Introduction
The expression of transforming growth factor-β1
(TGF-β1) is upregulated in the course of multiple renal fibrosis
and acts as an important cytokine in the renal fibrosis disease
process (1–4). It induces the transition of renal
tubular epithelial cells (RTECs) to mesenchymal cells and finally
into myofibroblasts, increasing the deposition of extracellular
matrix (ECM) (5,6). In recent years, many studies have
demonstrated that transcriptional corepressor Ski-related novel
protein N (SnoN), an important negative regulatory factor, inhibits
the transcription activation of Smads-mediated TGF-1 target gene
(7,8). It is well accepted that the
diminution of SnoN protein is involved in the development and
progression of renal fibrosis, and the downregulation of SnoN
protein facilitates renal fibrotic lesions while the upregulation
of which slows down the development of renal fibrosis (1,8).
Studies found that bone morphogenetic protein-7
(BMP-7), a member of TGF-β1 superfamily, is highly expressed in
kidney and plays a renal protective effect in maintaining the
normal development of kidney, reversing epithelial-to-mesenchymal
transition (EMT) (3,9), and in restricting the deposition of
ECM (4,10), but the exact mechanism is still
unclear. Recent studies have shown that BMP-7 may play a role in
anti-renal fibrosis by upregulating the protein expression of SnoN,
which is subject to regulation strictly in activation of both
transcription and protein stability (4,11,12).
Therefore, this study aimed to explore the associations between and
among BMP-7, the expression of transcriptional corepressor SnoN and
the changes of tubulointerstitial fibrosis during the development
and progression of diabetic nephropathy (DN). Furthermore, this
study also aimed to demonstrate BMP-7 may have effect on the target
of anti-fibrotic and potential mechanism by observing its influence
on the expression of SnoN and fibrosis of RTECs while exogenous
rhBMP-7 was added to RTECs cultured under hyperglycemic
conditions.
Materials and methods
Animal model
A total of 20 healthy and specific pathogen-free
male Sprague-Dawley rats (weight: 180±20 g) were provided by
Beijing HFK Bioscience Co., Ltd. (Beijing, China), and housed in
the animal center of Guiyang Medical University, (Guizhou, China).
The study was conducted in accordance with the guidelines of the
National Health and Medical Research Council of China's Code for
the care and use of animals for scientific purpose. All rats were
randomly divided into diabetic group (DM group, n=10) and
nomal control group (NC group, n=10). Diabetic rats were
produced by injecting 0.01-mol/l streptozotocin (STZ, prepared with
sterile citric acid-sodium citrate buffer, pH 4.5; Sigma, St.
Louis, MO, USA) in the tail vein at a dose of 55-mg/kg. Fasting
blood glucose level of all rats was detected after 48-h. The blood
glucose level ≥16.7-mmol/l indicates that the diabetic rat model
was established successfully. Rats in NC group were age-matched and
injected an equal volume of solvent. Rats in each group were given
normal diet and unlimited drinking water. After 24-weeks, 24-h
urine of each rat was collected in metabolic cage, and the total
volume of urine was recorded before rats were sacrificed. The rats
were fasted for 6–8 h before being anesthetized by diethyl ether,
and their femoral arteries were punctured to collect blood samples,
which were centrifuged at 4°C to separate serum. Urine and serum
were stored at −20°C for measuring urine protein and biochemical
indices. Kidneys of each rats were harvested, one was fixed in 4%
paraformaldehyde for paraffin sections, and the other one was
snap-frozen in liquid nitrogen and stored at −80°C for RNA and
protein extractions.
Tests of biochemical markers
The oxidase method was used to measure serum
glucose, and the Coomassie Brilliant Blue method was used to
measure urine protein. All tests were analyzed by 1650 automatic
biochemical analyzer (Beckman Instruments, Inc., Brea, CA, USA),
according to the manufacturer's instruction. Urine protein
excretion (mg/24-h) was assessed as follows: urine protein (mg/ml)
× urine volume (ml)/24-h.
Histopathological analysis
Kidneys were fixed in paraformaldehyde, embedded in
paraffin, and cut by transversing transverse sections (4-µm) for
staining. Renal tissue morphology was observed under a light
microscope by hematoxylin-eosin (H&E) stain, while renal tissue
fibrosis was observed by Masson's trichrome and Periodic
Acid-Schiff (PAS) stain.
Cell culture and treatments
RTECs (NRK-52E cells) were cultured in Dulbeccos's
modified Eagle's medium (Hyclone, Logan, UT, USA) supplemented with
5% fetal bovine serum (Gibco; Invitrogen, Carlsbad, CA, USA)
containing normal glucose (5.5-mmol/l glucose), seeded in
25-cm2 culture flasks, and placed in the incubator with
5% CO2 at 37°C. Before changing in a serum-free medium
for 20-h to keep the same pace in growth, cells were treated with
the following mediums: i) normal-glucose control group (NG group,
5.5-mmol/l glucose); ii) high-glucose control group (HG group)
(5.5-mmol/l glucose +19.5-mmol/l D-glucose); iii) high glucose
+10-ng/ml BMP-7 (HG+10-ng/ml rhBMP-7 group); and (iv) high glucose
+20-ng/ml BMP-7 (HG +20-ng/ml rhBMP-7 group). Cells in each group
were cultured for 48-h for further study.
Immunohistochemistry and
immunofluorescence stain
The biotin-streptavidin-peroxidase method (ZSBIO,
Beijing, China) was used to stain tissue sections which were
incubated with different antibodies [rabbit-anti-BMP-7 1:200;
rabbit-anti-E-cadherin 1:100; mouse-anti-α-SMA 1:100;
rabbit-anti-Collagen III (Col-III) 1:150; and rabbit-anti-SnoN
1:200] (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) to
show the distribution and expression of protein in kidney tissues.
