Introduction
Chemotherapy is considered an important approach for
the treatment of malignancies, however, several types of cancer
develop resistance to multiple antineoplastic drugs, which appears
to be primarily mediated by P-glycoprotein (P-gp) (1,2).
P-gp is encoded by the multidrug resistance (mdr1) gene, which
belongs to the ATP-binging cassette superfamily of transporters. It
is a large membrane-spanning protein, which acts as a
representative efflux pump (3–5). The
inhibition of P-gp transporters and modulation of multidrug
resistance (MDR) are important strategies in reversing MDR.
Astragaloside IV (ASIV) is a saponin isolated from
the rhizome of Radix Astragali, which is widely used in traditional
Chinese medicine (6). Our previous
studies reported that ASIV reverses the resistance of Bel-7402/FU
cells to drugs by downregulating the expression levels of mdr1 and
P-gp (7). However, the molecular
mechanism underlying the action of ASIV in downregulating the
expression of mdr1 remains to be fully elucidated.
The c-Jun N-terminal kinase (JNK) signaling pathway,
one of the mitogen-activated protein kinase (MAPK) pathways, may be
critical in the MDR phenotype (8–10).
The activation of JNK is increased in several types of human MDR
cancer cell following treatment with different chemotherapeutic
agents (11,12), whereas the inhibition of JNK or
combined treatment of a JNK inhibitor with anticancer drugs may
prevent the development of MDR (13). The present study aimed to
investigate the involvement of the JNK/c-Jun/activator protein-1
(AP-1) signaling pathway in ASIV-induced downregulated expression
of mdr1.
Materials and methods
Extraction, isolation and preparation
of ASIV
The ASIV was extracted and separated from the
Astragalus root (Gansu, SDIC Pharmaceutical Anhui Co., Ltd.,
Xuancheng, China) as previously described (14). ASIV preparation was performed
according to a previously published method (7).
Cell culture
The 5-fluorouracil (5-FU)-resistant Bel-7402/FU
human hepatic cancer cell line, purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China), was cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with the addition
of 20,000 ng/ml 5-FU (Tianjin Taihe Pharmaceutical Co., Ltd.,
Tianjin, China) and 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) in a CO2 incubator at 37°C.
Determination of the cytotoxicity of
SP600125
The in vitro cytotoxicity of the JNK
inhibitor, SP600125, was investigated using an MTT assay. The
Bel-7402/FU cells were seeded into 96-well plates at a density of
5×103 cells per well. Following incubation for 24 h,
SP600125 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
concentrations of 1, 2, 5, 10, 20, 40 and 80 µM were added. The
cells were then incubated for 24 h at 37°C in 5% CO2,
followed by the addition of MTT (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) solution (0.5 mg/ml) to each well and
incubation for 4 h at 37°C. The medium was discarded and formazan
crystals were dissolved in 150 µl DMSO. The plates were read at 570
nm with an automated microplate reader (680; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The concentrations of the assayed enzymes
required to inhibit cell proliferation by 50% (IC50
values) were calculated using SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA).
Western blot analysis
The Bel-7402/FU cells (1×106 cells/ml)
were treated with 0.1 mM ASIV or 11 µM SP600125 for 24 h at 37°C.
Cell extracts were prepared in RIPA buffer with the proteinase
inhibitor cocktail (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany). Protein concentration was quantified using a Bradford
assay (Beyotime Institute of Biotechnology, Inc., Haimen, China). A
20 µg per lane protein sample was separated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Bedford, MA,
USA). The membranes were blocked with 5% non-fat milk for 2 h at
room temperature, then probed with appropriate antibodies at 4°C
overnight in 5% BSA, including rabbit polyclonal antibodies against
P-gp (1:1,000, cat. no. ab98322, Abcam, Cambridge, UK),
phosphorylated (p-)-JNK (1:5,000, cat. no. 4668, Cell Signaling
Technology, Inc., Beverly, MA, USA) and JNK (1:5,000, cat. no.
9258, Cell Signaling Technology, Inc.), p-c-Jun (1:500, cat. no.
3270, Cell Signaling Technology, Inc.) and c-Jun (1:5,000, cat. no.
