Introduction
Post-translational modifications (PTMs) are highly
regulated and necessary for complex neuronal processing (1–4). In
addition, the regulation of PTMs is one of a number of well-known
mechanisms that is required for learning and memory, and for
long-term synaptic plasticity (5–8).
SUMOylation is one type of PTM, and involves the small
ubiquitin-like modifier (SUMO) family, the members of which are
ubiquitously expressed in all tissues, including the brain, and has
at least three paralogs (SUMO-1, SUMO-2 and SUMO-3) (9). SUMO-1 shares ~50% homology with
SUMO-2 and SUMO-3, with some overlap regarding target proteins
(10). The pool of unconjugated
SUMO-2 and SUMO-3 is generally much larger than that of SUMO-1 in
mammalian cells (10).
The hippocampus is considered one of the important
regions in the brain that is responsible for spatial memory and
navigation (11,12). The hippocampus is of particular
interest, as new cells are generated throughout a lifetime and may
proliferate and differentiate into neurons in the dentate gyrus
(13). SUMOylation is highly
regulated through the spatiotemporal expression of SUMO proteins
and the SUMO conjugation machinery. The SUMO signaling pathway is
involved in important molecular and biological processes, including
development and senescence (14,15),
and SUMO has been reported to be involved in a number of neuronal
pathways, including synaptic formation and transmission,
excitability, axonal trafficking and axonal guidance (16–19).
The brain is a unique organ with its own security
system comprising a network of blood vessels and astrocyte end-feet
that allow the selective entry of essential nutrients and block
other substances (20). However,
these unique structures may also limit medications from entering
and repairing the injured or diseased brain. Cell-penetrating
peptides have been a focus of research in neuroscience due to of
their ability to penetrate the blood-brain barrier (BBB) (21–23).
Transactivator of transcription (Tat) transduction proteins have
been revealed to be able to penetrate the BBB and reach the
hippocampus 8 h following treatment with Tat-fusion proteins in
mice (24). In addition, Tat
transduction proteins reportedly have no effects on motor
coordination, vision or motivation (25).
In the present study, a Tat-SUMO-1 fusion
transduction protein was constructed that was able to penetrate the
BBB and allowed for the investigation of the effects of SUMO-1 on
hippocampal functions, such as novel object recognition, and on
cell proliferation and neuroblast differentiation.
Immunohistochemical analysis of the proliferation marker protein
Ki67 (a marker for active cell cycles, which is absent during
G0 and early G1) and the neuronal migration
protein doublecortin (DCX, a marker for differentiating
neuroblasts) was performed to analyze the expression of these
proteins in the dentate gyrus. The results of the present study
will allow the role of SUMO-1 in hippocampal neurogenesis in
neurological disorders such as stroke and traumatic brain injury to
facilitate the rehabilitation of damage in the brain.
Materials and methods
Experimental animals
Six-week-old male C57BL/6 mice (n=14) were purchased
from Japan SLC, Inc. (Shizuoka, Japan). They were housed in a
conventional state at room temperature (23°C) and humidity (60%),
controlled with a 12-h light/dark cycle. The mice provided with
access to food and tap water ad libitum. Handling and caring
of the animals conformed to the guidelines established to comply
with current international laws and policies (NIH Guide for the
Care and Use of Laboratory Animals; National Institutes of Health,
Bethesda, MD, USA), and were approved by the Institutional Animal
Care and Use Committee of Seoul National University (Seoul, South
Korea). All experiments were conducted with an effort to minimize
the number of animals used and the suffering caused by the
procedures used in the present study.