Phosphate-buffered saline (PBS) was substituted for the primary
antibody, and the secondary antibodies used were affinity-purified
biotinylated goat anti-rabbit or anti-mouse immunoglobulin G (IgG).
The proteins were visualized by using diaminobenzidine
tetrahydrochloride as a chromogen. Tissue sections were
counterstained with Mayer's hematoxylin.
Cells cultured on coverslips were washed with PBS
twice and fixed with cold methanol: acetone (1:1) for 10 mins on
ice. After washing extensively, cells were blocked with bovine
serum antigen for 30 mins at room temperature and then incubated
with the specific primary antibodies rabbit-anti-cytokeratin-18
(CK-18, 1:100; Biosynthesis, China), rabbit-anti-E-cadherin
(1:200), and mouse-anti-α-SMA (1:100) overnight at 4°C. Then cells
were stained with fluorescein isothiocyanate (FITC)-conjugated
goat-anti-rabbit IgG (1:400) or cyanine-3 (Cy3)-conjugated
goat-anti-mouse IgG (1:400) (both from Beyotime, Haimen, China).
After washing again, the cells were stained with
4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei, and
then observed under an inverted fluorescence microscope. For
colocalization of the tubular marker E-cadherin and α-SMA on
NRK-52E cells, immunofluorescence stain was performed. After being
incubated with primary antibodies rabbit-anti-E-cadherin (1:200) at
4°C overnight, the slides were then stained with FITC-conjugated
goat-anti-rabbit IgG (1:400; Beyotime). After this, the slides were
incubated with primary antibodies mouse-anti-α-SMA (1:100) at 4°C
overnight, and then with Cy3-conjugated goat-anti-mouseIgG (1:400;
Beyotime). The nucleus were re-dyed with DAPI. These stained slides
were observed under an inverted fluorescence microscope and
photographed.
Western blot analysis
The protein expression in renal cortex and cultured
cells was analyzed by western blot analysis. Renal tissue and
cultured cells were lysed in ice-cold lysis buffer. The protein
concentration was determined using the BCA protein assay kit
(Beyotime). Samples were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. Then the proteins were
electro transferred onto a polyvinylidene difluoride membrane
(Millipore, Germany). Non-specific binding to the membrane was
blocked in 5% nonfat milk for 1 h at room temperature. The
membranes were then incubated were different primary antibodies
including BMP-7 (1:150), E-cadherin (1:200), α-SMA (1:150), Col-III
(1:200), SnoN (1:200), Smad ubiquitin regulatory factor 2 (Smurf2;
1:600; Abcam, Cambridge, UK), Arkadia (1:300) and β-actin (1:400)
(both from Santa Cruz Biotechnology) for 16 h at 4°C. After washing
extensively in Tris-buffered saline (TBS), the membranes were
incubated with a secondary horseradish peroxidase-conjugated
antibody (Santa Cruz Biotechnology) in TBS containing 1% nonfat
milk for 1 h at room temperature. The membranes were then washed
with TBS buffer again. The signals were visualized by using the
enhanced chemiluminescence system (Beyotime) and then detected on
x-ray films. The Bio-Rad gel imaging system (Bio-Rad, Hercules, CA,
USA) was used for image acquisition. The band intensity was
quantified by using the Quantity One 4.6 software (Bio-Rad).
Quantitative real-time polymerase
chain reaction (qRT-PCR)
Total RNA in rat renal cortex or NRK-52E cells was
extracted by using the TRIzol (Tiangen, Beijing, China) method
according to the manufacturer's protocol. First-strand cDNA, which
was stored at −20°C, was synthesized from 5-µg total RNA in renal
tissues or 3-µg total RNA in NRK-52E cells by using the revert Aid
First Strand cDNA Synthesis kit (Fermentas, Lithuania). All
quantitative real-time polymerase chain reaction (qRT-PCR)
amplification was performed by iQ SYBR-Green SuperMix (Bio-Rad).
The primer sequences were as follows: SnoN (ForteBio, Shanghai,
China) 5′-CCATTCAATGCCCCATCCT-3′ (sense) and
5′-AGTTCGTGGCCGCAATAAAG-3′ (antisense) (the size of the product was
81 bp); β-actin (ForteBio) 5′-GCCAACACAGTGCTGTCT-3′ (sense) and
5′-AGGAGCAATGATCTTGATCTT-3′ (antisense) (the size of the product
was 114 bp). After 45 cycles of amplification, data was collected
to plot kinetic curves, and the Ct value was acquired. The
aforementioned steps were repeated for three times, and the ΔΔCt
method was used to calculate the mRNA/β-actin ratio for each
group.
Statistical analysis
The SPSS 13.0 software (IBM Corp., Armonk, NY, USA)
was used for statistical analysis. All statistics were expressed as
mean ± standard deviation (mean ± SD). Statistical analysis between
the groups was performed using an unpaired Students t-test, while
comparison among multiple groups was performed using one-way
analysis of variance followed by Student-Newman-Keuls q-test.
Differences were considered significantly at P<0.05.
Results
Enhanced renal fibrosis in diabetic
rats
The symptoms of diabetic rats, which had being
injected with STZ, including polydipsia, diuresis, and polyphagia.
The levels of 24-h urine protein and blood glucose were
significantly higher in diabetic rats than those in normal rats
(24-h protein: 305.36±42.49 vs. 39.41±11.31 mg; blood glucose:
27.42±3.930 vs. 6.84±1.58 mmol/l) (P<0.05).