9165, Cell Signaling Technology, Inc.) and mouse monoclonal
antibody against β-actin (1:500, cat. no. sc-47778, Santa Cruz
Biotechnology, Inc.). The membranes were washed and incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin G (1:50,000, cat. no. ZB-2301, Zhongshan Jinqiao
Biotech, Co., Ltd., Beijing, China) or HRP-conjugated goat
anti-mouse immunoglobulin G (1:10,000, cat. no. ZB-2305, Zhongshan
Jinqiao Biotech, Co., Ltd.), and then detected using enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The Bel-7402/FU cells (1×106 cells/ml)
were treated with 0.1 mM ASIV or 11 µM SP600125 for 24 h at 37°C.
Total RNA was extracted from the cells using TRIzol reagent
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA (1 µg) was reverse transcribed using a
reverse transcription kit (Thermo Fisher Scientific, Inc.). The
RT-qPCR was performed using a Bio-Rad Real-time system (CFX96;
Bio-Rad Laboratories, Inc.) with a SYBR Green PCR kit (Qiagen GmbH,
Hilden, Germany). For RT-qPCR analysis, 1 µl cDNA samples, 1 µl
target primers (5 µM) were used to a total reaction volume of 10
µl. The primers were as follows: mdr1, forward
5′-GCTGTTCGTTTCCTTTAGGTCTTTC-3′ and reverse
5′-AGTTCTTCTTCTTTGCTCCTCCATT-3′; β-actin, forward
5′-GGGAAATCGTGCGTGACATTAAGG-3′ and reverse
5′-CAGGAAGGAAGGCTGGAAGAGTG-3′. PCR reaction conditions were as
follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 15 sec
and 60°C for 30 sec. Relative quantification of the mRNA expression
of mdr1 was normalized to the housekeeping gene β-actin using the
2−ΔΔCq method (15).
Nuclear protein extraction and
electrophoretic mobility shift assays (EMSA)
The Bel-7402/FU cells (1×106 cells/ml)
were incubated with 0.1 mM ASIV or 11 µM SP600125 for 24 h. The
cells were then treated with ice-cold lysis buffer and incubated
for 5 min. The nuclei were centrifuged at 13,225 × g for 1 min at
4°C. The nuclear pellet was resuspended in nuclear protein
extraction buffer for 30 min, and then centrifuged at 20,034 × g
for 10 min at 4°C. EMSA was performed using a non-radioactive EMSA
kit according to the manufacturer's protocol (Pierce; Thermo Fisher
Scientific, Inc.). Nuclear extracts (8 µg) were incubated with
32P-labeled double-stranded oligonucleotide with a
specific AP-1 binding sequence
(5′-GGAATCAGCATTCAGTCAATCCGGGCCGGG-3′) for 20 min at room
temperature. The specific competitor unlabeled oligonucleotide was
added in a competing system (cold competition) and incubated for 20
min. The protein-DNA complexes were resolved by electrophoresis at
4°C on a 6.5% non-denaturing acrylamide gel and subjected to
autoradiography. The electrophoresis was performed at 175 V in
0.25X TBE [1X TBE contained 89 mM Tris-HCl, 89 mM boric acid and 5
mM EDTA (pH 8.0)] at 4°C for 1 h. The gels were transferred to the
banding membrane at 394 mA in 0.5X TBE at room temperature for 40
min. Crosslinking of the membrane was performed using an
ultraviolet crosslink apparatus for 10 min (immobilization),
followed by blocking for 30 min at room temperature,
streptavidin-HRP labeling the membrane for 30 min at room
temperature, washing the membrane 4 times for 5 min at room
temperature and equilibrating the membrane for 5 min, obtaining the
images through the imager apparatus (Alpha Flurechemical; Alpha
Innotech Corporation, San Leandro, CA, USA).