Tat-SUMO-1 fusion protein expression
and purification
The Tat expression vector was prepared as previously
described (26). Briefly, the cDNA
of human SUMO-1 was amplified by polymerase chain reaction (PCR)
from a human liver cDNA library. The sense primer
(5′-CTCGAGATGTCTGACCAGGAGGCA-3′) contained an XhoI
restriction site; the antisense primer
(5′-GGATCCCTAAACTGTTGAATGACCCC-3′) contained a BamHI
restriction site. The sequences were ligated into the TA-cloning
vector (Promega Corporation, Madison, WI, USA), excised, and
inserted into the Tat pET-15b (Novagen; Merck KGaA, Darmstadt,
Germany) expression vector using T4 DNA ligase (Takara Bio. Inc.,
Otsu, Japan). The Tat-SUMO-1 plasmid was subsequently cloned into
Escherichia coli DH5α cells (Novagen; Merck KGaA) to generate the
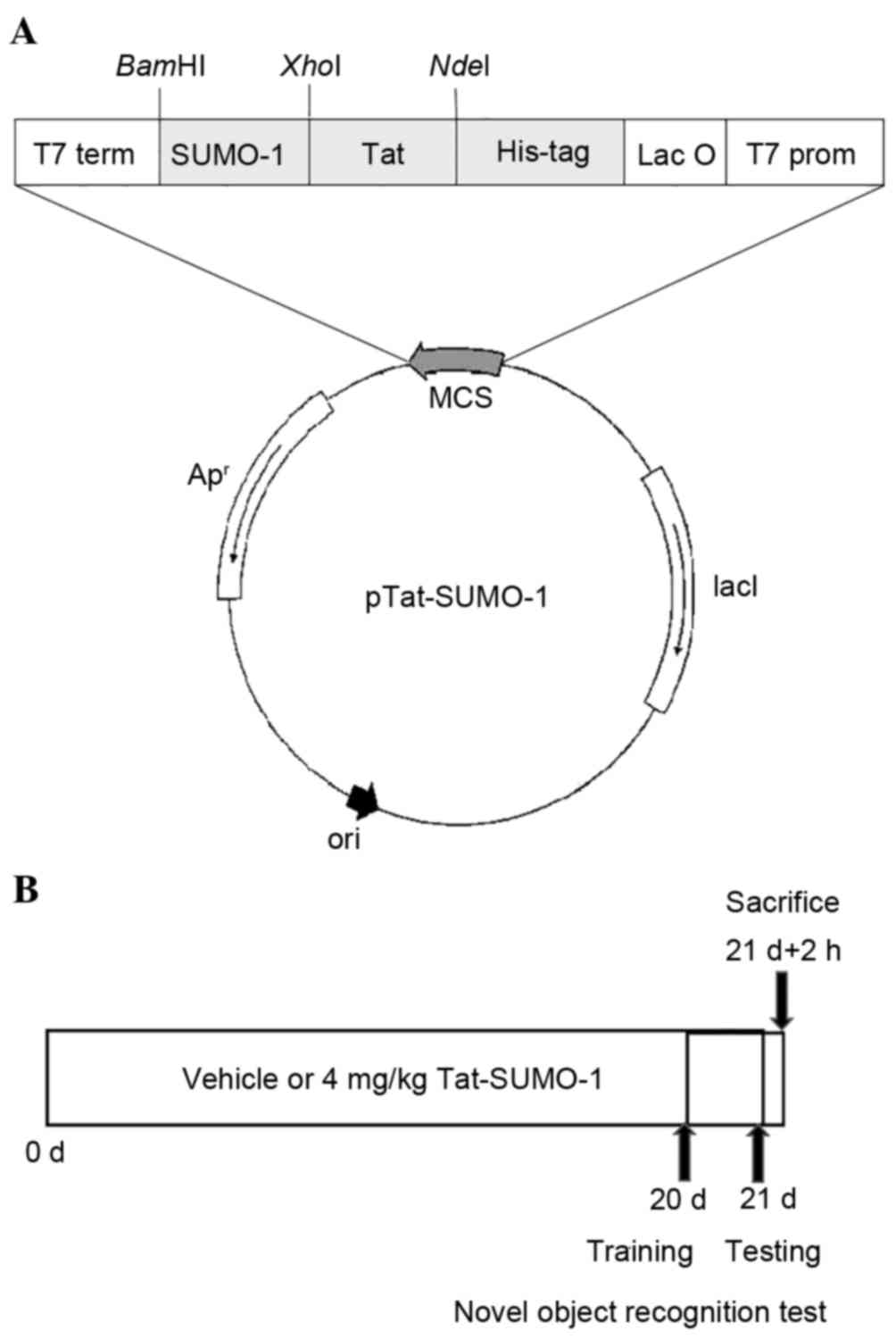
Tat-SUMO-1 fusion protein (Fig.
1A). Control SUMO-1 was generated without the Tat peptide. The
Tat peptide was synthesized by Peptron Inc. (Daejeon, South Korea).
The recombinant Tat-SUMO-1 plasmid (1 µg) was transformed into
Escherichia coli BL21 (DE3) competent cells (Novagen; Merck
KGaA) and induced with 0.5 mM isopropyl-β-D-thio-galactoside
(Duchefa Biochemie B.V., Haarlem, The Netherlands) at 18°C for
>24 h.
Cells were harvested by centrifugation (6,000 × g,
10 min, 4°C) and sonicated (3 passes; 4°C) in lysis buffer (5 mM
imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH7.9). The recombinant
his-tagged Tat-SUMO-1 fusion protein was purified using a
Ni2+-nitrilotriacetic acid Sepharose affinity column
(Qiagen, Valencia, CA, USA) according to the manufacturer's
protocol. Briefly, clarified cell extracts were loaded at 4°C. The
column was washed with 10 volumes of binding buffer (5 mM
imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9) and 6 volumes of a
wash buffer (60 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9).