H&E, PAS, and Masson stain showed an increase of
mesangial matrix and thickness of glomerular basement membrane in
24-week diabetic rats. Moreover, part of RTECs were atrophied or
lost, and the inflammatory cells increased and infiltrated into
wider tubulointerstitium (Fig.
1A).
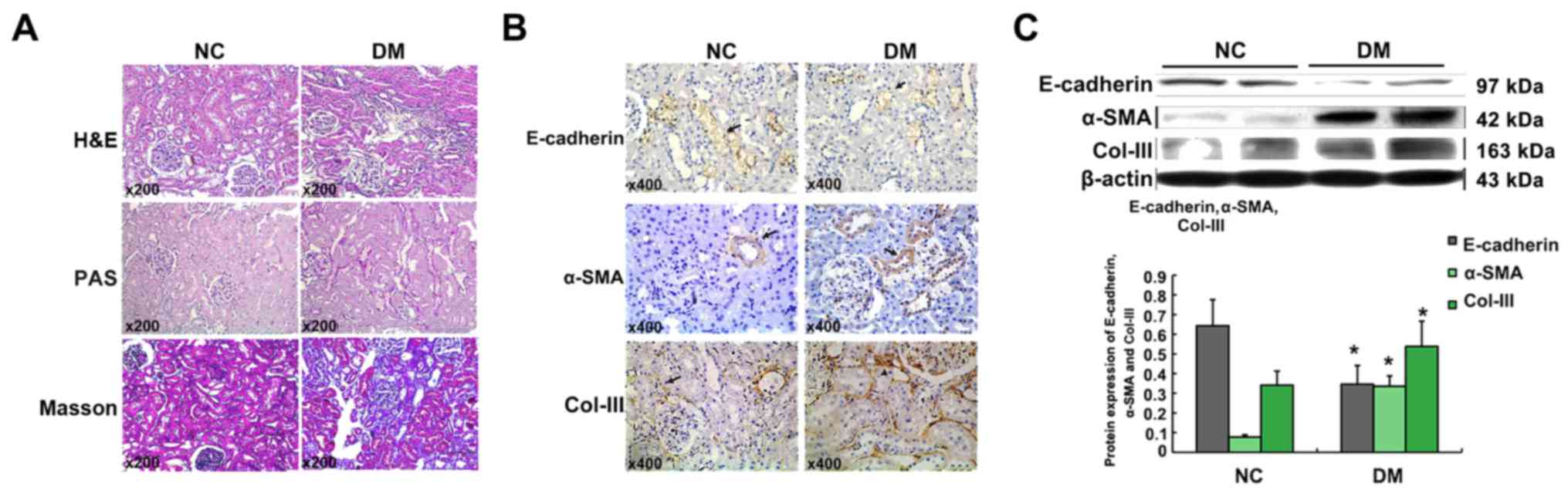 | Figure 1.Enhanced renal fibrosis in diabetic
rats. Histological changes of kidneys in the NC group and DM group
(hematoxylin-eosin staining, Periodic Acid-Schiff staining, and
Masson stains, magnification, ×200) (A). Immunohistochemical
staining of E-cadherin, α-SMA, and Col-III in the kidney tissues
(magnification, ×200). Arrows (→) indicate positive expression (B).
Graphical presentations show the protein expression of E-cadherin,
α-SMA, and Col-III. Mean ± SD, n=10, *P<0.05 vs. NC group
(C). |
The immunohistochemical analysis revealed that no
positive α-SMA expression was detected in renal tubules except
around the blood vessels in tubulointerstitium of normal kidneys,
but it was remarkable in renal tubules of diabetic rats. Moreover,
Col-III deposition was much more confined within the areas of
tubulointerstitium in kidneys of diabetic rats when comparing with
NC group. However, epithelial cell marker, E-cadherin, was much
lower in the tubular epithelium of DM groups when comparing with NC
group (Fig. 1B). Being consistent
with the immunohistochemical analysis, the Western blot analysis
revealed that the expression of α-SMA and Col-III increased, but
E-cadherin in the kidneys of DM group decreased when comparing with
NC group (Fig. 1C). This
observation may indicate that RTECs show the transition from the
epithelial cells to the mesenchymal cells.
Effect of diabetes on BMP-7, SnoN,
Smurf2, and Arkadia expression in renal tissues of rats
The results of immunohistochemistry showed that, in
rat renal tissues, BMP-7 mainly expressed in RTECs. Western blot
indicated that the BMP-7 protein expression in the renal cortex of
the DM group was lower than that in NC group (Fig. 2A). As shown in Fig. 2B and C, immunohistochemical stain
and Western blot analyses showed that, in diabetic conditions, the
SnoN protein expression was largely lower in vivo when
comparing with that in normal rats. In contrast to the significant
decrease of SnoN protein, the expression of SnoN mRNA was
upregulated in kidneys of diabetic rats when comparing with that in
normal rats. The western blot analysis revealed that Smurf2 and
Arkadia proteins were weakly expressed in renal extracts from
normal rat kidneys. In kidneys of diabetic rats, increased
expression of Smurf2 and Arkadia was notable at the protein level
(Fig. 2D).