Flow cytometric analysis
The Bel-7402/FU cells (5×105 cells/ml)
were incubated with 0.1 mM ASIV or 11 µM SP600125 for 24 h. A total
of 106 cells per well were incubated with 5 µg/ml
rhodamine 123 (Rh123; Sigma-Aldrich; Merck Millipore) for 1 h at
37°C and washed twice with cold PBS. Cell fluorescence was
evaluated using a flow cytometer (FC500; Beckman Coulter, Miami,
FL, USA) at an excitation wavelength of 488 nm and emission
wavelength of 525 nm.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical analysis of
difference was performed using one-way analysis of variance with
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
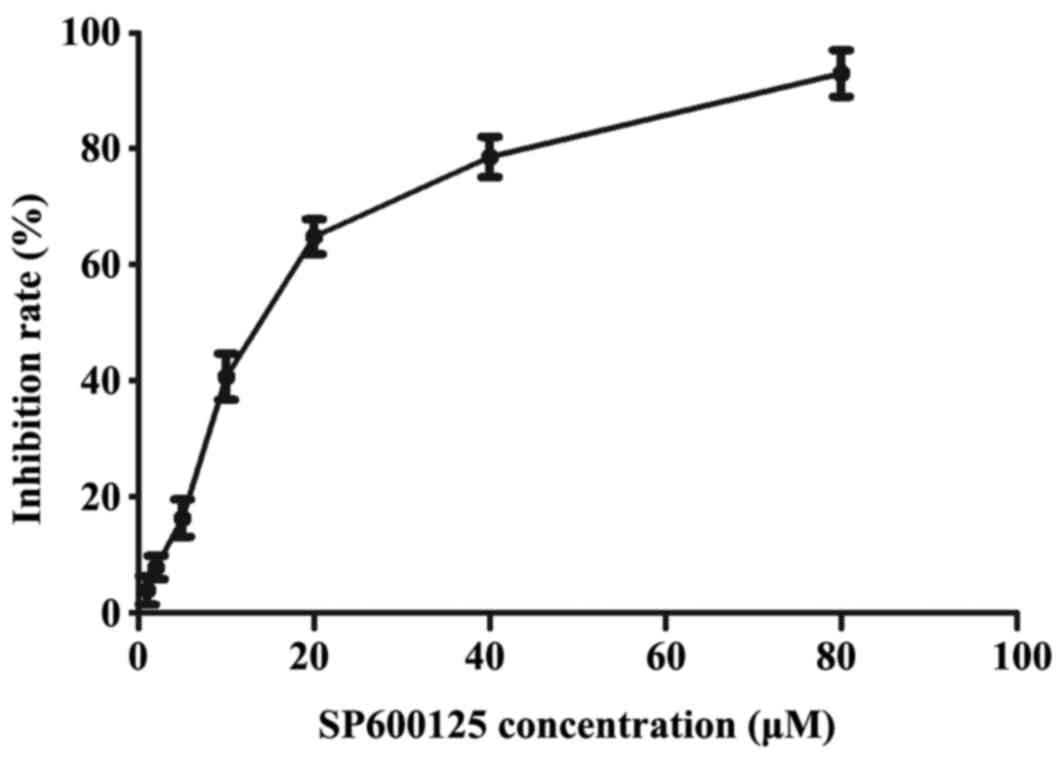
Cytotoxicity assay of SP600125
To further investigate the effect of the JNK
inhibitor, SP600125, in Bel-7402/FU cells, a weak cytotoxic
concentration of SP600125 was required. The cytotoxic effects of
SP600125 were measured using an MTT assay (Fig. 1). The IC50 of SP600125
in the Bel-7402/FU cells was 11.10 µM, which exhibited weak
cytotoxicity (inhibition rate <50%). Therefore, an 11 µM
concentration of SP600125 was used as the concentrations for the
following experiments.
ASIV inhibits the phosphorylation of
JNK and c-Jun in Bel-7402/FU cells
To examine the possible role of the JNK/c-Jun
signaling pathway in the ASIV-mediated reversal of MDR, the present
study examined the proteins levels of total-JNK, p-JNK,
total-c-Jun, p-c-Jun and P-gp in Bel-7402/FU cells using western
blot analysis. As shown in Fig. 2,
the JNK, p-JNK, c-Jun, p-c-Jun and P-gp proteins were detected in
the Bel-7402/FU cells. The results demonstrated that activation of
the JNK pathway was involved in the MDR of Bel-7402/FU cells. When
the Bel-7402/FU cells were treated with 0.1 mM ASIV or 11 µM
SP600125, the protein levels of P-gp, p-JNK and p-c-Jun were
decreased, whereas no significant change in the protein levels of
total JNK or c-Jun were observed. These results indicated that
SP600125 partially reversed the drug resistance of Bel-7402/FU
cells, and that activation of the JNK pathway was crucial in the
mechanism underlying the ASIV-mediated reversal of MDR.