The fusion proteins were subsequently eluted using elution buffer
(500 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9), followed
by desalting with a PD10 column (Amersham, Braunschweig, Germany)
according to the manufacturer's protocol. Bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used as a
standard and protein concentration was measured by Bradford assay
(27).
Treatment with Tat peptide or
Tat-SUMO-1
Seven-week-old mice were divided into 2 groups
(n=7/group): i) The vehicle-treatment group, which received a daily
intraperitoneal (i.p.) injection of glycerol with Tat peptide (4
mg/kg) for 3 weeks; and ii) the Tat-SUMO-1-treatment group, which
received a daily i.p. injection of Tat-SUMO-1 (4 mg/kg) for 3
weeks. This schedule was adopted as DCX is expressed exclusively in
the immature neurons between 1 and 28 days of cell age (28,29).
Novel object recognition test
One hour following vehicle or Tat-SUMO-1 treatment
on day 20 (Fig. 1B), the training
trial was performed. Mice (n=7/group) were placed in a 45×45×30 cm
acrylic box with three opaque walls and one transparent wall, and
allowed to explore two identical objects for 5 min each. This
training process was repeated once. A total of 24 h following the
training open-field trial, the test trial was performed; one of the
two familiar objects was replaced with a new one, and the mice were
allowed to explore them for 5 min. Relative exploration time was
calculated as follows: Relative exploration time in first and
second day trials=[time observed each object (familiar or new)/time
observing both objects]x100. The animals were sacrificed 2 h
following completion of the novel object recognition test.
Tissue processing
For histological analysis, mice from the vehicle and
Tat-SUMO-1-treated groups (n=7/group) were anesthetized with
urethane (2 mg/kg; Sigma-Aldrich; Merck KGaA) at 2 h following the
novel object recognition test and perfused transcardially with PBS
(0.1 M; pH 7.4), followed by 4% paraformaldehyde in phosphate
buffer (0.1 M; pH 7.4). Brains were removed and post-fixed in 4%
paraformaldehyde in phosphate buffer for 12 h at 4°C. Brain tissues
were cryoprotected by infiltration with 30% sucrose overnight at
4°C. Brain sections of (30 µm) were serially cut in the coronal
plane using a cryostat (Leica Microsystems GmbH, Wetzlar, Germany).
Sections were collected in six-well plates containing PBS until
further processing.
Immunohistochemistry
To obtain accurate data for immunohistochemistry,
free-floating brain sections were carefully processed under the
same conditions. For each animal, tissue sections were selected
between −1.46 and −2.46 mm posterior to bregma by referring to the
mouse atlas by Franklin and Paxinos (30). A total of 10 sections, 90 µm apart
from each other, were sequentially treated with 0.3% hydrogen
peroxide in PBS for 30 min at 25°C and 10% normal goat or rabbit
serum (Vector Laboratories, Inc., Burlingame, CA, USA) in 0.05 M
PBS for 1 h at 25°C. Sections were incubated overnight with rabbit
anti-Ki67 antibody (1:1,000; ab15580, Abcam, Cambridge, UK) or goat
anti-DCX antibody (1:50; sc-8066, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C, and subsequently exposed to biotinylated
rabbit anti-goat or goat anti-rabbit immunoglobulin G at 25°C for 2
h (1:200; BA-5000 or BA-1000, Vector Laboratories, Inc.) and
streptavidin peroxidase complex (1:200, Vector Laboratories, Inc.)
at 25°C for 2 h. The sections were visualized by reaction with
3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich; Merck
KGaA).