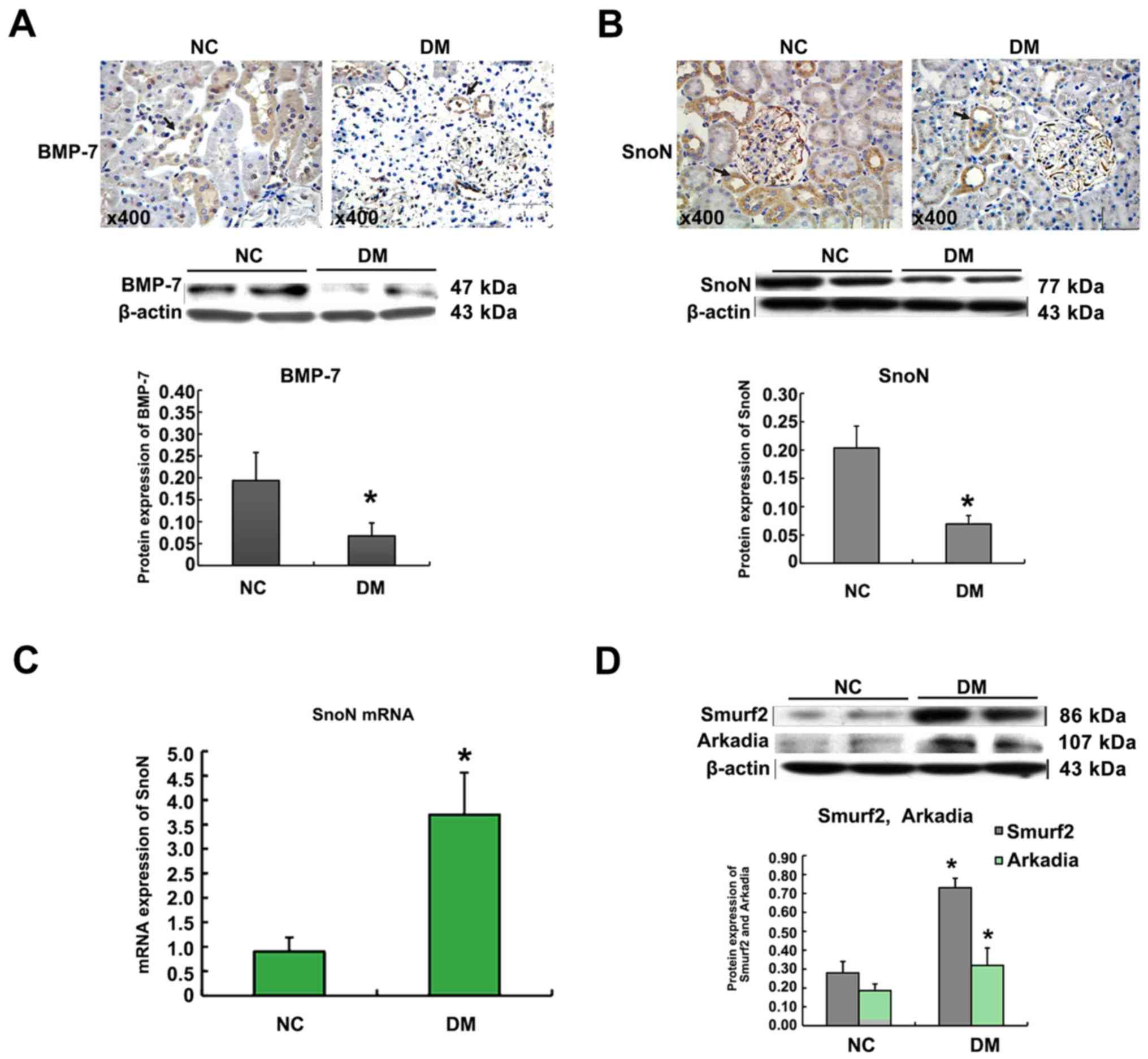 | Figure 2.Effect of diabetes on BMP-7, SnoN,
Smurf2, and Arkadia expression in the renal tissues of rats.
Immunohistochemical staining of BMP-7 in the kidney tissues
(magnification, ×200). Arrows (→) indicate positive expression.
Graphical presentations show the protein expression of BMP-7. Mean
± SD, n=10, *P<0.05 vs. NC group (A). Immunohistochemical
staining of SnoN in the kidney tissues (magnification, ×200).