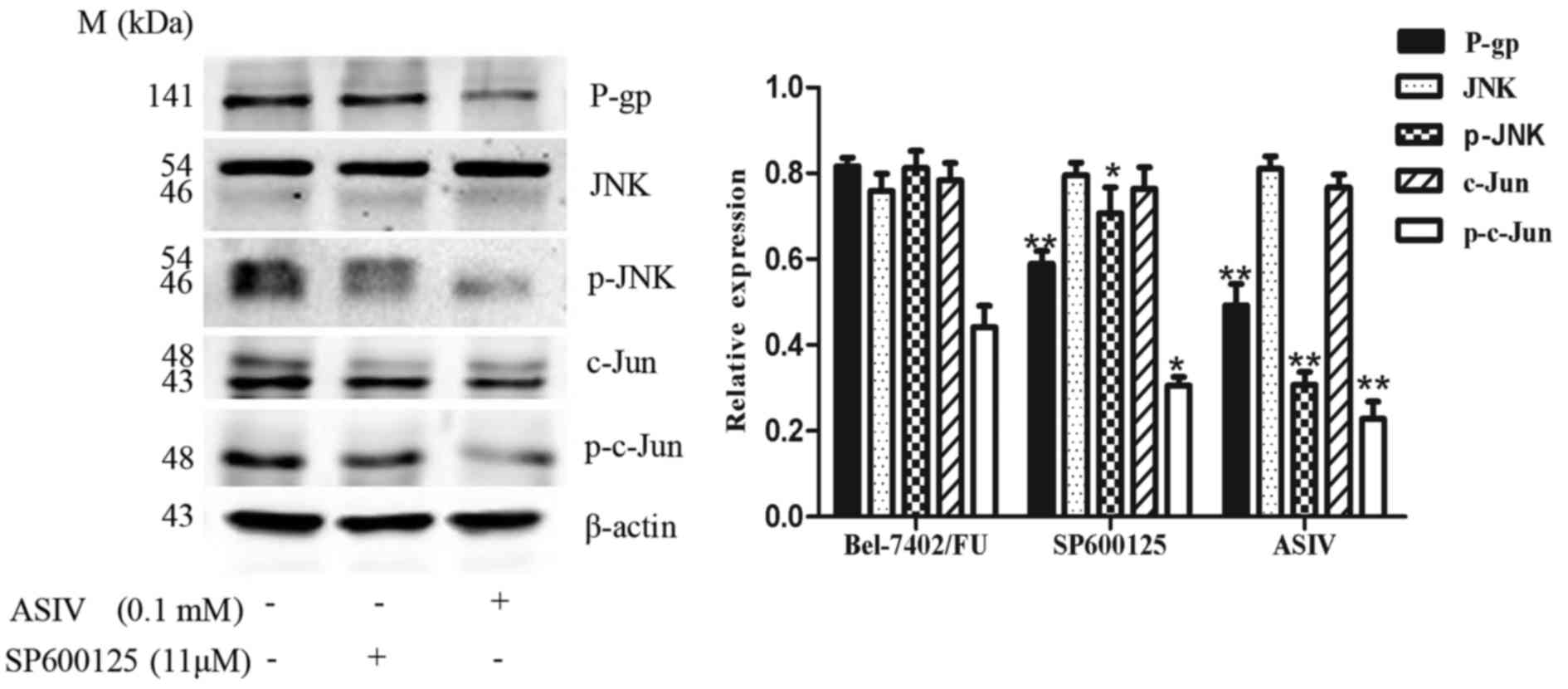 | Figure 2.Effect of ASIV and JNK pathway
inhibitor SP600125 on the expression of P-gp in Bel-7402/FU cells.
Western blot analysis of the expression levels of P-gp, JNK, p-JNK,
c-Jun, p-c-Jun and β-actin were determined in Bel-7402/FU cells
treated with 0.1 mM ASIV or 11 µM SP600125 for 24 h. Relative
protein expression levels of P-gp, p-JNK and p-c-Jun were
quantified following normalization to β-actin. Data are presented
as the mean ± standard deviation of three independent experiments.