Ki67- and DCX-positive cell counts were performed in
the hippocampal subgranular zone for each section of the dentate
gyrus using a computer-based CCD camera and Optimas v 6.5 image
analysis software (CyberMetrics, Phoenix, AZ, USA). Cell counts
from all sections from each of the mice were averaged.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Differences among the means were statistically
analyzed by two-way analysis of variance followed by Bonferroni's
post-hoc test to elucidate the effects of Tat-SUMO-1 on novel
object recognition. A paired Student's t-test was employed to
observe alterations in cell proliferation and neuroblast
differentiation in mice. All statistical tests were performed using
GraphPad Prism software v 5.01 (GraphPad Software, Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of Tat-SUMO-1 on object
recognition memory
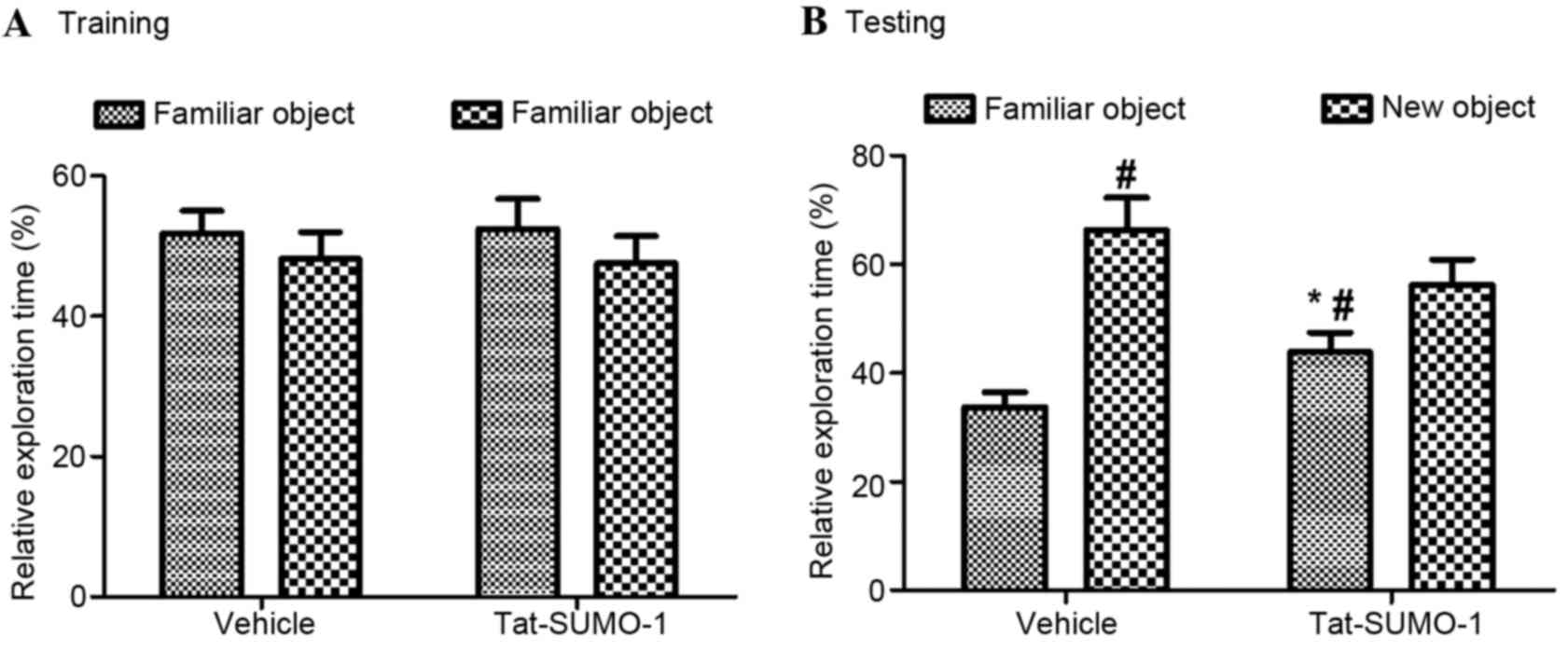
During the training period, mice from the vehicle
and Tat-SUMO-1-treated groups spent similar amounts of time (18.74
vs. 19.01 sec, respectively; Fig.
2) exploring two identical objects (F=0.03, P=0.8734). During
the test period, mice in both the vehicle- and the
Tat-SUMO-1-treated groups spent significantly more time exploring
the new object than the familiar one, compared with time spent with
the objects in the training period (F=5.20, P=0.03128). The
relative exploration time in the Tat-SUMO-1-treated group was less
than that for the vehicle-treated group and statistical
significance was identified between vehicle and Tat-SUMO-1-treated
groups (P<0.05).