Arrows (→) indicate positive expression. Graphical presentations
show the protein expression of SnoN. Mean ± SD, n=10, *P<0.05
vs. NC group (B). Graphical presentations show the relative
abundance of SnoN mRNA after normalization with β-actin mRNA. Mean
± SD, n=10, *P<0.05 vs. NC group (C). Graphical presentations
show the protein expression of Smurf2 and Arkadia. Mean ± SD, n=10,
*P<0.05 vs. NC group (D). |
Exogenous rhBMP-7 inhibited the
transition from tubular epithelial cells to mesenchymalcells and
the expression of Col-III in vitro
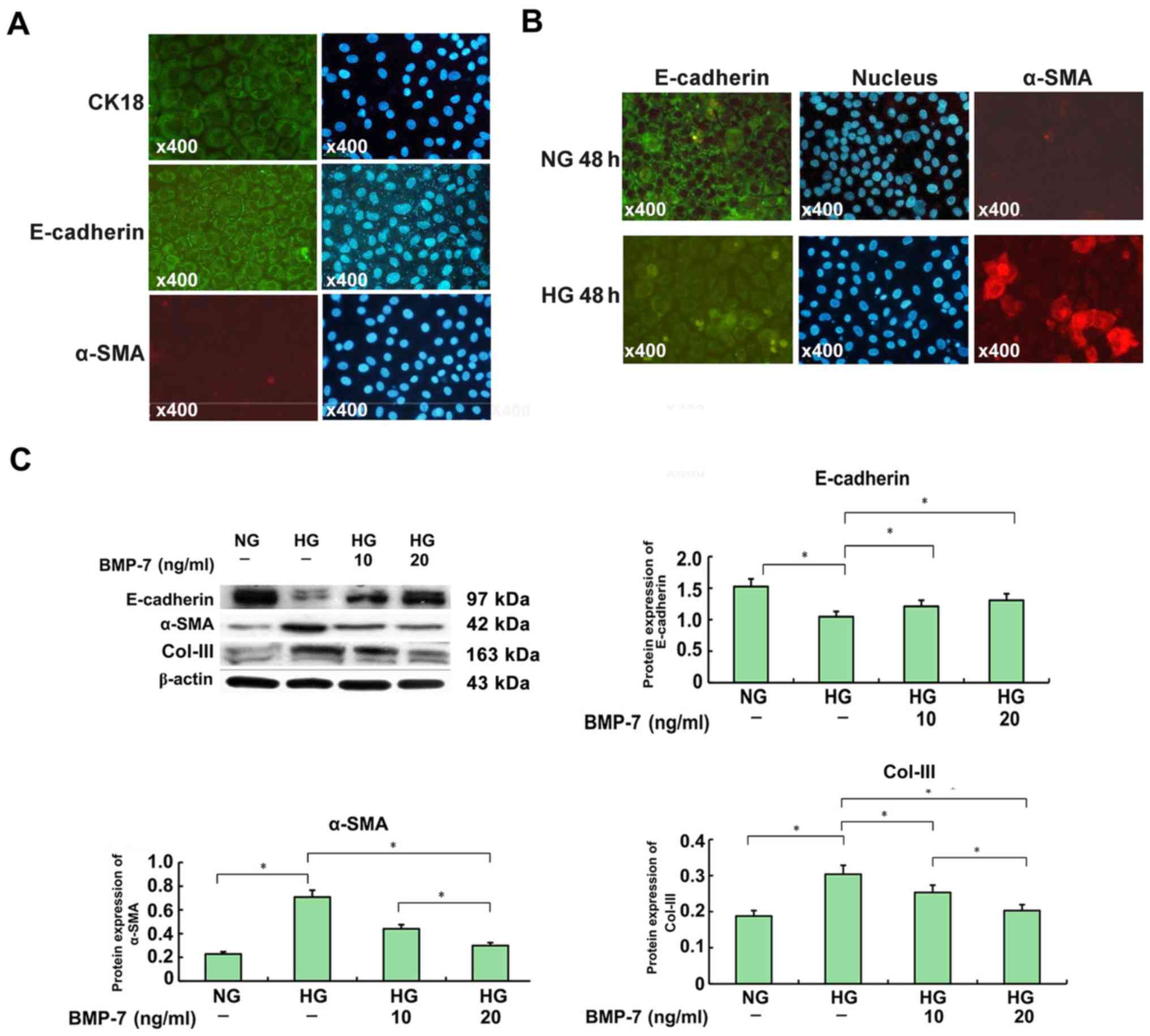
The immunofluorescence analysis showed that
E-cadherin and CK-18 predominantly localized in the cytomembrane of
NRK-52E cells. However, staining for α-SMA was hardly seen in
NRK-52E cells (Fig. 3A). These
evidences confirmed that the shape and growth of NRK-52E cells were
good. As shown in Fig. 3B,
E-cadherin progressively diminished in NRK-52E cells cultured in
high-glucose medium, when comparing with those cultured in
normal-glucose medium for 48 h. However, the α-SMA expression
increased significantly after high-glucose medium incubation.
Western blot analysis showed that renal fibrosis
occurred in the high-glucose group (Fig. 3C). This was demonstrated by a
significant upregulation of α-SMA and Col-III, and a remarkable
downregulation of E-cadherin in NRK-52E cells cultured in
high-glucose medium for 48-h. In contrast, these fibrotic changes
were largely attenuated in NRK-52E cells co-treated with exogenous
rhBMP-7. In high glucose conditions, treating NRK-52E cells with
rhBMP-7 suppressed the expression of both α-SMA and Col-III, and
restored the E-cadherin expression in a dose-dependent manner,
peaking at a dose of 20-ng/ml.