*P<0.05 and **P<0.01, compared with the Bel-7402/FU cell
control group. ASIV, astragaloside IV; JNK, c-Jun N-terminal
kinase; P-gp, P-glycoprotein; p-, phosphorylated. |
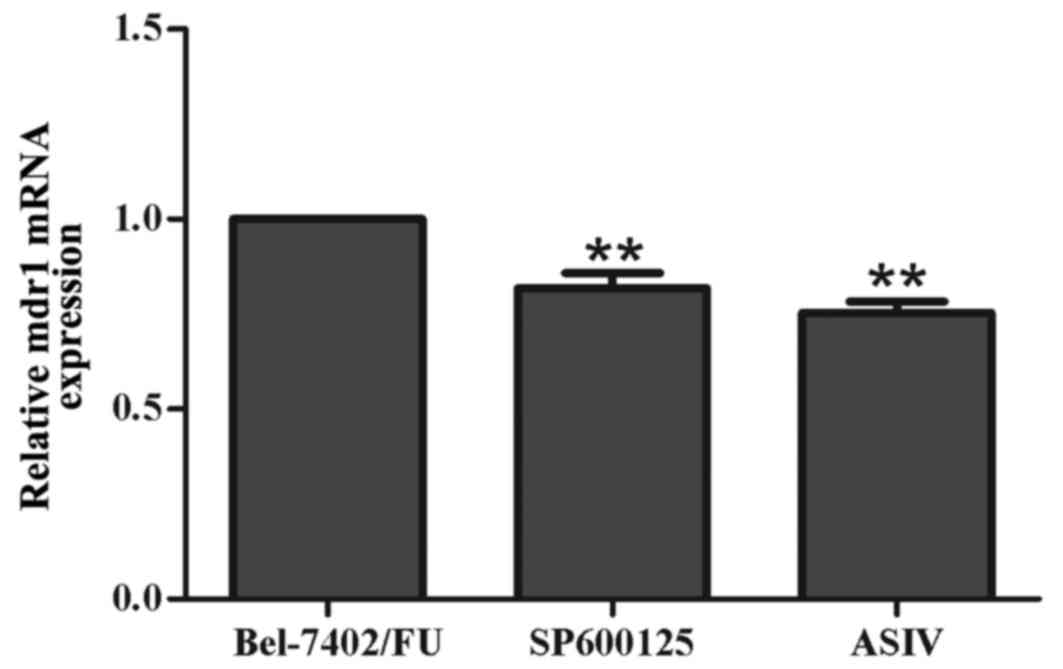
SP600125 downregulates the mRNA
expression of mdr1 in Bel-7402/FU cells
To investigate whether the JNK pathway inhibitor,
SP600125, downregulated mdr1 at the mRNA level, the mRNA expression
of mdr1 was examined using RT-qPCR analysis. As shown in Fig. 3, the mRNA levels of mdr1 were
decreased by 11 µM SP600125 and by 0.1 mM ASIV. These results
suggested that inhibiting the activation of the JNK pathway
prevented the development of MDR by downregulating the expression
of mdr1 in Bel-7402/FU cells.
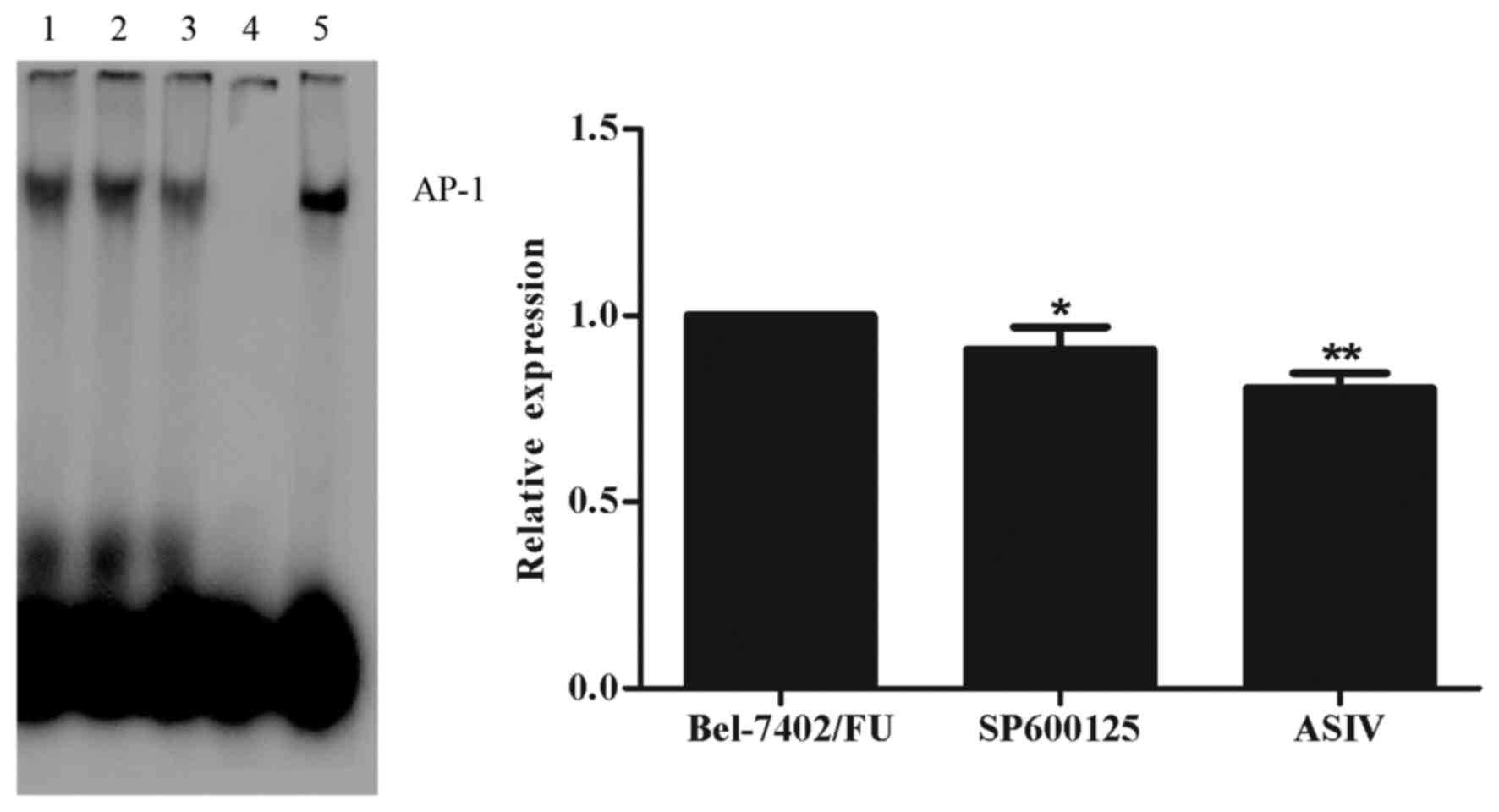
ASIV decreases AP-1 DNA binding
activity
In order to investigate the role of AP-1 in
regulating the gene expression of mdr1, an EMSA was performed. As
shown in Fig. 4, the DNA-binding
activity of AP-1 was decreased by 0.1 mM ASIV and by 11 µM SP600125
in the Bel-7402/FU cells. These results indicated that the
ASIV-induced downregulated expression of mdr1 was mediated by the
JNK/c-Jun/AP-1 signal transduction pathway.
ASIV and SP600125 inhibit the function
of P-gp
To determine the effect of ASIV and SP600125 on the
function of P-gp as an efflux pump, the fluorescence intensity of
P-gp substrate, Rh123, was examined in Bel-7402/FU cells treated
with ASIV or SP600125. As shown in Fig. 3, treatment with 0.1 mM ASIV and
treatment with 11 µM SP600125 increased the intracellular
accumulation of fluorescent Rh123.
Discussion
MDR in tumor cells involves complex intracellular
mechanisms, including decreased drug uptake and increased drug
efflux, which reduce intracellular drug concentrations (16,17).
P-gp, encoded by the mdr1 gene, functions in a manner similar to a
pump, to extrude chemotherapy drugs from cancer cells. The
inhibition of P-gp transporter function or inhibition of its
expression may reverse the MDR phenotype (18). Our previous investigations revealed
that ASIV not only reduced the protein expression of P-gp and gene
expression of mdr1, but also inhibited P-gp-mediated drug efflux in
the MDR Bel-7402/FU human hepatic cancer cell line (7). However, which signaling pathway was
involved in the ASIV-induced downregulated expression of mdr1
remained to be elucidated. Studies have indicated that activation
of the JNK signaling pathway or the transcription factor, c-Jun,
has a principal role in mdr1-induced MDR (19,20).