Effects of Tat-SUMO-1 on cell
proliferation
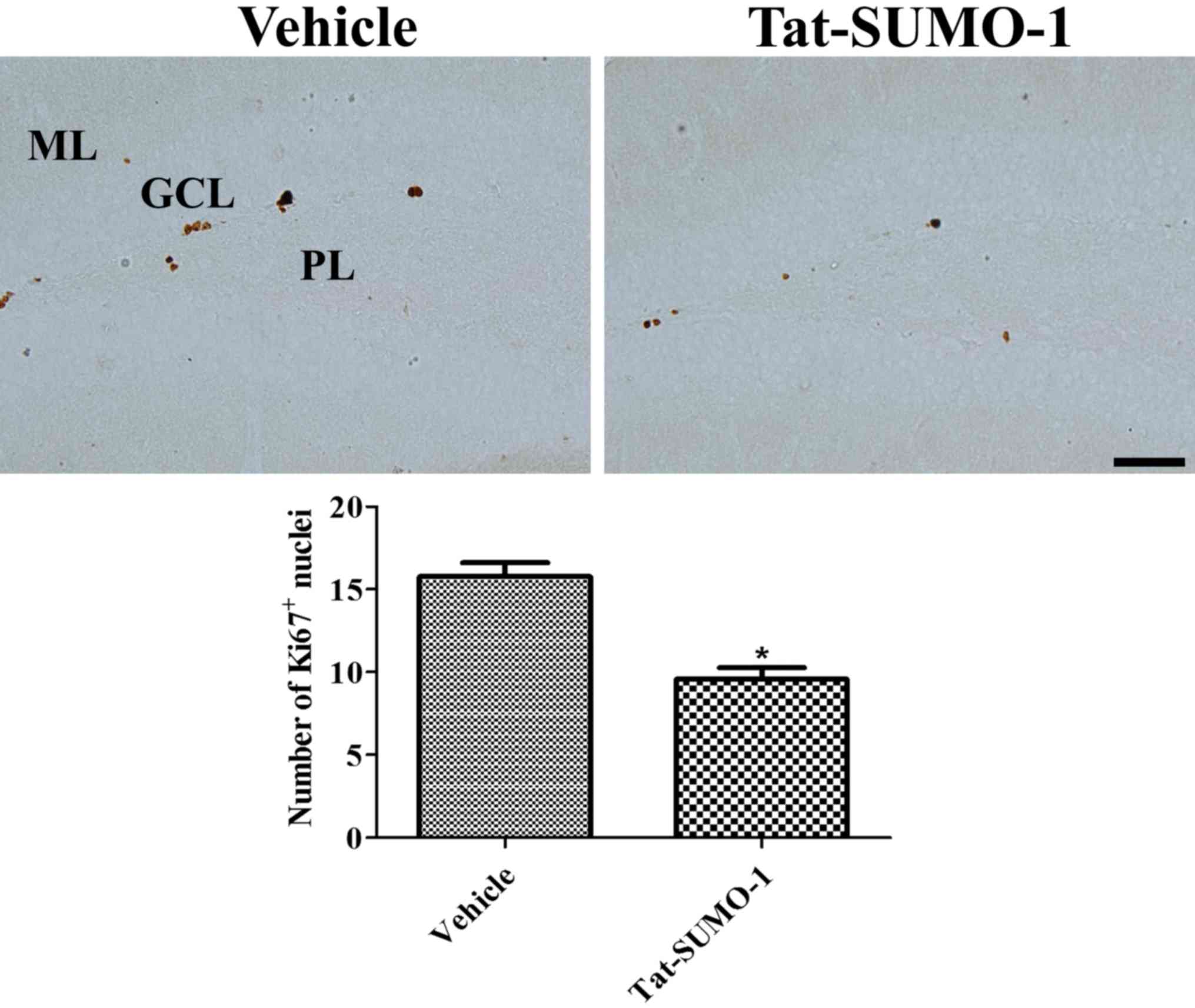
Ki67 immunoreactive-positive nuclei were observed in
the subgranular zone of the dentate gyrus. In the
Tat-SUMO-1-treated group, significantly fewer Ki67-positive nuclei
were detected compared with the vehicle-treated group (9.6 vs.
15.8, respectively; Fig. 3).
Effects of Tat-SUMO-1 on neuroblast
differentiation
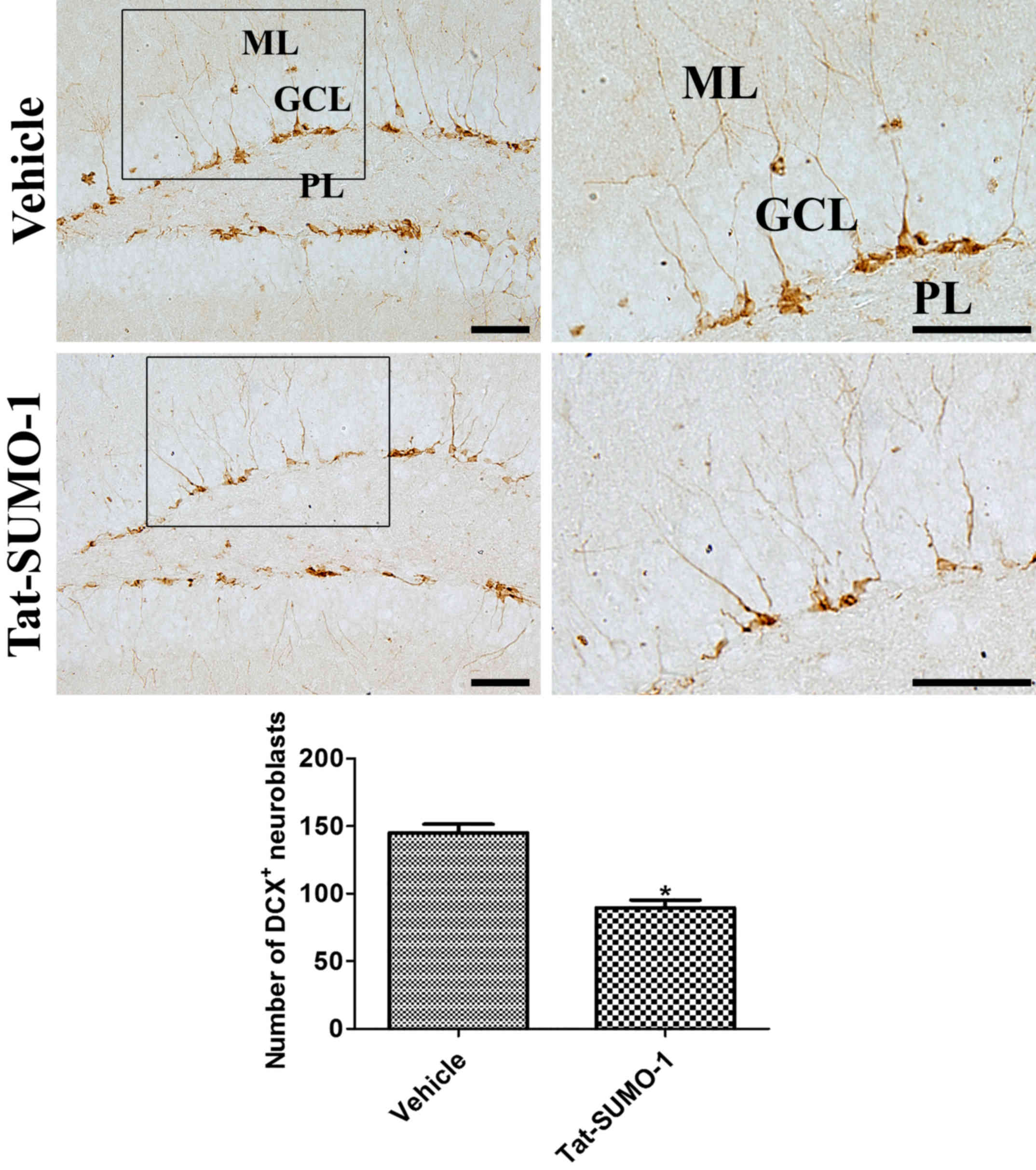
DCX immunoreactive-positive neuroblasts were
detected in the dentate gyrus. Many of the DCX-positive neuroblasts
had cytoplasm in the subgranular zone with dendrites extended
across the granule cell layer into molecular layer of the dentate
gyrus. In the Tat-SUMO-1-treated group, DCX-positive neuroblasts
were less abundant and had poorly developed dendrites in the
dentate gyrus. The average number of DCX immunoreactive neuroblasts
was significantly decreased in the Tat-SUMO-1-treated group
compared with the vehicle-treated group (89.5 vs. 145.0 per
section, respectively; Fig.
4).
Discussion
Modulation of PTMs is a crucial process for
homeostasis, development and metabolism in several organs,
including the brain. Several previous studies have suggested that
SUMOylation is one of the key factors in synaptic plasticity in the
brain (31,32). SUMOylation has been demonstrated to
induce important changes in Alzheimer's disease-associated
proteins, such as tau and amyloid-β precursor protein (33,34).
SUMO-1 mRNA levels, as well as SUMO-1-ylation, are increased in the