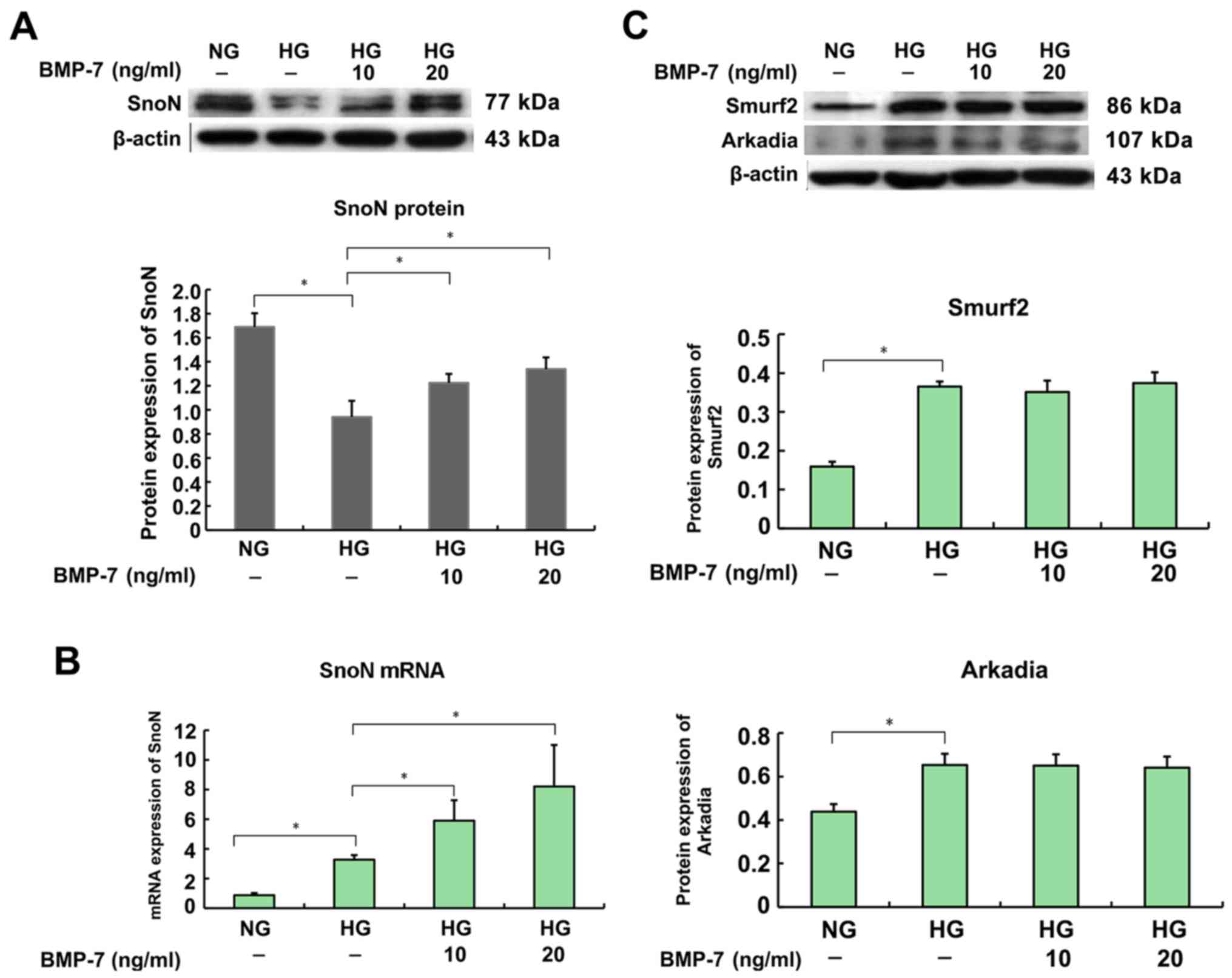
Exogenous rhBMP-7 upregulated the
expression of SnoN protein by promoting gene transcription
independent of Smurf2 and Arkadia
qRT-PCR demonstrated that the upregulation of SnoN
mRNA was inconsistent with the downregulation of SnoN protein as
revealed by the Western blot analysis in NRK-52E cells in
high-glucose medium when comparing with those in normal-glucose
medium (Fig. 4A and B). Moreover,
a significant increase in protein expression of Smurf2 and Arkadia
was seen in the cells exposed to high glucose, similar to that in
DM group (Fig. 4C). There results
from Western blot analysis demonstrated that rhBMP-7 induced SnoN
protein expression in a dose-dependent manner, peaking at 20-ng/ml
dose. In contrast, there was no effect on the expression of Smurf2
and Arkadia proteins in NRK-52E cells which were co-treated with or
without exogenous rhBMP-7 exposed to high glucose (Fig. 4C). Further studies by qRT-PCR
indicated that gene transcription of SnoN resulted in higher levels
of SnoN mRNA in NRK-52E cells co-treated with exogenous BMP-7 when
exposure to high glucose (Fig.
4B). All these results strongly suggested that BMP-7
upregulated the SnoN expression in NRK-52E cells.
Discussion
Numerous studies have suggested that the
TGF-β1/Smads signaling pathway activates Smad2/3, which
subsequently combine with Smad4 and translocate into the cell
nucleus, and finally control the transcription of TGF-β1 gene,
exerting its fibrotic effect on progression of DN (1,6).
Thus, how to inhibit or block the TGF-β1/Smads signaling pathway
has become the focus of recent research. SnoN was discovered early
as an oncoprotein, due to the induction of transdifferentiation in
chicken embryo fibroblasts (7).
The most important mechanism of SnoN is switching the function of
TGF-β1/Smads signal transduction pathways (6,7).
Recently, SnoN was knocked down by using siRNA following
high-glucose stimulation, which caused a significant reduction of
E-cadherin in primary RTECs, while the upregulation of fibronectin
(FN) and α-SMA could further increase RTECs fibrosis. On the
contrary, the overexpression of SnoN could reduce high
glucose-mediated RTECs fibrosis (1). So, the downregulation of SnoN protein
may be an important pathogenesis of DN renal fibrosis. In this
study, type 1 DM rat model was established by injecting STZ into
tail vein. Continuous DM status combined with significant
proteinuria and renal histological observation showed significant
renal tubulointerstitial fibrosis, were regarded as the model with
DN. The expression of SnoN and E-cadherin reduced in renal tissue
of DM group rats, while α-SMA and Col-III increased significantly
in renal tissue. This confirms that the reduction of SnoN proteins
could promote the development of DN, which is the same as the
preliminary findings of our study (1).
The present study found that the increase in the
mRNA level of SnoN was not consistent with the protein expression
in DM group. It was found that TGF-β1 upregulated the transcription
of SnoN by binding directly to sno promoter's SBE
(Smad-binding element) through the p-smad2/Smad4 complex. This
induced the expression of SnoN mRNA (13), which acted as a negative feedback
inhibitor for the TGF-β1/Smads pathway. Another research showed
that TGF-β1 activated the PI3K/AKT pathway and upregulated the
expression of SnoN mRNA (14).