Inhibition of the JNK signaling pathway enhances the sensitivity of
hepatocellular carcinoma cells to cisplatin by downregulating the
expression of P-gp (13). In the
present study, the protein expression levels of p-JNK and p-c-Jun
were decreased when the Bel-7402/FU cells were treated with ASIV.
In addition, SP600125, an inhibitor of the JNK signaling pathway,
downregulated the expression of P-gp and mdr1 in the Bel-7402/FU
cells. These results showed that the activation of JNK may be
essential for the induction of mdr1 in Bel-7402/FU cells.
To further elucidate the molecular mechanism
underlying the effect of ASIV on the reversal of drug resistance in
Bel-7402/FU cells; the present study examined the DNA binding
activity of AP-1 to the mdr1 promoter. The AP-1 transcription
factor, belonging to the leucine zipper family, consists of dimers
of Jun/Jun (c-Jun, JunB and JunD) or Jun/Fos (c-Fos, FosB,
Fos-related antigen 1, Fos-related antigen 2, activating
transcription factor 2 and cAMP response element binding protein)
proteins (21,22). Activated c-Jun homodimers and/or
heterodimers with c-Fos form the AP-1 transcription complex,
recognize TPA-responsive elements (5′-TGAG/CTCA-3′) and activate
the transcription of target genes. Previous studies have
demonstrated that the promoter region of the mdr1 gene contains a
putative binding site for AP-1 (23). To investigate whether the decreased
expression of p-c-Jun in Bel-7402/FU cells treated with ASIV has a
functional effect on AP-1 binding activity, the present study used
an EMSA to determine the binding activity of AP-1 to an
oligonucleotide probe containing the relevant mdr1 promoter
sequences. When the Bel-7402/FU cells were treated with ASIV or
SP600125, AP-1 binding activity was decreased. Therefore, AP-1 may
be involved in the ASIV-mediated downregulated expression of
mdr1.
The results of the present study are consistent with
previous reports that ASIV reverses the MDR of Bel-7402/FU cells by
downregulating the mRNA expression of mdr1 and protein expression
of P-gp (7). In the present study,
the JNK inhibitor, SP600125, reduced the expression of mdr1 and
P-gp, and increased the intracellular accumulation of Rh123 in
Bel-7402/FU cells. The results showed that activation of the JNK
signaling pathway promoted MDR, whereas SP600125 partially reversed
MDR in Bel-7402/FU cells.
Previous studies have reported that astragaloside II
(ASII) reverses the MDR of Bel-7402/FU cells through inhibiting the
phosphorylation of extracellular signal-regulated kinases (ERKs),
p38 and JNK (14). The results in
the present study demonstrated that ASIV may reverse the drug
resistance of Bel-7402/FU cells by downregulating the expression of
mdr1 via inhibition of the JNK/c-Jun/AP-1 signaling pathway.
However, whether other MAPK signaling pathways, including ERK and
p38 MAPK kinases, are involved in the ASIV-induced downregulation
of mdr1 requires elucidation in further investigations.
Acknowledgements
The present study was supported by the Anhui
Provincial Natural Science Major Research Project (grant no.
KJ2014A274).
References
|
1
|
Aouali N, Eddabra L, Macadré J and Morjani
H: Immunosuppressors and reversion of multidrug-resistance. Crit
Rev OncolHematol. 56:61–70. 2005. View Article : Google Scholar
|
|
2
|
Chen CY, Liu NY, Lin HC, Lee CY, Hung CC
and Chang CS: Synthesis and bioevaluation of novel benzodipyranone
derivatives as P-glycoprotein inhibitors for multidrug resistance
reversal agents. Eur J med Chem. 118:219–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safa AR: Identification and
characterization of the binding sites of P-glycoprotein for
multidrug resistance-related drugs and modulators. Curr Med Chem
Anti Cancer Agents. 4:1–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan WQ, Zhang RR, Wang J, Ma Y, Li WX,
Jing RW and Cai SH: Asclepiasterol, a novel C21 steroidal glycoside
derived from Asclepias curassavica, reverses tumor multidrug
resistance by downregulating P-glycoprotein expression. Oncotarget.