hippocampus of 6-month-old Tg2576 Alzheimer's disease model mice as
compared with wild-type mice (35). In amyloid-β precursor protein
transgenic (Tg6799) mice, free SUMO-1 protein levels are
significantly increased in the cortical tissue at 18 months old
(36). In subjects with dementia,
SUMO-1 levels were significantly increased in the serum as compared
with the levels of SUMO-1 in similarly aged healthy controls
(37).
In the present study, the effects of Tat-SUMO-1 on
hippocampal function were analyzed using the novel object
recognition paradigm, as the test depends on the integrity of the
hippocampus (38) and that newly
generated adult-born neurons of the dentate gyrus contribute to
hippocampus-dependent memory (39). Chronic administration of Tat-SUMO-1
significantly decreased novel object recognition memory. In SUMO-1
transgenic mice, genetic overexpression of SUMO-1 did not result in
alterations to the behavioral phenotype or open field responses,
except for fear conditioning (40). By contrast, the inhibition of
SUMOylation by infusions of dominant negative Tat-Ubc9
significantly impaired hippocampus-dependent reference memory,
based on the Morris water-maze test (25). In addition, knockdown of SUMO led
to impairment in episodic memory processes, contextual and cued
fear conditioning, and fear-potentiated startle (41). These conflicting results suggest
that the overexpression or lack of SUMO may affect memory
processing and have chronic deleterious effects.