Especially, TGF-β1 increased the SnoN transcription, strongly
inducing the expression of SnoN mRNA in cells and kidney tissues of
unilateral ureteral obstruction (UUO) rats (15). However, the level of protein was
not coupled with the expression of SnoN mRNA, as TGF-β1 protein led
to a reduction of SnoN rapidly and significantly. This decrease was
mainly connected with Arkadia and Smurf2, an E3 ubiquitin enzyme,
which specifically recognized SnoN and involved in activating
Smads-mediated ubiquitination and degradation of SnoN (15–17).
During DN, high expression of Smurf2 and Arkadia was observed in
renal tissue, resulting in the TGF-β1-mediated interaction of
Smad2, SnoN, and Smurf2, leading to the ubiquitin degradation of
SnoN protein (17,18). However, TGF-β1-activated Smad3
interacted with Arkadia, inducing the degradation of SnoN and
enhancing biological effects of p-smad3 (12,17,19).
Thus, the expression of SnoN protein facilitates the balance of
gene expression and degradation of ubiquitin: The protein level of
SnoN, due to degradation by the ubiquitin-proteasome system,
reverses the promotion of TGF-β1/Smads pathway on SnoN
transcriptional activation in renal disease processes, which
induces fibrosis cascade effects that enhance the DN fibrosis,
similar to the report by Fukasawa et al in UUO (15).
BMP-7 has been proved to be anti-fibrotic as it
counteracts the TGF-β1/Smads pathway in many fibrotic diseases
including pulmonary fibrosis (20), liver fibrosis (21), and renal fibrosis (3–5,22).
However, in a variety of fibrotic diseases, BMP-7 protein
downregulation prompts the development of fibrosis. The present
study showed that the expression of BMP-7 reduced gradually with
the progression of DN. Transfection of recombinant BMP-7 with
adenoviral gene or direct addition of rhBMP-7 into RTECs could
reduce or counteract the occurrence of EMT and the synthesis of ECM
induced by TGF-β1 (9,10,23).
However, the mechanism of BMP-7 inhibiting the TGF-β1/Smad
signaling pathway is not clear, only few studies explained the
mechanism that BMP-7 does not affect the synthesis of TGF-β1
induced by high glucose in RTECs (24). And Luo et al found that
SnoN, a negative regulator protein of the TGF-β1 signaling pathway,
may be involved in the mechanism, and when SnoN was knocked down,
the effect of BMP-7 inhibiting the TGF-β1/Smad signaling pathway
was weakened (4). But the specific
patterns and mechanisms were not clear. So, in this study, the
tubular epithelial cells were cultivated in vitro (NRK-52E
cells) in high glucose with various doses of rhBMP-7 (10- and
20-ng/ml) simultaneously, and the effects of rhBMP-7 were observed
on the transcription and protein levels of SnoN and the expression
of E3 ubiquitin ligases, Smurf2 and Arkadia, to clarify the
influence and mechanism of BMP-7 on the expression of SnoN.
BMP-7 has a variety of biological effects, and the
doses of rhBMP-7 that are applied for treating fibrotic diseases
range from 5- to 400-ng/ml. In the present study, 10- and 20-ng/ml
doses of rhBMP-7 were added to high glucose-cultured RTECs to
observe whether the fibrosis effect is being delayed. The results
showed that rhBMP-7 depressed the phenotype transition of RTECs and
synthesis of Col-III in a dose-dependent manner. The results
suggested that 10- and 20-ng/ml rhBMP-7 have therapeutic
implications. Moreover, although the high dose of glucose (in the
high-glucose group) increased the expression of SnoN mRNA and
Smurf2 and Arkadia proteins, exogenous rhBMP-7 (in the rhBMP-7 plus
high-glucose group) further enhanced the level of SnoN mRNA
significantly in NRK-52E cells under high glucose. And no
significant difference was found between the expression of Smurf2
and Arkadia in the rhBMP-7 plus high-glucose group compared with
the high-glucose group. In other words, BMP-7 could not inhibit the
expression of Smurf2 and Arkadia, but upregulated SnoN at the
transcriptional level significantly and even reversed part of the
degraded protein, eventually restoring the expression of SnoN
protein to block the TGF-β1/Smads signal pathway.
In conclusion, the results showed that BMP-7
increased the expression of SnoN protein to inhibit the
TGF-β1/Smads signaling pathway and delay the process of DN. And the
effect of BMP-7 in upregulating the expression of SnoN protein is
not mitigating E3 ubiquitin ligase protein-induced SnoN protein
degradation, but enhancing the SnoN mRNA expression. The mechanism
of BMP-7 regulating the expression of SnoN still requires further
study.
Acknowledgements
This research is funded by the National Natural
Science Foundation of China (no. 81160094 and 81541102), the
Guizhou Province Science and Technology Foundation [no. Guizhou
Branch J (2011) 2120], and the Guiyang medical school graduate
student education innovation plan special funds (contract no.
B201103). We thank Professor Limin Lu of Fudan University for
kindly gifting the NRK-52E cells.
References
|
1
|
Liu R, Wang Y, Xiao Y, Shi M, Zhang G and
Guo B: SnoN as a key regulator of the high glucose-induced
epithelial-mesenchymal transition in cells of the proximal tubule.
Kidney Blood Press Res. 35:517–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang YY, Liu RX, Guo B, Xiao Y, Shi MJ, Pi
MJ, Wen QY and Zhang GZ: Down-regulation of PTEN expression in
kidney and its role in development of diabetic nephropathy in rats.
