Su J, Yu HM, Xiang
XY, Li XN, Zhang ZC and Sun LK: The BH3 mimetic S1 induces
autophagy through ER stress and disruption of Bcl-2/Beclin 1
interaction in human glioma U251 cells. Cancer Lett. 323:180–187.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
16
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petherick KJ, Williams AC, Lane JD,
Ordonez-Moran P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik
K, Paraskeva C and Greenhough A: Autolysosomal β-catenin
degradation regulates Wnt-autophagy-p62 crosstalk. Embo J.
32:1903–1916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheen JH, Zoncu R, Kim D and Sabatini DM:
Defective regulation of autophagy upon leucine deprivation reveals
a targetable liability of human melanoma cells in vitro and in
vivo. Cancer Cell. 19:613–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun T, Li X, Zhang P, Chen WD, Zhang HL,
Li DD, Deng R, Qian XJ, Jiao L, Ji J, et al: Acetylation of Beclin
1 inhibits autophagosome maturation and promotes tumour growth. Nat
Commun. 6:72152015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim G, Kim JY, An HJ, Kang H, Kim TH, Shim
JY, Heo JH, Park HL and Choi YK: The loss of E-cadherin is
associated with the Epigenetic Alteration of CDH1 in breast cancer
and it is also associated with an Abnormal beta-catenin expression
in lobular carcinoma. Korean J Pathol. 43:400–407. 2009. View Article : Google Scholar
|
|
22
|
Luo S and Rubinsztein DC: Apoptosis blocks
Beclin 1-dependent autophagosome synthesis: An effect rescued by
Bcl-xL. Cell Death Differ. 17:268–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by Beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boya P, Gonzaléz-Polo RA, Casares N,
Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D,
Souquere S, Yoshimori T, et al: Inhibition of macroautophagy
triggers apoptosis. Mol Cell Biol. 25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the Beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yorimitsu T and Klionsky DJ: Eating the
endoplasmic reticulum: Quality control by autophagy. Trends Cell
Biol. 17:279–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aita VM, Liang XH, Murty VV, Pincus DL, Yu
W, Cayanis E, Kalachikov S, Gilliam TC and Levine B: Cloning and
genomic organization of Beclin 1, a candidate tumor suppressor gene
on chromosome 17q21. Genomics. 59:59–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garrington GE, Scofield HH, Cornyn J and
Hooker SP: Osteosarcoma of the jaws. Analysis of 56 cases. Cancer.
20:377–391. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: Where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang C, Li H, Shen C, Lai J, Shi Z, Liu B
and Tao HM: Genistein potentiates the anti-cancer effects of
gemcitabine in human osteosarcoma via the downregulation of Akt and
nuclear factor-κB pathway. Anticancer Agents Med Chem. 12:554–563.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mukubou H, Tsujimura T, Sasaki R and Ku Y:
The role of autophagy in the treatment of pancreatic cancer with
gemcitabine and ionizing radiation. Int J Oncol. 37:821–828.
2010.PubMed/NCBI
|
|
32
|
Teiten MH, Gaascht F, Cronauer M, Henry E,
Dicato M and Diederich M: Anti-proliferative potential of curcumin
in androgen-dependent prostate cancer cells occurs through
modulation of the Wingless signaling pathway. Int J Oncol.
38:603–611. 2011.PubMed/NCBI
|
|
33
|
Hope C, Planutis K, Planutiene M, Moyer
MP, Johal KS, Woo J, Santoso C, Hanson JA and Holcombe RF: Low
concentrations of resveratrol inhibit Wnt signal throughput in
colon-derived cells: Implications for colon cancer prevention. Mol
Nutr Food Res. 52 Suppl 1:S52–S61. 2008.PubMed/NCBI
|