SUMOylation has been implicated in
neurodevelopmental and neurodegenerative processes in the brain
(16–19). In a recent study, SUMO-1 transgenic
mice exhibited impairment in both short-term synaptic plasticity,
based on paired pulse facilitation measurements, and basal synaptic
transmission, based on the input-output relationship plots
(40). In addition, synaptic
density in pyramidal cells was reduced by 70% in the SUMO-1
transgenic mice based on Golgi staining as compared with that in
the wild-type mice (40). In the
present study, the effects of Tat-SUMO-1 on cell proliferation and
neuroblast differentiation in the dentate gyrus were observed.
Administration of Tat-SUMO-1 significantly reduced the number of
proliferating cells and differentiated neuroblasts in the dentate
gyrus, and significantly decreased the time spent exploring a new
object in the novel object recognition test. This study suggests
that SUMO-1 is closely involved in hippocampal neurogenesis and
functioning; controlling neurogenesis by modulation of SUMOylation
may be new strategy to overcome neurological disorders.
Acknowledgements
The present study was supported by National Research
Foundation of Korea (NRF) grant, funded by the Korean government
Ministry of Science, ICT & Future Planning (MSIP) (grant no.
NRF-2016R1A2B4009156). This research was also supported by a
Priority Research Centers Program grant from the NRF of Korea
(grant no. NRF-2009-00,93812), as funded by MSIP in the Republic of
Korea.
References
|
1
|
Gwizdek C, Cassé F and Martin S: Protein
sumoylation in brain development, neuronal morphology and
spinogenesis. Neuromolecular Med. 15:677–691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo J, Ashikaga E, Rubin PP, Heimann MJ,
Hildick KL, Bishop P, Girach F, Josa-Prado F, Tang LT, Carmichael
RE, et al: Receptor trafficking and the regulation of synaptic
plasticity by SUMO. Neuromolecular Med. 15:692–706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho Y and Cavalli V: HDAC signaling in
neuronal development and axon regeneration. Curr Opin Neurobiol.
27:118–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Santos AI, Martínez-Ruiz AI and Araújo IM:
S-nitrosation and neuronal plasticity. Br J Pharmacol.
172:1468–1478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soderling TR and Derkach VA: Postsynaptic
protein phosphorylation and LTP. Trends Neurosci. 23:75–80. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DiAntonio A and Hicke L:
Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci.
27:223–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukata Y and Fukata M: Protein
palmitoylation in neuronal development and synaptic plasticity. Nat
Rev Neurosci. 11:161–175. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Routtenberg A and Rekart JL:
Post-translational protein modification as the substrate for
long-lasting memory. Trends Neurosci. 28:12–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Droescher M, Chaugule VK and Pichler A:
SUMO rules: Regulatory concepts and their implication in neurologic
functions. Neuromolecular Med. 15:639–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saitoh H and Hinchey J: Functional
heterogeneity of small ubiquitin-related protein modifiers SUMO-1
versus SUMO-2/3. J Biol Chem. 275:6252–6258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moser MB and Moser EI: Functional
differentiation in the hippocampus. Hippocampus. 8:608–619. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmajuk NA: Role of the hippocampus in
temporal and spatial navigation: An adaptive neural network. Behav
Brain Res. 39:205–229. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gage FH, Kempermann G, Palmer TD, Peterson
DA and Ray J: Multipotent progenitor cells in the adult dentate
gyrus. J Neurobiol. 36:249–266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andreou AM and Tavernarakis N: Roles for
SUMO modification during senescence. Adv Exp Med Biol. 694:160–171.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lomelí H and Vázquez M: Emerging roles of
the SUMO pathway in development. Cell Mol Life Sci. 68:4045–4064.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin S, Wilkinson KA, Nishimune A and
Henley JM: Emerging extranuclear roles of protein SUMOylation in
neuronal function and dysfunction. Nat Rev Neurosci. 8:948–959.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Craig TJ and Henley JM: Protein
SUMOylation in spine structure and function. Curr Opin Neurobiol.