Sheng Li Xue Bao. 63:325–332. 2011.(In Chinese). PubMed/NCBI
|
|
3
|
Wang Z, Zhao J, Zhang J, Wei J, Zhang J
and Huang Y: Protective effect of BMP-7 against aristolochic
acid-induced renal tubular epithelial cell injury. Toxicol Lett.
198:348–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo DD, Phillips A and Fraser D: Bone
morphogenetic protein-7 inhibits proximal tubular epithelial cell
Smad3 signaling via increased SnoN expression. Am J Pathol.
176:1139–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, de Caestecker M, Kopp J, Mitu G,
Lapage J and Hirschberg R: Renal bone morphogenetic protein-7
protects against diabetic nephropathy. J Am Soc Nephrol.
17:2504–2512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
López-Hernández FJ and López-Novoa JM:
Role of TGF-β in chronic kidney disease: An integration of tubular,
glomerular and vascular effects. Cell Tissue Res. 347:141–154.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deheuninck J and Luo K: Ski and SnoN,
potent negative regulators of TGF-beta signaling. Cell Res.
19:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Yu N, Zhang XL, Chen XQ and Tang
LQ: Regulatory effect of berberine on unbalanced expressions of
renal tissue TGF-beta1/SnoN and smad signaling pathway in rats with
early diabetic nephropathy. Zhongguo Zhong Yao Za Zhi.
37:3604–3610. 2012.(In Chinese). PubMed/NCBI
|
|
9
|
Xu YF, Wan JX and Jiang DW: Effects of
bone morphogenic protein-7 on transdifferentiation and the
expression of connective tissue growth factor of human renal
tubular epithelial cells induced by transforming growth
factor-beta1. Zhonghua Yi Xue Za Zhi. 89:1639–1644. 2009.(In
Chinese). PubMed/NCBI
|
|
10
|
Xu Y, Wan J, Jiang D and Wu X: BMP-7
counteracts TGF-beta1-induced epithelial-to-mesenchymal transition
in human renal proximal tubular epithelial cells. J Nephrol.
22:403–410. 2009.PubMed/NCBI
|
|
11
|
Jahchan NS and Luo K: SnoN in mammalian
development, function and diseases. Curr Opin Pharmacol.
10:670–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan R, Zhang J, Tan X, Zhang X, Yang J and
Liu Y: Downregulation of SnoN expression in obstructive nephropathy
is mediated by an enhanced ubiquitin-dependent degradation. J Am
Soc Nephrol. 17:2781–2791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Q, Pearson-White S and Luo K:
Requirement for the SnoN oncoprotein in transforming growth factor
beta-induced oncogenic transformation of fibroblast cells. Mol Cell
Biol. 25:10731–10744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nanjundan M, Cheng KW, Zhang F, Lahad J,
Kuo WL, Schmandt R, Smith-McCune K, Fishman D, Gray JW and Mills
GB: Overexpression of SnoN/SkiL, amplified at the 3q26.2 locus, in
ovarian cancers: A role in ovarian pathogenesis. Mol Oncol.
2:164–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukasawa H, Yamamoto T, Togawa A, Ohashi
N, Fujigaki Y, Oda T, Uchida C, Kitagawa K, Hattori T, Suzuki S, et
al: Ubiquitin-dependent degradation of SnoN and Ski is increased in
renal fibrosis induced by obstructive injury. Kidney Int.
69:1733–1740. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyazono K and Koinuma D: Arkadia-beyond
the TGF-β pathway. J Biochem. 149:1–3. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakairi T, Hiromura K, Takahashi S,
Hamatani H, Takeuchi S, Tomioka M, Maeshima A, Kuroiwa T and Nojima
Y: Effects of proteasome inhibitors on rat renal fibrosis in vitro
and in vivo. Nephrology (Carlton). 16:76–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonni S, Wang HR, Causing CG, Kavsak P,
Stroschein SL, Luo K and Wrana JL: TGF-beta induces assembly of a
Smad2-Smurf2 ubiquitin ligase complex that targets SnoN for
degradation. Nat Cell Biol. 3:587–595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagano Y, Mavrakis KJ, Lee KL, Fujii T,
Koinuma D, Sase H, Yuki K, Isogaya K, Saitoh M, Imamura T, et al:
Arkadia induces degradation of SnoN and c-Ski to enhance
transforming growth factor-beta signaling. J Biol Chem.
282:20492–20501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang G, Zhu Z, Wang Y, Gao A, Niu P and
Tian L: Bone morphogenetic protein-7 inhibits silica-induced
pulmonary fibrosis in rats. Toxicol Lett. 220:103–108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang SL, Yang CQ, Qi XL, Yuan M, Chang YZ,
Yang L and Gao HJ: Inhibitory effect of bone morphogenetic
protein-7 on hepatic fibrosis in rats. Int J Clin Exp Pathol.
6:897–903. 2013.PubMed/NCBI
|
|
22
|
Zeisberg M, Bottiglio C, Kumar N, Maeshima
Y, Strutz F, Muller GA and Kalluri R: Bone morphogenic protein-7
inhibits progression of chronic renal fibrosis associated with two
genetic mouse models. Am J Physiol Renal Physiol. 285:F1060–F1067.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang SW, Wang W, Xu ZC and Zhu YY:
Construction of recombinant adenovirus containing BMP-7 gene and
its expression in proximal tubule epithelial cells. Zhejiang Da Xue
Xue Bao Yi Xue Ban. 39:71–78. 2010.(In Chinese). PubMed/NCBI
|
|
24
|
Wang SN, Lapage J and Hirschberg R: Loss
of tubular bone morphogenetic protein-7 in diabetic nephropathy. J
Am Soc Nephrol. 12:2392–2399. 2001.PubMed/NCBI
|