7:31466–31483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohseni M, Samadi N, Ghanbari P, Yousefi
B, Tabasinezhad M, Sharifi S and Nazemiyeh H: Co-treatment by
docetaxel and vinblastine breaks down P-glycoprotein mediated
chemo-resistance. Iran J Basic Med Sci. 19:300–309. 2016.PubMed/NCBI
|
|
6
|
Song JZ, Yiu HH, Qiao CF, Han QB and Xu
Hx: Chemical comparison and classification of Radix Astragali by
determination of isoflavonoids and astragalosides. J Pharm Biomed
Anal. 47:399–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang PP, Xu DJ, Huang C, Wang WP and Xu
WK: Astragaloside IV reduces the expression level of P-glycoprotein
in multidrug-resistant human hepatic cancer cell lines. Mol Med
Rep. 9:2131–2137. 2014.PubMed/NCBI
|
|
8
|
Osborn MT and Chambers TC: Role of the
stress-activated/c-Jun NH2-terminal protein kinase pathway in the
cellular response to adriamycin and other chemotherapeutic drugs. J
Biol Chem. 271:30950–30955. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han L, Wang Y, Guo X, Zhou Y, Zhang J,
Wang N, Jiang J, Ma F and Wang Q: Downregulation of MDR1 gene by
cepharanthine hydrochloride is related to the activation of
c-Jun/JNK in K562/ADR cells. Biomed Res Int. 2014:1643912014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu MM, Tong JL, Xu Q, Nie F, Xu XT, Xiao
SD and Ran ZH: Increased JNK1 signaling pathway is responsible for
ABCG2-mediated multidrug resistance in human colon cancer. PLoS
One. 7:e417632012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manov I, Bashenko Y, Eliaz-Wolkowicz A,
Mizrahi M, Liran O and Iancu TC: High-dose acetaminophen inhibits
the lethal effect of doxorubicin in HepG2 cells: The role of
P-glycoprotein and mitogen-activated protein kinase p44/42 pathway.
J Pharmacol Exp Ther. 322:1013–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shinoda C, Maruyama M, Fujishita T, Dohkan
J, Oda H, Shinoda K, Yamada T, Miyabayashi K, Hayashi R, Kawagishi
Y, et al: Doxorubicin induces expression of multidrug
resistance-associated protein 1 in human small cell lung cancer
cell lines by the c-jun N-terminal kinase pathway. Int J Cancer.
117:21–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XY, Liu SP, Jiang J, Zhang X and Zhang
T: Inhibition of the JNK signaling pathway increases sensitivity of
hepatocellular carcinoma cells to cisplatin by down-regulating
expression of P-glycoprotein. Eur Rev Med Pharmacol Sci.
20:1098–1108. 2016.PubMed/NCBI
|
|
14
|
Huang C, Xu DJ, Xia Q, Wang P, Rong C and
Su Y: Reversal of P-glycoprotein-mediated multidrug resistance of
human hepatic cancer cells by astragaloside II. J Pharm Pharmacol.
64:1741–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present, and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Liu M, Aneja R, Chandra R, Lage H
and Joshi HC: Reversal of P-glycoprotein-mediated multidrug
resistance in cancer cells by the c-Jun NH2-terminal
kinase. Cancer Res. 66:445–452. 2016. View Article : Google Scholar
|
|
19
|
Liu M, Li D, Aneja R, Joshi HC, Xie S,
Zhang C and Zhou J: PO2-dependent differential
regulation of multidrug resistance 1 gene expression by the c-Jun
NH2-terminal kinase pathway. J BiolChem. 282:17581–64.
2007.
|
|
20
|
Bark H and Choi CH: PSC833, cyclosporine
analogue, downregulates MDR1 expression by activating
JNK/c-Jun/AP-1 and suppressing NF-kappaB. Cancer Chemother
Pharmacol. 65:1131–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eferl R and Wagner EF: AP-1: A
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–E136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Daschner PJ, Ciolino HP, Plouzek CA and
Yeh GC: Increased AP-1 activity in drug resistant human breast
cancer MCF-7 cells. Breast Cancer Res Treat. 53:229–240. 1999.
View Article : Google Scholar : PubMed/NCBI
|