22:480–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilkinson KA, Nakamura Y and Henley JM:
Targets and consequences of protein SUMOylation in neurons. Brain
Res Rev. 64:195–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Plant LD, Dowdell EJ, Dementieva IS, Marks
JD and Goldstein SA: SUMO modification of cell surface Kv2.1
potassium channels regulates the activity of rat hippocampal
neurons. J Gen Physiol. 137:441–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ballabh P, Braun A and Nedergaard M: The
blood-brain barrier: An overview: Structure, regulation and
clinical implications. Neurobiol Dis. 16:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kilic E, Kilic U and Hermann DM: TAT
fusion proteins against ischemic stroke: Current status and future
perspectives. Front Biosci. 11:1716–1721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fonseca SB, Pereira MP and Kelley SO:
Recent advances in the use of cell-penetrating peptides for medical
and biological applications. Adv Drug Deliv Rev. 61:953–964. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stalmans S, Bracke N, Wynendaele E,
Gevaert B, Peremans K, Burvenich C, Polis I and De Spiegeleer B:
Cell-penetrating peptides selectively cross the blood-brain barrier
in vivo. PLoS One. 10:e01396522015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eum WS, Kim DW, Hwang IK, Yoo KY, Kang TC,
Jang SH, Choi HS, Choi SH, Kim YH, Kim SY, et al: In vivo protein
transduction: Biologically active intact pep-1-superoxide dismutase
fusion protein efficiently protects against ischemic insult. Free
Radic Biol Med. 37:1656–1669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee L, Dale E, Staniszewski A, Zhang H,
Saeed F, Sakurai M, Fa' M, Orozco I, Michelassi F, Akpan N, et al:
Regulation of synaptic plasticity and cognition by SUMO in normal
physiology and Alzheimer's disease. Sci Rep. 4:71902014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon HY, Eum WS, Jang HW, Kang JH, Ryu J,
Lee B Ryong, Jin LH, Park J and Choi SY: Transduction of Cu,
Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic
domain into mammalian cells. FEBS Lett. 485:163–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bradford MM: A rapid and sensitive method
for the quantification of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brown JP, Couillard-Després S, Cooper-Kuhn
CM, Winkler J, Aigner L and Kuhn HG: Transient expression of
doublecortin during adult neurogenesis. J Comp Neurol. 467:1–10.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Couillard-Despres S, Winner B, Schaubeck
S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG
and Aigner L: Doublecortin expression levels in adult brain reflect
neurogenesis. Eur J Neurosci. 21:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Franklin KBJ and Paxinos G: The Mouse
Brain In Stereotaxic Coordinates. 3rd. San Diego: Academic Press;
1997
|
|
31
|
Chamberlain SE, González-González IM,
Wilkinson KA, Konopacki FA, Kantamneni S, Henley JM and Mellor JR:
SUMOylation and phosphorylation of GluK2 regulate kainate receptor
trafficking and synaptic plasticity. Nat Neurosci. 15:845–852.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin S, Nishimune A, Mellor JR and
Henley JM: SUMOylation regulates kainate-receptor-mediated synaptic
transmission. Nature. 447:321–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Georgopoulou N, McLaughlin M, McFarlane I
and Breen KC: The role of post-translational modification in
beta-amyloid precursor protein processing. Biochem Soc Symp. 23–36.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marcus JN and Schachter J: Targeting
post-translational modifications on tau as a therapeutic strategy
for Alzheimer's disease. J Neurogenet. 25:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nisticò R, Ferraina C, Marconi V, Blandini
F, Negri L, Egebjerg J and Feligioni M: Age-related changes of
protein SUMOylation balance in the AβPP Tg2576 mouse model of
Alzheimer's disease. Front Pharmacol. 5:632014.PubMed/NCBI
|
|
36
|
Yun SM, Cho SJ, Song JC, Song SY, Jo SA,
Jo C, Yoon K, Tanzi RE, Choi EJ and Koh YH: SUMO1 modulates Aβ
generation via BACE1 accumulation. Neurobiol Aging. 34:650–662.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho SJ, Yun SM, Lee DH, Jo C, Park M Ho,
Han C and Koh Y Ho: Plasma SUMO1 protein is elevated in Alzheimer's
disease. J Alzheimers Dis. 47:639–643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Squire LR, Wixted JT and Clark RE:
Recognition memory and the medial temporal lobe: A new perspective.
Nat Rev Neurosci. 8:872–883. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leuner B and Gould E: Structural
plasticity and hippocampal function. Annu Rev Psychol. 61:111–140.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuzaki S, Lee L, Knock E, Srikumar T,
Sakurai M, Hazrati LN, Katayama T, Staniszewski A, Raught B,
Arancio O and Fraser PE: SUMO1 affects synaptic function, spine
density and memory. Sci Rep. 5:107302015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang L, Rodriguiz RM, Wetsel WC, Sheng H,
Zhao S, Liu X, Paschen W and Yang W: Neuron-specific Sumo1-3
knockdown in mice impairs episodic and fear memories. J Psychiatry
Neurosci. 39:259–266. 2014. View Article : Google Scholar : PubMed/NCBI
|